Utilization of Fish Farm Effluent for Irrigation Short Rotation Willow (Salix alba L.) under Lysimeter Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Climatic Conditions
2.2. The Plant Material
2.3. Experimental Design for Effluent Water Irrigation
2.4. Determination of Phenological Paramteres and Mineral Content
2.5. Statistical Analyses
3. Results
3.1. Results of Chemical Analyses—Changing of Sodium and Nitrogen Content of Soil
3.2. Phenological Results
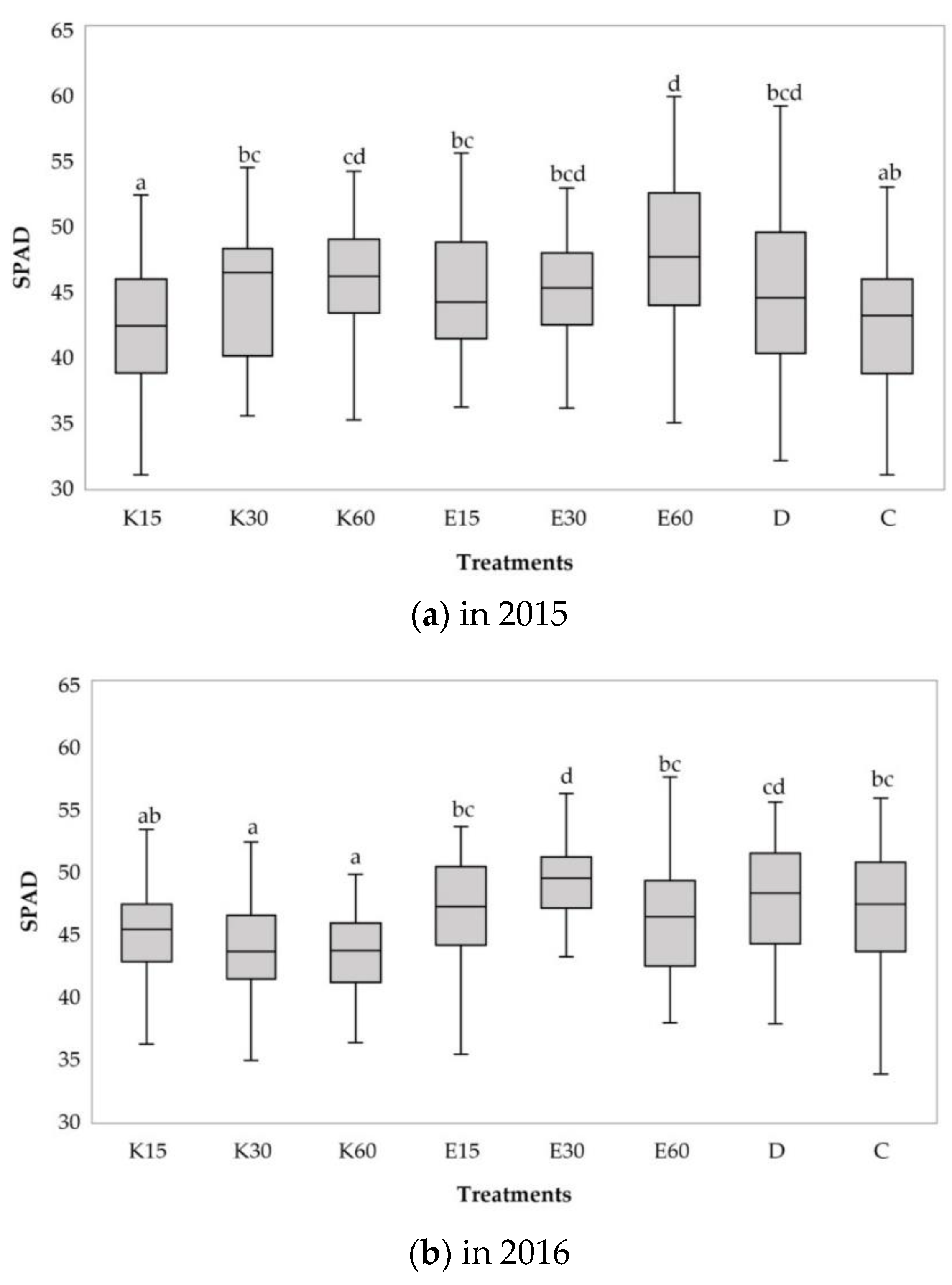
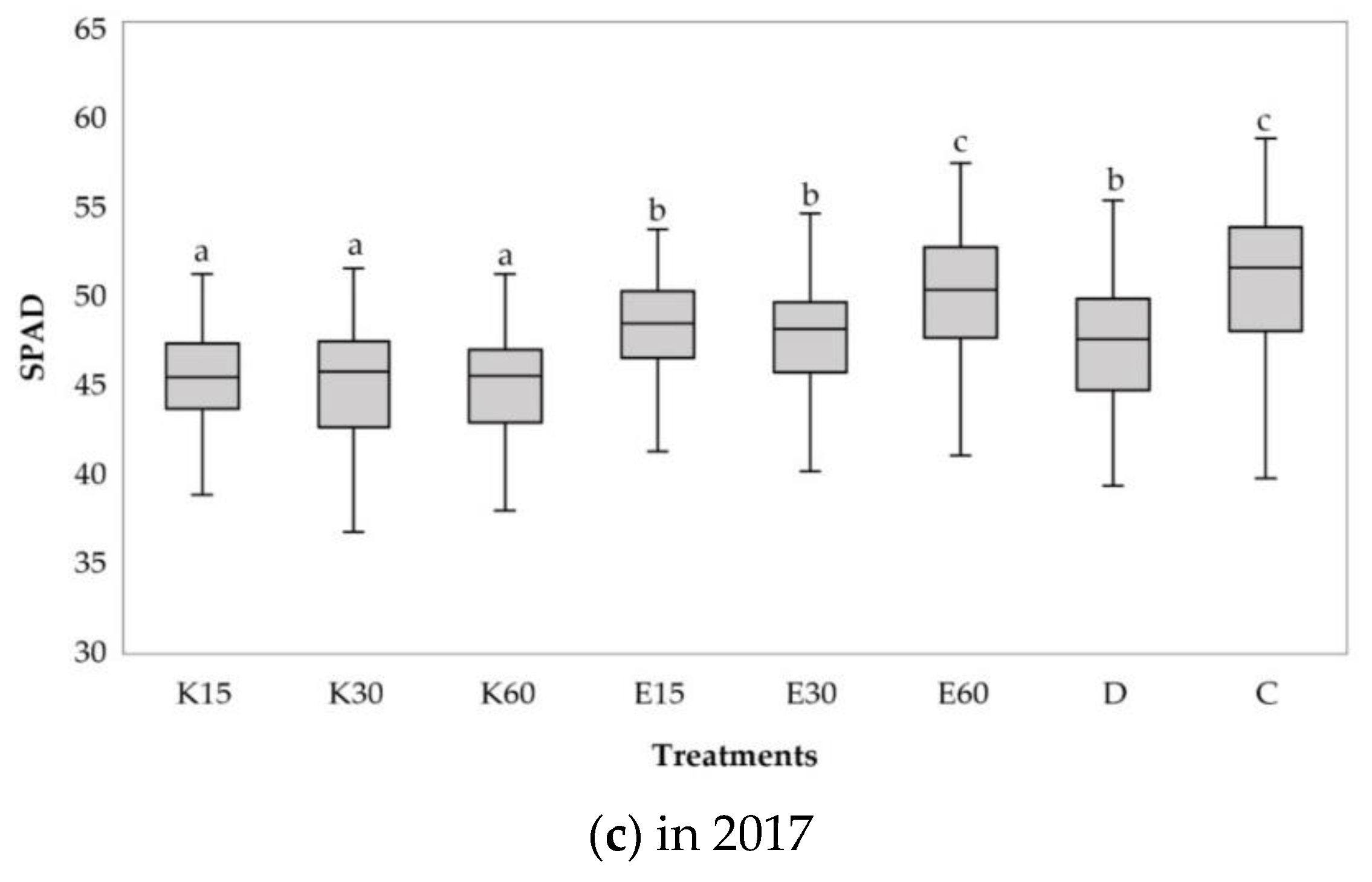
3.2.1. Changes of Relative Chlorophyll Content
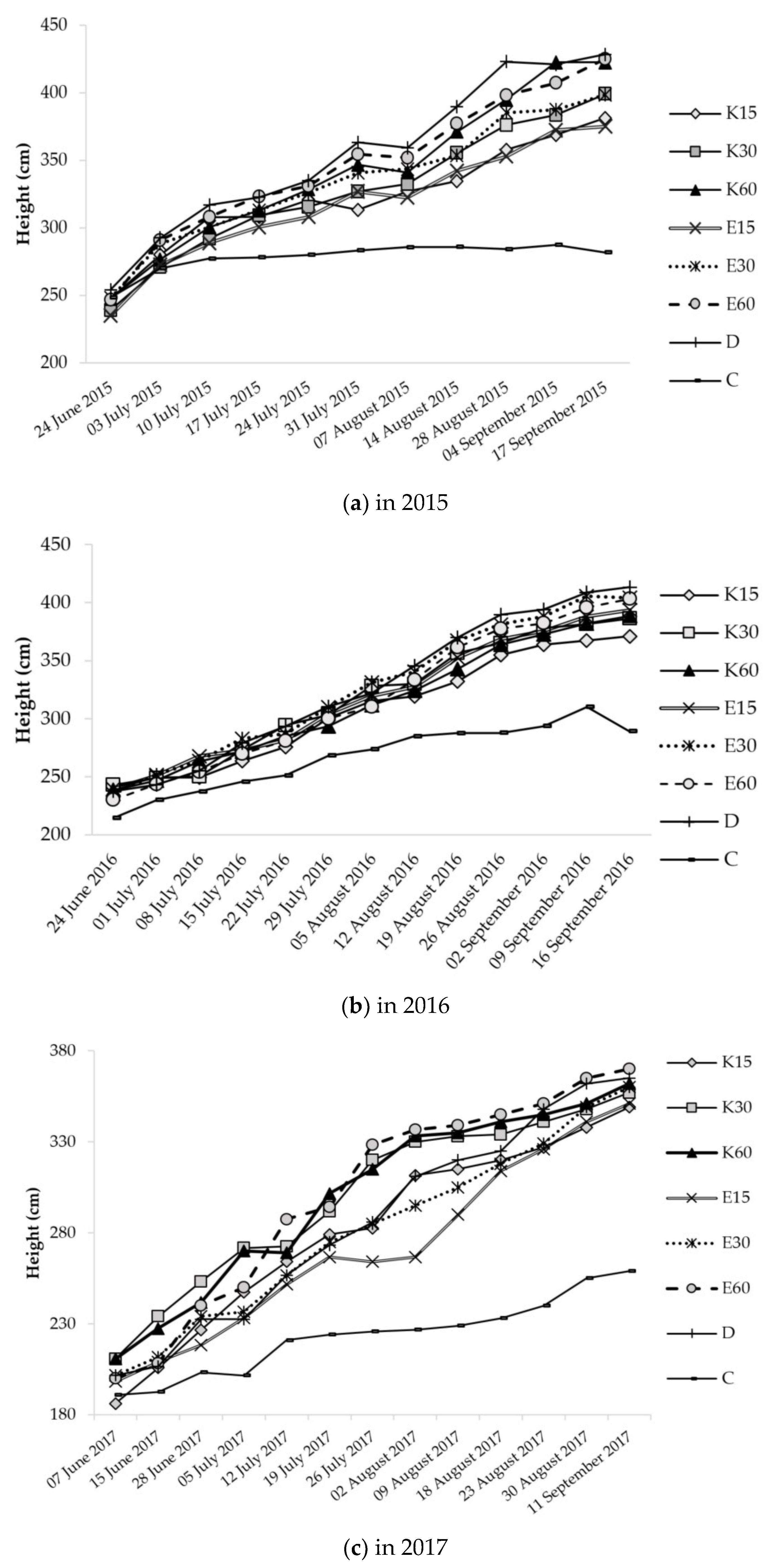
3.2.2. Growth of Test Plants during the Seasons
3.3. Results of Mineral Content
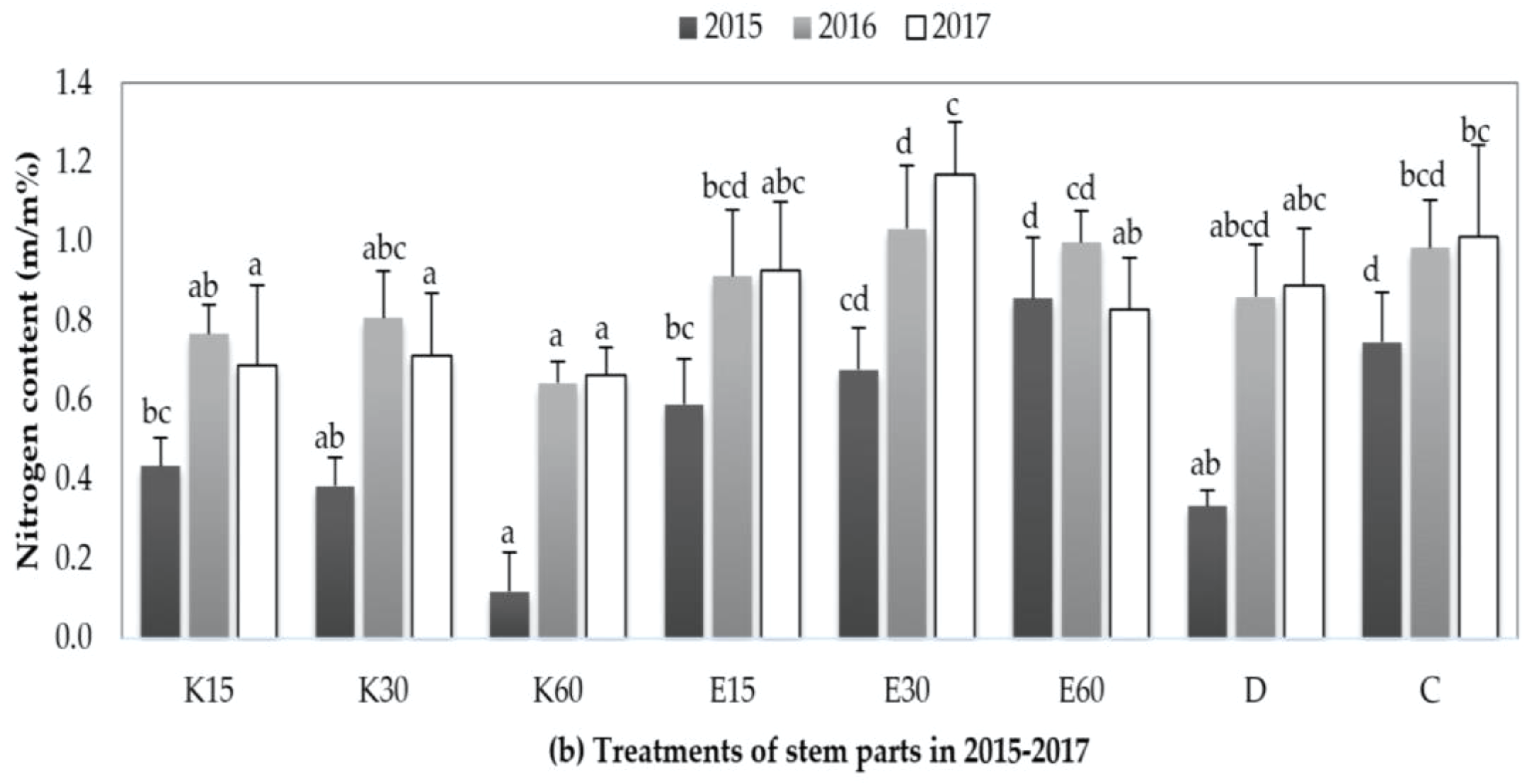
3.3.1. Changing of Nitrogen Content in Plant Parts
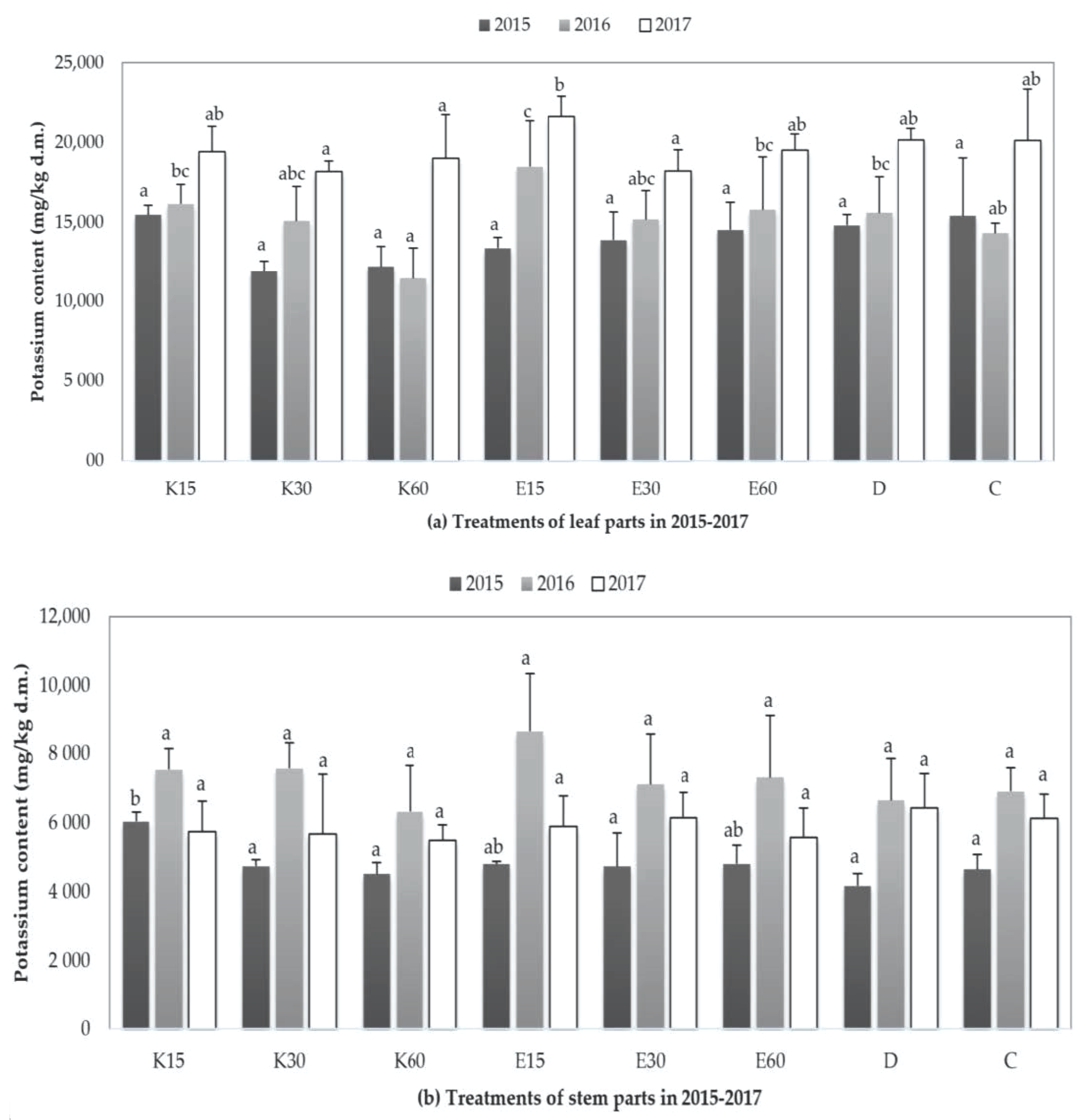
3.3.2. Changing of Potassium Content in Plant Parts
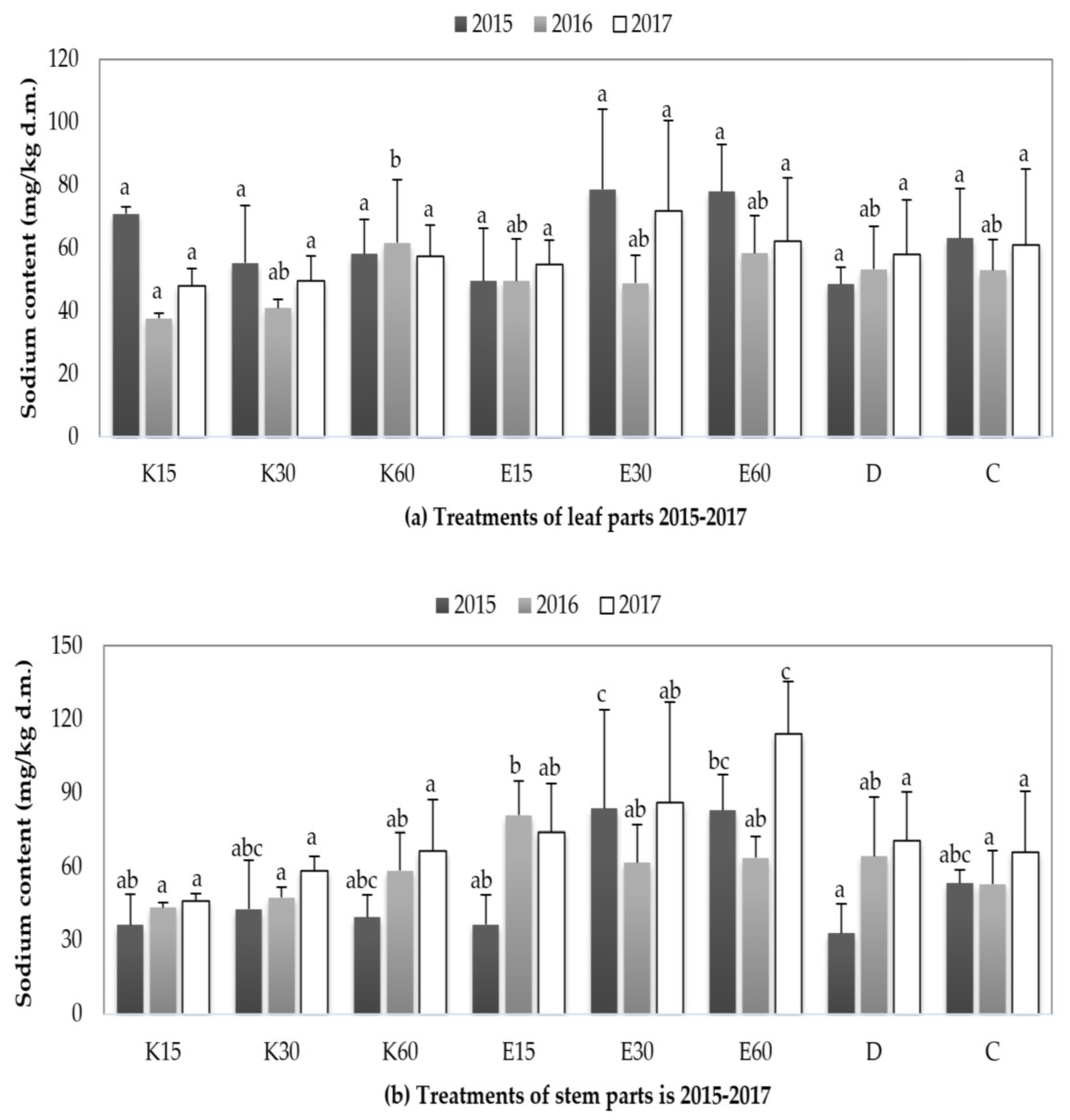
3.3.3. Changing of Sodium Content in Plant Parts
3.3.4. Changing of Phosphorus Content in Plant Parts
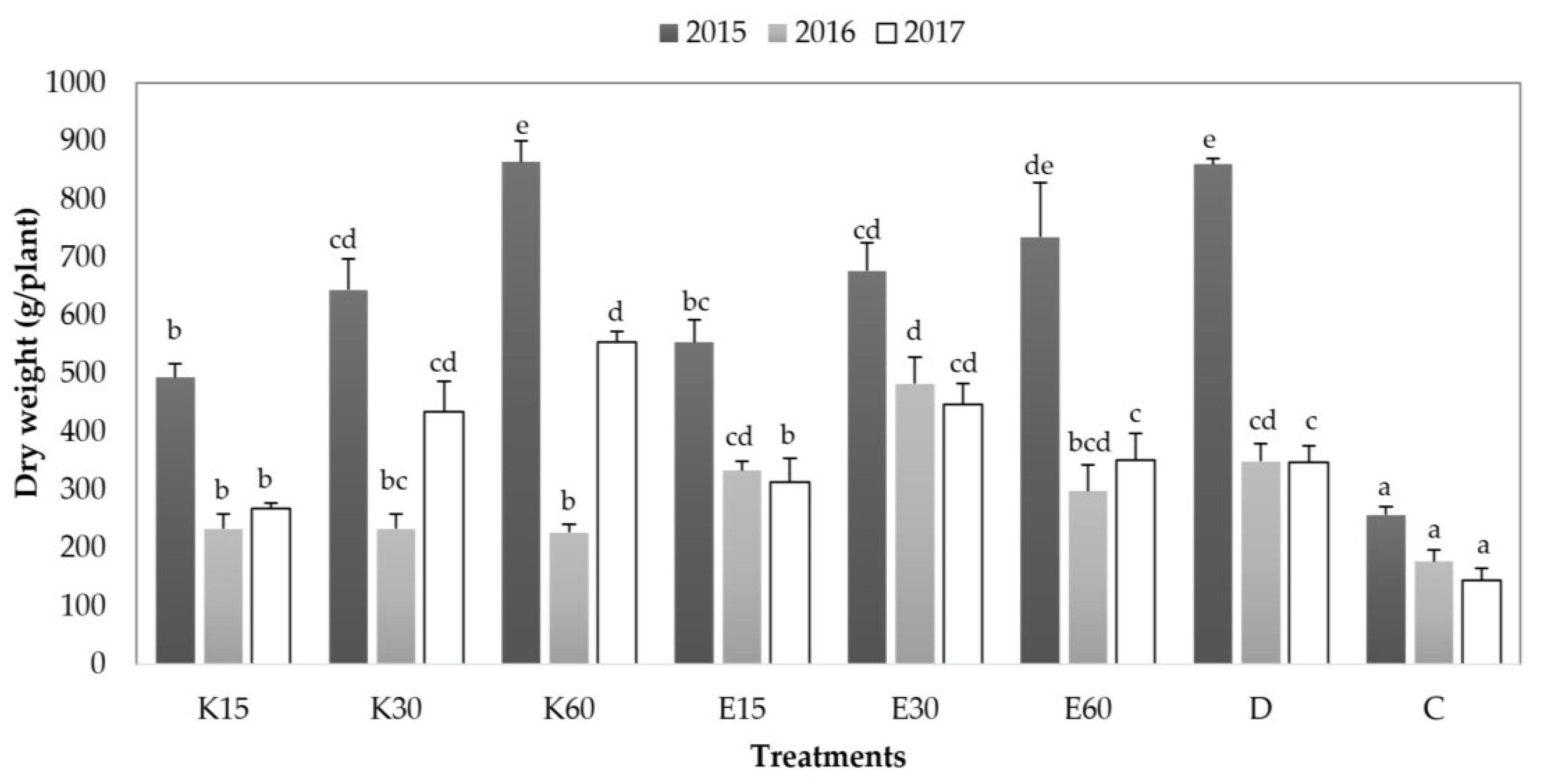
3.4. Biomass Changing over the Three Years of the Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barczi, A.; Joó, K.; Pető, Á.; Bucsi, T. Survey of the buried paleosoil under the Lyukas-mound in Hungary. Eurasian Soil Sci. 2006, 39, 133–140. [Google Scholar] [CrossRef]
- Bosco, C.; de Rigo, D.; Dewitte, O.; Poesen, J.; Panagos, P. Corrigendum to modelling soil erosion at European scale: Towards harmonization and reproducibility. Nat. Hazards Earth Syst. Sci. 2015, 15, 225–245. [Google Scholar] [CrossRef]
- Mikó, P.; Kovács, G.P.; Alexa, L.; Balla, I.; Póti, P.; Gyuricza, C. Biomass production of energy willow under unfavourable field conditions. Appl. Ecol. Environ. Res. 2014, 12, 1–11. [Google Scholar] [CrossRef]
- Hanley, S.J.; Karp, A. Genetic strategies for dissecting complex traits in biomass willows (Salix spp.). Tree Physiol. 2014, 34, 1167–1180. [Google Scholar] [CrossRef]
- Gencsi, L.; Vancsura, R. Dendrology; Mezőgazda Publisher: Budapest, Hungary, 1992. (In Hungarian) [Google Scholar]
- Koop, H. Vegetative reproduction of trees in some European natural forests. Vegetatio 1987, 72, 103–110. [Google Scholar]
- Blaskó, L.; Balogh, I.; Ábrahám, É.B. Possibilities of sweet sorghum production for ethanol on the Hungarian plain. Cereal Res. Commun. 2008, 36, 1251–1254. [Google Scholar]
- Szalay, D.; Papp, V.; Hodúr, C.; Czupy, I. Examination of bark content for different species of short rotation coppice. In Proceedings of the IOP Conference Series: Earth and Environmental Science, 5th International Conference on Environment and Renewable Energy, Ho Chi Minh City, Vietnam, 25–28 February 2019; Volume 307. [Google Scholar] [CrossRef]
- Shara, M.; Stohs, J.S. Efficacy and safety of white willow bark (Salix alba) extracts. Phytother. Res. 2015, 29, 1112–1116. [Google Scholar] [CrossRef]
- Gyuricza, C.; Nagy, L.; Ujj, A.; Miko, P.; Alexa, L. The impact of composts on the heavy metal content of the soil and plants in energy willow plantations (Salix sp.). Cereal Res. Commun. 2008, 36, 279–282. Available online: https://www.jstor.org/stable/90002695 (accessed on 12 March 2021).
- Smart, B.L.; Cameron, K.D. Shrub willow. In Handbook of Bioenergy Crop Plants; Kole, C., Joshi, C.P., Shonnard, D.R., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2012; pp. 687–708. [Google Scholar]
- Aronsson, P.; Perttu, K. Willow vegetation filters for wastewater treatment and soil remediation combined with biomass production. For. Chonicle 2001, 77, 293–299. [Google Scholar] [CrossRef]
- Tóth, F.; Zsuga, K.; Kerepeczki, É.; Nagy-Berzi, L.; Körmöczi, L.; Lövei, L.G. Seasonal differences in taxonomic diversity of rotifer communities in a Hungarian lowland oxbow lake esposed to aquaculture effluent. Water 2020, 12, 1300. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and improvement of saline and alkali soils. Agric. Handb. 1954, 60. [Google Scholar] [CrossRef]
- Castro, R.S.; Borges Azevedo, C.M.S.; Bezerra-Neto, F. Increasing cherry tomato yield using fish effluent as irrigation water in Northeast Brazil. Sci. Hortic. 2006, 110, 44–50. [Google Scholar] [CrossRef]
- Miranda, F.R.; Lima, R.N.; Crisóstomo, L.A.; Santana, M.G.S. Reuse of inland low-salinity shrimp farm effluent for melon irrigation. Aquac. Eng. 2008, 39, 1–5. [Google Scholar] [CrossRef]
- Al-Jaloud, A.A.; Hussain, G.; Alsadon, A.A.; Siddiqui, A.Q.; Al-Najada, A. Use of aquaculture effluent as a supplemental source of nitrogen fertilizer to wheat crop. Arid Soil Res. Rehabil. 1993, 7, 233–241. [Google Scholar] [CrossRef]
- Dhawan, A.; Sehdev, R.S. Present status and scope of integrated fish farming in the north-west plains of India. In Integrated Fish Farming; Mathias, J.A., Charles, A.T., Baotong, H., Eds.; CRC Press: Boca Raton, FL, USA; New York, NY, USA, 1994; pp. 295–306. [Google Scholar]
- Jahany, M.; Rezapour, S. Assessment of the quality indices of soils irrigated with treated wastewater in a calcareous semi-arid environment. Ecol. Indic. 2020, 109, 105800. [Google Scholar] [CrossRef]
- Malash, N.; Flowers, T.J.; Ragab, R. Effect of irrigation systems and water management practices using saline and non-saline water on tomato production. Agric. Water Manag. 2005, 78, 25–38. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, B.; Yang, Y.; Lu, Z.H. The effects of treated wastewater irrigation on soil health. Adv. Mater. Res. 2011, 393–395, 1545–1549. [Google Scholar] [CrossRef]
- Shilpi, S.; Seshadri, B.; Sarkar, B.; Bolan, B.; Lamb, D.; Naidu, R. Comparative values of various wastewater streams as a soil nutrient source. Chemosphere 2018, 192, 272–281. [Google Scholar] [CrossRef]
- Purves, D. Waste materials deliberately added to the soil. In Trace-Element Contamination of the Environment; Elsevier: Amsterdam, The Netherlands, 1977; Volume 93. [Google Scholar]
- Hopkins, B.G.; Horneck, D.A.; Stevens, R.G.; Ellsworth, J.W.; Sullivan, D.M. Managing Irrigation Water Quality for Crop Production in the Pacific Northwest; Technical Report; Oregon State University Extension Service: Corvallis, OR, USA, 2007; Available online: https://catalog.extension.oregonstate.edu/sites/catalog/files/project/pdf/pnw597.pdf (accessed on 8 April 2021).
- Sheoran, P.; Basak, N.; Kumar, A.; Yadav, R.K.; Singh, R.; Sharma, R.; Kumar, S.; Singh, R.K.; Sharma, P.C. Ameliorants and salt tolerant varieties improve rice-wheat production in soils undergoing sodification with alkali water irrigation in Indo–Gangetic Plains of India. Agric. Water Manag. 2021, 243, 106492. [Google Scholar] [CrossRef]
- Truua, M.; Truua, J.; Heinsoo, K. Changes in soil microbial community under willow coppice: The effect of irrigation with secondary-treated municipal wastewater. Ecol. Eng. 2009, 35, 1011–1020. [Google Scholar] [CrossRef]
- Kun, Á.; Bozán, C.; Oncsik, B.M.; Barta, K. Calculation nitrogen and sodium budget from lysimeter-grown short-rotation willow coppice experiment. Columella 2018, 5, 43–51. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Yoder, B.J.; Pettigrew-Crosby, R.E. Predicting nitrogen and chlorophyll content and concentrations from reflectance spectra (400–2500 nm) at leaf and canopy scales. Remote Sens. Environ. 1995, 53, 199–211. [Google Scholar] [CrossRef]
- Peng, Y.; Gitelson, A.A. Application of chlorophyll-related vegetation indices for remote estimation of maize productivity. Agric. For. Meteorol. 2011, 151, 1267–1276. [Google Scholar] [CrossRef]
- Niinemets, U.; Tenhunen, J.D. A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ. 1997, 20, 845–866. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Pinkard, E.A.; Patel, V.; Mohammed, C. Chlorophyll and nitrogen determination for plantation-grown Eucalyptus nitens and E. globulus using a non-destructive meter. For. Ecol. Manag. 2006, 223, 211–217. [Google Scholar] [CrossRef]
- Chang, S.X.; Robinson, D.J. Nondestructive and rapid estimation of hardwood foliar nitrogen status using the SPAD-502 chlorophyll meter. For. Ecol. Manag. 2003, 181, 331–338. [Google Scholar] [CrossRef]
- Geirth, M.; Mäser, P. Potassium transporters in plants—Involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett. 2007, 581, 2348–2356. [Google Scholar] [CrossRef]
- Freitas, S.W.; Oliveira, B.A.; Mesquita, O.R.; Carvalho, H.H.; Prisco, T.J.; Gomes-Filho, E. Sulfur-induced salinity tolerance in lettuce is due to a better P and K uptake, lower Na/K ratio and an efficient antioxidative defense system. Sci. Hortic. 2019, 257, 108764. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Al Shiblawi, F.R.; Sentenac, H. Roles and transport of sodium and potassium in plants. In The Alkali Metal Ions: Their Role for Life; Sigel, A., Sigel, H., Sigel, R., Eds.; Springer: Cham, Switzerland, 2016; Volume 16, pp. 291–324. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Kronzucker, H.J.; Coskun, D.; Schulze, M.L.; Wong, J.R.; Britto, T.D. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef]
- Jerbi, A.; Brereton, N.J.B.; Sas, E.; Amiot, S.; Lacsapelle-T, X.; Comeau, Y.; Pitre, F.E.; Labrecque, M. High biomass yield increases in a primary effluent wastewater phytofiltration are associated to altered leaf morphology and stomal size in Salix miyabeana. Sci. Total Environ. 2020, 738, 139728. [Google Scholar] [CrossRef] [PubMed]
- Aasamaa, K.; Heinsoo, K.; Holm, B. Biomass production, water use and photosynthesis of Salix clones grown in a wastewater purification system. Biomass Bioenergy 2010, 34, 897–905. [Google Scholar] [CrossRef]
- Oddiraju, V.G.; Beyl, C.A.; Barker, P.A.; Stutte, G.W. Container size alters root growth of western black cherry as measured via image analysis. Hort. Sci. 1994, 29, 910–913. [Google Scholar] [CrossRef]

| Average Temperature (°C) | Precipitation Amount (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1981–2010 | 2015 | 2016 | 2017 | 1981–2010 | 2015 | 2016 | 2017 | |
| January | −1.0 | 2.2 | −0.9 | −6.7 | 29.1 | 58.8 | 61.6 | 28.3 |
| February | 0.5 | 2.4 | 6.0 | 2.6 | 29.9 | 17.3 | 88.5 | 30.2 |
| March | 5.6 | 7.4 | 7.3 | 9.4 | 27.8 | 25.5 | 20.0 | 13.4 |
| April | 11.5 | 11.5 | 13.4 | 11.0 | 42.0 | 8.2 | 12.3 | 49.7 |
| May | 16.8 | 17.1 | 16.6 | 17.2 | 50.6 | 53.7 | 18.8 | 40.9 |
| June | 19.8 | 21.2 | 21.3 | 22.1 | 61.3 | 21.0 | 124.4 | 69.3 |
| July | 21.9 | 24.4 | 22.5 | 22.8 | 57.5 | 31.4 | 123.6 | 31.8 |
| August | 21.4 | 24.2 | 21.1 | 23.7 | 50.7 | 40.9 | 50.5 | 33.3 |
| September | 16.6 | 18.7 | 18.3 | 16.6 | 47.8 | 64.0 | 9.8 | 74.2 |
| October | 11.2 | 10.4 | 9.8 | 11.6 | 32.4 | 105.2 | 72.7 | 33.7 |
| November | 5.0 | 6.3 | 5.0 | 6.0 | 41.3 | 3.2 | 49.6 | 39.6 |
| December | 0.3 | 2.6 | −1.2 | 2.7 | 44.8 | 4.5 | 1.0 | 89.2 |
| Average/Summa | 10.8 | 12.4 | 11.6 | 11.6 | 515.3 | 433.7 | 633.6 | 533.6 |
| Irrigation Water Doses | Possibility of Irrigation during the Investigated Period | Amount of Water Applied by Irrigation (mm) | Precipitation during the Investigated Period (mm) | Amount of Available Water Quantity during the Investigated Period (mm) | |
|---|---|---|---|---|---|
| 2015 | 15 mm | 15 * | 310 | 105 | 415 |
| 30 mm | 520 | 625 | |||
| 60 mm | 940 | 1045 | |||
| 2016 | 15 mm | 6 | 90 | 308 | 398 |
| 30 mm | 180 | 488 | |||
| 60 mm | 360 | 668 | |||
| 2017 | 15 mm | 9 | 135 | 184 | 319 |
| 30 mm | 270 | 454 | |||
| 60 mm | 540 | 724 |
| EC | NH4-N | N | P | K | Na | SAR | |
|---|---|---|---|---|---|---|---|
| (µS/cm) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | ||
| Effluent water | 1306.7 | 21.9 | 29 | 3.9 | 7.2 | 273.5 | 11.9 |
| Körös River water | 388.3 | 0.4 | 1.2 | 0.2 | 4.3 | 31.3 | 1.2 |
| Diluted water | 1073.0 | 10.3 | 13.3 | 1.7 | 5.4 | 132.3 | 3.5 |
| Exchangeable Sodium Percentage ΔESP (2015–2017) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Depth of Soil Layer | Irrigation Water | Irrigation Water Amount | ||||||
| 15 mm | p-Value 1 | 30 mm | p-Value 1 | 60 mm | p-Value 2 | Non-Irrigated | ||
| Mean ± Std. Deviation | ||||||||
| 0–20 cm | Effluent water | 4.66 ± 0.6 | *** | 5.9 ± 0.77 | *** | 6.85 ± 0.10 c | *** | Non-irrigated 0–20 cm: 0.36 ± 0.2 |
| Körös River water | 0.05 ± 0.1 | 0.5 ± 0.35 | −0.62 ± 0.16 a | |||||
| Diluted water | - | - | - | - | 2.19 ± 0.30 b | |||
| 20–40 cm | Effluent water | 2.85 ± 1.1 | * | 3.5 ± 1.10 | ** | 5.82 ± 0.64 c | *** | Non-irrigated 20–40 cm: 0.33 ± 0.1 |
| Körös River water | 0.14 ± 0.1 | 0.4 ± 0.36 | −0.68 ± 0.08 a | |||||
| Diluted water | - | - | - | - | 1.85 ± 0.45 b | |||
| 40–60 cm | Effluent water | 1.02 ± 0.8 | n.s. | 1.8 ± 0.05 | *** | 4.38 ± 0.74 c | *** | Non-irrigated 20–40 cm: 0.32 ± 0.4 |
| Körös River water | 0.02 ± 0.2 | 0.4 ± 0.09 | −0.53 ± 0.23 a | |||||
| Diluted water | - | - | - | - | 1.19 ± 0.13 b | |||
| Available N2017 (mg/kg) | |||
|---|---|---|---|
| Irrigation water | Irrigation water amount | ||
| 15 mm | 30 mm | 60 mm | |
| Mean ± Std. Deviation | |||
| Effluent water | 13.43 ± 7.71 a | 16.65 ± 4.04 b | 15.46 ± 3.29 c |
| Diluted water | - | - | 7.52 ± 3.85 b |
| Körös River water | 7.02 ± 3.85 a | 3.65 ± 0.78 a * | 2.96 ± 0.28 a * |
| Non-irrigated control | 11.70 ± 4.53 | ||
| E15 | E30 | E60 | D | C | ||
|---|---|---|---|---|---|---|
| 2015 | leaf | 3023 ± 241 b | 2737 ± 95 ab | 2180 ± 370 a | 1990 ± 144 a | 3340 ± 419 b |
| 2016 | 2643 ± 57 ab | 1865 ± 210 a | 2007 ± 519 ab | 2850 ± 365 b | 1855 ± 219 a | |
| 2017 | 2428 ± 19 ab | 2272 ± 127 ab | 2532 ± 196 ab | 2723 ± 118 b | 2123 ± 66 a | |
| 2015 | stem | 1537 ± 35 c | 1050 ± 221 ab | 1330 ± 193 bc | 813 ± 79 a | 1647 ± 146 c |
| 2016 | 2192 ± 201 bc | 2010 ± 172 ab | 1788 ± 244 a | 2457 ± 201 c | 1740 ± 146 a | |
| 2017 | 1616 ± 209 a | 1596 ± 169 a | 1422 ± 266 a | 2003 ± 239 b | 1693 ± 90 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolozsvári, I.; Kun, Á.; Jancsó, M.; Bakti, B.; Bozán, C.; Gyuricza, C. Utilization of Fish Farm Effluent for Irrigation Short Rotation Willow (Salix alba L.) under Lysimeter Conditions. Forests 2021, 12, 457. https://doi.org/10.3390/f12040457
Kolozsvári I, Kun Á, Jancsó M, Bakti B, Bozán C, Gyuricza C. Utilization of Fish Farm Effluent for Irrigation Short Rotation Willow (Salix alba L.) under Lysimeter Conditions. Forests. 2021; 12(4):457. https://doi.org/10.3390/f12040457
Chicago/Turabian StyleKolozsvári, Ildikó, Ágnes Kun, Mihály Jancsó, Beatrix Bakti, Csaba Bozán, and Csaba Gyuricza. 2021. "Utilization of Fish Farm Effluent for Irrigation Short Rotation Willow (Salix alba L.) under Lysimeter Conditions" Forests 12, no. 4: 457. https://doi.org/10.3390/f12040457
APA StyleKolozsvári, I., Kun, Á., Jancsó, M., Bakti, B., Bozán, C., & Gyuricza, C. (2021). Utilization of Fish Farm Effluent for Irrigation Short Rotation Willow (Salix alba L.) under Lysimeter Conditions. Forests, 12(4), 457. https://doi.org/10.3390/f12040457






