Effects of Large-Scale Nitrogen Fertilization on Insect–Plant Interactions in the Canopy of Tall Alder Trees with N2-Fixing Traits in a Cool Temperate Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Study Species
2.2. Large-Scale N Fertilization Experiment
2.3. Estimation of Leaf Traits
2.4. Insect Herbivore Feeding Guilds
2.5. Statistical Analyses
3. Results
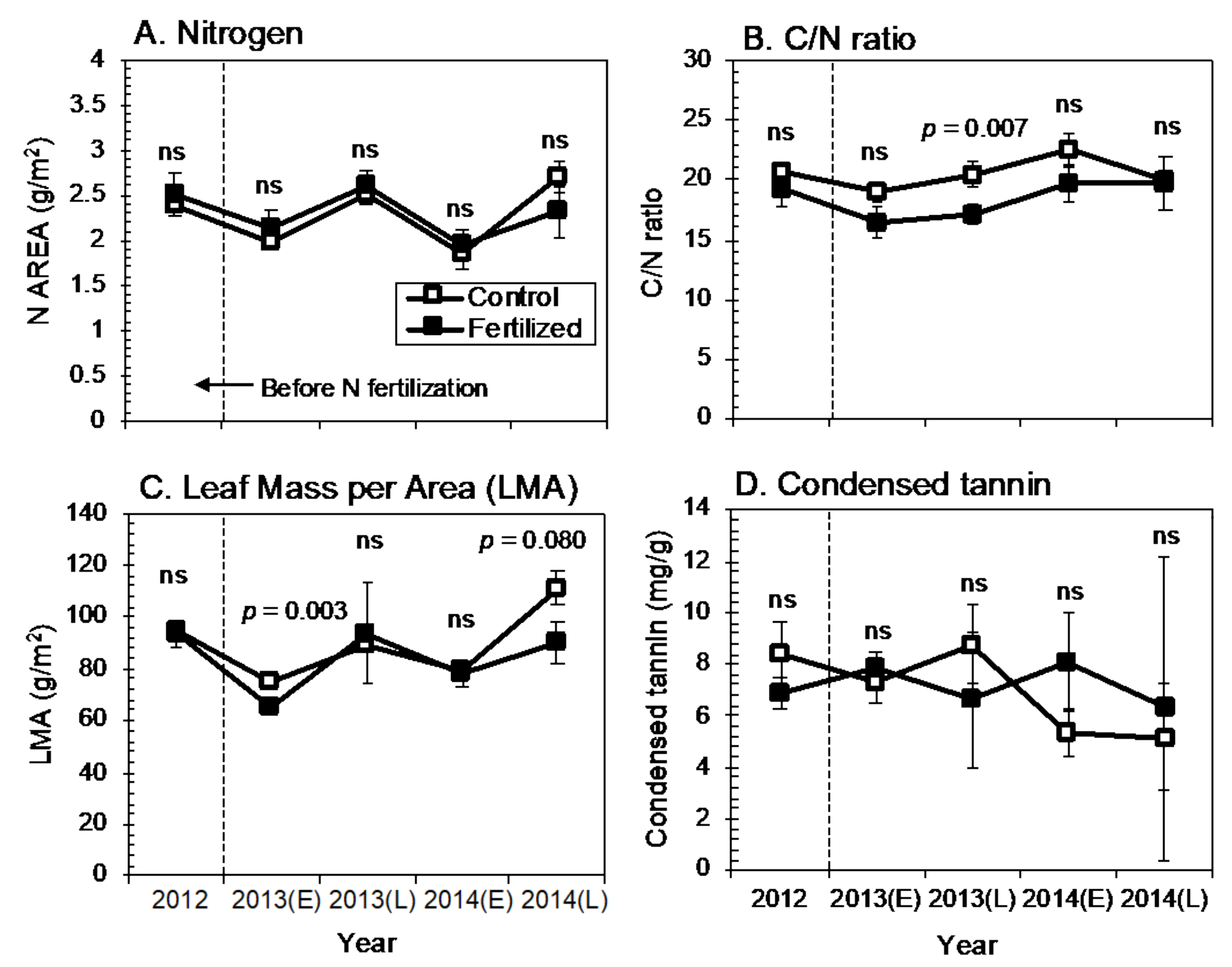
3.1. Effect of N Fertilization on Leaf Traits
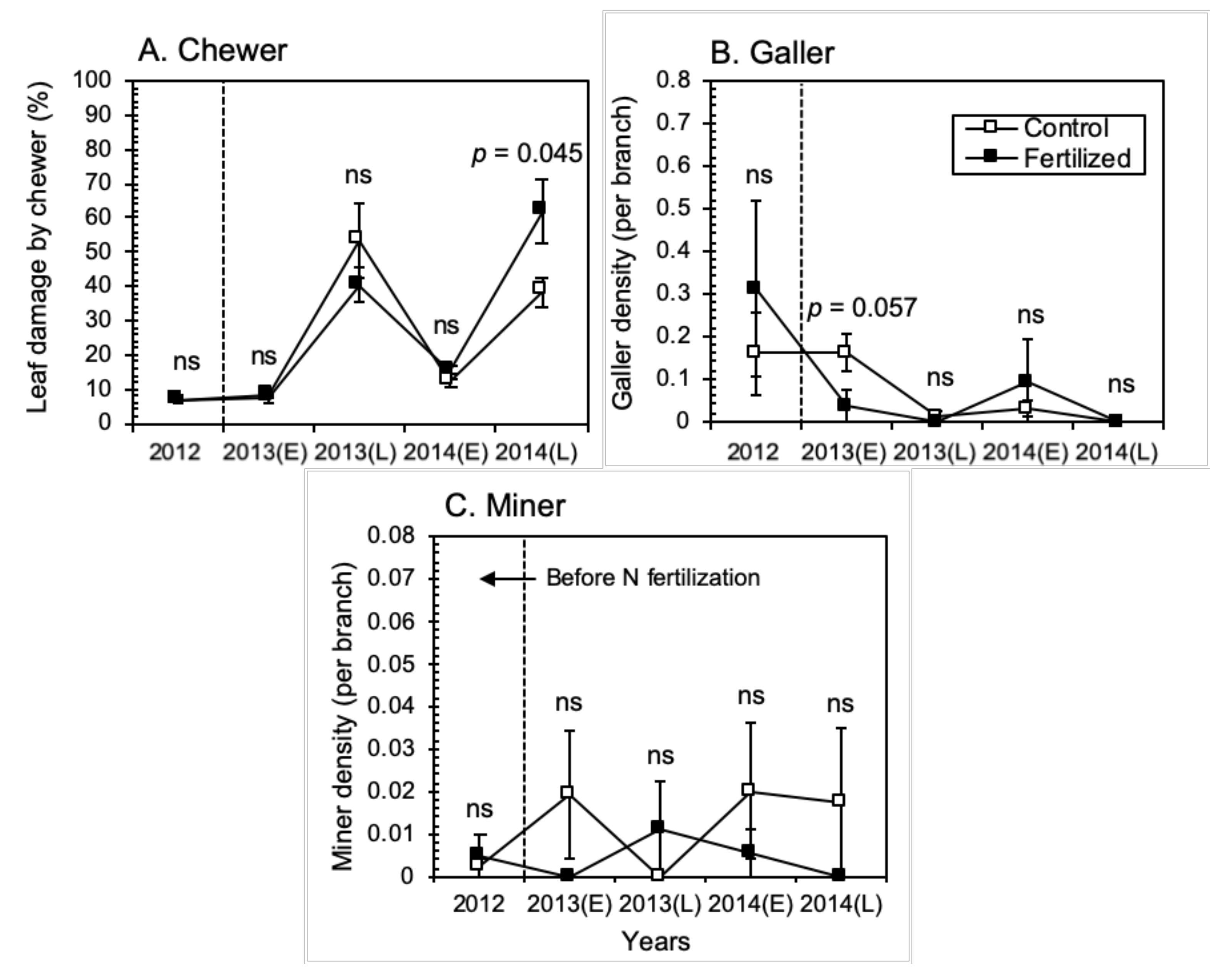
3.2. Insect Herbivore Responses
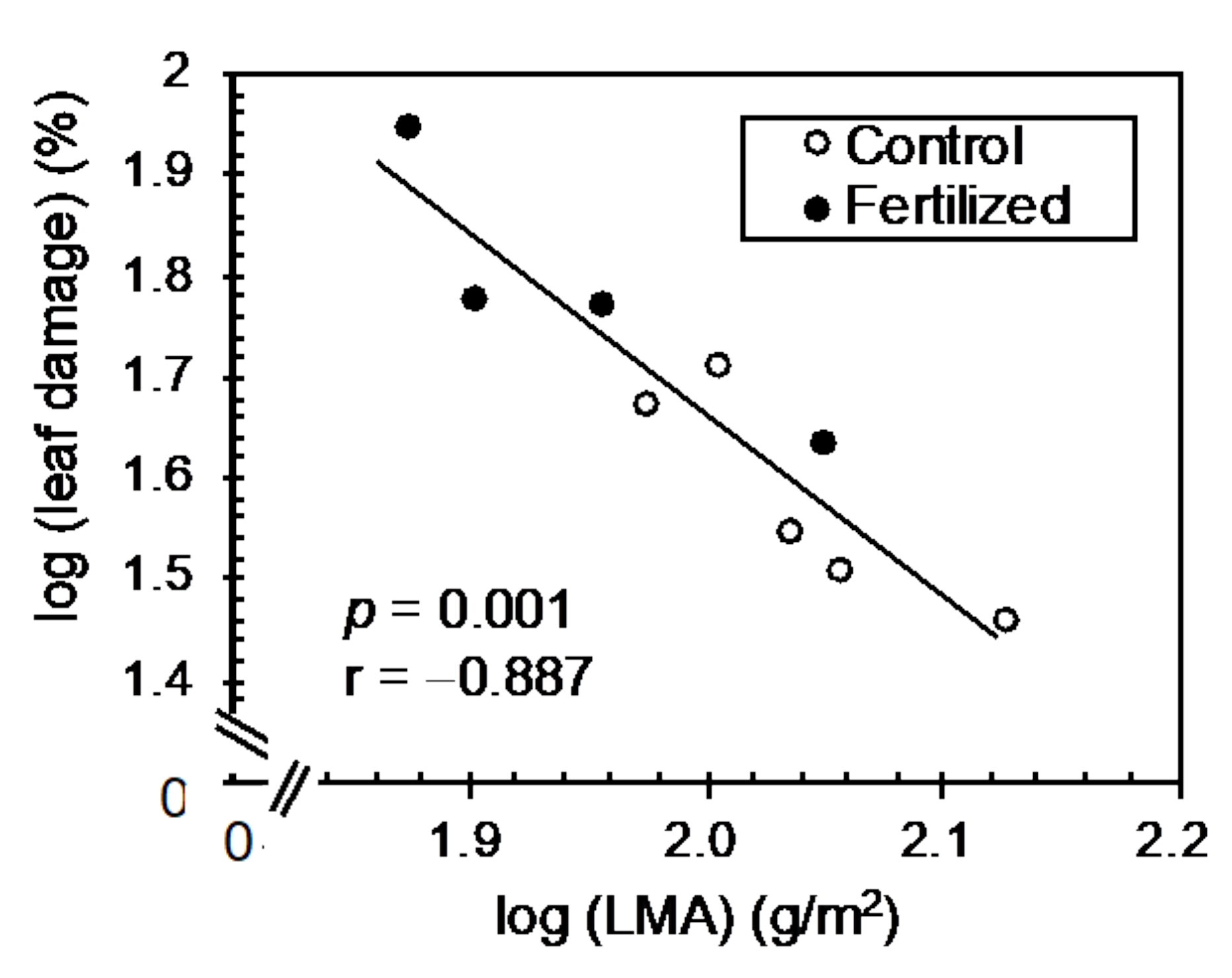
3.3. Correlation Analysis between Leaf Traits and Insect Herbivory
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bobbink, R.; Hornung, M.; Roelofs, J.G. The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. J. Ecol. 1998, 86, 717–738. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, A.; Chapin, F.S., III; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar]
- Aber, J.D.; Magill, A.; Mcnulty, S.G.; Boone, R.D.; Nadelhoffer, K.J.; Downs, M.; Hallett, R. Forest biogeochemistry and primary production altered by nitrogen saturation. Water Air Soil Pollut. 1995, 85, 1665–1670. [Google Scholar] [CrossRef]
- Fenn, M.E.; Baron, J.S.; Allen, E.B.; Rueth, H.M.; Nydick, K.R.; Geiser, L.; Bowman, W.D.; Sickman, J.O.; Meixner, T.; Johnson, D.W.; et al. Ecological effects of nitrogen deposition in the western United States. BioScience 2003, 53, 404–420. [Google Scholar] [CrossRef]
- Jones, M.E.; Paine, T.D.; Fenn, M.E. The effect of nitrogen additions on oak foliage and herbivore communities at sites with high and low atmospheric pollution. Envion. Pollut. 2008, 151, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Secondary succession and the pattern of plant dominance along experimental nitrogen gradients. Ecol. Monogr. 1987, 57, 189–214. [Google Scholar] [CrossRef]
- Throop, H.L.; Lerdau, M.T. Effects of nitrogen deposition on insect herbivory: Implications for community and ecosystem processes. Ecosystems 2004, 7, 109–133. [Google Scholar] [CrossRef]
- Chen, Y.; Olson, D.M.; Ruberson, J.R. Effects of nitrogen fertilization on tritrophic interactions. Arthropod Plant Interact. 2010, 4, 81–94. [Google Scholar] [CrossRef]
- Andrew, N.R.; Hughes, L. Herbivore damage along a latitudinal gradient: Relative impacts of different feeding guilds. Oikos 2005, 108, 176–182. [Google Scholar] [CrossRef]
- Dittrich, A.D.; Helden, A.J. Experimental sward islets: The effect of dung and fertilisation on Hemiptera and Araneae. Insect Conserv. Divers. 2012, 5, 46–56. [Google Scholar] [CrossRef]
- Hargrove, W.W.; Crossley, D.A., Jr.; Seastedt, T.R. Shifts in insect herbivory in the canopy of black locust, Robinia pseudacacia, after fertilization. Oikos 1984, 43, 322–328. [Google Scholar] [CrossRef]
- Jones, M.E.; Fenn, M.E.; Paine, T.D. The effect of nitrogen additions on bracken fern and its insect herbivores at sites with high and low atmospheric pollution. Arthropod Plant Interact. 2011, 5, 163–173. [Google Scholar] [CrossRef][Green Version]
- Kytö, M.; Niemelä, P. Larsson S Insects on trees: Population and individual response to fertilization. Oikos 1996, 75, 148–159. [Google Scholar] [CrossRef]
- Scriber, J.M.; Slansky, F., Jr. The nutritional ecology of immature insects. Annu. Rev. Entomol. 1981, 26, 183–211. [Google Scholar] [CrossRef]
- Koike, T.; Tobita, H.; Shibata, T.; Matsuki, S.; Konno, K.; Kitao, M.; Yamashita, N.; Maruyama, Y. Defense characteristics of several deciduous broad-leaved tree seedlings grown under differing levels of CO2 and nitrogen. Popul. Ecol. 2006, 48, 23–29. [Google Scholar] [CrossRef]
- Moise, E.R.; Henry, H.A. Like moths to a street lamp: Exaggerated animal densities in plot−level global change field experiments. Oikos 2010, 119, 791–795. [Google Scholar] [CrossRef]
- Englund, G.; Cooper, S.D. Scale effects and extrapolation in ecological experiments. Adv. Ecol. Res. 2003, 33, 161–213. [Google Scholar]
- Fay, T.M.; Turner, E.C.; Basset, Y.; Ewers, R.M.; Glen, R.; Novotny, V. Whole-ecosystem experimental manipulations of tropical forests. Trends Ecol. Evol. 2015, 30, 334–346. [Google Scholar]
- Southwood, T. Insect/plant relationship—An evolutionary perspective. In Insect-Plant Relationships; Blackwell: Oxford, UK, 1972; pp. 3–30. [Google Scholar]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Bryant, J.P.; Chapin, F.S., III; Klein, D.R. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef]
- Coley, P.D.; Bryant, J.P.; Chapin, F.S. Resource availability and plant antiherbivore defense. Science 1985, 230, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J. Villar R Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Webber, B.L.; Woodrow, I.E. In Intra-plant variation in cyanogenesis and the continuum of foliar plant defense traits in the rainforest tree Ryparosa kurrangii (Achariaceae). Tree Physiol. 2008, 28, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Webber, B.L.; Woodrow, I.E. Chemical and physical plant defence across multiple ontogenetic stages in a tropical rain forest understorey tree. J Ecol. 2009, 97, 761–771. [Google Scholar] [CrossRef]
- Stefan, K.; Fürst, A.; Hacker, R.; Bartels, U. Forest Foliar Condition in Europe. Results of Large-Scale Foliar Chemistry Surveys 1995; EC-UN/ECE-FBVA: Brussels, Belgium; Geneva, Switzerland, 1997. [Google Scholar]
- Perakis, S.S.; Matkins, J.J.; Hibbs, D.E. N2-fixing red alder indirectly accelerates ecosystem nitrogen cycling. Ecosystems 2012, 15, 1182–1193. [Google Scholar] [CrossRef]
- Rytter, L.; Arveby, A.S.; Granhall, U. Dinitrogen (C2H2) fixation in relation to nitrogen fertilization of grey alder [Alnus incana (L.) Moench.] plantations in a peat bog. Biol. Fertil. Soils 1991, 10, 233–240. [Google Scholar] [CrossRef]
- Gaulke, L.S.; Henry, C.L.; Brown, S.L. Nitrogen fixation and growth response of Alnus rubra following fertilization with urea or biosolids. Sci. Agric. 2006, 63, 361–369. [Google Scholar] [CrossRef]
- Troelstra, S.R.; Wagenaar, R.; Smant, W. Growth of actinorhizal plants as influenced by the form of nitrogen with special reference to Myrica gale and Alnus glutinosa. J. Exp. Bot. 1992, 43, 1349–1359. [Google Scholar] [CrossRef]
- Simberloff, D.; Dayan, T. The Guild Concept and the Structure of Ecological Communities. Annu. Rev. Eco. Syst. 1991, 22, 115–143. [Google Scholar] [CrossRef]
- Landsberg, J.; Smith, M.S. A functional scheme for predicting the outbreak potential of herbivorous insects under global atmospheric change. Aust. J. Bot. 1992, 40, 565–577. [Google Scholar] [CrossRef]
- Hartley, S.E.; Lawton, J.H. Host-plant manipulation by gall-insects: A test of the nutrition hypothesis. J. Anim. Ecol. 1992, 61, 113–119. [Google Scholar] [CrossRef]
- Faeth, S.H.; Mopper, S.; Simberloff, D. Abundances and diversity of leaf-mining insects on three oak host species: Effects of host-plant phenology and nitrogen content of leaves. Oikos 1981, 37, 238–251. [Google Scholar] [CrossRef]
- Shibata, H.; Kirikae, M.; Tanaka, Y.; Sakuma, T.; Hatano, R. Proton budgets of forest ecosystems on volcanogenous regosols in Hokkaido, Northern Japan. Water Air Soil Pollut. 1998, 105, 63–72. [Google Scholar] [CrossRef]
- Hiura, T. Stochasticity of species assemblage of canopy trees and understory plants in a temperate secondary forest created by major disturbances. Ecol. Res. 2001, 16, 887–893. [Google Scholar] [CrossRef]
- Kikuzawa, K. Leaf survival and evolution in Betulaceae. Ann. Bot. 1982, 50, 345–353. [Google Scholar] [CrossRef]
- Ataka, M.; Sun, L.; Nakaji, T.; Katayama, A.; Hiura, T. Five-year nitrogen addition affects fine root exudation and its correlation with root respiration in a dominant species, Quercus crispula, of a cool temperate forest, Japan. Tree Physiol. 2020, 40, 367–376. [Google Scholar] [CrossRef]
- Oksanen, L. Logic of experiments in ecology: Is pseudoreplication a pseudoissue? Oikos 2001, 94, 27–38. [Google Scholar] [CrossRef]
- Colegrave, N.; Ruxton, G.D. Using biological insight and pragmatism when thinking about pseudoreplication. Trends Ecol Evol. 2018, 33, 28–35. [Google Scholar] [CrossRef]
- Yoshida, K. Seasonal fluctuation of moth community in Tomakomai Experiment Forest of Hokkaido University. Res. Bull. Hokkaido Univ. For. 1980, 37, 675–685. [Google Scholar]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Barton, K.E.; Valkama, E.; Vehviläinen, H.; Ruohomäki, K.; Knight, T.M.; Koricheva, J. Additive and non-additive effects of birch genotypic diversity on arthropod herbivory in a long-term field experiment. Oikos 2015, 124, 697–706. [Google Scholar] [CrossRef]
- Kudo, G. Herbivory pattern and induced responses to simulated herbivory in Quercus mongolica var. grosseserrata. Ecol. Res. 1996, 11, 283–289. [Google Scholar] [CrossRef]
- Nakamura, M.; Hina, T.; Nabeshima, E.; Hiura, T. Do spatial variation in leaf traits and herbivory within a canopy respond to selective cutting and fertilization? Can. J. For. Res. 2008, 38, 1603–1610. [Google Scholar] [CrossRef]
- R Development Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Augspurger, C.K.; Bartlett, E.A. Differences in leaf phenology between juvenile and adult trees in a temperate deciduous forest. Tree Physiol. 2003, 23, 517–525. [Google Scholar] [CrossRef]
- Delagrange, S.; Messier, C.; Lechowicz, M.J.; Dizengremel, P. Physiological, morphological and allocational plasticity in understory deciduous trees: Importance of plant size and light availability. Tree Physiol. 2004, 24, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Vitasse, Y. Ontogenic changes rather than difference in temperature cause understory trees to leaf out earlier. New Phytol. 2013, 198, 149–155. [Google Scholar] [CrossRef]
- Weiner, J.; Thomas, S.C. The nature of tree growth and the “age-related decline in forest productivity”. Oikos 2001, 94, 374–376. [Google Scholar] [CrossRef]
- Struve, D.K. A review of shade tree nitrogen fertilization research in the United States. J. Arboric. 2002, 28, 252–263. [Google Scholar]
- Coley, P.D. Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol. Monogr. 1983, 53, 209–234. [Google Scholar] [CrossRef]
- Bussotti, F.; Borghini, F.; Celesti, C.; Leonzio, C.; Bruschi, P. Leaf morphology and macronutrients in broadleaved trees in central Italy. Trees 2000, 14, 361–368. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Rodà, F. Plasticity of leaf morphological traits, leaf nutrient content, and water capture in the Mediterranean evergreen oak Quercus ilex subsp. ballota in response to fertilization and changes in competitive conditions. Ecoscience 2006, 13, 258–270. [Google Scholar]
- Ruess, R.W.; Anderson, M.D.; McFarland, J.M.; Kielland, K.; Olson, K.; Taylor, D.L. Ecosystem−level consequences of symbiont partnerships in an N-fixing shrub from interior Alaskan floodplains. Ecol Monogr. 2013, 83, 177–194. [Google Scholar] [CrossRef]
- Lee, J.; Nakumara, M.; Hiura, T. Does large-scale N fertilization have time-delayed effect on insects community structure by changing oak quantity and quality. Arthropod Plant Interact. 2017, 11, 515–523. [Google Scholar] [CrossRef]
- Witkowski, E.T.F.; Lamont, B.B. Leaf specific mass confounds leaf density and thickness. Oecologia 1991, 88, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Clissold, F.J.; Sanson, G.D.; Read, J. The paradoxical effects of nutrient ratios and supply rates on an outbreaking insect herbivore, the Australian plague locust. J. Anim. Ecol. 2006, 75, 1000–1013. [Google Scholar] [CrossRef]
- Clissold, F.J.; Sanson, G.D.; Read, J.; Simpson, S.J. Gross vs. net income: How plant toughness affects performance of an insect herbivore. Ecology 2009, 90, 3393–3405. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Nakamura, M.; Hiura, T. Effects of Large-Scale Nitrogen Fertilization on Insect–Plant Interactions in the Canopy of Tall Alder Trees with N2-Fixing Traits in a Cool Temperate Forest. Forests 2021, 12, 210. https://doi.org/10.3390/f12020210
Lee J, Nakamura M, Hiura T. Effects of Large-Scale Nitrogen Fertilization on Insect–Plant Interactions in the Canopy of Tall Alder Trees with N2-Fixing Traits in a Cool Temperate Forest. Forests. 2021; 12(2):210. https://doi.org/10.3390/f12020210
Chicago/Turabian StyleLee, Jin, Masahiro Nakamura, and Tsutom Hiura. 2021. "Effects of Large-Scale Nitrogen Fertilization on Insect–Plant Interactions in the Canopy of Tall Alder Trees with N2-Fixing Traits in a Cool Temperate Forest" Forests 12, no. 2: 210. https://doi.org/10.3390/f12020210
APA StyleLee, J., Nakamura, M., & Hiura, T. (2021). Effects of Large-Scale Nitrogen Fertilization on Insect–Plant Interactions in the Canopy of Tall Alder Trees with N2-Fixing Traits in a Cool Temperate Forest. Forests, 12(2), 210. https://doi.org/10.3390/f12020210





