Nutrient Dynamics Assessment of Coarse Wood Debris Subjected to Successional Decay Levels of Three Forests Types in Northeast, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Experiment Design and Sample Collection
2.3. Sample Processing and Analyses of Nutrients
2.4. Soil Sampling and Analysis
2.5. Statistical Analyses
3. Results
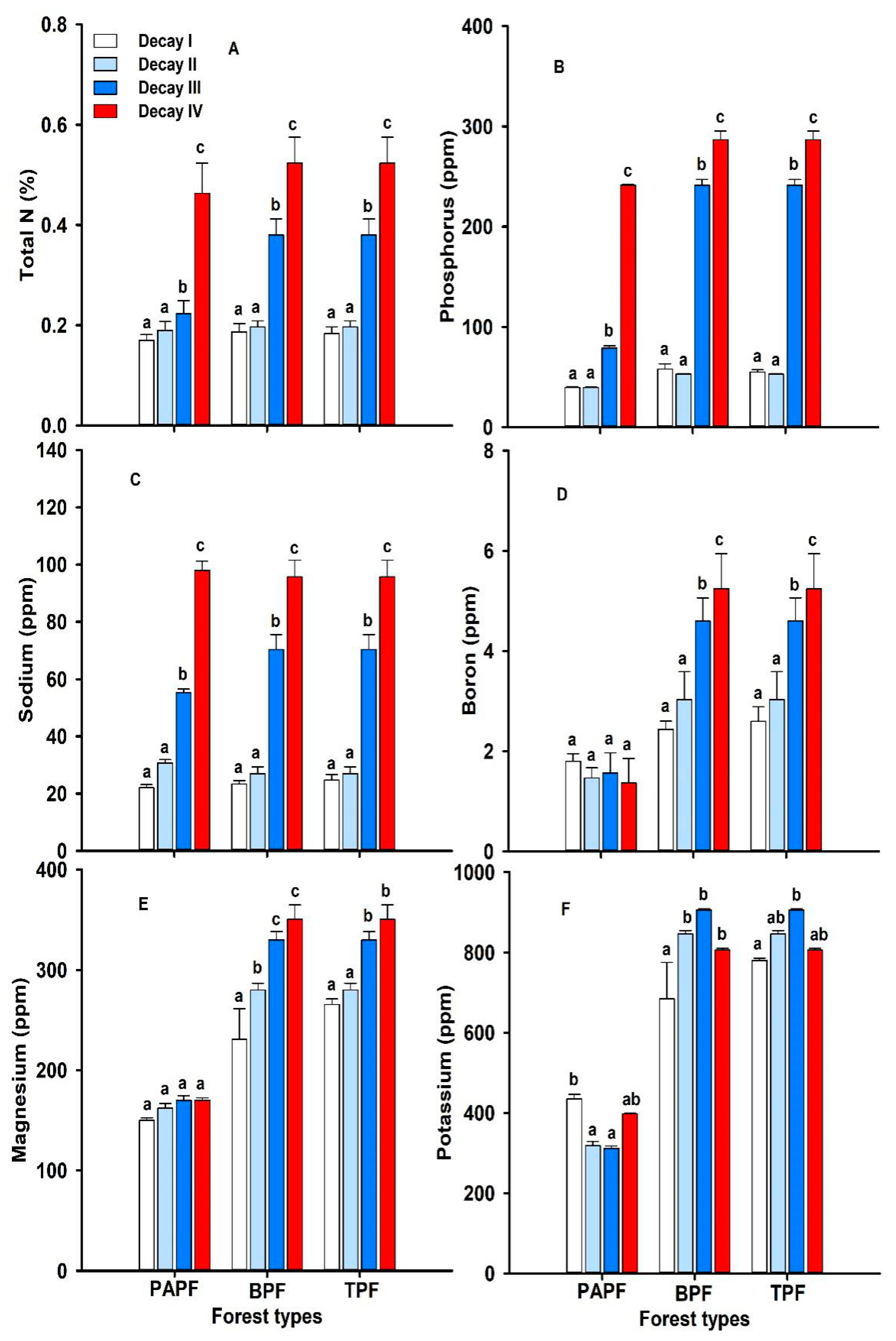
3.1. Nutrient Concentration across Different Decay Classes
3.2. Nutrient Concentration across Different Forest Types
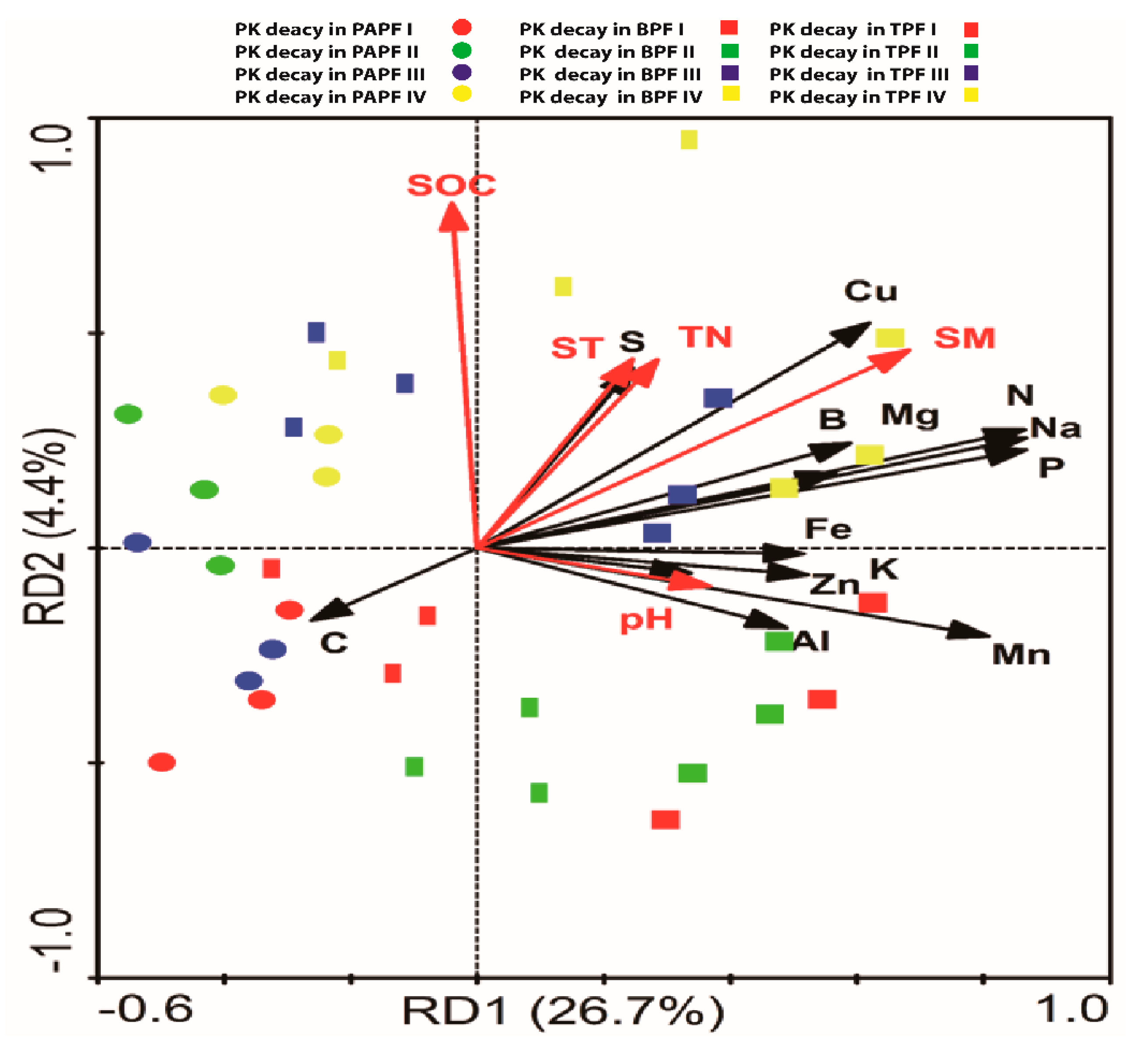
3.3. Interrelations of the Nutrient Composition of CWD with Environmental Factors
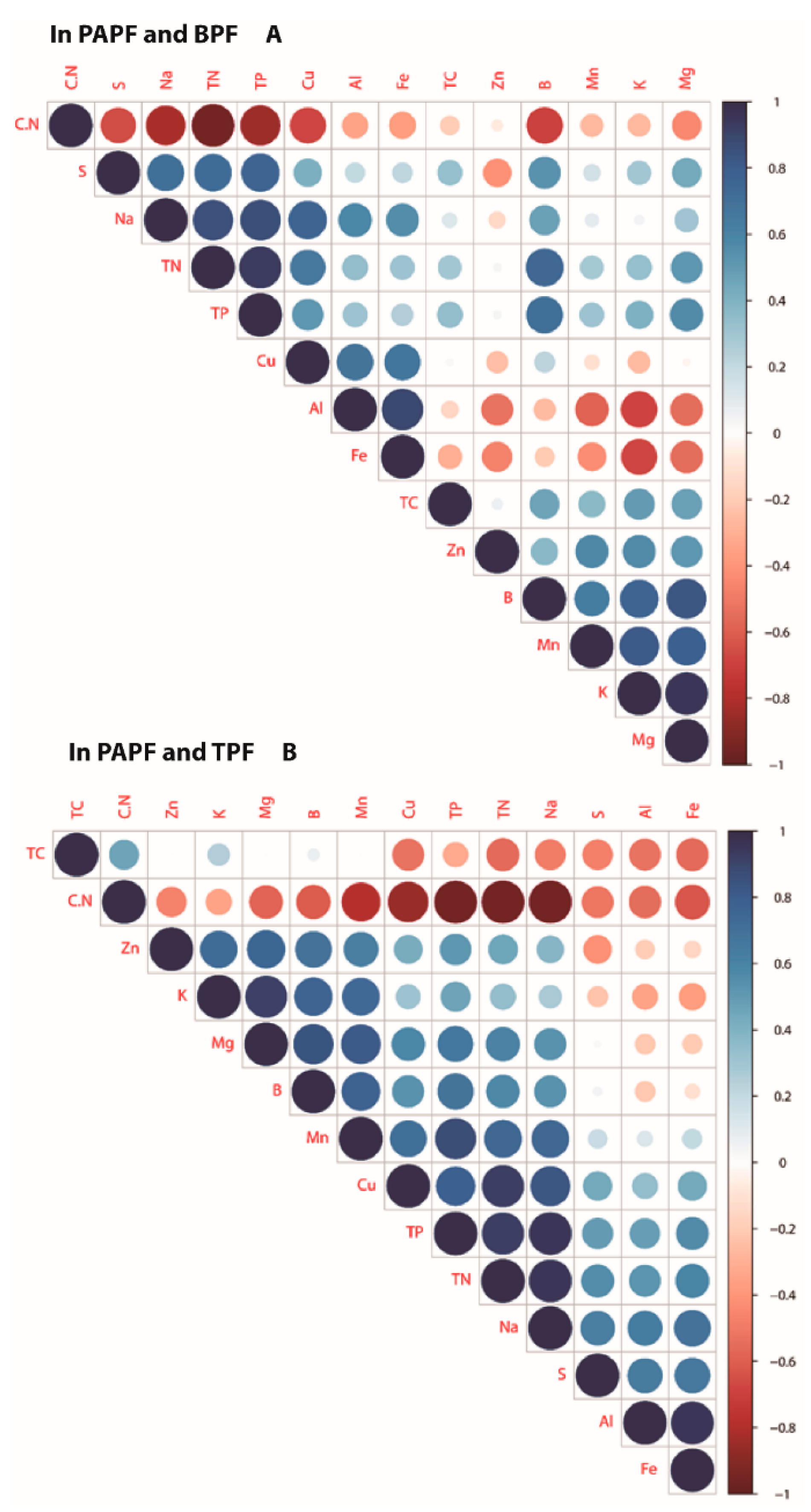
3.4. Correlations between the Nutrient Compositions of CWD across Forest Types
4. Discussion
4.1. Decay Class’s Effect on Nutrient Dynamics
4.2. Effect of Species on Nutrient Dynamics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunnell, F.L.; Houde, I. Down wood and biodiversity—Implications to forest practices. Environ. Rev. 2010, 18, 397–421. [Google Scholar] [CrossRef]
- Siitonen, J. Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecol. Bull. 2001, 49, 11–41. [Google Scholar]
- Bolton, N.W.; D’Amato, A.W. Regeneration responses to gap size and coarse woody debris within natural disturbance-based silvicultural systems in northeastern Minnesota, USA. For. Ecol. Manag. 2011, 262, 1215–1222. [Google Scholar] [CrossRef]
- Åström, M.; Dynesius, M.; Hylander, K.; Nilsson, C. Effects of slash harvest on bryophytes and vascular plants in southern boreal forest clear-cuts. J. Appl. Ecol. 2005, 42, 1194–1202. [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.; Lattin, J.; Anderson, N.; Cline, S.; Aumen, N.; Sedell, J. Ecology of coarse woody debris in temperate ecosystems. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 1986; Volume 15, pp. 133–302. [Google Scholar]
- Clark, D.B.; Clark, D.A.; Brown, S.; Oberbauer, S.F.; Veldkamp, E. Stocks and flows of coarse woody debris across a tropical rain forest nutrient and topography gradient. For. Ecol. Manag. 2002, 164, 237–248. [Google Scholar] [CrossRef]
- Noh, N.J.; Yoon, T.K.; Kim, R.-H.; Bolton, N.W.; Kim, C.; Son, Y. Carbon and nitrogen accumulation and decomposition from coarse woody debris in a naturally regenerated Korean red pine (Pinus densiflora S. et Z.) forest. Forests 2017, 8, 214. [Google Scholar] [CrossRef]
- Yoon, T.K.; Noh, N.J.; Kim, S.; Han, S.; Son, Y. Coarse woody debris respiration of Japanese red pine forests in Korea: Controlling factors and contribution to the ecosystem carbon cycle. Ecol. Res. 2015, 30, 723–734. [Google Scholar] [CrossRef]
- Russell, M.B.; Fraver, S.; Aakala, T.; Gove, J.H.; Woodall, C.W.; D’Amato, A.W.; Ducey, M.J. Quantifying carbon stores and decomposition in dead wood: A review. For. Ecol. Manag. 2015, 350, 107–128. [Google Scholar] [CrossRef]
- Magnússon, R.Í.; Tietema, A.; Cornelissen, J.H.; Hefting, M.M.; Kalbitz, K. Tamm Review: Sequestration of carbon from coarse woody debris in forest soils. For. Ecol. Manag. 2016, 377, 1–15. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.; Allison, S.D.; Bauhus, J.; Eggleton, P.; Preston, C.M.; Scarff, F.; Weedon, J.T.; Wirth, C.; Zanne, A.E. Plant traits and wood fates across the globe: Rotted, burned, or consumed? Glob. Chang. Biol. 2009, 15, 2431–2449. [Google Scholar] [CrossRef]
- Brovkin, V.; Van Bodegom, P.; Kleinen, T.; Wirth, C.; Cornwell, W.; Cornelissen, J.; Kattge, J. Plant-driven variation in decomposition rates improves projections of global litter stock distribution. Biogeosciences 2012, 9, 565–576. [Google Scholar] [CrossRef]
- Herrero, C.; Krankrina, O.; Monleon, V.J.; Bravo Oviedo, F. Amount and distribution of coarse woody debris in pine ecosystems of north-western Spain, Russia and the United States. iFor. Biogeosci. For. 2013, 7, 53–60. [Google Scholar] [CrossRef]
- Filipiak, M. Nutrient dynamics in decomposing dead wood in the context of wood eater requirements: The ecological stoichiometry of saproxylophagous insects. In Saproxylic Insects; Springer: Berlin/Heidelberg, Germany, 2018; pp. 429–469. [Google Scholar]
- Walczynska, A. How does a xylem-feeder maximize its fitness? Bull. Entomol. Res. 2012, 102, 644. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, M.; Weiner, J. How to make a beetle out of wood: Multi-elemental stoichiometry of wood decay, xylophagy and fungivory. PLoS ONE 2014, 9, e115104. [Google Scholar] [CrossRef] [PubMed]
- Dighton, J. Fungi in Ecosystem Processes; CRC Press: Boca Raton, FL, USA, 2016; Volume 31. [Google Scholar]
- Cotrufo, M.F.; Ineson, P. Does elevated atmospheric CO2 concentrations affect wood decomposition? Plant Soil 2000, 224, 51–57. [Google Scholar] [CrossRef]
- Creed, I.; Morrison, D.; Nicholas, N. Is coarse woody debris a net sink or source of nitrogen in the red spruce Fraser fir forest of the southern Appalachians, USA? Can. J. For. Res. 2004, 34, 716–727. [Google Scholar] [CrossRef]
- Krzyszowska-Waitkus, A.; Vance, G.F.; Preston, C.M. Influence of coarse wood and fine litter on forest organic matter composition. Can. J. Soil Sci. 2006, 86, 35–46. [Google Scholar] [CrossRef]
- Laiho, R.; Prescott, C.E. Decay and nutrient dynamics of coarse woody debris in northern coniferous forests: A synthesis. Can. J. For. Res. 2004, 34, 763–777. [Google Scholar] [CrossRef]
- Saunders, M.R.; Fraver, S.; Wagner, R.G. Nutrient concentration of down woody debris in mixedwood forests in central Maine, USA. Silva Fennica. 2011, 45, 197–210. [Google Scholar] [CrossRef]
- Gonzalez-Polo, M.; Fernández-Souto, A.; Austin, A.T. Coarse woody debris stimulates soil enzymatic activity and litter decomposition in an old-growth temperate forest of Patagonia, Argentina. Ecosystems 2013, 16, 1025–1038. [Google Scholar] [CrossRef]
- Lombardi, F.; Cherubini, P.; Tognetti, R.; Cocozza, C.; Lasserre, B.; Marchetti, M. Investigating biochemical processes to assess deadwood decay of beech and silver fir in Mediterranean mountain forests. Ann. For. Sci. 2013, 70, 101–111. [Google Scholar] [CrossRef]
- Silva, L.F.S.G.; de Castilho, C.V.; de Oliveira Cavalcante, C.; Pimentel, T.P.; Fearnside, P.M.; Barbosa, R.I. Production and stock of coarse woody debris across a hydro-edaphic gradient of oligotrophic forests in the northern Brazilian Amazon. For. Ecol. Manag. 2016, 364, 1–9. [Google Scholar] [CrossRef]
- Sefidi, K.; Esfandiary Darabad, F.; Azaryan, M. Effect of topography on tree species composition and volume of coarse woody debris in an Oriental beech (Fagus orientalis Lipsky) old growth forests, northern Iran. iFor. Biogeosci. For. 2016, 9, 658. [Google Scholar] [CrossRef]
- Palviainen, M.; Finér, L.; Laiho, R.; Shorohova, E.; Kapitsa, E.; Vanha-Majamaa, I. Carbon and nitrogen release from decomposing Scots pine, Norway spruce and silver birch stumps. For. Ecol. Manag. 2010, 259, 390–398. [Google Scholar] [CrossRef]
- Yan, E.; Wang, X.; Huang, J. Concept and classification of coarse woody debris in forest ecosystems. Front. Biol. China 2006, 1, 76–84. [Google Scholar] [CrossRef]
- Wang, W.-J.; Chang, Y.; Liu, Z.-H.; Chen, H.-W.; Jing, G.-Z.; Zhang, H.-X.; Wang, J.-H. Coarse woody debris loading capacity and its environmental gradient in Huzhong forest area of Great Xing‘an Mountains. J. Appl. Ecol. 2009, 20, 773–778. [Google Scholar]
- Yuan, J.; Cheng, F.; Zhao, P.; Qiu, R.; Wang, L.; Zhang, S. Characteristics in coarse woody debris mediated by forest developmental stage and latest disturbances in a natural secondary forest of Pinus tabulaeformis. Acta Ecol. Sin. 2014, 34, 232–238. [Google Scholar] [CrossRef]
- Yang, F.-F.; Li, Y.-L.; Zhou, G.-Y.; Wenigmann, K.; Zhang, D.-Q.; Wenigmann, M.; Liu, S.-Z.; Zhang, Q.-M. Dynamics of coarse woody debris and decomposition rates in an old-growth forest in lower tropical China. For. Ecol. Manag. 2010, 259, 1666–1672. [Google Scholar] [CrossRef]
- Cardoso Filho, J.A.; Leal, G.A., Jr. Soil microbial ecology and its role in soil carbon sequestration in sustainable agroecosystems under climate change. In Carbon and Nitrogen Cycling in Soil; Springer: Singapore, 2020; pp. 249–291. [Google Scholar]
- Zeng, L.; He, W.; Teng, M.; Luo, X.; Yan, Z.; Huang, Z.; Zhou, Z.; Wang, P.; Xiao, W. Effects of mixed leaf litter from predominant afforestation tree species on decomposition rates in the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 639, 679–686. [Google Scholar] [CrossRef]
- Forrester, D.I. Transpiration and water-use efficiency in mixed-species forests versus monocultures: Effects of tree size, stand density and season. Tree Physiol. 2015, 35, 289–304. [Google Scholar] [CrossRef]
- Gonzalez de Andres, E.; Seely, B.; Blanco, J.A.; Imbert, J.B.; Lo, Y.H.; Castillo, F.J. Increased complementarity in water-limited environments in Scots pine and European beech mixtures under climate change. Ecohydrology 2017, 10, e1810. [Google Scholar] [CrossRef]
- Fichtner, A.; Von Oheimb, G.; Härdtle, W.; Wilken, C.; Gutknecht, J. Effects of anthropogenic disturbances on soil microbial communities in oak forests persist for more than 100 years. Soil Biol. Biochem. 2014, 70, 79–87. [Google Scholar] [CrossRef]
- Prescott, C.E. The influence of the forest canopy on nutrient cycling. Tree Physiol. 2002, 22, 1193–1200. [Google Scholar] [CrossRef]
- Mayor, Á.G.; Goirán, S.B.; Vallejo, V.R.; Bautista, S. Variation in soil enzyme activity as a function of vegetation amount, type, and spatial structure in fire-prone Mediterranean shrublands. Sci. Total Environ. 2016, 573, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Bayranvand, M.; Kooch, Y.; Rey, A. Earthworm population and microbial activity temporal dynamics in a Caspian Hyrcanian mixed forest. Eur. J. For. Res. 2017, 136, 447–456. [Google Scholar] [CrossRef]
- Lindén, M.; Agestam, E. Increment and yield in mixed and monoculture stands of Pinus sylvestris and Picea abies based on an experiment in southern Sweden. Scand. J. For. Res. 2003, 18, 155–162. [Google Scholar] [CrossRef]
- Felton, A.; Nilsson, U.; Sonesson, J.; Felton, A.M.; Roberge, J.-M.; Ranius, T.; Ahlström, M.; Bergh, J.; Björkman, C.; Boberg, J. Replacing monocultures with mixed-species stands: Ecosystem service implications of two production forest alternatives in Sweden. Ambio 2016, 45, 124–139. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L.; Vanclay, J.K. Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: A review. For. Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef]
- Pretzsch, H.; Bielak, K.; Block, J.; Bruchwald, A.; Dieler, J.; Ehrhart, H.-P.; Kohnle, U.; Nagel, J.; Spellmann, H.; Zasada, M. Productivity of mixed versus pure stands of oak (Quercus petraea (M att.) L iebl. and Quercus robur L.) and European beech (Fagus sylvatica L.) along an ecological gradient. Eur. J. For. Res. 2013, 132, 263–280. [Google Scholar] [CrossRef]
- Richards, A.E.; Forrester, D.I.; Bauhus, J.; Scherer-Lorenzen, M. The influence of mixed tree plantations on the nutrition of individual species: A review. Tree Physiol. 2010, 30, 1192–1208. [Google Scholar] [CrossRef] [PubMed]
- Klemmedson, J.O. Influence of oak in pine forests of central Arizona on selected nutrients of forest floor and soil. Soil Sci. Soc. Am. J. 1987, 51, 1623–1628. [Google Scholar] [CrossRef]
- Staff, S. Keys to Soil Taxonomy, 12th ed.; Natural Resources Conservation Service, United States Department of Agriculture: Washington, DC, USA, 2014. [Google Scholar]
- Carmona, M.R.; Armesto, J.J.; Aravena, J.C.; Pérez, C.A. Coarse woody debris biomass in successional and primary temperate forests in Chiloé Island, Chile. For. Ecol. Manag. 2002, 164, 265–275. [Google Scholar] [CrossRef]
- R CoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 10 March 2021).
- Parkin, E. The digestive enzymes of some wood-boring beetle larvae. J. Exp. Biol. 1940, 17, 364–377. [Google Scholar]
- Pettersen, R.C. The chemical composition of wood. Chem. Solid Wood 1984, 207, 57–126. [Google Scholar]
- Ragland, K.; Aerts, D.; Baker, A. Properties of wood for combustion analysis. Bioresour. Technol. 1991, 37, 161–168. [Google Scholar] [CrossRef]
- Johnston, S.R.; Boddy, L.; Weightman, A.J. Bacteria in decomposing wood and their interactions with wood-decay fungi. FEMS Microbiol. Ecol. 2016, 92, fiw179. [Google Scholar] [CrossRef]
- Pastorelli, R.; Paletto, A.; Agnelli, A.E.; Lagomarsino, A.; De Meo, I. Microbial communities associated with decomposing deadwood of downy birch in a natural forest in Khibiny Mountains (Kola Peninsula, Russian Federation). For. Ecol. Manag. 2020, 455, 117643. [Google Scholar] [CrossRef]
- Sommer, R.; Vlek, P.L.; de Abreu Sá, T.D.; Vielhauer, K.; Coelho, R.d.F.R.; Fölster, H. Nutrient balance of shifting cultivation by burning or mulching in the Eastern Amazon–evidence for subsoil nutrient accumulation. Nutr. Cycl. Agroecosyst. 2004, 68, 257–271. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Horn, S.; Brownie, C.; Strickland, M.S.; Wurzburger, N.; Zanne, A. Comparison of decay rates between native and non-native wood species in invaded forests of the southeastern US: A rapid assessment. Biol. Invasions 2020, 15, 1263. [Google Scholar]
- Klockow, P.A. Impacts of biomass harvesting on biomass, carbon, and nutrient stocks in Populus tremuloides forests of northern Minnesota, USA. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, February 2012. [Google Scholar]
- Islam, W.; Noman, A.; Naveed, H.; Huang, Z.; Chen, H.Y. Role of environmental factors in shaping the soil microbiome. Environ. Sci. Pollut. Res. 2020, 27, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Hueso, R.; Arca, V.; Delgado-Baquerizo, M.; Hamonts, K.; Piñeiro, J.; Serrano-Grijalva, L.; Shawyer, J.; Power, S.A. Links between soil microbial communities, functioning, and plant nutrition under altered rainfall in Australian grassland. Ecol. Monogr. 2020, 90, e01424. [Google Scholar] [CrossRef]
- Busse, M.D. Downed bole-wood decomposition in lodgepole pine forests of central Oregon. Soil Sci. Soc. Am. J. 1994, 58, 221–227. [Google Scholar] [CrossRef]
- Bütler, R.; Patty, L.; Le Bayon, R.-C.; Guenat, C.; Schlaepfer, R. Log decay of Picea abies in the Swiss Jura Mountains of central Europe. For. Ecol. Manag. 2007, 242, 791–799. [Google Scholar] [CrossRef]
- Swift, M. Animal-microbial interactions in wood decomposition. In Invertebrate-Microbial Interactions. Joint Symposium of the British Mycological Society and the British Entomological Society, University of Exeter, September 1982; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Smythe, R.V.; Carter, F.L.; Baxter, C.C. Influence of wood decay on feeding and survival of the eastern subterranean termite, Reticulitermes flavipes (Isoptera: Rhinotermitidae). Ann. Entomol. Soc. Am. 1971, 64, 59–62. [Google Scholar] [CrossRef]
- Biedermann, P.H.; Vega, F.E. Ecology and evolution of insect–fungus mutualisms. Annu. Rev. Entomol. 2020, 65, 431–455. [Google Scholar] [CrossRef]
- Sornakili, A.; Thankappan, S.; Sridharan, A.; Nithya, P.; Uthandi, S. Antagonistic fungal endophytes and their metabolite-mediated interactions against phytopathogens in rice. Physiol. Mol. Plant Pathol. 2020, 112, 101525. [Google Scholar] [CrossRef]
- Boddy, L. Fungal community ecology and wood decomposition processes in angiosperms: From standing tree to complete decay of coarse woody debris. Ecol. Bull. 2001, 49, 43–56. [Google Scholar]
- Kovoor, J. Entomologie-modifications chimiques dune sciure de bois de peupleir sous laction dun termitide-microcerotermes edentatus (wasmann). Comptes Rendus Hebd. Seances Acad. Sci. 1964, 258, 2887. [Google Scholar]
- Gentry, J.; Whitford, W.G. The relationship between wood litter infall and relative abundance and feeding activity of subterranean termites Reticulitermes spp. in three southeastern coastal plain habitats. Oecologia 1982, 54, 63–67. [Google Scholar] [CrossRef]
- Becker, G. Versuche über den Einfluß von Braunfäulepilzen auf Wahl und Ausnutzung der Holznahrung durch Termiten; Duncker und Humblot: Berlin, Germany, 1965. [Google Scholar]
- Hendee, E. The role of fungi in the diet of the common damp-wood termite, Zootermopsis angusticollis. Hilgardia 1935, 9, 499–525. [Google Scholar] [CrossRef][Green Version]
- Filipiak, M.; Weiner, J. Nutritional dynamics during the development of xylophagous beetles related to changes in the stoichiometry of 11 elements. Physiol. Entomol. 2017, 42, 73–84. [Google Scholar] [CrossRef]
- Filipiak, M. Pollen stoichiometry may influence detrital terrestrial and aquatic food webs. Front. Ecol. Evol. 2016, 4, 138. [Google Scholar] [CrossRef]
- Johnson, D.W.; Turner, J. Tamm Review: Nutrient cycling in forests: A historical look and newer developments. For. Ecol. Manag. 2019, 444, 344–373. [Google Scholar] [CrossRef]
- Bryanin, S.; Kondratova, A.; Abramova, E. Litter decomposition and nutrient dynamics in fire-affected larch forests in the russian far east. Forests 2020, 11, 882. [Google Scholar] [CrossRef]
- Arthur, M.A.; Fahey, T.J. Mass and nutrient content of decaying boles in an Engelmann spruce–subalpine fir forest, Rocky Mountain National Park, Colorado. Can. J. For. Res. 1990, 20, 730–737. [Google Scholar] [CrossRef]
- Mladenoff, D.; Forrester, J.; Schatz, J. Impacts of Biomass Removal on Carbon and Nutrient Pools in Wisconsin Northern Hardwood Forests: Establishment of a Long-Term Study; Public Service Commision of Wisconsin: Madison, WI, USA, 2010. [Google Scholar]
- Brais, S.; Paré, D.; Lierman, C. Tree bole mineralization rates of four species of the Canadian eastern boreal forest: Implications for nutrient dynamics following stand-replacing disturbances. Can. J. For. Res. 2006, 36, 2331–2340. [Google Scholar] [CrossRef]
- Dhiedt, E.; De Keersmaeker, L.; Vandekerkhove, K.; Verheyen, K. Effects of decomposing beech (Fagus sylvatica) logs on the chemistry of acidified sand and loam soils in two forest reserves in Flanders (northern Belgium). For. Ecol. Manag. 2019, 445, 70–81. [Google Scholar] [CrossRef]
- Lo Monaco, A.; Luziatelli, G.; Latterini, F.; Tavankar, F.; Picchio, R. Structure and Dynamics of Deadwood in Pine and Oak Stands and their Role in CO2 Sequestration in Lowland Forests of Central Italy. Forests 2020, 11, 253. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, J.; Zhao, X.; Chao, Z.; Wang, Y.; Zhang, X.; Wang, D. The linkages of plant, litter and soil C: N: P stoichiometry and nutrient stock in different secondary mixed forest types in the Qinling Mountains, China. PeerJ 2020, 8, e9274. [Google Scholar] [CrossRef]
- Haggerty, C.J.; Crisman, T.L.; Rohr, J.R. Direct and indirect effects of pine silviculture on the larval occupancy and breeding of declining amphibian species. J. Appl. Ecol. 2019, 56, 2652–2662. [Google Scholar] [CrossRef]
- Shik, J.Z.; Kooij, P.W.; Donoso, D.A.; Santos, J.C.; Gomez, E.B.; Franco, M.; Crumière, A.J.; Arnan, X.; Howe, J.; Wcislo, W.T. Nutritional niches reveal fundamental domestication trade-offs in fungus-farming ants. Nat. Ecol. Evol. 2021, 5, 122–134. [Google Scholar] [CrossRef] [PubMed]
| Forest Type | Decay Classes | Soil Temperature (°C) | Soil Moisture (%) | Soil Organic C (g/kg) | Total N (g/kg) | Soil pH |
|---|---|---|---|---|---|---|
| PAPF * | I | 11.70 | 35.57 | 52.03 | 6.58 | 5.48 |
| II | 11.52 | 35.78 | 56.47 | 6.37 | 5.33 | |
| III | 11.96 | 37.52 | 59.50 | 8.32 | 5.23 | |
| IV | 12.17 | 38.86 | 62.09 | 8.33 | 5.11 | |
| TPF ** | I | 13.23 | 41.43 | 63.06 | 8.75 | 5.82 |
| II | 13.44 | 41.06 | 55.87 | 8.85 | 5.72 | |
| III | 13.72 | 42.30 | 66.59 | 9.03 | 5.57 | |
| IV | 13.96 | 44.47 | 67.66 | 9.27 | 5.48 | |
| BPF *** | I | 12.4 | 41.72 | 52.44 | 7.55 | 5.74 |
| II | 12.47 | 41.63 | 53.26 | 7.87 | 5.68 | |
| III | 12.57 | 42.38 | 59.16 | 8.22 | 5.42 | |
| IV | 12.73 | 44.71 | 61.66 | 8.70 | 5.46 |
| Forest Type | Decay Classes | C | C:N | Al | Cu | Fe | Zn | S | Mn |
|---|---|---|---|---|---|---|---|---|---|
| PAPF * | I | 51.5 ± 0.2 a | 305.7 ± 20.3 a | 45.3 ± 1.2 c | 0.6 ± 0.1 b | 90.0 ± 1.5 c | 14.0 ± 1.2 b | 125.3 ± 0.3 c | 105.0 ± 1.5 b |
| II | 50.8 ± 0.4 a | 271.6 ± 23.5 b | 40.7 ± 0.9 c | 1.0 ± 0.1 b | 75.0 ± 1.2 d | 10.7 ± 0.9 b | 180.0 ± 0.6 b | 70.3 ± 1.8 c | |
| III | 50.7 ± 0.4 a | 232.9 ± 25.7 c | 55.0 ± 1.2 b | 2.7 ± 0.9 a | 130.3 ± 1.9 b | 28.3 ± 2.0 a | 190.0 ± 1.0 b | 105.7 ± 0.9 b | |
| IV | 49.7 ± 0.6 a | 111.0 ± 14.4 d | 371.0 ± 5.5 a | 1.9 ± 0.7 a | 270.7 ± 1.5 a | 30.0 ± 1.2 a | 340.0 ± 1.2 a | 250.0 ± 7.9 a | |
| BPF ** | I | 51.0 ± 0.8 a | 278.0 ± 26.0 a | 31.0 ± 1.2 c | 0.4 ± 0.0 b | 30.7 ± 1.7 d | 210.3 ± 5.2 c | 33.7 ± 1.5 d | 140.3 ± 1.2 d |
| II | 51.4 ± 0.1 a | 263.2 ± 14.9 a | 25.3 ± 0.9 c | 0.6 ± 0.0 b | 48.4 ± 1.9 c | 215.7 ± 3.8 c | 45.7 ± 3.5 c | 355.0 ± 1.5 c | |
| III | 52.1 ± 0.4 a | 139.2 ± 12.9 b | 44.3 ± 1.2 b | 2.1 ± 0.5 a | 85.0 ± 1.6 b | 250.3 ± 6.4 b | 87.0 ± 5.5 b | 510.0 ± 1.7 a | |
| IV | 50.2 ± 0.6 a | 98.2 ± 11.3 c | 100.3 ± 4.5 a | 2.5 ± 0.6 a | 120.3 ± 1.3 a | 390.7 ± 7.2 a | 112.0 ± 11.4 a | 390.7 ± 1.8 b | |
| TPF *** | I | 51.4 ± 0.6 a | 283.2 ± 20.9 a | 33.0 ± 3.1 c | 0.5 ± 0.1 b | 56.0 ± 1.0 c | 72.7 ± 40.2 c | 102.3 ± 37.2 d | 183.0 ± 23.2 d |
| II | 51.4 ± 0.1 a | 263.2 ± 14.9 a | 25.3 ± 0.9 c | 0.6 ± 0.0 b | 48.4 ± 1.9 c | 45.7 ± 3.5 d | 355.0 ± 1.5 c | 215.7 ± 3.8 c | |
| III | 52.1 ± 0.4 a | 139.2 ± 12.9 b | 44.3 ± 1.2 b | 2.1 ± 0.5 a | 85.0 ± 1.6 b | 87.0 ± 5.5 b | 510.0 ± 1.7 a | 250.3 ± 6.4 b | |
| IV | 50.2 ± 0.6 a | 98.2 ± 11.3 c | 100.3 ± 4.5 a | 2.5 ± 0.6 a | 120.3 ± 1.3 a | 112.0 ± 11.4 a | 390.7 ± 1.8 b | 390.7 ± 7.2 a |
| Source of Variation | df | N | C | C:N | P | Al | B | Cu |
|---|---|---|---|---|---|---|---|---|
| Forest Type(FT) | 2 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Decay classes (Decay) | 3 | 0.024 | 0.189 | 0.016 | <0.001 | <0.001 | <0.001 | 0.900 |
| FT× Decay | 6 | 0.233 | 0.647 | 0.191 | <0.001 | <0.001 | 0.021 | 0.812 |
| Source of variation | df | Fe | K | Mg | Zn | Na | S | Mn |
| Forest Type(FT) | 2 | <0.001 | 0.022 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Decay classes (Decay) | 3 | <0.001 | <0.001 | <0.001 | <0.001 | 0.462 | <0.001 | <0.001 |
| FT × Decay | 6 | <0.001 | <0.001 | 0.006 | <0.001 | 0.122 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, K.; Tuyen, T.T.; Chen, L.; Duan, W.; Hussain, A.; Jamil, M.A.; Li, C.; Guo, Q.; Qu, M.; Wang, Y.; et al. Nutrient Dynamics Assessment of Coarse Wood Debris Subjected to Successional Decay Levels of Three Forests Types in Northeast, China. Forests 2021, 12, 401. https://doi.org/10.3390/f12040401
Khan K, Tuyen TT, Chen L, Duan W, Hussain A, Jamil MA, Li C, Guo Q, Qu M, Wang Y, et al. Nutrient Dynamics Assessment of Coarse Wood Debris Subjected to Successional Decay Levels of Three Forests Types in Northeast, China. Forests. 2021; 12(4):401. https://doi.org/10.3390/f12040401
Chicago/Turabian StyleKhan, Kashif, Tran Thi Tuyen, Lixin Chen, Wenbiao Duan, Anwaar Hussain, Muhammad Atif Jamil, Changzhun Li, Qiwen Guo, Meixue Qu, Yafei Wang, and et al. 2021. "Nutrient Dynamics Assessment of Coarse Wood Debris Subjected to Successional Decay Levels of Three Forests Types in Northeast, China" Forests 12, no. 4: 401. https://doi.org/10.3390/f12040401
APA StyleKhan, K., Tuyen, T. T., Chen, L., Duan, W., Hussain, A., Jamil, M. A., Li, C., Guo, Q., Qu, M., Wang, Y., & Khan, A. (2021). Nutrient Dynamics Assessment of Coarse Wood Debris Subjected to Successional Decay Levels of Three Forests Types in Northeast, China. Forests, 12(4), 401. https://doi.org/10.3390/f12040401








