Synergistic Ability of Chitosan and Trichoderma harzianum to Control the Growth and Discolouration of Common Sapstain Fungi of Pinus radiata
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fungal Cultures and Wood Material
2.2. Wood Treatment and Infection
2.3. Assessments and Statistical Analysis
3. Results
3.1. Observation of Fungal Growth on the Surface of Wafers
3.1.1. Sphaeropsis sapinea
3.1.2. Leptographium procerum
3.1.3. Ophiostoma piceae
3.2. Microscopic Observations of Sapstain Penetration into Wafers
3.2.1. Sphaeropsis sapinea
3.2.2. Leptographium procerum
3.2.3. Ophiostoma piceae
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCarthy, J.K.; Brockerhoff, E.G.; Didham, R.K. An Experimental Test of Insect-Mediated Colonisation of Damaged Pinus radiata Trees by Sapstain Fungi. PLoS ONE 2013, 8, e55692. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, J.M.; Farrell, R.L.; Duncan, S.M.; Reay, S.D.; Blanchette, R.A.; Hadar, E.; Hadar, Y.; Harrington, T.C.; McNew, D. Survey of potential sapstain fungi on pinus radiata in New Zealand. N. Z. J. Bot. 2005, 43, 653–663. [Google Scholar] [CrossRef]
- Horgan, G. Cost–benefits of antisapstain treatment of New Zealand radiata pine for present and future markets. In Proceedings of the 2nd New Zealand Sapstain Symposium, Rotorua, New Zealand, 18–19 November 1999. [Google Scholar]
- Chittenden, C.; Singh, T. In vitro evaluation of combination of Trichoderma harzianum and chitosan for the control of sapstain fungi. Biol. Control 2009, 50, 262–266. [Google Scholar] [CrossRef]
- Hernandez, V.A.; Galleguillos, F.; Robinson, S. Fungal pigments from spalting fungi attenuating blue stain in Pinus spp. Int. Biodeter. Biodegr. 2016, 107, 154–157. [Google Scholar] [CrossRef]
- Bihon, W.; Slippers, B.; Burgess, T.; Wingfield, M.J.; Wingfield, B.D. Sources of Diplodia pinea endophytic infections in Pinus patula and P. radiata seedlings in South Africa. For. Pathol. 2011, 41, 370–375. [Google Scholar] [CrossRef]
- Kim, J.-J.; Ra, J.-B.; Kim, H.-J.; Kim, G.-H. Sapstain and mold control on radiata pine lumber: Laboratory and field tests of selected fungicides. Mycobiology 2002, 30, 37–40. [Google Scholar] [CrossRef]
- Singh, T.; Singh, A.P. A review on natural products as wood protectant. Wood Sci. Technol. 2012, 46, 851–870. [Google Scholar] [CrossRef]
- Benko, R.; Highley, T.L. Selection of media for screening interaction of wood-attacking fungi and antagonistic bacteria. Mater. Org. 1990, 25, 173–180. [Google Scholar]
- Gradinger, C.; Boisselet, T.; Stratev, D.; Ters, T.; Messner, K.; Fackler, K. Biological control of sapstain fungi: From laboratory experiments to field trials. Holzforschung 2009, 63, 751–759. [Google Scholar] [CrossRef]
- O’Callahan, D.R.; Singh, T.; McDonald, I.R. Evaluation of lactic acid bacterium from chilli waste as a potential antifungal agent for wood products. J. Appl. Microbiol. 2012, 112, 436–442. [Google Scholar] [CrossRef]
- Singh, T.; Chittenden, C. In-vitro antifungal activity of chilli extracts in combination with Lactobacillus casei against common sapstain fungi. Int. Biodeterior. Biodegrad. 2008, 62, 364–367. [Google Scholar] [CrossRef]
- Yang, D.Q.; Rossignol, L. Evaluation of Gliocladium roseum against wood-degrading fungi in vitro and on major Canadian wood species. Biocontrol Sci. Technol. 1999, 9, 409–420. [Google Scholar] [CrossRef]
- Byrne, A. Chemical control of biological stain: Past, present and future. In Proceedings of the Biology and Prevention of Sapstain; Forest Products Society: Madison, WI, USA, 1998; pp. 63–69. [Google Scholar]
- Ribera, J.; Fink, S.; Bas, M.D.C.; Schwarze, F.W.M.R. Integrated control of wood destroying basidiomycetes combining Cu-based wood preservatives and Trichoderma spp. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Vanneste, J.L.; Hill, R.A.; Kay, S.J.; Farrell, R.L.; Holland, P.T. Biological control of sapstain fungi with natural products and biological control agents: A review of the work carried out in New Zealand. Mycol. Res. 2002, 106, 228–232. [Google Scholar] [CrossRef]
- Held, B.W.; Thwaites, J.M.; Farrell, R.L.; Blanchette, R.A. Albino strains of Ophiostoma species for biological control of sapstaining fungi. Holzforschung 2003, 57, 237–242. [Google Scholar] [CrossRef]
- Yang, D.Q. Integrated Method for Protecting Logs and Green Lumber from Sapstain and Mold. U.S. Patent 6083537, 4 July 2000. [Google Scholar]
- Hussain, I.; Chittenden, C.; Singh, T. Scoping antifungal activities of various forms of chitosan oligomers and their potential fixation in wood. J. Chem. Chem. Eng. 2013, 7, 1175. [Google Scholar]
- Singh, T.; Vesentini, D.; Singh, A.P.; Daniel, G. Effect of chitosan on physiological, morphological, and ultrastructural characteristics of wood-degrading fungi. Int. Biodeterior. Biodegrad. 2008, 62, 116–124. [Google Scholar] [CrossRef]
- Xuan Du, D.; Xuan Vuong, B. Study on Preparation of Water-Soluble Chitosan with Varying Molecular Weights and Its Antioxidant Activity. Adv. Mater. Sci. Eng. 2019, 2019, 8781013. [Google Scholar] [CrossRef]
- Vårum, K.M.; Ottøy, M.H.; Smidsrød, O. Water-solubility of partially N-acetylated chitosans as a function of pH: Effect of chemical composition and depolymerisation. Carbohydr. Polym. 1994, 25, 65–70. [Google Scholar] [CrossRef]
- Minh, N.C.; Nguyen, V.H.; Schwarz, S.; Stevens, W.F.; Trung, T.S. Preparation of water soluble hydrochloric chitosan from low molecular weight chitosan in the solid state. Int. J. Biol. Macromol. 2019, 121, 718–726. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, T.; Chittenden, C. Preparation of chitosan oligomers and characterization: Their antifungal activities and decay resistance. Holzforschung. 2012, 66, 119–125. [Google Scholar] [CrossRef]
- Liu, J.; Morrell, J. The Role of Chitinase in Bioprotectant Activity against Wood Staining Fungi; The International Research Group on Wood Preservation: Stockholm, Sweden, 1996; p. 11. [Google Scholar]
- Messner, K.; Fleck, V.; Buergel, J. Strains of the Trichoderma Fungus, Fungicide Derived Therefrom and Process for Using the Same. International Application WO 9308694, 13 May 1993. [Google Scholar]
- Roco, A.; Pérez, L.M. In vitro biocontrol activity of Trichoderma harzianum on Alternaria alternata in the presence of growth regulators. Electron. J. Biotechnol. 2001, 4, 68–73. [Google Scholar]
- Uzunovic, A.; O’Callahan, D.; Kreber, B. Mechanical tree havesters spread fungal inoculum onto freshly felled Canadian and New Zealand pine logs. For. Prod. J. 2004, 54, 34–40. [Google Scholar]
- Harrington, T. Cycloheximide sensitivity as a taxonomic character in Ceratocystis. Mycologia 1981, 73, 1123–1129. [Google Scholar] [CrossRef]
- Xiao, Y.; Morrell, J.; Ciuffetti, L. Effects of Prior Establishment of Trichoderma Harzianum on Ophiostoma Picea Growth in Freshly Sawn Douglas Fir Sapwood; International Research Group on Wood Preservation: Brisbane, Australia, 2003; p. 03-10476. [Google Scholar]
- Vesentini, D.; Steward, D.; Singh, A.P.; Ball, R.; Daniel, G.; Franich, R. Chitosan-mediated changes in cell wall composition, morphology and ultrastructure in two wood-inhabiting fungi. Mycol. Res. 2007, 111, 875–890. [Google Scholar] [CrossRef]
- Witkowska, D.; Maj, A. Production of lytic enzymes by Trichoderma spp. and their effect on the growth of phytopathogenic fungi. Folia Microbiol. 2002, 47, 279–282. [Google Scholar] [CrossRef]
- Takashi, K.; Masato, I. Agent for Controlling Black Spot of Pear. Japanese Patent 62198604, 14 September 1987. [Google Scholar]
- Silva-Castro, I.; Casados-Sanz, M.; Alonso-Cortés, A.L.; Martín-Ramos, P.; Martín-Gil, J.; Acuña-Rello, L. Chitosan-based coatings to prevent the decay of Populus spp. Wood caused by trametes versicolor. Coatings 2018, 8, 415. [Google Scholar] [CrossRef]
- da Silva, J.A.T.; de Medeiros, E.V.; da Silva, J.M.; Tenório, D.A.; Moreira, K.A.; da Silva Nascimento, T.C.E.; Souza-Motta, C. Antagonistic activity of Trichoderma spp. against Scytalidium lignicola CMM 1098 and antioxidant enzymatic activity in cassava. Phytoparasitica 2017, 45, 219–225. [Google Scholar] [CrossRef]
- Mazrou, Y.S.A.; Makhlouf, A.H.; Elseehy, M.M.; Awad, M.F.; Hassan, M.M. Antagonistic activity and molecular characterization of biological control agent Trichoderma harzianum from Saudi Arabia. Egypt. J. Biol. Pest Control 2020, 30. [Google Scholar] [CrossRef]
- Schubert, M.; Fink, S.; Schwarze, F.W.M.R. Evaluation of Trichoderma spp. as a biocontrol agent against wood decay fungi in urban trees. Biol. Control 2008, 45, 111–123. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R.; Yu, J.; Saravanakumar, K.; Chen, J. Antagonistic and Biocontrol Potential of Trichoderma asperellum ZJSX5003 against the Maize Stalk Rot Pathogen Fusarium graminearum. Indian J. Microbiol. 2016, 56, 318–327. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Dennis, C.; Webster, J. Antagonistic properties of species groups of Trichoderma I: Production of non-volatile antibiotics. J. Agric. Food Chem. 1971, 20, 437–438. [Google Scholar]
- Dotson, B.R.; Soltan, D.; Schmidt, J.; Areskoug, M.; Rabe, K.; Swart, C.; Widell, S.; Rasmusson, A.G. The antibiotic peptaibol alamethicin from Trichoderma permeabilises Arabidopsis root apical meristem and epidermis but is antagonised by cellulase-induced resistance to alamethicin. BMC Plant Biol. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Kong, S. Effects of Trichoderma asperellum and its siderophores on endogenous auxin in Arabidopsis thaliana under iron-deficiency stress. Int. Microbiol. 2020, 23, 501–509. [Google Scholar] [CrossRef]
- Humphris, S.N.; Wheatley, R.E.; Bruce, A. The effects of specific volatile organic compounds produced by Trichoderma spp. on the growth of wood decay basidiomycetes. Holzforschung 2001, 55, 233–237. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K. Beneficial effects of Trichoderma secondary metabolites on crops. Phytother. Res. 2020, 34, 2835–2842. [Google Scholar] [CrossRef]
- Kredics, L.; Antal, Z.; Szekeres, A.; Hatvani, L.; Manczinger, L.; Vágvölgyi, C.; Nagy, E. Extracellular proteases of Trichoderma species: A review. Acta Microbiol. Immunol. Hung. 2005, 52, 169–184. [Google Scholar] [CrossRef]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of microbial enzymes in food industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef]
- Eagen, R.; Brisson, A.; Breuil, C. The sap-staining fungus Ophiostoma piceae synthesizes different types of melanin in different growth media. Can. J. Microbiol. 1997, 43, 592–595. [Google Scholar] [CrossRef]
- Jankowiak, R.; Bilański, P.; Chyrzyński, Ł.; Strzałka, B. Identification of sapstain fungi from Scots pine pallets and assessment of their staining ability. Eur. J. Plant Pathol. 2018, 150, 307–322. [Google Scholar] [CrossRef]
- Sajitha, K.L.; Dev, S.A.; Maria Florence, E.J. Biocontrol potential of Bacillus subtilis B1 against sapstain fungus in rubber wood. Eur. J. Plant Pathol. 2018, 150, 237–244. [Google Scholar] [CrossRef]


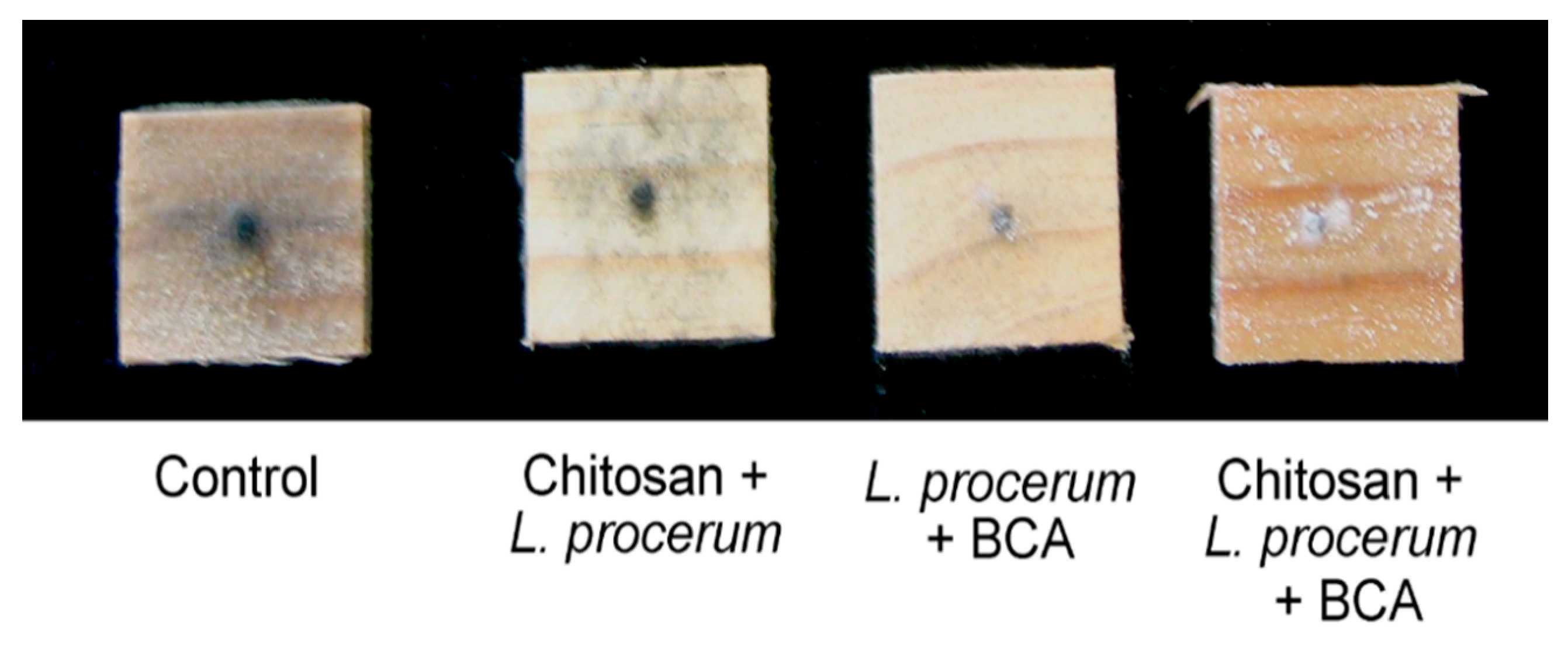
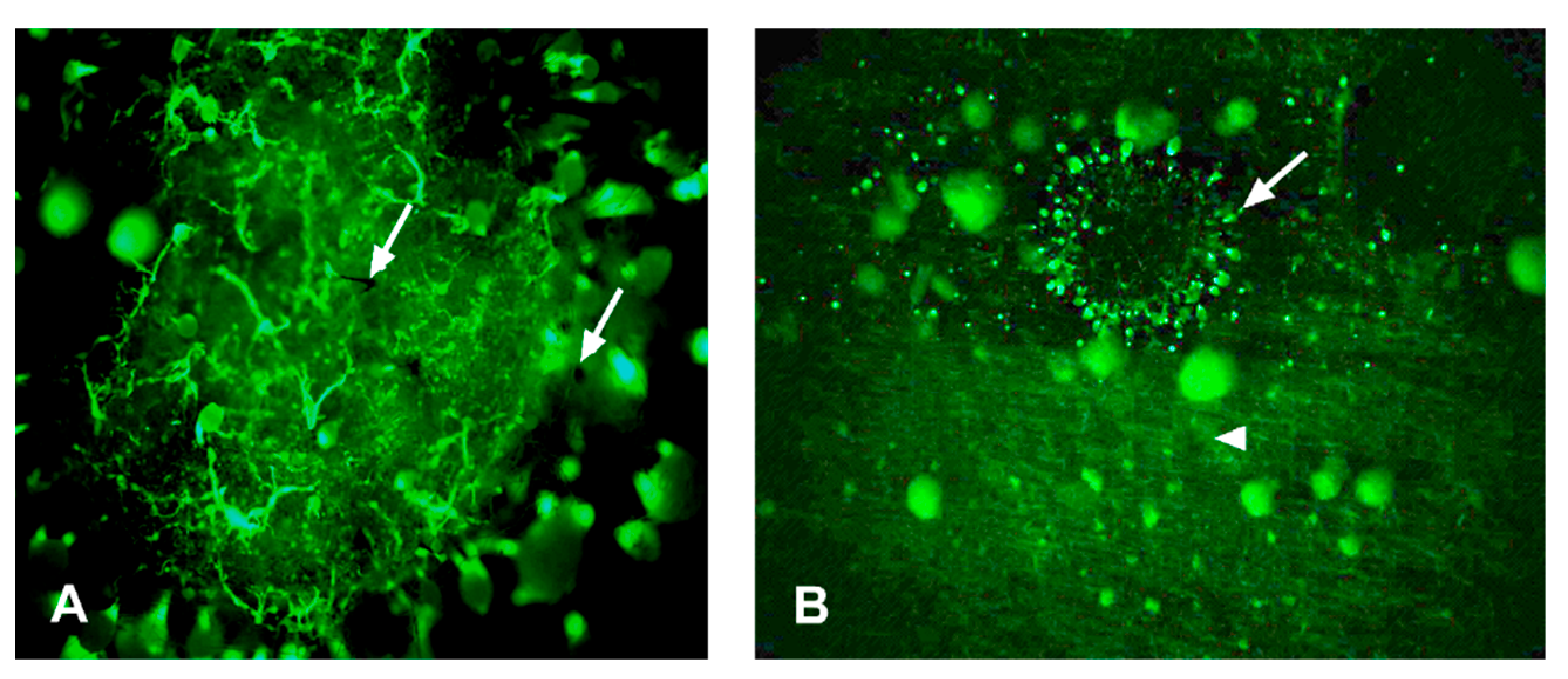
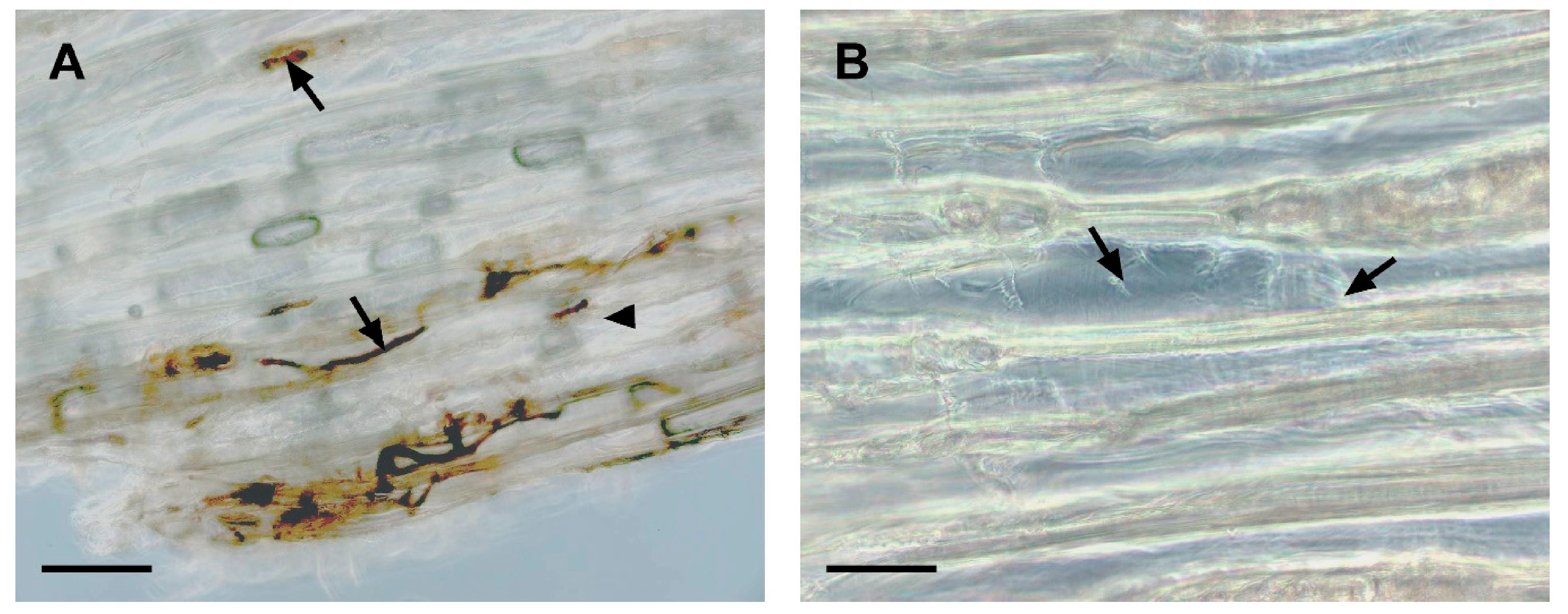
| Rating | Surface Cover (%) |
|---|---|
| 0 | 0 (no growth) |
| 1 | 1–5 |
| 2 | 6–25 |
| 3 | 26–50 |
| 4 | 51–75 |
| 5 | 76–100 |
| Treatments | S. sapinea | L. procerum | O. picea |
|---|---|---|---|
| Control | >270 a* | >270 a | >270 a |
| Challenge fungus + BCA | >270 ab | Up to 270 ab | Up to 180 de |
| Chitosan + Challenge fungus | >270 a | >270 ab | Up to 90 gh |
| Chitosan + Challenge fungus + BCA | Up to 90 gh | 0 i | 0 i |
| Challenge fungus + BCA1 | Up to 270 b | Up to 270 b | >270 ab |
| Chitosan + Challenge fungus + BCA1 | Up to 180 de | 0 hi | 0 i |
| Challenge fungus + BCA2 | >270 a | >270 a | >270 a |
| Chitosan + Challenge fungus + BCA2 | Up to 180 d | Up to 90 fg | 0 i |
| Challenge fungus + BCA3 | >270 a | >270 a | >270 a |
| Chitosan + Challenge fungus + BCA3 | Up to 270 c | Up to 90 ef | 0 hi |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, T.; Chittenden, C. Synergistic Ability of Chitosan and Trichoderma harzianum to Control the Growth and Discolouration of Common Sapstain Fungi of Pinus radiata. Forests 2021, 12, 542. https://doi.org/10.3390/f12050542
Singh T, Chittenden C. Synergistic Ability of Chitosan and Trichoderma harzianum to Control the Growth and Discolouration of Common Sapstain Fungi of Pinus radiata. Forests. 2021; 12(5):542. https://doi.org/10.3390/f12050542
Chicago/Turabian StyleSingh, Tripti, and Colleen Chittenden. 2021. "Synergistic Ability of Chitosan and Trichoderma harzianum to Control the Growth and Discolouration of Common Sapstain Fungi of Pinus radiata" Forests 12, no. 5: 542. https://doi.org/10.3390/f12050542
APA StyleSingh, T., & Chittenden, C. (2021). Synergistic Ability of Chitosan and Trichoderma harzianum to Control the Growth and Discolouration of Common Sapstain Fungi of Pinus radiata. Forests, 12(5), 542. https://doi.org/10.3390/f12050542







