Inferences on the Susceptibility of Wood of Different Tree Species to Heterobasidion annosum Sensu Lato Primary Infections and on the Range of Pathogen Spores Dispersal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Comparative Susceptibility of Wood Discs of Seven Coniferous Tree Species to Inoculation with H. annosum and H. parviporum Conidial Suspensions in Controlled Conditions
2.2. Comparative Susceptibility of Wood Discs of Seven Coniferous Tree Species to Heterobasidion spp. Natural Airborne Infections and Rates of Infection at Increasing Distance from Spore Sources
2.2.1. Study Areas and Characterization of Heterobasidion annosum s.l. Disease Centres
2.2.2. Samplings to Determine the Susceptibility of Wood Discs to Heterobasidion annosum s.l. Natural Airborne Infections and the Rates of Spore Deposition at Increasing Distance from Spore Sources
2.3. Calculations and Statistics
3. Results
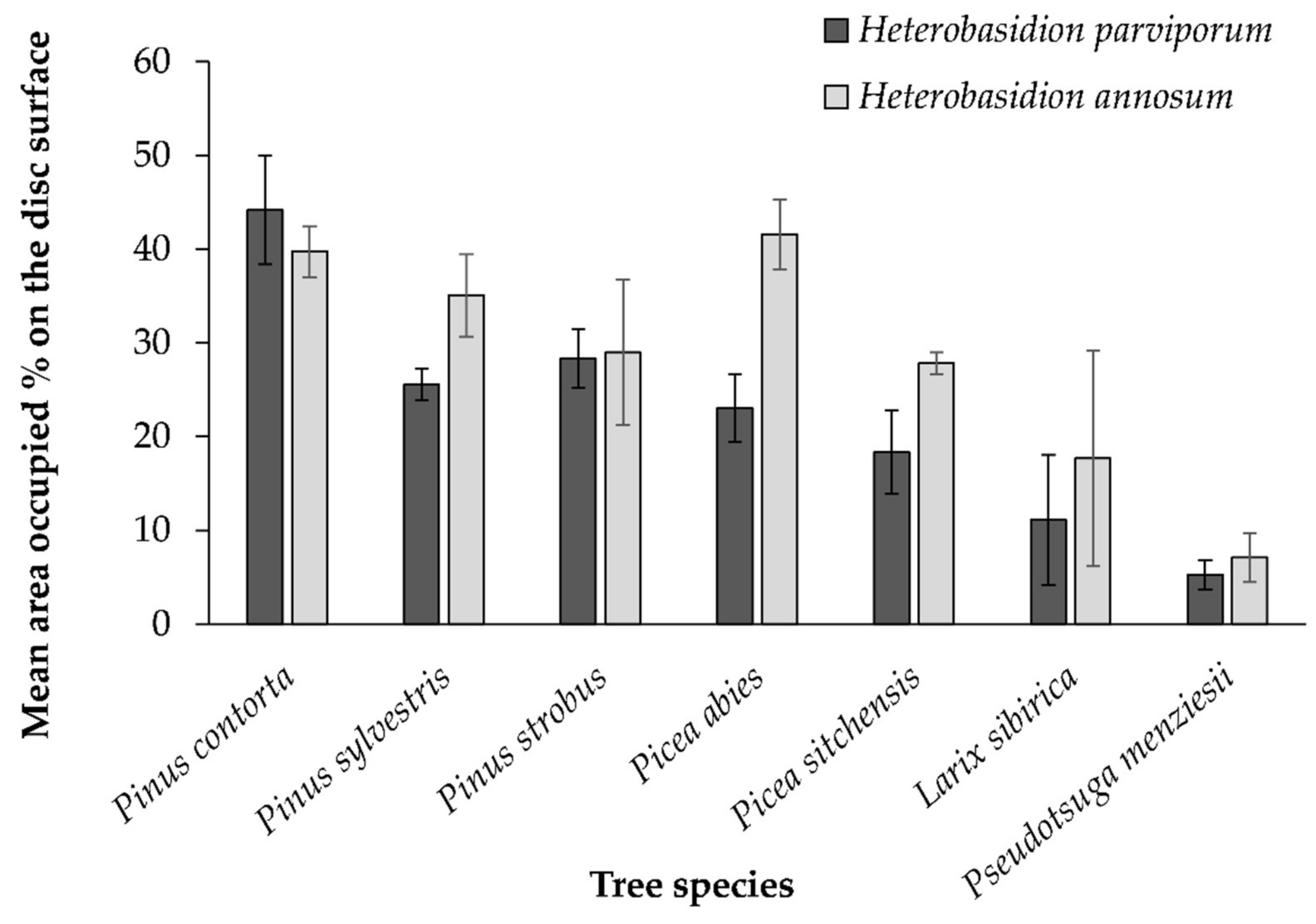
3.1. Comparative Susceptibility of Wood Discs of Seven Coniferous Tree Species to H. annosum and H. parviporum Conidial Suspensions in Controlled Conditions
3.2. Comparative Susceptibility of Wood Discs of Seven Coniferous Tree Species to H. annosum s.l. Natural Airborne Infections
3.3. Rates of Infection at Increasing Distance from Spore Sources in the Field
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garbelotto, M.; Gonthier, P. Biology, Epidemiology, and Control of Heterobasidion Species Worldwide. Annu. Rev. Phytopathol. 2013, 51, 39–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korhonen, K.; Holdenrieder, O. Neue Erkenntnisse über den Wurzelschwamm (Heterobasidion annosum s.l.). Forst Holz 2005, 5, 206–211. (In German) [Google Scholar]
- Korhonen, K.; Bobko, I.; Hanso, S.; Piri, T.; Vasiliauskas, A. Intersterility groups of Heterobasidion annosum in some spruce and pine stands in Byelorussia, Lithuania and Estonia. Eur. J. For. Pathol. 1992, 22, 384–391. [Google Scholar] [CrossRef]
- Witzell, J.; Berglund, M.; Rönnberg, J. Does temperature regime govern the establishment of Heterobasidion annosum in Scandinavia? Int. J. Biometeorol. 2011, 55, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, K.; Capretti, P.; Karjalainen, R.; Stenlid, J. Distribution of Heterobasidion intersterility groups in Europe. In Heterobasidion annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 93–104. [Google Scholar]
- Lygis, V.; Vasiliauskas, R.; Stenlid, J. Planting Betula pendula on pine sites infested by Heterobasidion annosum: Disease transfer, silvicultural evaluation, and community of wood-inhabiting fungi. Can. J. For. Res. 2004, 34, 120–130. [Google Scholar] [CrossRef]
- Asiegbu, F.O.; Adomas, A.; Stenlid, J. Conifer root and butt rot caused by Heterobasidion annosum (Fr.) Bref. s.l. Mol. Plant. Pathol. 2005, 6, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, P.; Warner, R.; Nicolotti, G.; Mazzaglia, A.; Garbelotto, M.M. Pathogen introduction as a collateral effect of military activity. Mycol. Res. 2004, 108, 468–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonthier, P.; Nicolotti, G.; Linzer, R.; Guglielmo, F.; Garbelotto, M.M. Invasion of European pine stands by a North American forest pathogen and its hybridization with a native interfertile taxon. Mol. Ecol. 2007, 16, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, P.; Anselmi, N.; Capretti, P.; Bussotti, F.; Feducci, M.; Giordano, L.; Honorati, T.; Lione, G.; Luchi, N.; Michelozzi, M.; et al. An integrated approach to control the introduced forest pathogen Heterobasidion irregulare in Europe. Forestry 2014, 87, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Gonthier, P.; Thor, M. Annosus root and butt rots. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CAB International: Wallingford, UK; New York, NY, USA, 2013; pp. 128–158. [Google Scholar]
- Greig, B.J.W. Species susceptibility to Fomes butt-rot. Q. J. For. 1979, 73, 21–25. [Google Scholar]
- Pratt, J.E.; Greig, B.J.W. Heterobasidion annosum: Development of butt rot following thinning in two young first rotation stands of Norway spruce. Forestry 1988, 61, 339–347. [Google Scholar] [CrossRef]
- Rönnberg, J.; Vollbrecht, G.; Thomsen, I.M. Incidence of butt rot in a tree species experiment in Northern Denmark. Scand. J. For. Res. 1999, 14, 234–239. [Google Scholar] [CrossRef]
- Greig, B.J.W.; Gibbs, J.N.; Pratt, J.E. Experiments on the susceptibility of conifers to Heterobasidion annosum in Great Britain. For. Pathol. 2001, 31, 219–228. [Google Scholar] [CrossRef]
- Lygis, V.; Vasiliauskaite, I.; Stenlid, J.; Vasaitis, R. Impact of forest fire on occurrence of Heterobasidion annosum s.s. root rot and other wood-inhabiting fungi in roots of Pinus mugo. Forestry 2010, 83, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Gonthier, P.; Brun, F.; Lione, G.; Nicolotti, G. Modelling the incidence of Heterobasidion annosum butt rots and related economic losses in alpine mixed naturally regenerated forests of northern Italy. For. Pathol. 2012, 42, 57–68. [Google Scholar] [CrossRef]
- Gonthier, P. Frequency of stump infections by Heterobasidion annosum s.l. and benefits from urea treatments vary with tree species and season in European Alpine forests. For. Ecol. Manag. 2019, 434, 76–86. [Google Scholar] [CrossRef]
- Kenigsvalde, K.; Brauners, I.; Korhonen, K.; Zaļuma, A.; Mihailova, A.; Gaitnieks, T. Evaluation of the biological control agent Rotstop in controlling the infection of spruce and pine stumps by Heterobasidion in Latvia. Scand. J. For. Res. 2016, 31, 254–261. [Google Scholar] [CrossRef]
- Rishbeth, J. Dispersal of Fomes annosus Fr. and Peniophora gigantea (Fr.) Massee. Trans. Br. Mycol. Soc. 1959, 42, 243–260. [Google Scholar] [CrossRef]
- Möykkynen, T.; Von Weissenberg, K.; Pappinen, A. Estimation of dispersal gradients of S- and P-type basidiospores of Heterobasidion annosum. Eur. J. For. Pathol. 1997, 27, 291–300. [Google Scholar] [CrossRef]
- La Porta, N.; Ambrosi, P.; Grillo, R.; Korhonen, K. A study on the inoculum potential of Heterobasidion annosum in conifer stands of Alpine forests. In Proceedings of the 5th Congress of European Foundation for Plant Pathology: Biodiversity in Plant Pathology, Taormina, Italy, 18–22 September 2000; Catara, A., Albanese, G., Catara, V., La Rosa, R., Polizzi, G., Tessitori, M., Eds.; Societa Italiana di Patologia Vegetele: Pisa, Itay, 2001; pp. 289–294. [Google Scholar]
- Gonthier, P.; Garbelotto, M.M.; Varese, G.C.; Nicolotti, G. Relative abundance and potential dispersal range of intersterility groups of Heterobasidion annosum in pure and mixed forests. Can. J. Bot. 2001, 79, 1057–1065. [Google Scholar] [CrossRef]
- Gonthier, P.; Garbelotto, M.M.; Nicolotti, G. Seasonal patterns of spore deposition of Heterobasidion species in four forests of the western Alps. Phytopathology 2005, 95, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Möykkynen, T.; Kontiokari, J. Spore deposition of Heterobasidion annosum coll. in Picea abies stands of North Karelia, eastern Finland. For. Pathol. 2001, 31, 107–114. [Google Scholar] [CrossRef]
- Wang, L.Y.; Pålsson, H.; Ek, E.; Rönnberg, J. The effect of Phlebiopsis gigantea and urea stump treatment against spore infection of Heterobasidion spp. on hybrid larch (Larix × eurolepis) in southern Sweden. For. Pathol. 2012, 42, 420–428. [Google Scholar] [CrossRef]
- Pellicciaro, M.; Lione, G.; Giordano, L.; Gonthier, P. Biocontrol potential of Pseudomonas protegens against Heterobasidion species attacking conifers in Europe. Biol. Control. 2021, 157, 104583. [Google Scholar] [CrossRef]
- Redfern, D.B. Infection of Picea sitchensis and Pinus contorta stumps by basidiospores of Heterobasidion annosum. Eur. J. For. Pathol. 1982, 12, 11–25. [Google Scholar] [CrossRef]
- Thomsen, I.M.; Jacobsen, J.B. Testing of Rotstop on Sitka spruce, Douglas-fir and larch. In Root and Butt Rots of Forest Trees, Proceedings of the 10th International Conference on Root and Butt Rots: IUFRO WP 7.02.01, Québec City, QC, Canada, 16–22 September 2001; Laflamme, G., Bérubé, J.A., Bussières, G., Eds.; Canadian Forest Service: Sainte-Foy, QC, Canada, 2003; pp. 216–220. [Google Scholar]
- Dimitri, L.; Zycha, H.; Kliefoth, R. Untersuchungen über die Bedeutung der Stubbeninfektion durch Fomes annosus für die Ausbreitung der Rotfäule der Fichte. Forstwiss. Cent. 1971, 90, 104–117. (In German) [Google Scholar] [CrossRef]
- Jansons, J. Statistical Inventory of Resources of Forests of Latvia: Results of Cycle IV. Available online: http://www.silava.lv/petijumi/nacionlais-mea-monitorings.aspx (accessed on 25 March 2021). (In Latvian).
- Zaluma, A.; Bruna, L.; Klavina, D.; Burnevica, N.; Kenigsvalde, K.; Lazdins, A.; Gaitnieks, T. Growth of Phlebiopsis gigantea in wood of seven conifer species. For. Pathol. 2019, 49, e12555. [Google Scholar] [CrossRef]
- Brandtberg, P.-O.; Johansson, M.; Seeger, P. Effects of season and urea treatment on infection of stumps of Picea abies by Heterobasidion annosum in stands on former arable land. Scand. J. For. Res. 1996, 11, 261–268. [Google Scholar] [CrossRef]
- Kallio, T. Aerial distribution of the root-rot fungus Fomes annosus (Fr.) Cooke in Finland. Acta For. Fenn. 1970, 107, 7541. [Google Scholar] [CrossRef] [Green Version]
- Stenlid, J. Regional differentiation in Heterobasidion annosum. In Proceedings of the 8th international Conference on Root and Butt Rots, Wik, Sweden and Haikko, Finland, 9–16 August 1993; Johansson, M., Stenlid, J., Eds.; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1994; pp. 243–248. [Google Scholar]
- Gonthier, P.; Lione, G.; Giordano, L.; Garbelotto, M. The American forest pathogen Heterobasidion irregulare colonizes unexpected habitats after its introduction in Italy. Ecol. Appl. 2012, 22, 2135–2143. [Google Scholar] [CrossRef] [Green Version]
- Bendz-Hellgren, M.; Brandtberg, P.O.; Johansson, M.; Swedjemark, G.; Stenlid, J. Growth rate of Heterobasidion annosum in Picea abies established on forest land and arable land. Scand. J. For. Res. 1999, 14, 402–407. [Google Scholar] [CrossRef]
- Piri, T. Early development of root rot in young Norway spruce planted on sites infected by Heterobasidion in southern Finland. Can. J. For. Res. 2003, 33, 604–611. [Google Scholar] [CrossRef]
- Bruna, L.; Klavina, D.; Zaluma, A.; Kenigsvalde, K.; Burneviča, N.; Nikolajeva, V.; Gaitnieks, T.; Piri, T. Efficacy of Phlebiopsis gigantea against Heterobasidion conidiospore and basidiospore infection in spruce wood. iForest Biogeosci. For. 2020, 13, 369–375. [Google Scholar] [CrossRef]
- Arhipova, N.; Gaitnieks, T.; Donis, J.; Stenlid, J.; Vasaitis, R. Butt rot incidence, causal fungi, and related yield loss in Picea abies stands of Latvia. Can. J. For. Res. 2011, 41, 2337–2345. [Google Scholar] [CrossRef]
- Korhonen, K. Intersterility Groups of Heterobasidion Annosum; Metsäntutkimuslaitoksen Julkaisuja; Valtioneuvoston Kirjapaino: Helsinki, Finland, 1978; Volume 94, pp. 1–25. [Google Scholar]
- R CoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 3 September 2020).
- Vasiliauskas, R.; Stenlid, J. Spread of S and P group isolates of Heterobasidion annosum within and among Picea abies trees in central Lithuania. Can. J. For. Res. 1998, 28, 961–966. [Google Scholar] [CrossRef]
- Korhonen, K. Simulated stump treatment experiments for monitoring the efficacy of Phlebiopsis gigantea against Heterobasidion annosum. In Root and Butt Rots of Forest Trees, Proceedings of the 10th International Conference on Root and Butt Rots: IUFRO WP 7.02.01, Québec City, QC, Canada, 16–22 September 2001; Laflamme, G., Bérubé, J.A., Bussières, G., Eds.; Canadian Forest Service: Sainte-Foy, QC, Canada, 2003; pp. 206–210. [Google Scholar]
- Oliva, J.; Bendz-Hellgren, M.; Stenlid, J. Spread of Heterobasidion annosum s.s. and Heterobasidion parviporum in Picea abies 15 years after stump inoculation. FEMS Microbiol. Ecol. 2011, 75, 414–429. [Google Scholar] [CrossRef]
- Zaluma, A.; Gaitnieks, T.; Arhipova, N.; Vasaitis, R. Growth rates of Heterobasidion annosum s.s. and H. parviporum in functional sapwood of Pinus sylvestris and Picea abies. For. Pathol. 2015, 45, 437–439. [Google Scholar] [CrossRef]
- Gonthier, P.; Garbelotto, M.M.; Nicolotti, G. Swiss stone pine trees and spruce stumps represent an important habitat for Heterobasidion spp. in subalpine forests. For. Pathol. 2003, 33, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Daniel, G.; Asiegbu, F.O.; Johansson, M. The saprotrophic wood-degrading abilities of Heterobasidium annosum intersterility groups P and S. Mycol. Res. 1998, 102, 991–997. [Google Scholar] [CrossRef]
- Gaitnieks, T.; Brauners, I.; Kenigsvalde, K.; Zaļuma, A.; Brūna, L.; Jansons, J.; Burņeviča, N.; Lazdiņš, A.; Vasaitis, R. Infection of pre-commercially cut stumps of Picea abies and Pinus sylvestris by Heterobasidion spp.—A comparative study. Silva. Fenn. 2018, 52, 1–7. [Google Scholar] [CrossRef]
- Gaitnieks, T.; Zaļuma, A.; Kenigsvalde, K.; Brūna, L.; Kļaviņa, D.; Burņeviča, N.; Stenlid, J.; Jankovský, L.; Vasaitis, R. Natural infection and colonization of pre-commercially cut stumps of Picea abies and Pinus sylvestris by Heterobasidion rot and its biocontrol fungus Phlebiopsis gigantea. Biol. Control. 2020, 143, 104208. [Google Scholar] [CrossRef]
- Morrison, D.J.; Johnson, A.L.S. Incidence of Heterobasidion annosum in precommercial thinning stumps in coastal British Columbia. Eur. J. For. Pathol. 1999, 29, 1–16. [Google Scholar] [CrossRef]
- Rönnberg, J.; Petrylaite, E.; Nilsson, G.; Pratt, J. Two studies to assess the risk to Pinus sylvestris from Heterobasidion spp. in southern Sweden. Scand. J. For. Res. 2006, 21, 405–413. [Google Scholar] [CrossRef]
- Oliva, J.; Bernat, M.; Stenlid, J. Heartwood stump colonisation by Heterobasidion parviporum and H. annosum s.s. in Norway spruce (Picea abies) stands. For. Ecol. Manag. 2013, 295, 1–10. [Google Scholar] [CrossRef]
- Swedjemark, G.; Stenlid, J. Population Dynamics of the Root Rot Fungus Heterobasidion annosum Following Thinning of Picea abies. Oikos 1993, 66, 247. [Google Scholar] [CrossRef]
- Nicolotti, G.; Gonthier, P.; Varese, G.C. Effectiveness of some biocontrol and chemical treatments against Heterobasidion annosum on Norway spruce stumps. Eur. J. For. Pathol. 1999, 29, 339–346. [Google Scholar] [CrossRef]
- Johansson, S.M.; Pratt, J.E.; Asiegbu, F.O. Treatment of Norway spruce and Scots pine stumps with urea against the root and butt rot fungus Heterobasidion annosum—Possible modes of action. For. Ecol. Manag. 2002, 157, 87–100. [Google Scholar] [CrossRef]
- Berglund, M.; Rönnberg, J. Effectiveness of treatment of Norway spruce stumps with Phlebiopsis gigantea at different rates of coverage for the control of Heterobasidion. For. Pathol. 2004, 34, 233–243. [Google Scholar] [CrossRef]
- Rönnberg, J.; Sidorov, E.; Petrylaite, E. Efficacy of different concentrations of Rotstop® and Rotstop®S and imperfect coverage of Rotstop®S against Heterobasidion spp. spore infections on Norway spruce stumps. For. Pathol. 2006, 36, 422–433. [Google Scholar] [CrossRef]
- Redfern, D.B. The effect of wood moisture on infection of Sitka spruce stumps by basidiospores of Heterobasidion annosum. Eur. J. For. Pathol. 1993, 23, 218–235. [Google Scholar] [CrossRef]
- Redfern, D.B.; Pratt, J.E.; Gregory, S.C.; MacAskill, G.A. Natural infection of Sitka spruce thinning stumps in Britain by spores of Heterobasidion annosum and long-term survival of the fungus. Forestry 2001, 74, 53–72. [Google Scholar] [CrossRef] [Green Version]
- Gonthier, P. Controlling root and butt rot diseases in Alpine European forests. In Management of Fungal Plant Pathogens; Arya, A., Pereló, A.E., Eds.; CABI Internationl: Wallingford, UK, 2010; pp. 345–361. ISBN 9781845936037. [Google Scholar]
- Rishbeth, J. Observations on the Biology of Fomes annosus, with Particular Reference to East Anglian Pine Plantations: II. Spore production, stump infection, and a saprophytic activity in stumps. Ann. Bot. 1951, 15, 1–22. [Google Scholar] [CrossRef]
- Yde-Anersen, A. Seasonal Incidence of Stump Infection in Norway Spruce by Air-Borne Fomes annosus Spores. For. Sci. 1962, 8, 98–103. [Google Scholar] [CrossRef]
- Redfern, D.B.; Pratt, J.E.; Whiteman, A. Stump treatment against Heterobasidion annosum: A re-appraisal. In Proceedings of the 8th International Conference on Root and Butt Rots, Wik, Sweden and Haikko, Finland, 9–16 August 1993; Johansson, M., Stenlid, J., Eds.; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1994; pp. 656–661. [Google Scholar]




| Sample Plot | Date of Experiment in 2008 | Analyzed Directions | Distance from the Group of Fruit Bodies, m | Air Temperature, °C | Air Humidity, % | Speed (m/s) and Direction of Wind |
|---|---|---|---|---|---|---|
| Kalsnava | 15.07–16.07 | N, S, E, W | 1 *, 5, 10, 20, 30, 40, 50 | 16 | 80 | 1–3, SW |
| 03.08–04.08 | N, S, E, W | 1, 10, 30 | 18 | 80 | 1–4, SW, W | |
| 11.08–12.08 | N, S, E, W | 1, 10, 30 | 17 | 89 | 1–3, S, SW | |
| 21.08–22.08 | N, S, E, W | 1, 20, 40 | 15 | 89 | 3–4, SW | |
| Tireli | 30.08–31.08 | NW | 1, 5, 10, 30 | 12 | 86, heavy rain | 1–4, NW |
| SE | 10, 20, 40 | |||||
| 11.09–12.09 | NW | 1, 5, 10, 30 | 8 | 72 | 3–4, NE | |
| SE | 10, 20, 40 |
| Mean Area (%) Occupied by Heterobasidion spp. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Larix sibirica | Picea abies | Picea sitchensis | Pinus contorta | Pinus strobus | Pinus sylvestris | Pseudotsuga menziesii | ||
| H. parviporum | Sapwood | 24.9 | 43.4 | 25.8 | 48.4 | 71.5 | 67.8 | 11.0 |
| Heartwood | 0.1 | 2.1 | 4.7 | 0.0 | 0.2 | 0.0 | 0.1 | |
| H. annosum | Sapwood | 40.8 | 71.4 | 40.6 | 44.4 | 68.0 | 80.6 | 18.0 |
| Heartwood | 0.5 | 6.3 | 3.0 | 0.5 | 1.8 | 0.01 | 0.1 | |
| Tree Species | Kalsnava | Tireli | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 * | 2 | 3 | 4 | Average | 1 | 2 | Average | |
| Larix sibirica | 96 | 100 | 67 | 67 | 83 | 88 | 50 | 69 |
| Picea abies | 100 | 100 | 67 | 89 | 89 | 88 | 88 | 88 |
| Picea sitchensis | 98 | 100 | 72 | 78 | 87 | 100 | 69 | 85 |
| Pinus contorta | 92 | 100 | 78 | 72 | 86 | 94 | 69 | 82 |
| Pinus strobus | 100 | 100 | 100 | 100 | 100 | 75 | 100 | 88 |
| Pinus sylvestris | 96 | 100 | 89 | 100 | 96 | 100 | 100 | 100 |
| Pseudotsuga menziesii | 100 | 89 | 67 | 83 | 85 | 69 | 50 | 60 |
| Average | 97 | 98 | 77 | 84 | 89 | 88 | 75 | 82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brūna, L.; Lione, G.; Kenigsvalde, K.; Burņeviča, N.; Zaļuma, A.; Kļaviņa, D.; Gaitnieks, T.; Gonthier, P. Inferences on the Susceptibility of Wood of Different Tree Species to Heterobasidion annosum Sensu Lato Primary Infections and on the Range of Pathogen Spores Dispersal. Forests 2021, 12, 854. https://doi.org/10.3390/f12070854
Brūna L, Lione G, Kenigsvalde K, Burņeviča N, Zaļuma A, Kļaviņa D, Gaitnieks T, Gonthier P. Inferences on the Susceptibility of Wood of Different Tree Species to Heterobasidion annosum Sensu Lato Primary Infections and on the Range of Pathogen Spores Dispersal. Forests. 2021; 12(7):854. https://doi.org/10.3390/f12070854
Chicago/Turabian StyleBrūna, Lauma, Guglielmo Lione, Kristīne Kenigsvalde, Natālija Burņeviča, Astra Zaļuma, Dārta Kļaviņa, Tālis Gaitnieks, and Paolo Gonthier. 2021. "Inferences on the Susceptibility of Wood of Different Tree Species to Heterobasidion annosum Sensu Lato Primary Infections and on the Range of Pathogen Spores Dispersal" Forests 12, no. 7: 854. https://doi.org/10.3390/f12070854
APA StyleBrūna, L., Lione, G., Kenigsvalde, K., Burņeviča, N., Zaļuma, A., Kļaviņa, D., Gaitnieks, T., & Gonthier, P. (2021). Inferences on the Susceptibility of Wood of Different Tree Species to Heterobasidion annosum Sensu Lato Primary Infections and on the Range of Pathogen Spores Dispersal. Forests, 12(7), 854. https://doi.org/10.3390/f12070854








