The Seasonal Population Dynamics of Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae) and the Relationship between Meteorological Factors and the Diurnal Flight Intensity of the Adults in Romanian Oak Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Data Collection
2.1.1. Seasonal Dynamics of OLB Populations—2019
2.1.2. Dynamics of the Diurnal Activity of the OLB Population and Influential Weather Factors—2020
2.2. Data Analysis
3. Results
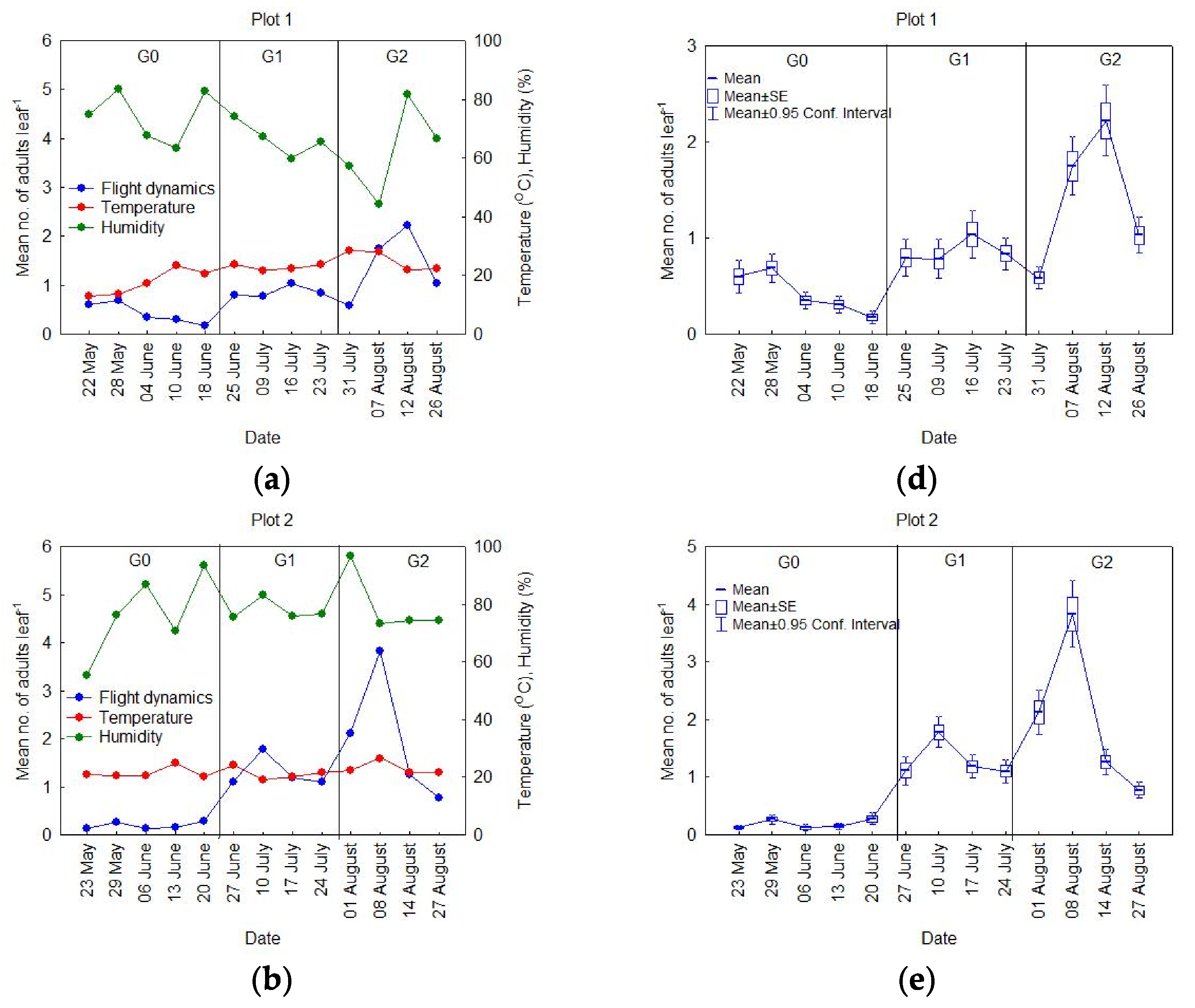
3.1. Seasonal Dynamics of the OLB Populations
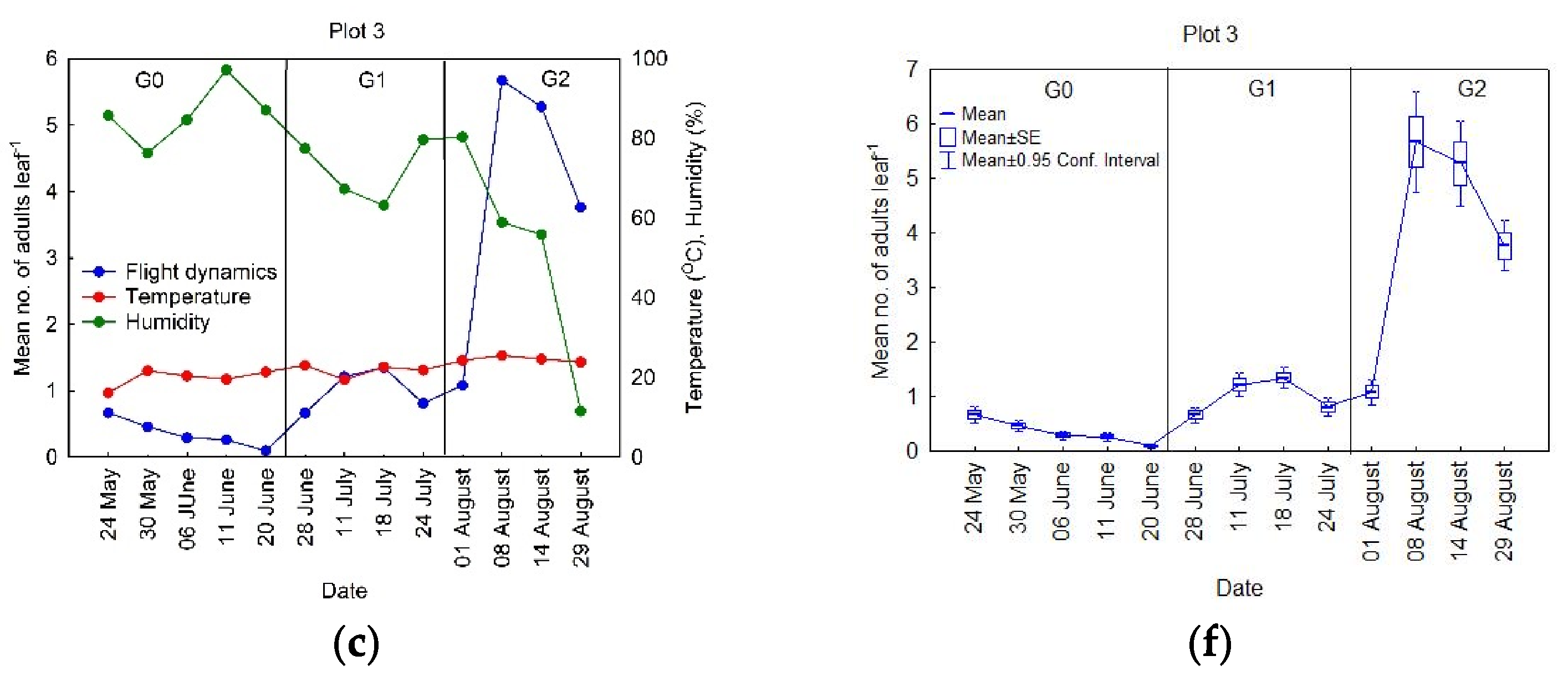
- Period 1, starting the second week of May until the second week of June (Figure 2a). It is worth mentioning that, when the observations began (May), the number of adults in flight was already at a maximum level for this period (<1 ), which decreased through time at Plots 1 and 2, while it remained relatively constant at Plot 3. The data analysis, using standard error values, showed that the variability was small during this period (Figure 2d).
- Period 2, between the last week of June and the second week of July (Figure 2b). During this period, the proportion of insects in flight began with similar values to those in Period 1, with an increase in the population to a peak in the middle of July, and then a decrease to low values. During this period, the standard error values indicated that the variability had begun to increase (Figure 2e).
- Period 3, beginning with the last week in July until the last week of August (Figure 2c). As in Period 2, there was an increase in the number of insects in flight, up to a peak, after which they decreased. The data analysis showed that, during this period, the standard error values were higher than in Periods 1 and 2 (Figure 2f), meaning that the variability in this period was the highest throughout the season.
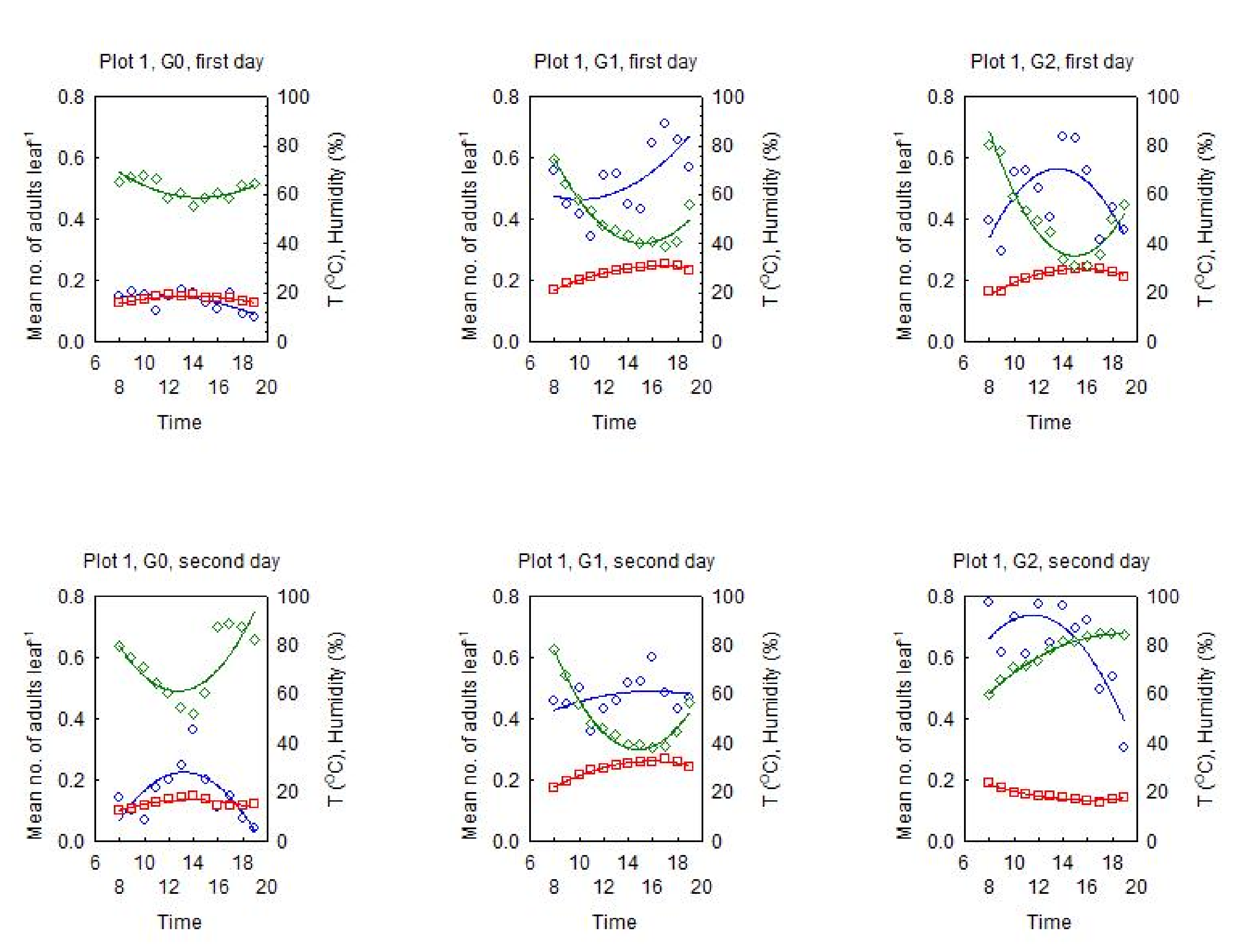
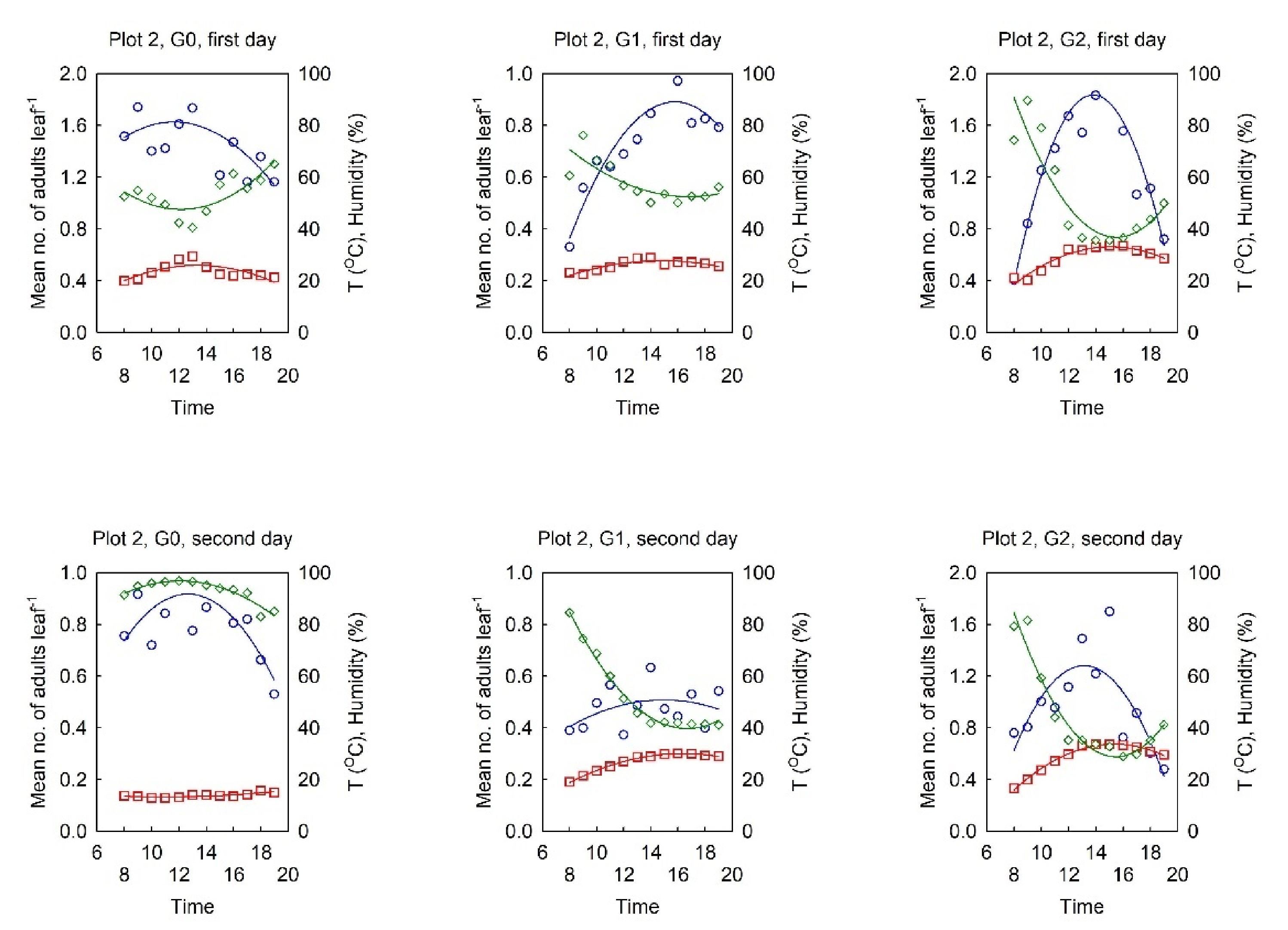
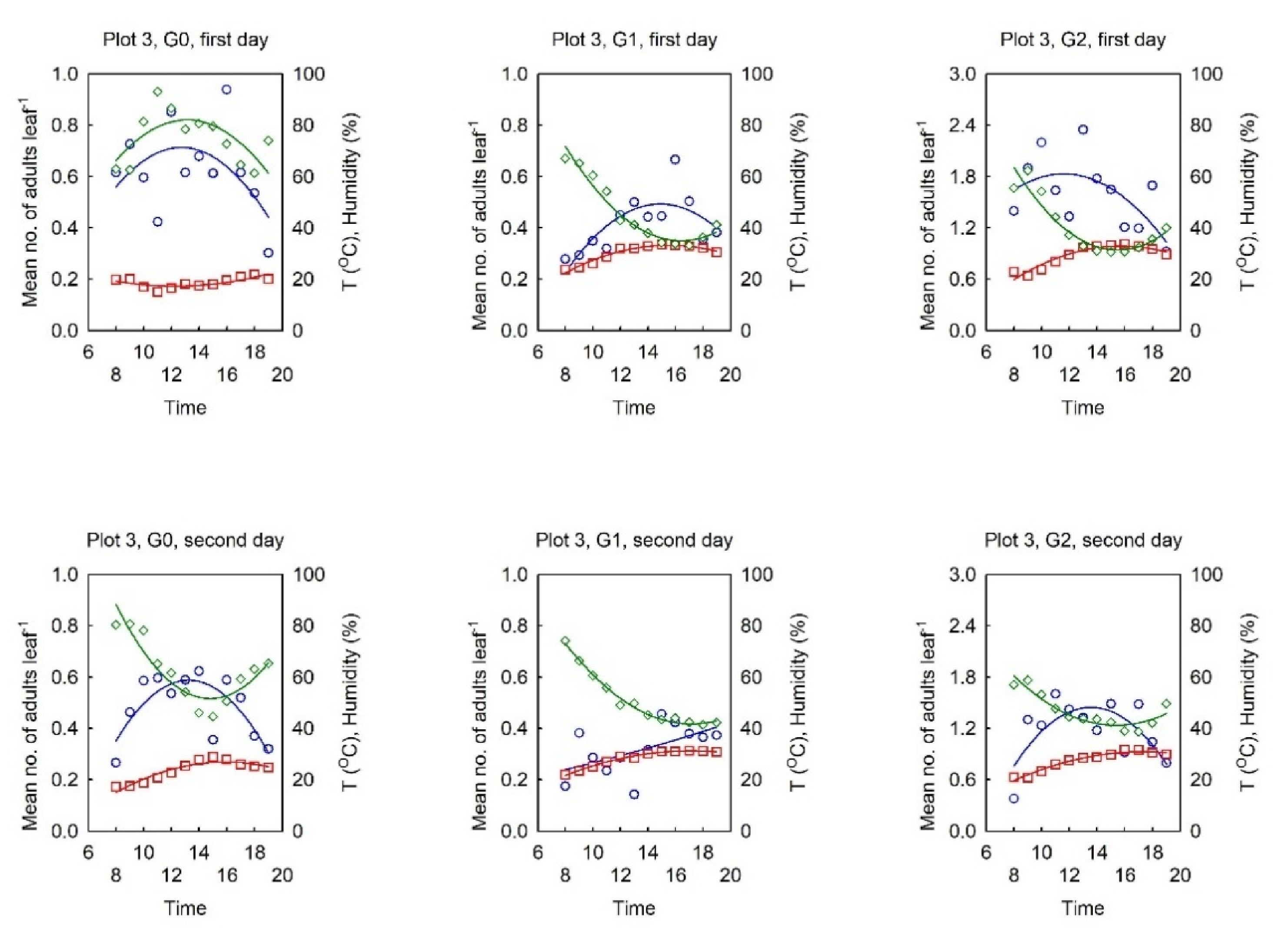
3.2. Dynamics of the Diurnal Activity in the OLB Population and Influential Factors
3.2.1. Relationship between the OLB Population Dynamics and the Influential Factors
3.2.2. Dynamics of the Diurnal Activity in the OLB Population
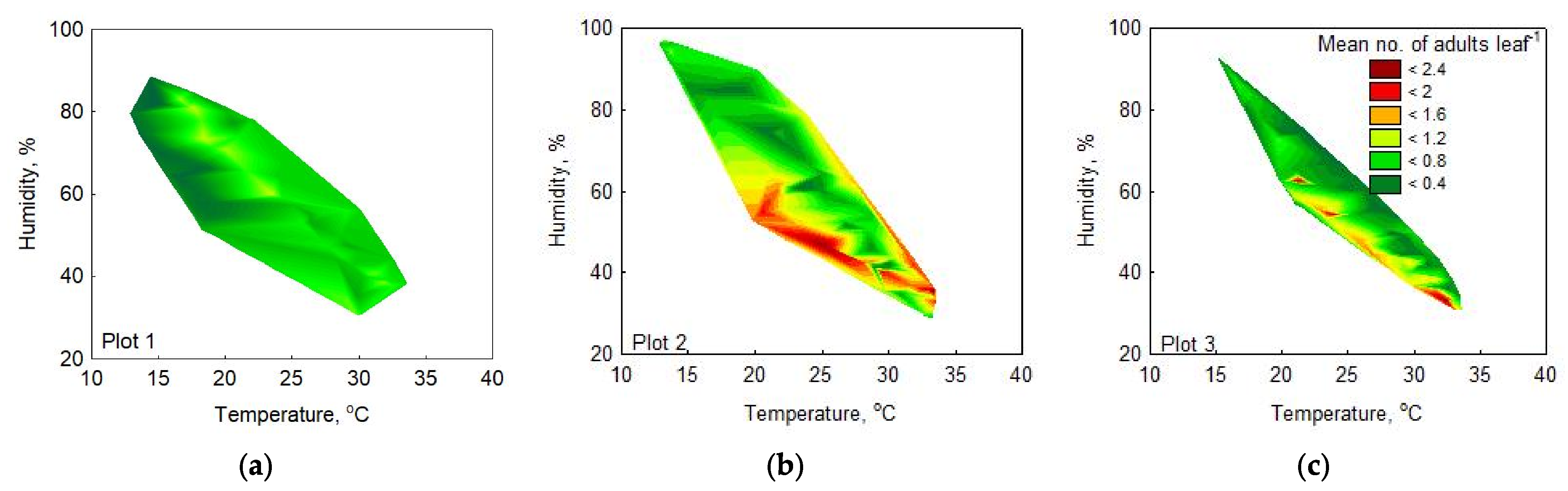
3.2.3. Range of Optimal Meteorological Factors for Activity in the OLB Population
4. Discussion
5. Conclusions
- Corythucha arcuata has a seasonal dynamic that shows two population peaks—one in July and the other in August;
- The diurnal dynamics of OLB activity have a pattern—the number of adults in flight reaches a peak at midday, which varies positively with the diurnal air temperature and negatively with the humidity;
- In many cases, air temperature and humidity significantly influence the dynamics of the OLB population;
- Even if the findings of this study are limited by the environmental conditions in southern Romania, they can be used to inform the direction of future studies on other meteorological or climatic factors that may influence OLB behaviour.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bjorkman, C.; Niemela, P. Climate Change and Insect Pests; CABI: Wallingford, UK, 2015; Volume 8, ISBN 1780643780. [Google Scholar]
- Arnaldo, P.S.; Oliveira, I.; Santos, J.; Leite, S. Climate change and forest plagues: The case of the pine processionary moth in Northeastern Portugal. For. Syst. 2011, 20, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Ge, X.; Zou, Y.; Guo, S.; Wang, T.; Zong, S. Climate change impacts on the potential distribution and range shift of Dendroctonus ponderosae (Coleoptera: Scolytidae). Forests 2019, 10, 860. [Google Scholar] [CrossRef] [Green Version]
- Sierpiñska, A. Towards an integrated management of Dendrolimus pini L. In Proceedings of the Population Dynamics, Impacts, and Integrated Management of Forest Defoliating Insects (NE-247), Banská Štiavnica, Slovakia, 18–23 August 1996; pp. 129–142. [Google Scholar]
- Ray, D.; Peace, A.; Moore, R.; Petr, M.; Grieve, Y.; Convery, C.; Ziesche, T. Improved prediction of the climate-driven outbreaks of Dendrolimus pini in Pinus sylvestris forests. Forestry 2016, 89, 230–244. [Google Scholar] [CrossRef] [Green Version]
- Kenis, M.; Branco, M. Impact of alien terrestrial arthropods in Europe. Chapter 5. BioRisk 2010, 4, 51–71. [Google Scholar] [CrossRef] [Green Version]
- Bernardinelli, I.; Zandigiacomo, P. Prima segnalazione di Corythucha arcuata (Say) (Heteroptera, Tingidae) in Europa. Inf. Fitopatol. 2000, 50, 47–49. Available online: http://hdl.handle.net/11390/710684 (accessed on 14 December 2021).
- Forster, B.; Giacalone, I.; Moretti, M.; Dioli, P.; Wermelinger, B. Die amerikanische Eichennetzwanze Corythucha arcuata (Say)(Heteroptera, Tingidae) hat die Sudschweiz erreicht. Mitt. Schweiz. Entomol. Ges. 2005, 78, 317. [Google Scholar]
- Mutun, S. First report of the oak lace bug, Corythucha arcuata (Say, 1832) (Heteroptera: Tingidae), from Bolu, Turkey. Isr. J. Zool. 2003, 49, 323–324. [Google Scholar]
- EPPO Reporting Service. Introduction of Corythucha arcuata in Italy. Addition to the EPPO Alert List. (No. 03—2001; Article 2001/057). 2001. Available online: https://gd.eppo.int/reporting/article-2882 (accessed on 14 December 2021).
- EPPO Global Database. Mini Data Sheet on Corythucha arcuate. 2007. Available online: https://gd.eppo.int/taxon/CRTHAR/documents (accessed on 14 December 2021).
- Dobreva, M.; Simov, N.; Georgiev, G.; Mirchev, P.; Georgieva, M. First record of Corythucha arcuata (Say) (Heteroptera: Tin-gidae) on the Balkan Peninsula. Acta. Zool. Bulg. 2013, 65, 409–412. [Google Scholar]
- Csóka, G.; Hirka, A.; Somlyai, M. A tölgy csipkéspoloska (Corythuca arcuata Say, 1832–Hemiptera, Tingidae) elsô észlelése magyarországon. Növényvédelem 2013, 49, 293–296. [Google Scholar]
- Hrašovec, B.; Posarić, D.; Lukić, I.; Pernek, M. Prvi nalaz hrastove mrežaste stjenice (Corythucha arcuata) u Hrvatskoj. Šumarski List 2013, 137, 499–503. [Google Scholar]
- Poljaković-Pajnik, L.; Drekić, M.; Pilipović, A.; Nikolić, N.; Pap, P.; Vasić, V.; Marković, M. Pojava velikih šteta od Corythucha arcuata (Say) (Heteroptera: Tingidae) u šumama hrasta u Vojvodini. XIII Savetovanje o Zaštiti Bilja. Zb. Radova Str. 2015, 63, 179–181. [Google Scholar]
- Pap, P.; Drekić, M.; Poljaković-Pajnik, L.; Marković, M.; Vasić, V. Monitoring zdravstvenog stanja šuma na teritoriji Voj-vodine u 2015. Godini. Topola 2015, 195, 117–133. [Google Scholar]
- Csóka, G.; Hirka, A.; Mutun, S.; Glavendekić, M.; Mikó, Á.; Szőcs, L.; Paulin, M.; Eötvös, C.B.; Gáspár, C.; Csepelényi, M.; et al. Spread and potential host range of the invasive oak lace bug [Corythucha arcuata (Say, 1832)—Heteroptera: Tingidae] in Eurasia. Agric. For. Èntomol. 2019, 22, 61–74. [Google Scholar] [CrossRef]
- Mutun, S.; Ceyhan, Z.; Sözen, C. Invasion by the oak lace bug, Corythucha arcuata (Say) (Heteroptera: Tingidae), in Turkey. Turk. J. Zool. 2009, 33, 263–268. [Google Scholar] [CrossRef]
- Connel, W.A.; Beacher, J.H. Life history and control of the oak lace bug. Del. Agric. Exp. Sta. Bull. 1947, 265, 28. [Google Scholar]
- Paulin, M.; Hirka, A.; Eötvös, C.B.; Gáspár, C.; Fürjes-Mikó, Á.; Csóka, G. Known and predicted impacts of the invasive oak lace bug (Corythucha arcuata) in European oak ecosystems—A review. Folia Oecologica 2020, 47, 131–139. [Google Scholar] [CrossRef]
- Bernardinelli, I. Distribution of the oak lace bug Corythucha arcuata (Say) in Northern Italy (Heteroptera Tingidae). Redia 2000, 83, 157–162. [Google Scholar]
- Csepelényi, M.; Hirka, A.; Szénási, Á.; Mikó, Á.; Szöcs, L.; Csóka, G. Az inváziós tölgy csipkéspoloska [Corythucha arcuata (Say, 1832)] gyors terjeszkedése és tömeges fellépése Magyarországon. Erdészettudományi Közlemények 2017, 7, 127–134. [Google Scholar] [CrossRef] [Green Version]
- May, R.M.; Hassell, M.P.; Anderson, R.; Tonkyn, D. Density dependence in host-parasitoid models. J. Anim. Ecol. 1981, 50, 855. [Google Scholar] [CrossRef]
- Jarosik, V.; Dixon, A.F.G. Population dynamics of a tree-dwelling aphid: Regulation and density-independent processes. J. Anim. Ecol. 1999, 68, 726–732. [Google Scholar] [CrossRef]
- Norris, R.F.; Caswell-Chen, E.P.; Kogan, M. Concepts in Integrated Pest Management; Prentice Hall: Hoboken, NJ, USA, 2002. [Google Scholar]
- Supriadi, K.; Mudjiono, G.; Abadi, A.L.; Karindah, S. The Influence of environmental factors to the abundance of scales (Hemiptera: Diaspididae) population on apple crop. J. Trop. Life Sci. 2015, 5, 20–24. [Google Scholar] [CrossRef]
- Uvarov, B.P. Insects and climate. Trans. R. Entomol. Soc. Lond. 1931, 79, 1–247. [Google Scholar] [CrossRef]
- Lindsey, A.A.; Newman, J.E. Use of official weather data in spring time: Temperature analysis of an Indiana phenological record. Ecology 1956, 37, 812–823. [Google Scholar] [CrossRef]
- Kocmánková, E.; Trnka, M.; Juroch, J.; Dubrovský, M.; Semerádová, D.; Možný, M.; Žalud, Z. Impact of climate change on the occurrence and activity of harmful organisms. Plant Prot. Sci. 2010, 45, S48–S52. [Google Scholar] [CrossRef] [Green Version]
- Skendžić, S.; Zovko, M.; Živković, I.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Pathak, H.; Aggarwal, P.K.; Singh, S.D. Climate Change Impact, Adaptation and Mitigation in Agriculture: Methodology for Assessment and Applications; Indian Agricultural Research Institute: New Delhi, India, 2012; p. 302. [Google Scholar]
- Shrestha, S. Effects of climate change in agricultural insect pest. Acta Sci. Agric. 2019, 3, 74–80. [Google Scholar] [CrossRef]
- Connor, E.F. Plant water deficits and insect responses: The preference of Corythucha arcuata (Heteroptera: Tingidae) for the foliage of white oak, Quercus Alba. Ecol. Èntomol. 1988, 13, 375–381. [Google Scholar] [CrossRef]
- Nikolic, N.; Pilipovic, A.; Drekic, M.; Kojic, D.; Poljakovic-Pajnik, L.; Orlovic, S.; Arsenov, D. Physiological responses of Pedunculate oak (Quercus robur L.) to Corythucha arcuata (Say, 1832) attack. Arch. Biol. Sci. 2019, 71, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Franjević, M.; Drvodelić, D.; Kolar, A.; Gradečki-Poštenjak, M.; Hrašovec, B. Impact of oak lace bug Corythucha arcuata (Heteroptera: Tingidae) on pedunculate oak (Quercus robur) seed quality. Nat. Resour. Green Technol. Sustain. Dev. 2018, 3, 161–165. [Google Scholar]
- Sönmez, E.; Demirbağ, Z.; Demir, I. Pathogenicity of selected entomopathogenic fungal isolates against the oak lace bug, Corythucha arcuata Say. (Hemiptera: Tingidae), under controlled conditions. Turk. J. Agric. For. 2016, 40, 715–722. [Google Scholar] [CrossRef]
- Kovač, M.; Gorczak, M.; Wrzosek, M.; Tkaczuk, C.; Pernek, M. Identification of Entomopathogenic Fungi as naturally occurring enemies of the invasive oak lace bug, Corythucha arcuata (Say) (Hemiptera: Tingidae). Insects 2020, 11, 679. [Google Scholar] [CrossRef]
- Kovač, M.; Linde, A.; Lacković, N.; Bollmann, F.; Pernek, M. Natural infestation of entomopathogenic fungus Beauveria pseudobassiana on overwintering Corythucha arcuata (Say) (Hemiptera: Tingidae) and its efficacy under laboratory conditions. For. Ecol. Manag. 2021, 491, 119193. [Google Scholar] [CrossRef]
- Bălăcenoiu, F.; Nețoiu, C.; Tomescu, R.; Simon, D.; Buzatu, A.; Toma, D.; Petrițan, I. Chemical control of Corythucha arcuata (Say, 1832), an invasive alien species, in oak forests. Forests 2021, 12, 770. [Google Scholar] [CrossRef]
- Tomescu, R.; Olenici, N.; Netoiu, C.; Balacenoiu, F.; Buzatu, A. Invasion of the oak lace bug Corythucha arcuata (Say.) in Romania: A first extended reporting. Ann. For. Res. 2018, 61, 161. [Google Scholar] [CrossRef]
- Shchurov, V.I.; Zamotajlov, A.S.; Bondarenko, A.S.; Shchurova, A.V.; Skvortsov, M.M.; Glushchenko, L.S. The oak lace bug Corythucha arcuata (Say, 1832) (Heteroptera: Tingidae) in the Northwestern Caucasus: Phenology, biology, monitoring of the territorial expansion, and harmfulness. Izv. St. Peterbg. Lesoteh. Akad. 2019. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; DebRoy, S.; Sarkar, D. R Package, version 3.1-147, Nlme: Linear and Nonlinear Mixed Effects Models. 2020. Available online: https://CRAN.R-project.org/package=nlme (accessed on 14 December 2021).
- Williams, D.; Hocht, G.; Csóka, G.; de Groot, M.; Hradil, K.; Chireceanu, C.; Hrašovec, B.; Castagneyrol, B. Corythucha arcuata (Heteroptera, Tingidae): Evaluation of the pest status in Europe and development of survey, control and management strategies. Euphresco 2021. [Google Scholar] [CrossRef]
- Hufnagel, L.; Ladányi, M.; Őszi, B. Population dynamics of the sycamore lace bug in Hungary. Appl. Ecol. Environ. Res. 2004, 4, 135–150. [Google Scholar]
- Ju, R.-T.; Wang, F.; Li, B. Effects of temperature on the development and population growth of the Sycamore lace bug, Corythucha ciliata. J. Insect Sci. 2011, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Willmer, P. Microclimate and the environmental physiology of insects. Adv. Insect Physiol. 1982, 16, 1–57. [Google Scholar] [CrossRef]
- Rahmathulla, V.K.; Kumar, C.M.K.; Angadi, B.S.; Sivaprasad, V. association of climatic factors on population dynamics of leaf roller, Diaphania pulverulentalis Hampson (Lepidoptera: Pyralidae) in mulberry plantations of sericulture seed farm. Psyche A J. Èntomol. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Maqbool, A.; Ahmad, H.; Srivastava, K.; Kumar, M.; Vir, V.; Jamwal, S. Effect of meteorological factors on the population dynamics of insect pests of tomato. Veg. Sci. 2013, 40, 90–92. [Google Scholar]
- Williams, C.B. An Analysis of Four Years Captures of Insects in a Light Trap. Part 1 I.I. The Effect of Weather Conditions on Insect Activity and the Estimation and Forecasting of Changes in the Insect Population. Ecol. Èntomol. 2009, 90, 227–306. [Google Scholar] [CrossRef]
- Williams, C.B.; Osman, M.F.H. A new approach to the problem of the optimum temperature for insect activity. J. Anim. Ecol. 1960, 29, 187–189. [Google Scholar] [CrossRef]
- Williams, C.B. Studies in the effect of weather conditions on the activity and abundance of insect populations. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1961, 244, 331–378. [Google Scholar] [CrossRef]
- Taylor, L.R. Analysis of the effect of temperature on insects in flight. J. Anim. Ecol. 1963, 32, 99–117. [Google Scholar] [CrossRef]
- Käfer, H.; Kovac, H.; Simov, N.; Battisti, A.; Erregger, B.; Schmidt, A.K.D.; Stabentheiner, A. Temperature tolerance and thermal environment of european seed bugs. Insects 2020, 11, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, R.-T.; Gao, L.; Zhou, X.-H.; Li, B. Tolerance to high temperature extremes in an invasive lace bug, Corythucha ciliata (Hemiptera: Tingidae), in subtropical China. PLoS ONE 2013, 8, e54372. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.-H.; Wei, M.-C.; Yuan, G.-J.; Cui, J.-X.; Gong, D.-F. Flight behavior of the sycamore lace bug, Corythucha ciliata, in relation to temperature, age, and sex. J. Integr. Agric. 2019, 18, 2330–2337. [Google Scholar] [CrossRef]
- Bălăcenoiu, F.; Japelj, A.; Bernardinelli, I.; Castagneyrol, B.; Csóka, G.; Glavendekić, M.; Hoch, G.; Hrašovec, B.; Ostoic, S.K.; Paulin, M.; et al. Corythucha arcuata (Say, 1832) (Hemiptera, Tingidae) in its invasive range in Europe: Perception, knowledge and willingness to act in foresters and citizens. NeoBiota 2021, 69, 133–153. [Google Scholar] [CrossRef]
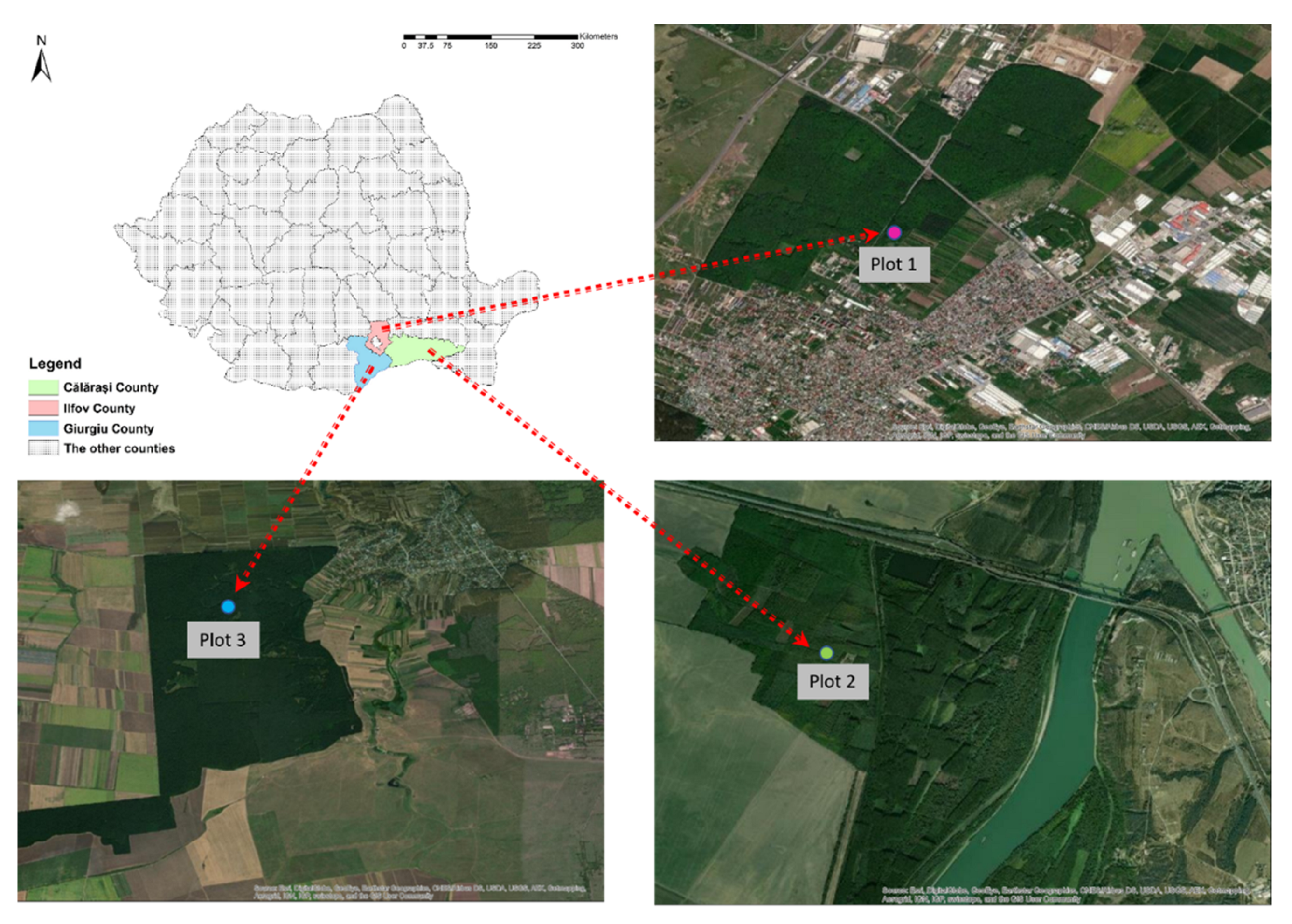
| Characteristic | Plot 1 | Plot 2 | Plot 3 |
|---|---|---|---|
| County | Ilfov | Călărași | Giurgiu |
| Forest district | I.N.C.D.S. | Lehliu | Giurgiu |
| Forest name | Ștefănești | Incinta | Ceagău |
| Coordinates | 44°30′13′′ N | 44°20′06′′ N | 44°08′11′′ N |
| 26°11′02′′ E | 27°59′22′′ E | 26°01′50′′ E | |
| Altitude | 80 m | 10 m | 90 m |
| First reported OLB | 2016 | 2018 | 2017 |
| Main species of stand 1 | Q.r. | Q.r. + Q.c. | Q.r. + Q.c. + Q.f. |
| Second species of stand 2 | T.t. + C.b. | U.m. | C.b. |
| Regeneration form 3 | N.R. | N.R. | N.R. |
| Average annual temperature | 10.9 °C | 13.3 °C | 11.3 °C |
| Average annual rainfall | 580 mm | 504 mm | 553 mm |
| Stand age (years) | 75 | 30 | 75 |
| Average stand diameter | 28 cm | 12 cm | 30 cm |
| Average stand height | 23 m | 10 m | 17 m |
| Vegetation limiting factor | poor humus content | poor water retention capacity | water deficiency |
| Description of the Sampling Procedure | 2019 | 2020 |
|---|---|---|
| Research period | 22 May–29 August | 18 May–8 September |
| Frequency of visits | 7–10 days | Two visits for each generation |
| Total number of visits | 13 for each plot | 6 for each plot |
| The purpose of each visit | Determining the proportion of in-flight adults | Determining the hourly proportion of in-flight adults (8:00 a.m.–7:00 p.m.) |
| Sampling methods | Mean number of adults on the leaf | Mean number of adults on the leaf |
| Number of samples | 300 leaves | 300 leaves |
| Period | Target OLB Generation | Day 1 | Day 2 | Plot/Forest |
|---|---|---|---|---|
| 18–26 May 2020 | Adults from the overwintering population | 20 May | 21 May | 2–Incinta |
| 18 May | 19 May | 3–Ceagău | ||
| 25 May | 26 May | 1–Ștefănești | ||
| 20–30 July 2020 | Adults from the first generation | 20 July | 21 July | 2–Incinta |
| 22 July | 23 July | 3–Ceagău | ||
| 28 July | 30 July | 1–Ștefănești | ||
| 31 August 31–8 September 2020 | Adults from the second generation | 31 August | 01 September | 2–Incinta |
| 02 September | 03 September | 3–Ceagău | ||
| 07 September | 08 September | 1–Ștefănești |
| Fixed Effects | Estimate | p-Value |
|---|---|---|
| Intercept (Time: 8 a.m.) | 0.17621 | 0.0000 |
| Temperature | +0.01936 | 0.0001 |
| Time: 9 a.m. | Time: 8 a.m. + 0.163521 | 0.0004 |
| Time: 10 a.m. | Time: 8 a.m. + 0.133903 | |
| Time: 11 a.m. | Time: 8 a.m. + 0.081469 | |
| Time: 12 p.m. | Time: 8 a.m. + 0.108626 | |
| Time: 1 p.m. | Time: 8 a.m. + 0.136774 | |
| Time: 2 p.m. | Time: 8 a.m. + 0.163615 | |
| Time: 3 p.m. | Time: 8 a.m. + 0.1688 | |
| Time: 4 p.m. | Time: 8 a.m. + 0.05681 | |
| Time: 5 p.m. | Time: 8 a.m. − 0.00516 | |
| Time: 6 p.m. | Time: 8 a.m. − 0.02147 | |
| Time: 7 p.m. | Time: 8 a.m. − 0.15106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bălăcenoiu, F.; Simon, D.C.; Nețoiu, C.; Toma, D.; Petrițan, I.C. The Seasonal Population Dynamics of Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae) and the Relationship between Meteorological Factors and the Diurnal Flight Intensity of the Adults in Romanian Oak Forests. Forests 2021, 12, 1774. https://doi.org/10.3390/f12121774
Bălăcenoiu F, Simon DC, Nețoiu C, Toma D, Petrițan IC. The Seasonal Population Dynamics of Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae) and the Relationship between Meteorological Factors and the Diurnal Flight Intensity of the Adults in Romanian Oak Forests. Forests. 2021; 12(12):1774. https://doi.org/10.3390/f12121774
Chicago/Turabian StyleBălăcenoiu, Flavius, Dieter Carol Simon, Constantin Nețoiu, Dragoș Toma, and Ion Cătălin Petrițan. 2021. "The Seasonal Population Dynamics of Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae) and the Relationship between Meteorological Factors and the Diurnal Flight Intensity of the Adults in Romanian Oak Forests" Forests 12, no. 12: 1774. https://doi.org/10.3390/f12121774
APA StyleBălăcenoiu, F., Simon, D. C., Nețoiu, C., Toma, D., & Petrițan, I. C. (2021). The Seasonal Population Dynamics of Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae) and the Relationship between Meteorological Factors and the Diurnal Flight Intensity of the Adults in Romanian Oak Forests. Forests, 12(12), 1774. https://doi.org/10.3390/f12121774






