1. Introduction
Climate warming, land use change and biodiversity loss now have become the leading three drivers of global changes, and biological invasion is a key cause of diversity loss [
1]. Alien plant invasion significantly affects local community biodiversity and stability, and ecosystem services and functions will accordingly be changed dramatically [
2,
3]. Therefore, research into local community invasibility mechanisms has become a critical scientific issue in invasion ecology.
Urban and rural ecotone refers to the transition zone between urban and rural areas, and its characteristics integrate some features of the two areas to form composite landscapes [
4], which play an important role in balancing ecological problems caused by urbanization. However, habitat loss and fragmentation caused by urbanization also leave urban and rural ecotones at higher invasion risk [
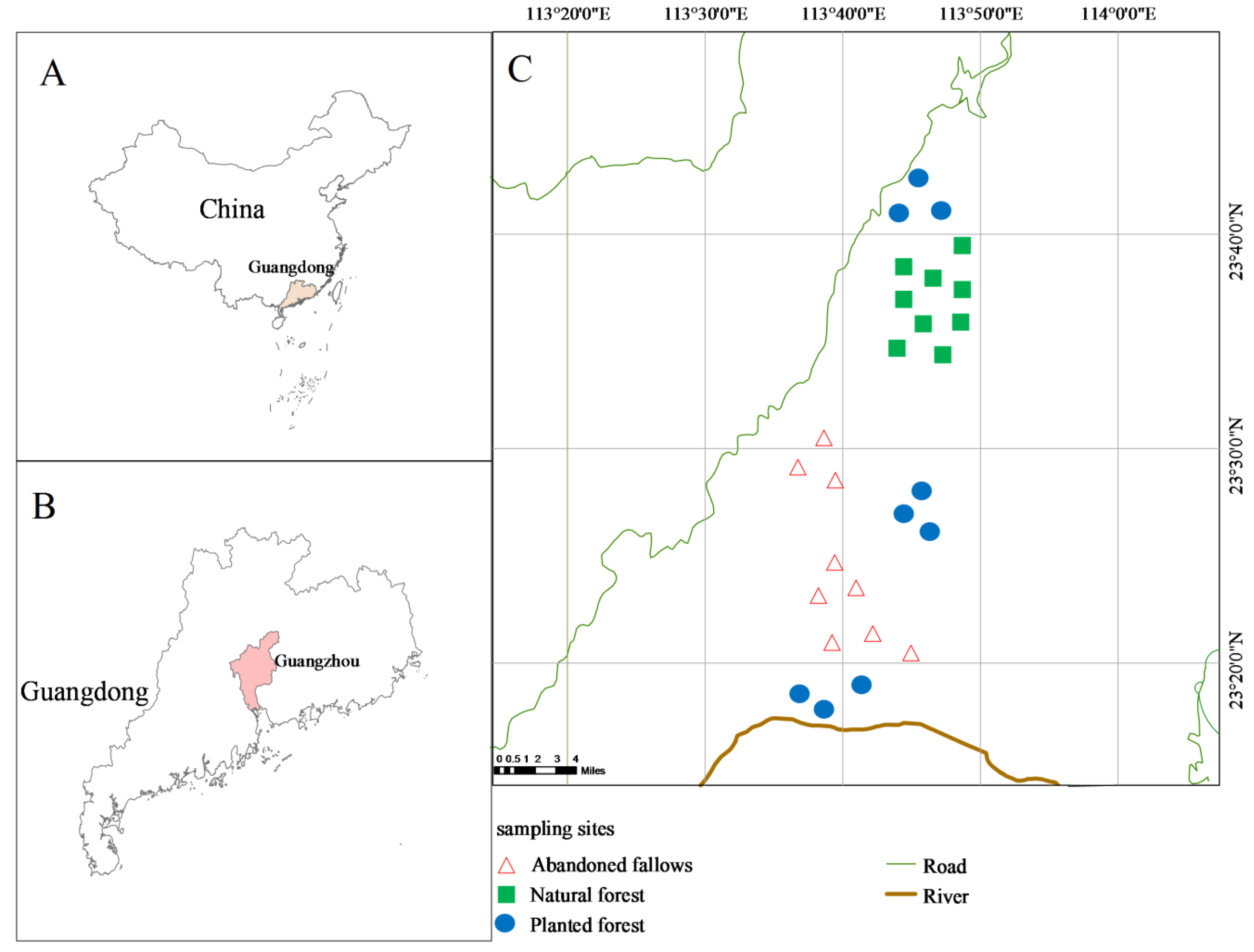
5]. The eastern suburb of Guangzhou is a typical urban and rural ecotone where agriculture and forestry are combined. It has a unique ecological patch landscape of orchards, cultivated land, natural forest and planted forest. Here, the alien species,
Borreria latifolia and
Mikania micrantha, are abundant.
Mikania micrantha spread rapidly from Hong Kong to Southern China in 1919, and
Borreria latifolia was introduced into Guangdong Province in 1937 as feed for army horses and also planted to stabilize soil. The rapid spread of these two alien species can quickly occupy native species’ living space [
6,
7,
8], which seriously changes understory species composition.
Invasibility refers to community sensitivity to invasion, which is an inherent community characteristic [
9]. It is generally believed that there are three hypotheses for the regulation of invasibility by community structure: (1) if the number of local species is large, the community is not easily invaded [
3,
10], (2) the higher the functional similarity between alien and native species, the easier it is for successful invasion by the alien species [
11], and (3) the limitation based on the principle of community construction depends on the number of vacant niches [
12]. However, it also largely depends on resource availability. The composition, structure and diversity of native species may be modified when faced with invasion, which, in turn, determines whether a community can accept the successful establishment of invaders [
13]. We found that these two invasive species were mainly distributed in the understory of abandoned fallows. This understory was investigated and compared with those of adjacent natural and planted forests in the structure, composition and diversity of native species under similar ecological conditions. Furthermore, exploring the causes and results of invasion in this area was an important step to evaluate the risk and prevention of invasion.
Successful invasion is not only associated with local community structure but also affected by environmental factors. Due to the different survival strategies between invasive and native species, differences in appearance (the composition, structure and life history strategy of the vegetation on the ground) and biomass and litter properties may greatly change soil properties. However, differences in soil mineral elements may also play an important role in determining species distribution [
14,
15]. It is generally believed that areas with higher resource levels are more likely to be invaded [
16,
17]. Indeed, in determining invasion success and changing the allocation of aboveground and belowground resources, soil mineral element concentrations play a leading role in promoting invasion [
18]. Related studies on soil physical and chemical properties changed by invasion have mainly focused on soil carbon, nitrogen and phosphorus. Various mechanisms have been identified, including the tendency for invasive species to have a higher nutrient utilization rate, litter accumulation and decomposition efficiency. Thus, microhabitats that favor invasion can be established. Likewise, soil microbial communities also promote invasion by positive feedback to regulate and control nutrient cycling and effective proportion [
19,
20,
21]. Native species may also reduce invasion by affecting the availability of soil mineral elements. Some studies have pointed out there is a significant relationship between local and alien plant niche space and soil phosphorus availability [
22]. Additionally, invasion could be reduced by lowering soil nitrogen availability [
23]. However, there are few studies on other mineral elements and invasion, and related research has mainly focused on riparian aquatic plants. Due to high human interference, and active and frequent river erosion and deposition, considerable heavy metal elements accumulate [
24]. A large number of factories are distributed in urban and rural Guangzhou, and the ecotones are threatened with urban and industrial pollution. Many mineral elements are deposited in soil under runoff, leaching and weathering [
25,
26]. Consequently, the growth and physiological functions of native and alien species have changed in this area, which may affect species distribution. Furthermore, the existence of alien species may also affect soil mineral element composition.
Therefore, our study aim was to compare the relationships between invasibility and changes in soil mineral elements and understory community structure in abandoned fallows and natural and planted forests. Abandoned fallows were seriously invaded by Mikania micrantha and Borreria latifolia in the eastern urban and rural ecotone of Guangzhou, whereas natural and planted forests were only slightly invaded. We asked the following three specific research questions: (1) What was the impact of invasion on soil mineral element composition? (2) How did species composition, structure, stability and diversity respond to invasion? (3) What were the distribution factors driving alien and native species, respectively? We hypothesized that there were differences in soil mineral element concentrations, understory species composition, structure and stability among the three communities and that invasibility was influenced by soil mineral elements and community structure. Our findings should be relevant to land managers and hopefully could be used as guidance for decision-makers in forest protection and management of invasive plants in the future.
4. Discussion
Variation in soil mineral element concentration is closely related to soil formation conditions and processes. It is influenced by the combination of soil-forming parent material, topographic factors and biological effects [
40,
41]. The soils in the study area had developed from granite and other soil-forming matrices intruded during the Yanshanian, and so local mineral elements are generally depleted [
42]. This may be due to the local high temperature and rainfall, as, during strong soil formation, easily mobile mineral elements such as K, Mg and Ca, are leached, while a few difficult elements, such as Fe and Al, are enriched in the soil. Alternatively, the site vegetation was severely damaged, soil erosion was exacerbated, and organic matter and clay particles were low, which reduced mineral element adsorption [
43]. The effects of these factors on soil mineral elements are extensive and therefore negligible in our study. We selected sites with the same parent material and soil type to minimize the mineral differences in the soil itself (
Table 1) and collected only the topsoil (0–25 cm depth) for analysis. Because of differences in aboveground litter and sediment input, mineral element concentrations are changed by root absorption and turnover of the shrub and grass community and, therefore, most obvious in the soil surface layer. This approach helped us to explore soil mineral element changes in different stands.
Our results indicated that there were significant differences in soil available Fe, available B and total Hg among the three stand types. Compared with the other two stand types, abandoned fallows had the highest soil concentrations of exchangeable Ca, available Cu, available Zn, available Fe and available B. However, abandoned fallows had a lower pH and organic matter concentration than the other stand types, although there were no significant differences between them (
Table S2). The differences in mineral concentrations between stands may be related to the land use history. Because the abandoned fallows was initially used as an agroforestry complex, tillage and fertilization may have caused a continuous increase in cation concentration in the soil [
44]. Although conversion to forest land has occurred due to abandonment and natural succession, such changes may still be detectable decades or even centuries after afforestation [
44,
45,
46]. It has been suggested that community composition may also alter soil mineral concentrations. Different species may directly or indirectly influence mineralization rates and alter soil mineral cycles through uptake rates [
47], litter quality and quantity [
48,
49] and synergistic interactions with soil microorganisms [
48,
50]. Additionally, other unmeasured factors in this study, such as soil temperature and humidity, density and porosity, may also affect soil mineral element concentrations [
51,
52].
The extensive occurrence of invasive species in abandoned lands is related to their specific environmental characteristics, such as light requirements [
53], but it could also be a preference for a certain soil element [
54]. Many studies have shown the limiting effect of nitrogen and phosphorus deficiency on the growth of invasive plants [
54,
55,
56]. In addition to these soil elements, other elements also play a role in the distribution of invasive plants. For example,
Solidago canadensis L. is abundantly distributed in soils with high zinc (Zn) and lead (Pb) concentrations, which may be the result of its ability to overcome the stress of high element concentrations by developing defense mechanisms while maintaining competitive ability under stressed conditions [
57]. The low number of invasive species in natural and planted forests may be due to their adaptation to low mineral concentrations in local soils. It has been suggested that high mineral element utilization (e.g., longer roots) may be a survival strategy for native species in low mineral concentration habitats. In contrast, invasive species have difficulty utilizing low mineral element concentrations and therefore have difficulty surviving [
58]. However, in the study area, the correlation between mineral concentration and invasive species requires verification by further experimental studies.
In our study, there were significant differences in species composition in the three stand types (MRPP test, p < 0.05). However, there were no common species with indicator values >25, indicating that no habitats could be differentiated by these species; for example, natural forest indicator species were all common and widely adaptable native species. Abandoned fallows were mainly invaded by Borreria latifolia and Mikania micrantha, both of which were photophilous, and associated native species tended to have two different survival strategies: (1) direct competition between associated native species and invasive plants for light resources, and (2) reduced competition for the same resources in low light environments where native species exist and invasive plants are less common. Planted forest indicator species were characterized by a wide tolerance and rapid growth. For planted and natural forest with lower invasion, the selected indicator species all played an important role in habitat protection.
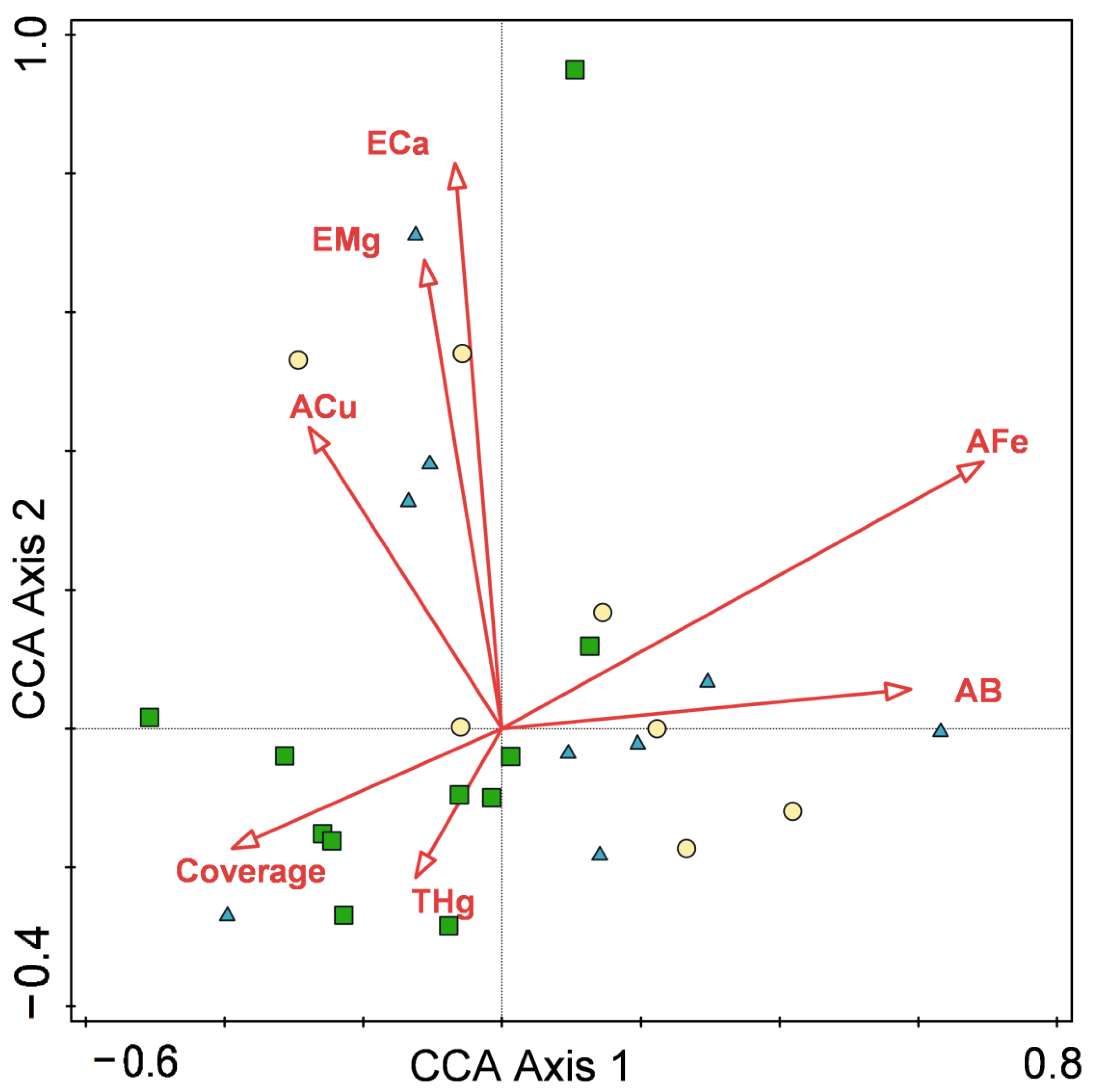
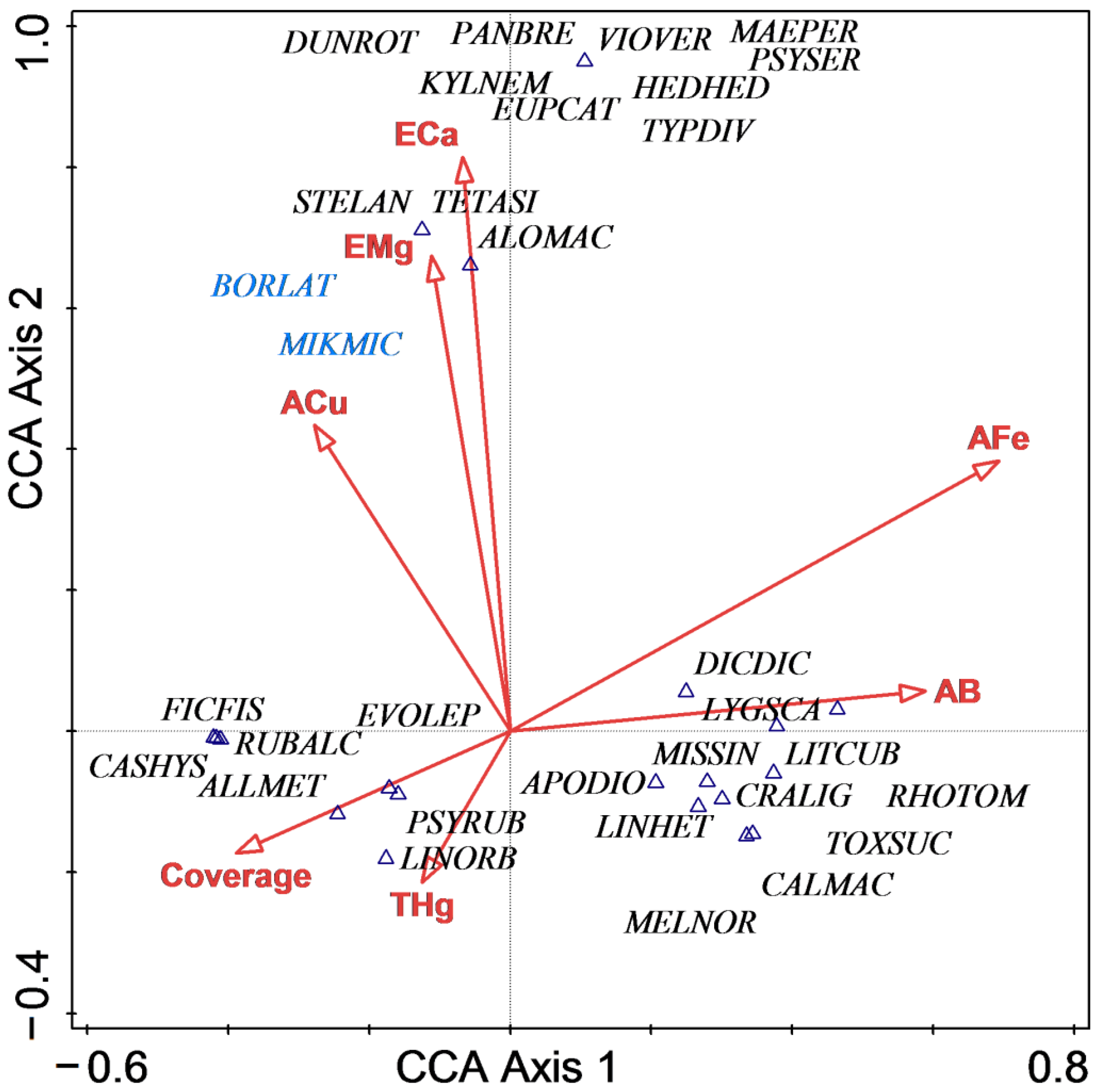
The aim of our ecological research was to confirm the factors controlling community distribution. Understory species composition and community structure are regulated by many environmental factors. Based on our CCA analysis, the important factors influencing quadrat distribution were available Fe, exchangeable Mg, exchangeable Ca, available B, available Cu, total Hg and community cover. However, quadrat distributions in the CCA sequencing diagram indicated that there were significant differences in the three stand types. Natural forest plots were tightly clustered, while abandoned fallows and planted forest plots had similar habitat requirements that were mainly affected by multiple mineral elements. The occurrence of invasive species Borreria latifolia and Mikania micrantha was closely related to available Cu and exchangeable Mg, but at the same time, many native species, such as Tetracera asiatica and Alocasia macrorrhiza, were similarly affected. The remaining native species were distributed in two areas: low cover and low total Hg concentration, or high available B and available Fe concentrations.
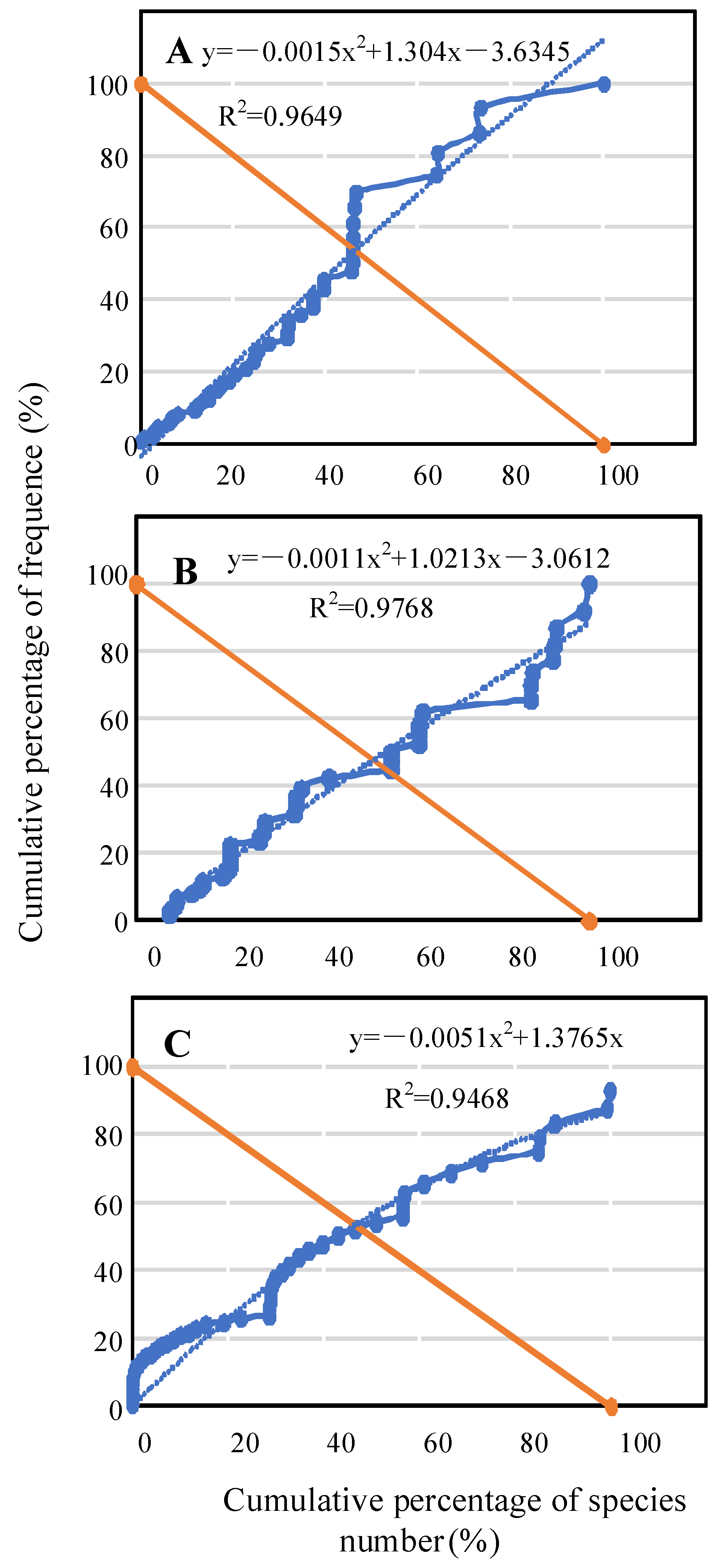
Community stability is the comprehensive performance of community dynamic balance achieved through population competition and symbiosis adjustment. Compared with a non-invaded community, an invasive community has limited living space and available resources. The stability of the invasive community represents its capacity to maintain homeostasis under positive and negative feedback to the alien species [
59].We reflected the homogeneity of community distribution with the fitted curve of species frequency shown in
Figure 6. The resulting fitted curve reflects uniformity of community distribution; the more uniform the distribution, the more stable the community [
60]. Compared with the traditional method of community succession and comparative analysis, it is possible to obtain current community stability from one time data, but the limitation is that it is not possible to predict community successional trends [
37,
38]. In our study, Euclidean square distances of the three stand types between intersection coordinates and stable point coordinates were distributed in the range of 37–46 and in the stability order: planted forest > natural forest > abandoned fallows. It may be that as invasive species increase plant height and cover, it helps them to compete with native plants for growing space and resources to maintain their dominant position and strong resistance to external interference [
61]. Furthermore, root allelopathic effect may increase [
62], which would aggravate competition between invasive and native species. Therefore, species replacement rate would be enhanced and stability decreased in the community. Moreover, changing trends in the stability index were consistent with changes in the species diversity index, Shannon–Wienerindex and Simpson index (
Figure S1). We can, therefore, conclude that the higher the community species diversity, the more stable the community.
Plant invasions are often considered to be related to three factors: propagule pressure, native community invasibility and land use history [
63]. The extent to which each factor plays a role in a given habitat may vary, and there may be some linkage between them [
64]. It has been suggested that invasibility is regulated by exposure to propagule pressure. Whether under high or low propagule pressure, communities change invasibility through different strategies (adjusting the density or diversity of the community), and invasion outcomes change as a result [
65]. In addition, during the natural recovery of cropland to forest, even with the influence of land use history, native communities can enhance resistance to invasion and reduce the probability of invasion success by changing to those species that can effectively use resources and become dominant [
66]. Although we could not completely exclude the effects of pressure and land use history in our study, community invasibility is still worth further study. In the future, we will consider the effects of integrating propagule pressure, land use practices and invasibility.
Invasibility is determined by a combination of community dominance, species diversity, community structure and other components. In past field investigations and controlled experiments on invasibility, native species diversity was considered as one of the main factors affecting the invasion process [
67]. However, there are few reports on the invasibility of community structure. In our study, although there was a size relationship between the diversity indices of stand types, it was statistically insignificant. Furthermore, there was no significant relationship between soil mineral element concentrations and diversity, a similar result to a study of
Galium verum L invasion. Regardless of native species diversity, alien species can use their resources as much as possible at different nutrient levels to increase their biomass. [
16]. Some mineral elements had significant relevance with average cover and height (
Table S3) in our study. There were also significant differences in average cover among the three stand types, indicating that plant height and cover play an important role in plant competitiveness and resistance to environments. Compared with the number of species, the relationship between diversity and invasiveness may be more dependent on species structure. When species with different survival strategies coexist, structurally rich communities may increase complexity and improve the utilization rate of available resources (especially light), while reducing the probability of invasion [
68,
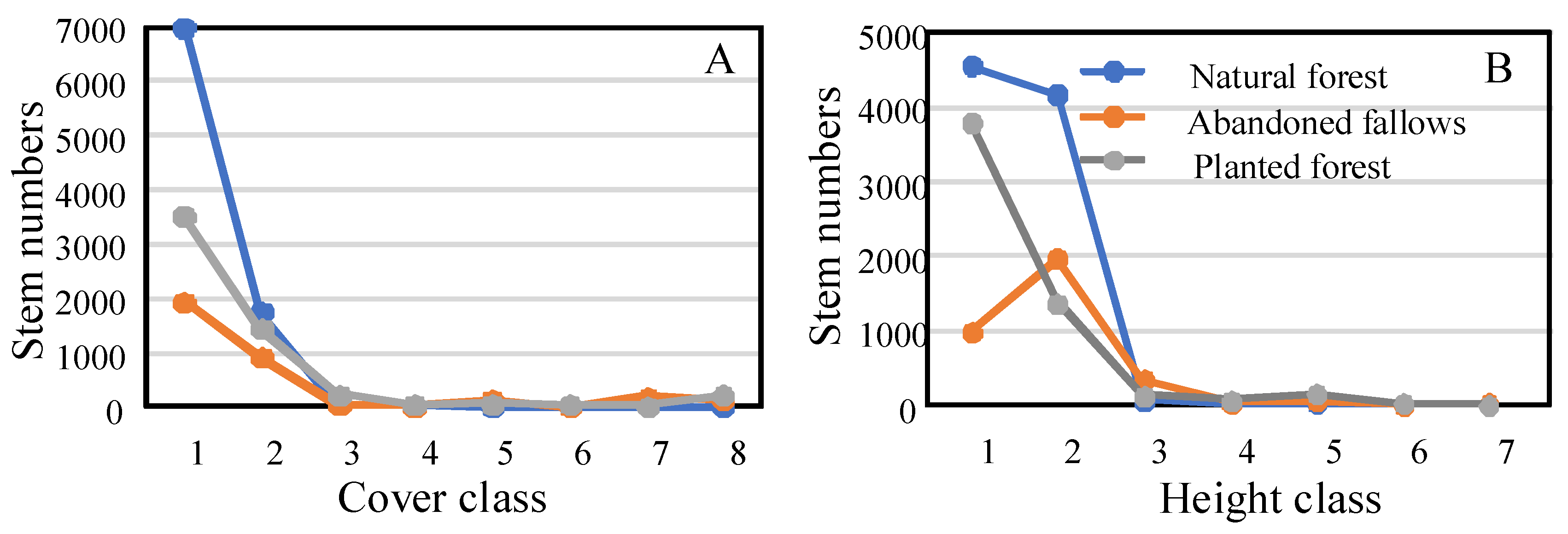
69]. The cover and height class distributions of the three stands were further analyzed, and it was found that there was little difference between stand types at height and cover classes >3. However, the number of stems in natural forest in height and cover classes 1 and 2 was clearly higher than in the other two stand types. There was only a slight difference between the stand types above class 3, which demonstrates that cover and height distribution tends to be stable among communities. In natural and planted forests, the level of invasion was lower, while the invasion level of abandoned fallows was higher, which may be due to the large number of plants with low cover class in the former habitats. This may be a difference in growth strategies: when species allocate resources to aboveground vegetative growth in a resource-rich environment, plants with different cover classes can make full use of the abundant resources in the environment, making it difficult for invasive plants to compete with them [
70,
71]. In studying the impact of alien plant invasion on native species, the relationship between community structure and invasibility should be paid more attention in the future. Considering the complexity of community structure, it may also be necessary to establish larger sample plots and increase time series to explore the relationships with invasibility.