Gypsy Moth Management with LdMNPV Baculovirus in Cork Oak Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Formulations
2.2. LdMNPV Applications from the Ground
2.3. Aerial Applications
2.4. Data Elaboration and Statistical Analysis
3. Results
3.1. LdMNPV Applications from the Ground
3.2. Aerial Applications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lentini, A.; Mannu, R.; Cocco, A.; Ruiu, P.A.; Carboneschi, A.; Luciano, P. Long-term monitoring and microbiological control programs against lepidopteran defoliators in Sardinian cork oak forests (Italy). Ann. Silvic. Res. 2020, 45, 21–30. [Google Scholar]
- Martin, J.C.; Bonneau, X. Bacillus thuringiensis 30 ans de lutte contre les chenilles defoliatrices en foret. Phytoma Def. Veg. 2006, 590, 4–7. [Google Scholar]
- Crickmore, N. Beyond the spore—Past and future developments of Bacillus thuringiensis as a biopesticide. J. Appl. Microbiol. 2006, 101, 616–619. [Google Scholar] [CrossRef]
- Scriber, J.M. Non-target impacts of forest defoliator management options: Decision for no spraying may have worse impacts on non-target Lepidoptera than Bacillus thuringiensis insecticides. J. Insect Conserv. 2004, 8, 243–263. [Google Scholar] [CrossRef]
- Cory, J.S.; Myers, J.H. The ecology and evolution of insect baculoviruses. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 239–272. [Google Scholar] [CrossRef]
- Clem, R.J.; Passarelli, A.L. Baculoviruses: Sophisticated pathogens of insects. PLoS Pathog. 2013, 9, e1003729. [Google Scholar] [CrossRef]
- Barber, K.N.; Kaupp, W.J.; Holmes, S.B. Specificity testing of the nuclear polyhedrosis virus of the gypsy moth, Lymantria dispar (L.) (Lepidoptera: Lymantriidae). Can. Entomol. 1993, 125, 1055–1066. [Google Scholar] [CrossRef]
- Elkinton, J.S.; Liebhold, A.M. Population dynamics of gypsy moth in North America. Annu. Rev. Entomol. 1990, 35, 571–596. [Google Scholar] [CrossRef]
- Liebhold, A.; Elkinton, J.; Williams, D.; Muzika, R.M. What causes outbreaks of the gypsy moth in North America? Popul. Ecol. 2000, 42, 257–266. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT 9.1: User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2004. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. Robust tests of equality of variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Olkin, I., Ed.; Stanford University Press: Palo Alto, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Therneau, T.M.; Grambsch, P.M. The cox model. In Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; pp. 39–77. [Google Scholar]
- Therneau, T.M. Coxme: Mixed Effects Cox Models. R Package Version 2.2-5. 2015. Available online: http://CRAN.R-project.org/package=coxme (accessed on 21 February 2020).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. J. Math. Methods Biosci. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Puntener, W. Manual for Field Trials in Plant Protection; Ciba-Geigy Limited: Basle, Switzerland, 1981; p. 205. [Google Scholar]
- Moscardi, F.; de Souza, M.L.; de Castro, M.E.B.; Moscardi, M.L.; Szewczyk, B. Baculovirus pesticides: Present state and future perspectives. In Microbes and Microbial Technology; Springer: New York, NY, USA, 2011; pp. 415–445. [Google Scholar]
- Ruiu, L.; Mannu, R.; Falchi, G.; Braggio, A.; Luciano, P. Evaluation of different Bacillus thuringiensis sv kurstaki formulations against Lymantria dispar and Malacosoma neustria larvae infesting Quercus suber trees. Redia 2013, 96, 27–31. [Google Scholar]
- McNeil, J.; Cox-Foster, D.; Gardner, M.; Slavicek, J.; Thiem, S.; Hoover, K. Pathogenesis of Lymantria dispar multiple nucleopolyhedrovirus in L. dispar and mechanisms of developmental resistance. J. Gen. Virol. 2010, 91, 1590–1600. [Google Scholar] [CrossRef]
- Scholefield, J.A.; Shikano, I.; Lowenberger, C.A.; Cory, J.S. The impact of baculovirus challenge on immunity: The effect of dose and time after infection. J. Invertebr. Pathol. 2019, 167, 107232. [Google Scholar] [CrossRef]
- Smitley, D.R.; Davis, T.W. Aerial application of Bacillus thuringiensis for suppression of Gypsy Moth (Lepidoptera: Lymantriidae) in Populus-Quercus Forests. J. Econ. Entomol. 1993, 86, 1178–1184. [Google Scholar] [CrossRef]
- Mannu, R.; Cocco, A.; Luciano, P.; Lentini, A. Influence of Bacillus thuringiensis application timing on population dynamics of gypsy moth in Mediterranean cork oak forests. Pest Manag. Sci. 2020, 76, 1103–1111. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Crickmore, N. Specificity determinants for Cry insecticidal proteins: Insights from their mode of action. J. Invertebr. Pathol. 2017, 142, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, L.; Injac, M. Influence des maladies virales sur le dynamisme des populations de Lymantria dispar L. et Hyphantria cunea Drury en Yougoslavie. Bull. Soc. Entomol. Fr. 1984, 89, 800–807. [Google Scholar]
- Anderson, R.M.; May, R.M. The population dynamics of microparasites and their invertebrate hosts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1981, 291, 451–524. [Google Scholar]
- Reilly, J.R.; Hajek, A.E. Density-dependent resistance of the gypsy moth Lymantria dispar to its nucleopolyhedrovirus, and the consequences for population dynamics. Oecologia 2008, 154, 691–701. [Google Scholar] [CrossRef]
- Akhanaev, Y.B.; Belousova, I.A.; Lebedeva, D.A.; Pavlushin, S.V.; Martemyanov, V.V. A Comparison of the Vertical Transmission of High- and Low-Virulence Nucleopolyhedrovirus Strains in Lymantria dispar L. Insects 2020, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.H.; Cory, J.S. Ecology and evolution of pathogens in natural populations of Lepidoptera. Evol. Appl. 2016, 9, 231–247. [Google Scholar] [CrossRef]

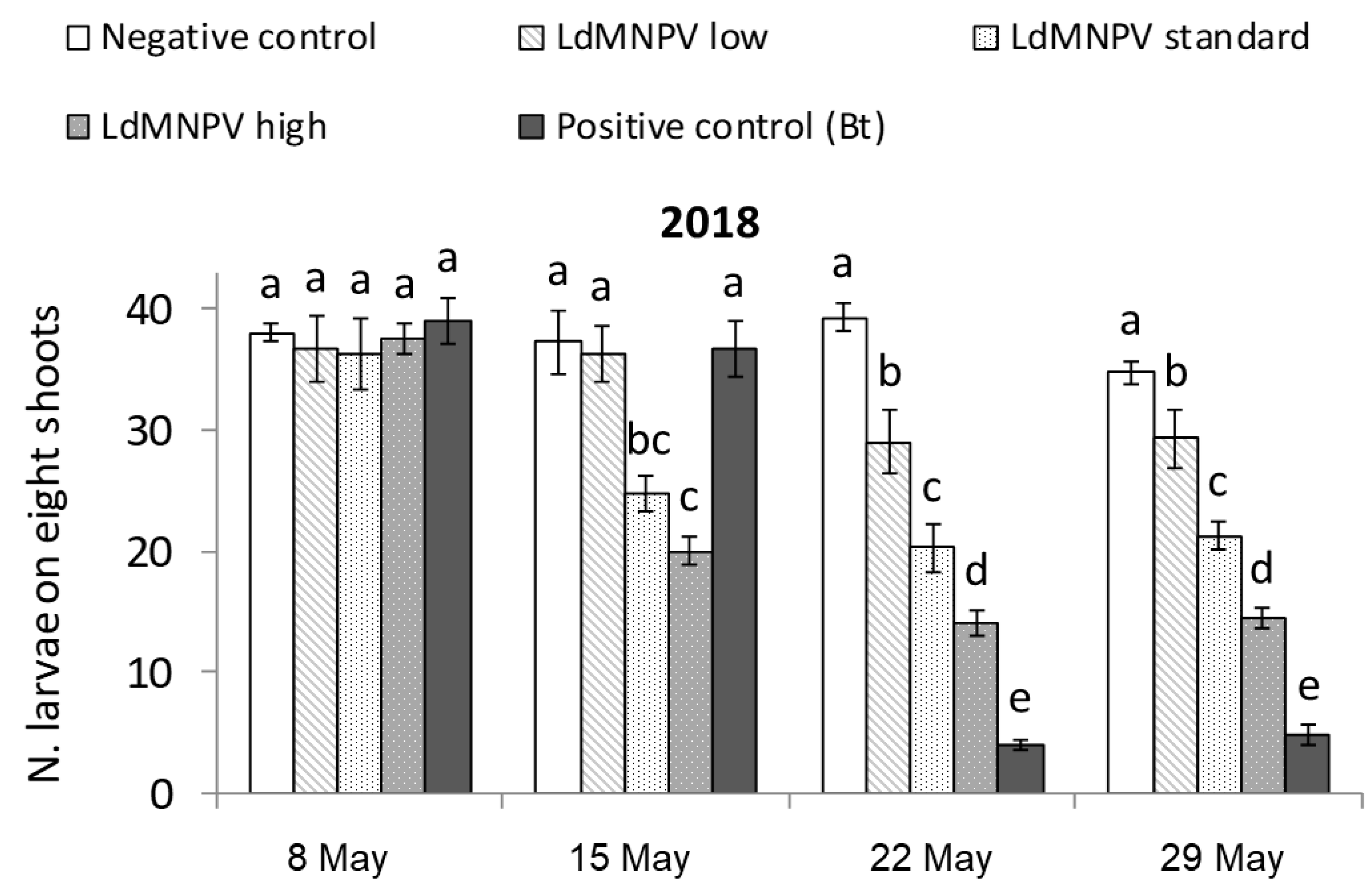
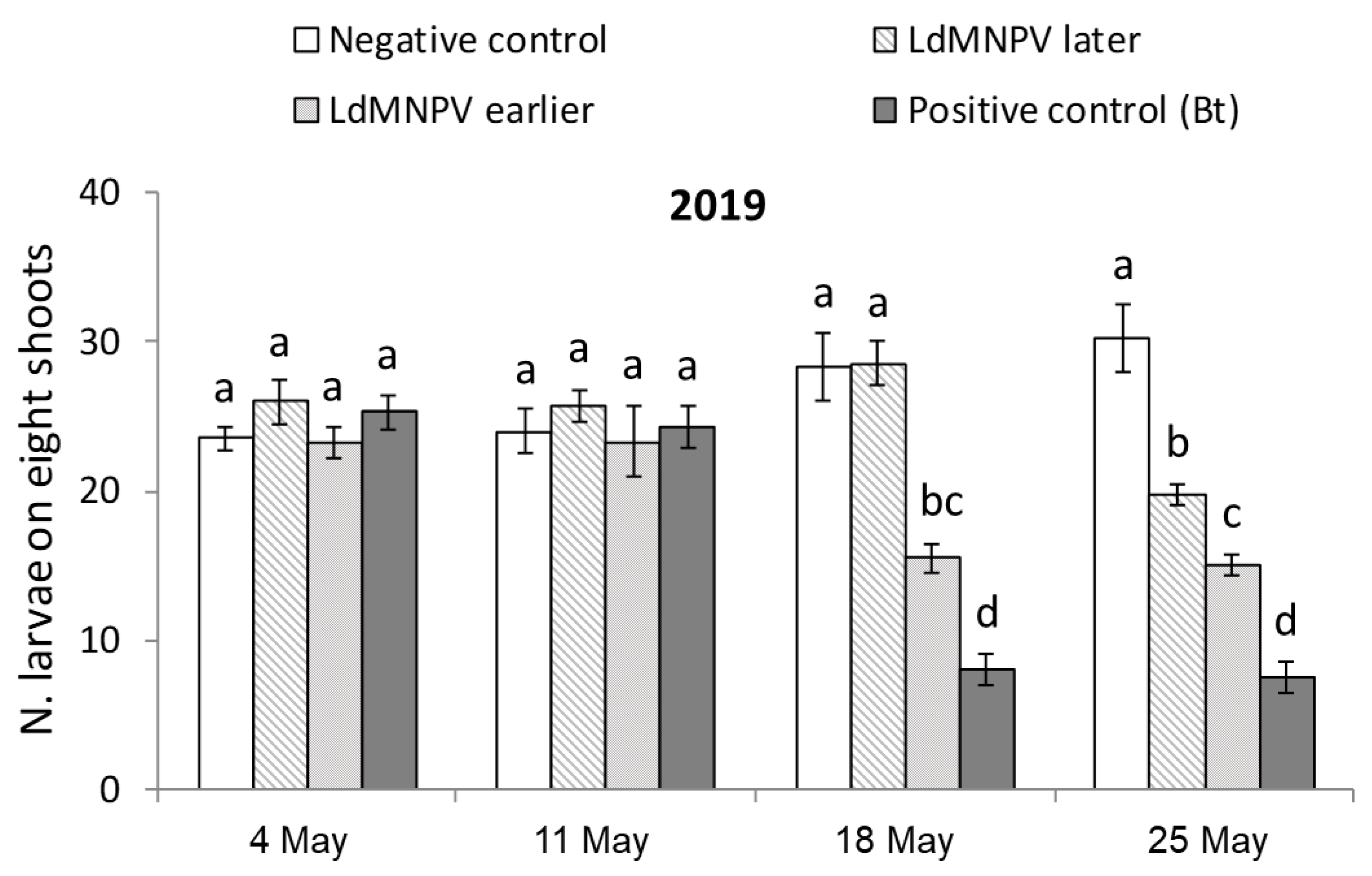



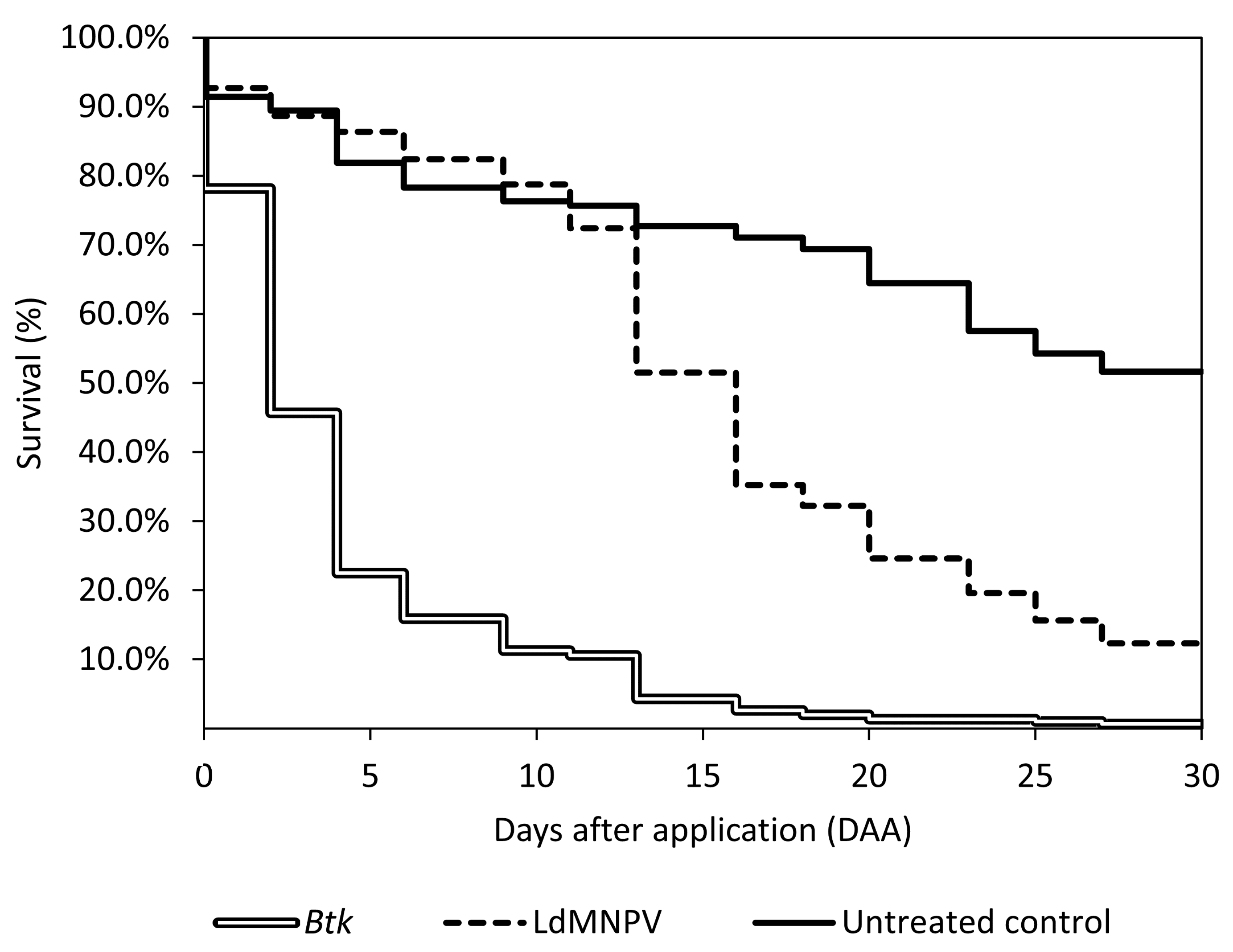
| Treatment | Description | Application Date | Application Rate | |
|---|---|---|---|---|
| 2018 | 2019 | |||
| Untreated Check | Not treated | - | - | - |
| LdMNPV Early | Earlier application | 9 May | 4 May | 2 L/ha |
| LdMNPV Later | Later application | 16 May | 11 May | 2 L/ha |
| Foray 76B | Reference product | 16 May | 11 May | 2 L/ha |
| Treatment a | Description | Application Rate |
|---|---|---|
| Untreated Check | Not treated | - |
| LdMNPV Low | 1/3 standard rate | 0.66 L/ha |
| LdMNPV Standard | Standard rate | 2 L/ha |
| LdMNPV High | 3× standard rate | 6 L/ha |
| Foray 76B | Reference product | 2 L/ha |
| Days a | Treatment | F | p | ||
|---|---|---|---|---|---|
| Foray 76B | LdMNPV | Untreated Check | |||
| 7 | 64.40 ± 8.82 b | 31.71 ± 4.00 | 39.66 ± 17.02 | 3.52 | 0.13 |
| 14 | 70.28 ± 8.94 | 34.34 ± 34.33 | 46.87 ± 18.57 | 0.95 | 0.46 |
| 21 | 75.30 ± 9.11 | 45.59 ± 5.83 | 50.58 ± 20.39 | 1.85 | 0.27 |
| Days a | Treatment b | t | p | |
|---|---|---|---|---|
| Foray 76B | LdMNPV | |||
| 7 | 75.44 ± 4.55 a | 10.05 ± 2.67 b | 7.16 | 0.004 |
| 14 | 86.74 ± 1.80 a | 21.94 ± 2.12 b | 13.44 | <0.001 |
| 21 | 96.33 ± 0.41 a | 70.19 ± 5.47 a | 2.75 | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiu, L.; Mannu, R.; Olivieri, M.; Lentini, A. Gypsy Moth Management with LdMNPV Baculovirus in Cork Oak Forest. Forests 2021, 12, 495. https://doi.org/10.3390/f12040495
Ruiu L, Mannu R, Olivieri M, Lentini A. Gypsy Moth Management with LdMNPV Baculovirus in Cork Oak Forest. Forests. 2021; 12(4):495. https://doi.org/10.3390/f12040495
Chicago/Turabian StyleRuiu, Luca, Roberto Mannu, Maurizio Olivieri, and Andrea Lentini. 2021. "Gypsy Moth Management with LdMNPV Baculovirus in Cork Oak Forest" Forests 12, no. 4: 495. https://doi.org/10.3390/f12040495
APA StyleRuiu, L., Mannu, R., Olivieri, M., & Lentini, A. (2021). Gypsy Moth Management with LdMNPV Baculovirus in Cork Oak Forest. Forests, 12(4), 495. https://doi.org/10.3390/f12040495








