Prediction of Natural Volatile Organic Compounds Emitted by Bamboo Groves in Urban Forests
Abstract
1. Introduction
2. Materials and Methods
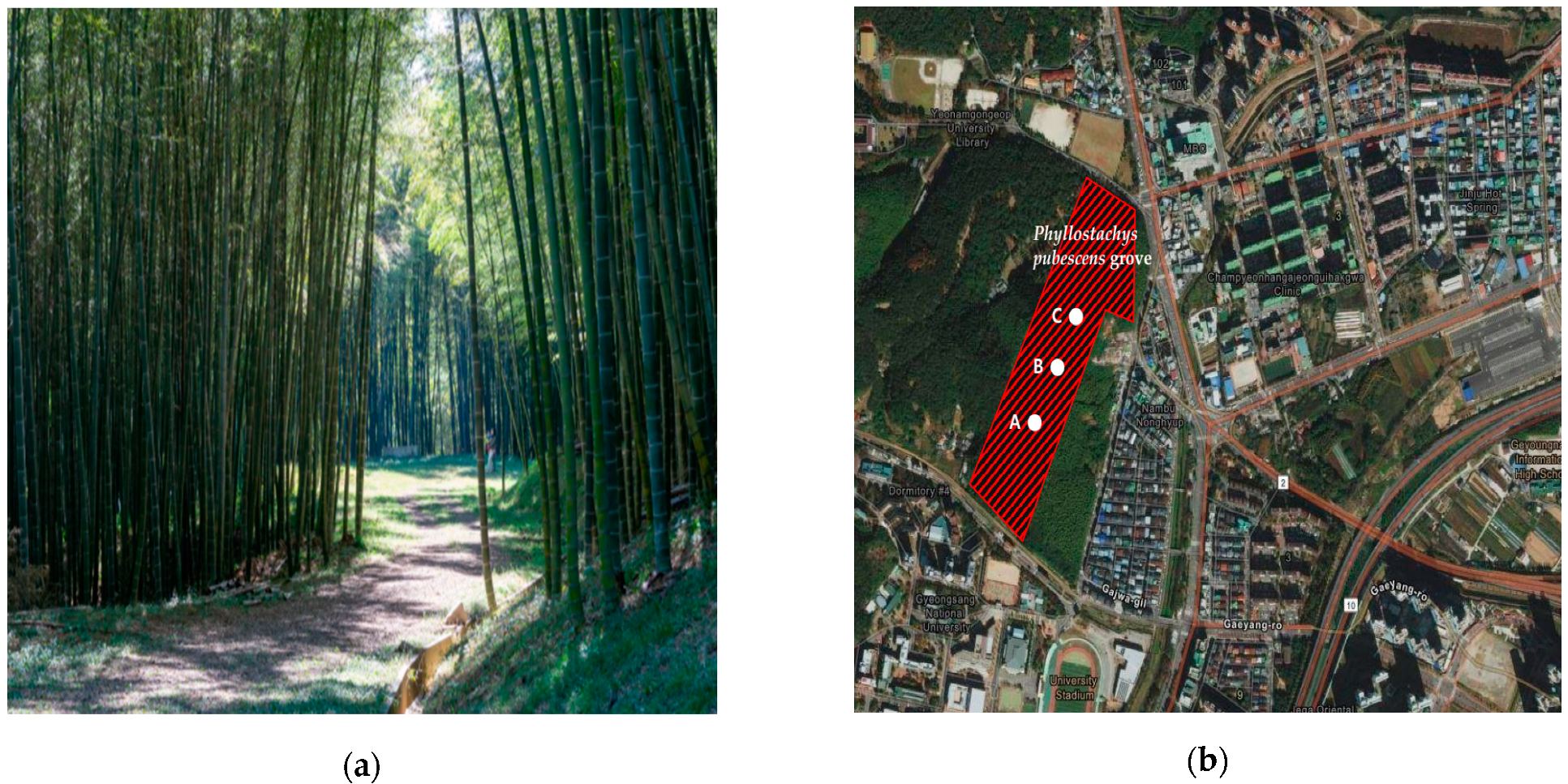
2.1. Study Site
2.2. Measurement Methods
2.2.1. NVOCs (Natural Volatile Organic Compounds)
2.2.2. Microclimate Environment
2.2.3. Calibration Curve
2.3. Analysis Methods
3. Results
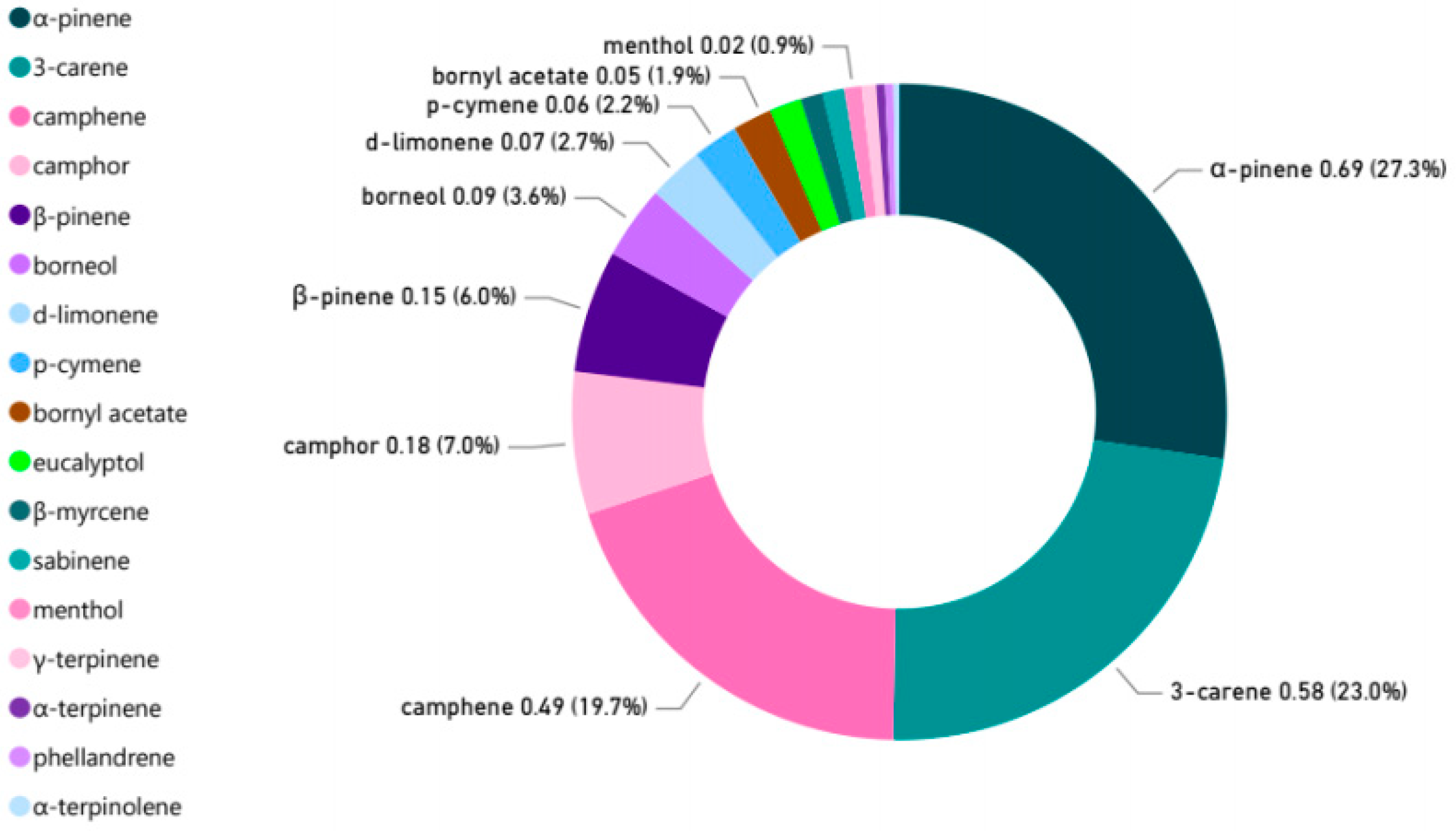
3.1. Characteristics of NVOCs at Bamboo Groves
3.2. The Variations of NVOC Concentration
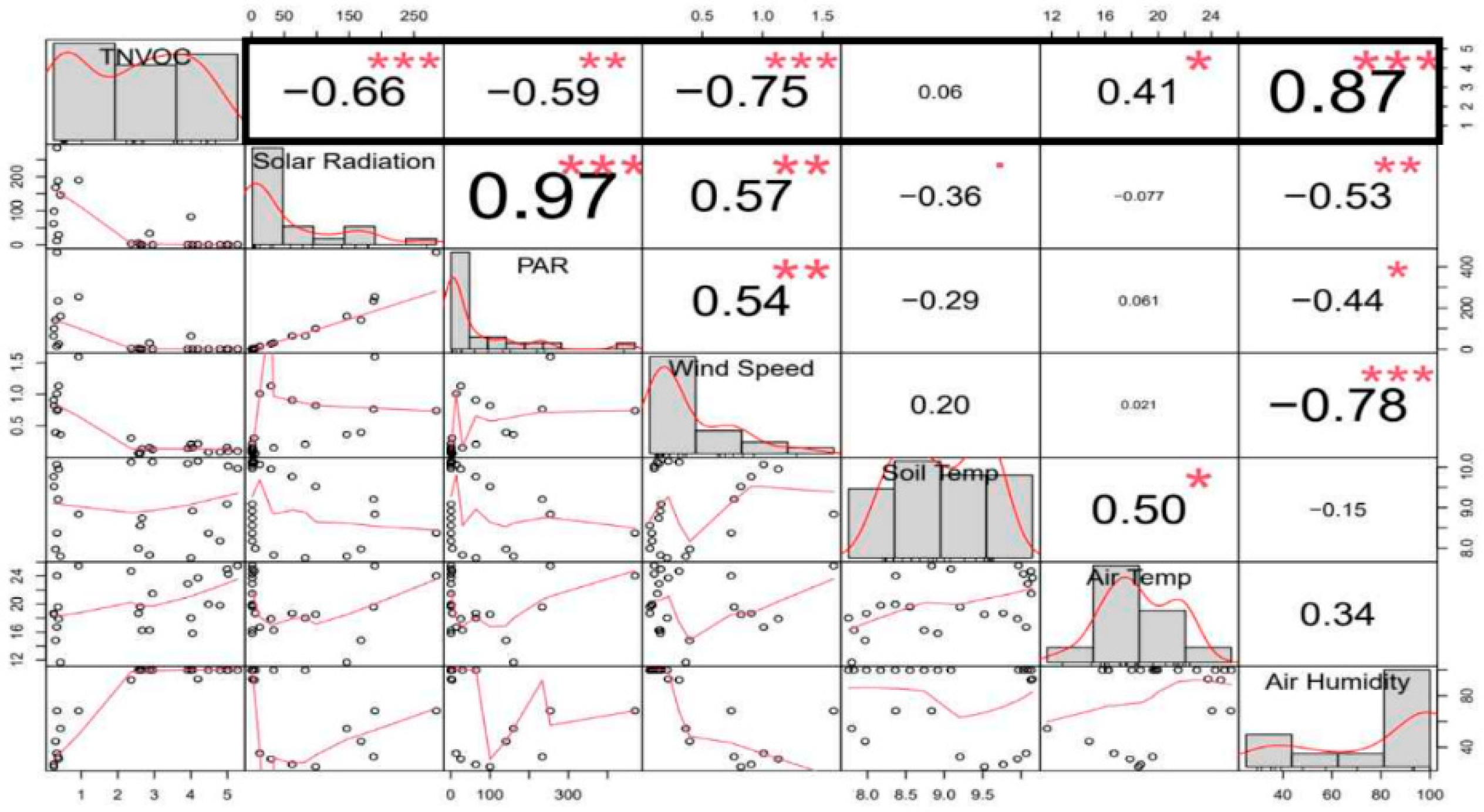
3.3. NVOCs and Physical Environments of Bamboo Groves
(Air Temperature) + 0.029 × (Air Humidity)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaplan, S. The Restorative Benefits of Nature: Toward an Integrative Framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Hartig, T.; van den Berg, A.E.; Hagerhall, C.M.; Tomalak, M.; Bauer, N.; Hansmann, R.; Ojala, A.; Syngollitou, E.; Carrus, G.; van Herzele, A. Health Benefits of Nature Experience: Psychological, Social and Cultural Processes. In Forests, Trees and Human Health; Springer: Berlin/Heidelberg, Germany, 2011; pp. 127–168. [Google Scholar]
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Gostin, L.O.; Wiley, L.F. Governmental Public Health Powers during the COVID-19 Pandemic: Stay-at-Home Orders, Business Closures, and Travel Restrictions. JAMA 2020, 323, 2137–2138. [Google Scholar] [CrossRef]
- Geng, D.C.; Innes, J.; Wu, W.; Wang, G. Impacts of COVID-19 Pandemic on Urban Park Visitation: A Global Analysis. J. For. Res. 2021, 32, 553–567. [Google Scholar] [CrossRef]
- Derks, J.; Giessen, L.; Winkel, G. COVID-19-Induced Visitor Boom Reveals the Importance of Forests as Critical Infrastructure. For. Policy Econ. 2020, 118, 102253. [Google Scholar] [CrossRef]
- KIM, G.; PARK, B.-J.; KOGA, S. Development of a Prediction Model for NVOC Concentration with Changing Microclimate in Camellia Japonica Temple Forest. Kyushu Univ. 2021, 66, 105–113. [Google Scholar]
- Tull, M.T.; Edmonds, K.A.; Scamaldo, K.M.; Richmond, J.R.; Rose, J.P.; Gratz, K.L. Psychological Outcomes Associated with Stay-at-Home Orders and the Perceived Impact of COVID-19 on Daily Life. Psychiatry Res. 2020, 289, 113098. [Google Scholar] [CrossRef]
- Ha, K.; Shin, W. Changes of the Forest Therapy Paradigm in the Post-Corona Era: Focusing on Analysis of News Search Words Related to Forest Therapy and COVID-19. J. Tour. Manag. Res. 2021, 25, 611–637. [Google Scholar]
- Fong, K.C.; Hart, J.E.; James, P. A Review of Epidemiologic Studies on Greenness and Health: Updated Literature through 2017. Curr. Environ. Health Rep. 2018, 5, 77–87. [Google Scholar] [CrossRef]
- Ulrich, R.S. Natural versus Urban Scenes: Some Psychophysiological Effects. Environ. Behav. 1981, 13, 523–556. [Google Scholar] [CrossRef]
- James, P.; Banay, R.F.; Hart, J.E.; Laden, F. A Review of the Health Benefits of Greenness. Curr. Epidemiol. Rep. 2015, 2, 131–142. [Google Scholar] [CrossRef]
- Hug, S.-M.; Hartig, T.; Hansmann, R.; Seeland, K.; Hornung, R. Restorative Qualities of Indoor and Outdoor Exercise Settings as Predictors of Exercise Frequency. Health Place 2009, 15, 971–980. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest Volatile Organic Compounds and Their Effects on Human Health: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2020, 17, 6506. [Google Scholar] [CrossRef]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Park, B.J. Effect of Phytoncide from Trees on Human Natural Killer Cell Function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef]
- Abe, T.; Hisama, M.; Tanimoto, S.; Shibayama, H.; Mihara, Y.; Nomura, M. Antioxidant Effects and Antimicrobial Activites of Phytoncide. Biocontrol. Sci. 2008, 13, 23–27. [Google Scholar] [CrossRef]
- Nam, E.-S.; Uhm, D.-C. Effects of Phytoncides Inhalation on Serum Cortisol Level and Life Stress of College Students. Korean J. Adult Nurs. 2008, 20, 697–706. [Google Scholar]
- Kawakami, K.; Kawamoto, M.; Nomura, M.; Otani, H.; Nabika, T.; Gonda, T. Effects of Phytoncides on Blood Pressure under Restraint Stress in SHRSP. Clin. Exp. Pharmacol. Physiol. 2004, 31, S27–S28. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.-J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of Forest Bathing on Physiological and Psychological Responses in Young Japanese Male Subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef]
- Kim, C.-G.; Cho, M.-K.; Kim, J.-I. Effects of Phytoncide Aromatherapy on Stress, Symptoms of Stress and Heart Rate Variability among Nursing Students. J. Korean Biol. Nurs. Sci. 2012, 14, 249–257. [Google Scholar] [CrossRef]
- Cheng, W.-W.; Lin, C.-T.; Chu, F.-H.; Chang, S.-T.; Wang, S.-Y. Neuropharmacological Activities of Phytoncide Released from Cryptomeria Japonica. J. Wood Sci. 2009, 55, 27–31. [Google Scholar] [CrossRef]
- Melnychenko, A.N.; Rosenstiel, T.N. Biogenic Volatile Organic Compound Emissions from Bamboo: Exploring Patterns of Diversity across Species. J. Am. Bamboo Soc. 2015, 28, 1–9. [Google Scholar]
- Kim, J.-S.; Lee, H.C.; Jo, J.-S.; Jung, J.Y.; Ha, Y.L.; Yang, J.-K. Evaluation of Antioxidant and Anticancer Activity of Steam Extract from the Bamboo Species. J. Korean Wood Sci. Technol. 2014, 42, 543–554. [Google Scholar] [CrossRef]
- Hassan, A.; Tao, J.; Li, G.; Jiang, M.; Aii, L.; Zhihui, J.; Zongfang, L.; Qibing, C. Effects of Walking in Bamboo Forest and City Environments on Brainwave Activity in Young Adults. Evid. Based Complementary Altern. Med. 2018, 2018, 9653857. [Google Scholar] [CrossRef]
- Ben-Zhi, Z.; Mao-Yi, F.; Jin-Zhong, X.; Xiao-Sheng, Y.; Zheng-Cai, L. Ecological Functions of Bamboo Forest: Research and Application. J. For. Res. 2005, 16, 143–147. [Google Scholar] [CrossRef]
- Lyu, B.; Zeng, C.; Xie, S.; Li, D.; Lin, W.; Li, N.; Jiang, M.; Liu, S.; Chen, Q. Benefits of a Three-Day Bamboo Forest Therapy Session on the Psychophysiology and Immune System Responses of Male College Students. Int. J. Environ. Res. Public Health 2019, 16, 4991. [Google Scholar] [CrossRef]
- Shin, W.S.; Kim, J.-J.; Lim, S.S.; Yoo, R.-H.; Jeong, M.-A.; Lee, J.; Park, S. Paradigm Shift on Forest Utilization: Forest Service for Health Promotion in the Republic of Korea. Net. J. Agric. Sci 2017, 5, 53–57. [Google Scholar] [CrossRef][Green Version]
- Geonwoo, K.I.M.; PARK, B.-J.; Joung, D.; YEOM, D.-G.; Shinya, K. Primary Concentration Measurements of Natural Volatile Organic Compounds in Atmosphere Using the Headspace Solid–Phase Microextraction Method within the Forest. J. Fac. Agr. Kyushu Univ. 2015, 60, 471–476. [Google Scholar]
- Kim, G.; Park, S.; Kwak, D. Is It Possible to Predict the Concentration of Natural Volatile Organic Compounds in Forest Atmosphere? Int. J. Environ. Res. Public Health 2020, 17, 7875. [Google Scholar] [CrossRef]
- Yoshida, N.; Takada, T.; Yamamura, Y.; Adachi, I.; Suzuki, H.; Kawakami, J. Inhibitory Effects of Terpenoids on Multidrug Resistance-Associated Protein 2-and Breast Cancer Resistance Protein-Mediated Transport. Drug Metab. Dispos. 2008, 36, 1206–1211. [Google Scholar] [CrossRef]
- Khoshnazar, M.; Bigdeli, M.R.; Parvardeh, S.; Pouriran, R. Attenuating Effect of A-pinene on Neurobehavioural Deficit, Oxidative Damage and Inflammatory Response Following Focal Ischaemic Stroke in Rat. J. Pharm. Pharmacol. 2019, 71, 1725–1733. [Google Scholar] [CrossRef]
- Rahbar, I.; Abbasnejad, M.; Haghani, J.; Raoof, M.; Kooshki, R.; Esmaeili-Mahani, S. The Effect of Central Administration of Alpha-pinene on Capsaicin-induced Dental Pulp Nociception. Int. Endod. J. 2019, 52, 307–317. [Google Scholar] [CrossRef]
- Bottoni, M.; Milani, F.; Colombo, L.; Nallio, K.; Colombo, P.S.; Giuliani, C.; Bruschi, P.; Fico, G. Using Medicinal Plants in Valmalenco (Italian Alps): From Tradition to Scientific Approaches. Molecules 2020, 25, 4144. [Google Scholar] [CrossRef]
- Girola, N.; Figueiredo, C.R.; Farias, C.F.; Azevedo, R.A.; Ferreira, A.K.; Teixeira, S.F.; Capello, T.M.; Martins, E.G.; Matsuo, A.L.; Travassos, L.R. Camphene Isolated from Essential Oil of Piper Cernuum (Piperaceae) Induces Intrinsic Apoptosis in Melanoma Cells and Displays Antitumor Activity in Vivo. Biochem. Biophys. Res. Commun. 2015, 467, 928–934. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.; Moreira, J.C.; Pasquali, M.A.; Rabie, S.; Pires, A.S.; Schröder, R.; Rabelo, T.K.; Santos, J.; Lima, P.S.; Cavalcanti, S.C. Antinociceptive Activity and Redox Profile of the Monoterpenes. Int. Sch. Res. Not. 2013, 2013, 459530. [Google Scholar]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Hadzopoulou-Cladaras, M. Camphene, a Plant-Derived Monoterpene, Reduces Plasma Cholesterol and Triglycerides in Hyperlipidemic Rats Independently of HMG-CoA Reductase Activity. PLoS ONE 2011, 6, e20516. [Google Scholar] [CrossRef]
- Bach, A.; Yáñez-Serrano, A.M.; Llusià, J.; Filella, I.; Maneja, R.; Penuelas, J. Human Breathable Air in a Mediterranean Forest: Characterization of Monoterpene Concentrations under the Canopy. Int. J. Environ. Res. Public Health 2020, 17, 4391. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Cheng, S.-S.; Chang, S.-T. Monitoring the Emission of Volatile Organic Compounds from the Leaves of Calocedrus Macrolepis Var. Formosana Using Solid-Phase Micro-Extraction. J. Wood Sci. 2010, 56, 140–147. [Google Scholar] [CrossRef]
- Llusia, J.; Peñuelas, J.; Seco, R.; Filella, I. Seasonal Changes in the Daily Emission Rates of Terpenes by Quercus Ilex and the Atmospheric Concentrations of Terpenes in the Natural Park of Montseny, NE Spain. J. Atmos. Chem. 2012, 69, 215–230. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Woo, J.-S.; Choi, S.-R.; Shin, E.-S. Comparison of Phytoncide (Monoterpene) Concentration by Type of Recreational Forest. J. Environ. Health Sci. 2015, 41, 241–248. [Google Scholar]
- Jing, X.; Lun, X.; Fan, C.; Ma, W. Emission Patterns of Biogenic Volatile Organic Compounds from Dominant Forest Species in Beijing, China. J. Environ. Sci. 2020, 95, 73–81. [Google Scholar] [CrossRef]
- Kim, B.-U.; Hyun, G.-W.; Choi, J.-H.; Hong, Y.-K.; Yi, G.-H.; Huh, I.-R.; Choi, S.-B. Survey of Emission Characteristics and Weather Factors for Application in Prediction Modeling for Phytoncide Weather Services. J. Environ. Health Sci. 2020, 46, 636–645. [Google Scholar]


| NVOCs | Microclimate Environment |
|---|---|
| 3-carene, borneol, bornyl acetate, camphene, camphor, d-limonene, eucalyptol, menthol, p-cymene, phellandrene, sabinene, α-pinene, α-terpinene, α-terpinolene, β-myrcene, β-pinene, γ-terpinene | air humidity, air temperature, soil temperature, soil humidity, gust speed, par (photosynthetically active radiation), solar radiation, wind speed |
| Parameters | Conditions | |||||
|---|---|---|---|---|---|---|
| Column | HP-INNOWAX (60 m × 0.25 mmL D × 0.25 μm, film thickness) 7890N-5975, Agilent (Santa Clara, CA, USA) | |||||
| Carrier gas flow | Helium at 1 mL/min | |||||
| Injection mode | Pulsed splitless | |||||
| Injection port temp. | 210 °C | |||||
| Transfer line temp. | 210 °C | |||||
| Over temp. program | Initial | Rate | Final | |||
| 40 °C | 3 min | 8 °C/min | 220 °C | 3 min | 40 °C | |
| Post run | 220 °C, 5 min | |||||
| Indicators | Estimate | Std. Error | t-Value 1 | p2 | Adjusted R2 | F 3 | p4 | |
|---|---|---|---|---|---|---|---|---|
| Model 1 | (Intercept) | 8.920 | 20.254 | 0.440 | 0.666 | 0.796 | 12.240 | 0.000 *** |
| Solar Radiation | 0.008 | 0.016 | 0.510 | 0.618 | ||||
| PAR | −0.010 | 0.009 | −1.095 | 0.291 | ||||
| Wind speed | −1.078 | 1.154 | −0.934 | 0.365 | ||||
| Gust speed | −0.039 | 1.300 | −0.030 | 0.976 | ||||
| Soil temp. | 0.022 | 0.672 | 0.033 | 0.974 | ||||
| Soil humidity | −80.648 | 174.771 | −0.461 | 0.651 | ||||
| Air temp. | 0.183 | 0.087 | 2.093 | 0.054 | ||||
| Air humidity | 0.026 | 0.022 | 1.163 | 0.263 | ||||
| Model 2 | (Intercept) | −1.704 | 0.986 | −1.729 | 0.100 | 0.829 | 28.930 | 0.000 *** |
| PAR | −0.004 | 0.002 | −2.682 | 0.015 * | ||||
| Wind speed | −1.075 | 0.720 | −1.493 | 0.152 | ||||
| Air temp. | 0.135 | 0.051 | 2.626 | 0.017 * | ||||
| Air humidity | 0.029 | 0.011 | 2.761 | 0.012 * | ||||
| Model | Variance Inflation Factor | Durbin–Watson Statistics | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 2 | PAR | Wind Speed | Air Temp. | Air Humidity | Lag | Auto-correlation | DW 1 | p |
| 1.43 | 3.60 | 1.49 | 3.76 | 1 | −0.03 | 1.95 | 0.50 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Kim, G.; Park, S.; Kim, E.; Kim, S. Prediction of Natural Volatile Organic Compounds Emitted by Bamboo Groves in Urban Forests. Forests 2021, 12, 543. https://doi.org/10.3390/f12050543
Choi Y, Kim G, Park S, Kim E, Kim S. Prediction of Natural Volatile Organic Compounds Emitted by Bamboo Groves in Urban Forests. Forests. 2021; 12(5):543. https://doi.org/10.3390/f12050543
Chicago/Turabian StyleChoi, Yeji, Geonwoo Kim, Sujin Park, Eunsoo Kim, and Soojin Kim. 2021. "Prediction of Natural Volatile Organic Compounds Emitted by Bamboo Groves in Urban Forests" Forests 12, no. 5: 543. https://doi.org/10.3390/f12050543
APA StyleChoi, Y., Kim, G., Park, S., Kim, E., & Kim, S. (2021). Prediction of Natural Volatile Organic Compounds Emitted by Bamboo Groves in Urban Forests. Forests, 12(5), 543. https://doi.org/10.3390/f12050543








