Water Availability Controls the Biomass Increment of Melia dubia in South India
Abstract
:1. Introduction
2. Materials and Methods
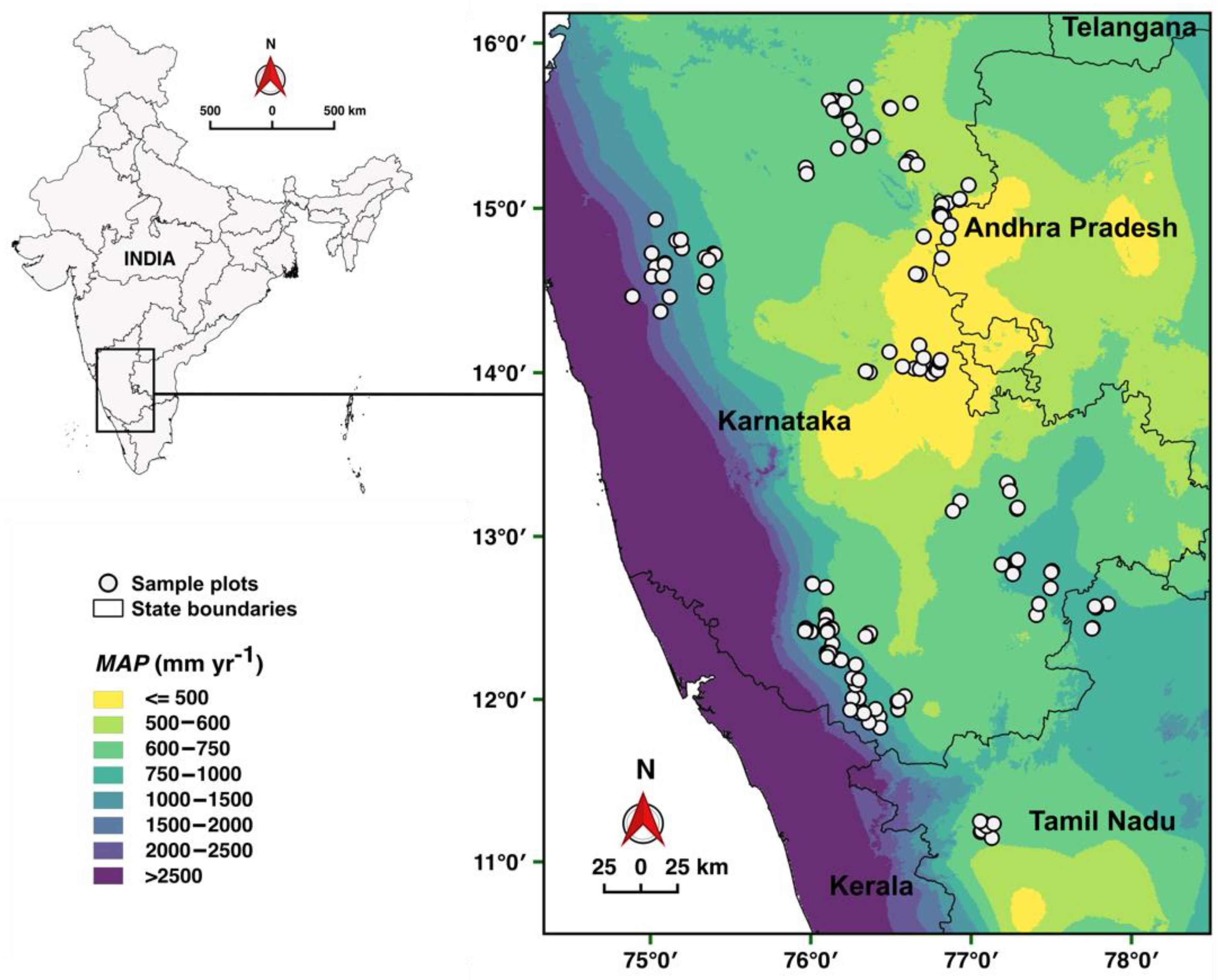
2.1. Study Region
2.2. Study Sites and Plot Design
2.3. Tree Observations
2.4. Wood Density
2.5. Aboveground Biomass Estimation
2.6. Bioclimatic Variables
2.7. Soil Variables
2.8. Statistical Analyses
3. Results
4. Discussion
4.1. Aboveground Biomass of M. dubia
4.2. Growth Potential of M. dubia
4.3. Controls of Biomass and Growth of M. dubia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghazoul, J.; Bugalho, M.; Keenan, R. Plantations take economic pressure off natural forests. Nature 2019, 570, 307. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, M.R.; Chazdon, R.L.; Brancalion, P.H.S.; David, L. Forests: When natural regeneration is unrealistic. Nature 2019, 570, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, S.L.; Wheeler, C.E.; Mitchard, E.T.A.; Koch, A. Regenerate natural forests to store carbon. Nature 2019, 568, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.; Maginnis, S.; Crouzeilles, R. Forests: Many benefits of the Bonn Challenge. Nature 2019, 570, 164. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Delgado-baquerizo, M.; García-gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; García-palacios, P.; et al. Europe PMC Funders Group Plant species richness and ecosystem multifunctionality in glob-al drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated dryland expansion under climate change. Nat. Clim. Chang. 2016, 6, 166–171. [Google Scholar] [CrossRef]
- Bastin, J.-F.; Berrahmouni, N.; Grainger, A.; Maniatis, D.; Mollicone, D.; Moore, R.; Patriarca, C.; Picard, N.; Sparrow, B.; Abra-ham, E.M.; et al. The extent of forest in dryland biomes. Science 2017, 356, 635–638. [Google Scholar] [CrossRef] [Green Version]
- Koh, L.P.; Levang, P.; Ghazoul, J. Designer landscapes for sustainable biofuels. Trends Ecol. Evol. 2009, 24, 431–438. [Google Scholar] [CrossRef]
- FAO; UNEP. The State of the World’s Forests 2020: Forests, Biodiversity and People; FAO: Rome, Italy; UNEP: Nairobi, Kenya, 2020; 188p, ISBN 978-92-5-132419-6. [Google Scholar]
- United Nations United Nations Population Division-Department of Economic and Social Affairs: World Population Prospects 2019. Available online: http://creativecommons.org/licenses/by/3.0/igo/ (accessed on 25 October 2021).
- Singh, S.J.; Krausmann, F.; Gingrich, S.; Haberl, H.; Erb, K.H.; Lanz, P.; Martinez-Alier, J.; Temper, L. India’s biophysical econ-omy, 1961–2008. Sustainability in a national and global context. Ecol. Econ. 2012, 76, 60–69. [Google Scholar] [CrossRef]
- World Bank. The World Bank Data. Available online: https://data.worldbank.org/country/india?view=chart (accessed on 25 October 2021).
- FAO. Forestry Production and Trade. FAOSTAT Database. Available online: http://fenix.fao.org/faostat/beta/en/#data/FO (accessed on 17 October 2021).
- OECD. Economic Outlook for Southeast Asia, China and India 2019: Towards Smart Urban Transportation; OECD Publishing: Paris, France, 2018; 280p. [Google Scholar] [CrossRef]
- Ghosh, M.; Sinha, B. Impact of forest policies on timber production in India: A review. Nat. Resour. Forum 2016, 40, 62–76. [Google Scholar] [CrossRef] [Green Version]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of degraded tropical forest landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef] [Green Version]
- FAO. Global Forest Resources Assessment 2010-Main Report; FAO Forestry Paper 163; FAO: Rome, Italy, 2010; 340p, Available online: http://www.fao.org/3/i1757e/i1757e.pdf (accessed on 25 October 2021).
- Amazonas, N.T.; Forrester, D.I.; Silva, C.C.; Almeida, D.R.A.; Rodrigues, R.R.; Brancalion, P.H.S. High diversity mixed planta-tions of Eucalyptus and native trees: An interface between production and restoration for the tropics. For. Ecol. Manage. 2018, 417, 247–256. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-species versus monocultures in plantation forestry: Development, benefits, ecosystem services and perspectives for the future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Rawat, V.; Negi, J.D.S. Biomass production of Eucalyptus tereticornis in different agroecological region. Indian For. 2004, 130, 762–770. [Google Scholar]
- Hughes, C.E. Risks of species introductions in tropical forestry. Commonw. For. Rev. 1994, 73, 243–252. [Google Scholar]
- Joshi, M.; Palanisami, K. Impact of Eucalyptus Plantations on Ground Water Availability in South Karnataka. In Proceedings of the ICID 21st International Congress on Irrigation and Drainage, Tehran, Iran, 15–23 October 2011; International Commission on Irrigation and Drainage: Tehran, Iran, 2011; pp. 255–262. [Google Scholar]
- Bilal, H.; Nisa, S.; Shahid Ali, S. Effects of Exotic Eucalyptus Plantation on the Ground and Surface Water of District Malakand, Pakistan. Int. J. Innov. Sci. Res. 2014, 8, 299–304. [Google Scholar]
- GOK. Government of Karnataka Notification No. FEE 37 FDP 2017 Dated 23.02.2017; Government of Karnataka: Bengaluru, India, 2017; 6p.
- Nasayao, E.E.; Nasayao, L.Z.; Zara, M.A.; Ulep, F.V. Bagalunga (Melia dubia Cav.): An indigenous fast-growing multipurpose tree species in Eastern Visayas, Philippines. Ann. Trop. Res. 1994, 16, 6–19. [Google Scholar]
- Sharma, S.K.; Shukla, S.R.; Sujatha, M.; Shashikala, S.; Kumar, P. Assessment of certain wood quality parameters of selected gen-otypes of Melia dubia Cav. grown in a seedling seed orchard. J. Indian Acad. Wood Sci. 2012, 9, 165–169. [Google Scholar] [CrossRef]
- Thakur, N.S.; Mohanty, S.; Gunaga, R.P.; Gajbhiye, N.A. Melia dubia Cav. spatial geometries influence the growth, yield and es-sential oil principles content of Cymbopogon flexuosus (Nees Ex Steud.) W.Watson. Agrofor. Syst. 2020, 94, 985–995. [Google Scholar] [CrossRef]
- Wunder, S.; Kaphengst, T.; Timeus, K.; Berzins, K. Impact of EU Bioenergy Policy on Developing Countries; EP/EXPO/B/DEVE/2011/FWC/2009-01/LOT 5/21; EU: Brussels, Belgium, 2012; 24p. [Google Scholar] [CrossRef]
- Warrier, R.R. Money Spinning Trees 2: Melia dubia Cav. Synonyms: Melia composita Willd., Melia superb Roxb; Director, Institute of Forest Genetics and Tree Breeding: Coimbatore, India, 2011; 16p. [Google Scholar]
- Nguyen, H.; Lamb, D.; Herbohn, J.; Firn, J. Designing mixed species tree plantations for the tropics: Balancing ecological attributes of species with landholder preferences in the Philippines. PLoS ONE 2014, 9, e95267. [Google Scholar] [CrossRef]
- Parthiban, K.T.; Bharathi, A.K.; Seenivasan, R.; Kamala, K.; Rao, M.G. Integrating Melia dubia in Agroforestry farms as an alter-nate pulpwood species. Asia-Pac. Agrofor. Newsl. 2009, 34, 3–4. [Google Scholar]
- Sinha, S.K.; Chaudhari, P.A.; Thakur, N.S.; Jha, S.K.; Patel, D.P.; Dhaka, R.K. Melia dubia Cav. wood properties vary with age and influence the pulp and paper quality. Int. Wood Prod. J. 2019, 10, 139–148. [Google Scholar] [CrossRef]
- Kirankumar, G.K.; Patil, H.Y. Growth and productivity of Melia dubia under different plant density. J. farm Sci. 2017, 30, 70–73. [Google Scholar]
- Toledo, M.; Poorter, L.; Peña-Claros, M.; Alarcón, A.; Balcázar, J.; Leaño, C.; Licona, J.C.; Llanque, O.; Vroomans, V.; Zuidema, P.; et al. Climate is a stronger driver of tree and forest growth rates than soil and disturbance. J. Ecol. 2011, 99, 254–264. [Google Scholar] [CrossRef]
- Becknell, J.M.; Kissing Kucek, L.; Powers, J.S. Aboveground biomass in mature and secondary seasonally dry tropical forests: A literature review and global synthesis. For. Ecol. Manag. 2012, 276, 88–95. [Google Scholar] [CrossRef]
- Wagner, F.; Rossi, V.; Stahl, C.; Bonal, D.; Hérault, B. Water availability is the main climate driver of neotropical tree growth. PLoS ONE 2012, 7, e34074. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.; Rossi, V.; Aubry-Kientz, M.; Bonal, D.; Dalitz, H.; Gliniars, R.; Stahl, C.; Trabucco, A.; Hérault, B. Pan-tropical analysis of climate effects on seasonal tree growth. PLoS ONE 2014, 9, e92337. [Google Scholar] [CrossRef]
- Guan, K.; Pan, M.; Li, H.; Wolf, A.; Wu, J.; Medvigy, D.; Caylor, K.K.; Sheffield, J.; Wood, E.F.; Malhi, Y.; et al. Photosynthetic seasonality of global tropical forests constrained by hydroclimate. Nat. Geosci. 2015, 8, 284–289. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; ter Steege, H.; Lopez-Gonzalez, G.; Monteagudo Mendoza, A.; Bri-enen, R.; Feldpausch, T.R.; Pitman, N.; et al. Seasonal drought limits tree species across the Neotropics. Ecography 2017, 40, 618–629. [Google Scholar] [CrossRef] [Green Version]
- Hunter, I. Above ground biomass and nutrient uptake of three tree species (Eucalyptus camaldulensis, Eucalyptus grandis and Dalbergia sissoo) as affected by irrigation and fertiliser, at 3 years of age, in southern India. For. Ecol. Manage. 2001, 144, 189–200. [Google Scholar] [CrossRef]
- Stape, J.L.; Binkley, D.; Ryan, M.G.; Fonseca, S.; Loos, R.A.; Takahashi, E.N.; Silva, C.R.; Silva, S.R.; Hakamada, R.E.; de A Ferreira, J.M.; et al. The Brazil Eucalyptus Potential Productivity Project: Influence of water, nutrients and stand uniformity on wood production. For. Ecol. Manag. 2010, 259, 1684–1694. [Google Scholar] [CrossRef]
- Campoe, O.C.; Stape, J.L.; Albaugh, T.J.; Lee Allen, H.; Fox, T.R.; Rubilar, R.; Binkley, D. Fertilization and irrigation effects on tree level aboveground net primary production, light interception and light use efficiency in a loblolly pine plantation. For. Ecol. Manage. 2013, 288, 43–48. [Google Scholar] [CrossRef]
- Minhas, P.S.; Yadav, R.K.; Lal, K.; Chaturvedi, R.K. Effect of long-term irrigation with wastewater on growth, biomass production and water use by Eucalyptus (Eucalyptus tereticornis Sm.) planted at variable stocking density. Agric. Water Manag. 2015, 152, 151–160. [Google Scholar] [CrossRef]
- Pérez-Cruzado, C.; Sanchez-Ron, D.; Rodríguez-Soalleiro, R.; Hernández, M.J.; Mario Sánchez-Martín, M.; Cañellas, I.; Sixto, H. Biomass production assessment from Populus spp. short-rotation irrigated crops in Spain. GCB Bioenergy 2014, 6, 312–326. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1 km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Pal, D.K.; Mandal, C.; Chandran, P.; Ray, S.K.; Sarkar, D.; Velmourougane, K.; Srivastava, A.; Sidhu, G.S.; Singh, R.S.; et al. Soils of India: Historical perspective, classification and recent advances. Curr. Sci. 2013, 104, 1308–1323. [Google Scholar]
- Reddy, C.S.; Jha, C.S.; Diwakar, P.G.; Dadhwal, V.K. Nationwide classification of forest types of India using remote sensing and GIS. Environ. Monit. Assess. 2015, 187, 1–30. [Google Scholar] [CrossRef]
- Ratnam, J.; Chengappa, S.K.; Machado, S.J.; Nataraj, N.; Osuri, A.M.; Sankaran, M. Functional Traits of Trees from Dry Decidu-ous “Forests” of Southern India Suggest Seasonal Drought and Fire Are Important Drivers. Front. Ecol. Evol. 2019, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.S.; Behera, M.D.; Murthy, M.S.R.; Roy, A.; Singh, S.; Kushwaha, S.P.S.; Jha, C.S.; Sudhakar, S.; Joshi, P.K.; Reddy, C.S.; et al. New vegetation type map of India prepared using satellite remote sensing: Comparison with global vegetation maps and utili-ties. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 142–159. [Google Scholar] [CrossRef]
- GOI. Pocket Book of Agricultural Statistics; Ministry of Agriculture & Farmers Welfare Department of Agriculture, Cooperation & Farmers Welfare, Directorate of Economics & Statistics, Government of India: New Delhi, India, 2019; 122p. Available online: https://eands.dacnet.nic.in/PDF/Pocket%20Book%202019.pdf (accessed on 25 October 2021).
- Trabucco, A.; Zomer, R. Global Aridity Index and Potential Evapotranspiration (ET0) Climate Database v2. Figshare Dataset 2019. [Google Scholar] [CrossRef]
- Chauhan, S.; Arun Kumar, A.N. Assessment of variability in morphological and wood quality traits in Melia dubia Cav. for selec-tion of superior trees. J. Indian Acad. Wood Sci. 2014, 11, 25–32. [Google Scholar] [CrossRef]
- Reyes, G.; Brown, S.; Chapman, J.; Lugo, A.E. Wood Densities of Tropical Tree Species: General Technical Report SO-88; U.S. Dept of Agriculture, Forest Service, Southern Forest: New Orleans, LA, USA, 1992; 15p. [CrossRef]
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Data from: Towards a Worldwide Wood Economics Spectrum. Available online: https://datadryad.org/stash/dataset/doi:10.5061/dryad.234 (accessed on 25 October 2021).
- Osuri, A.M.; Gopal, A.; Raman, T.R.S.; Defries, R.; Cook-Patton, S.C.; Naeem, S. Greater stability of carbon capture in spe-cies-rich natural forests compared to species-poor plantations. Environ. Res. Lett. 2020, 15, 034011. [Google Scholar] [CrossRef]
- Sales-come, R.; Baldos, A.P. Carbon Stocks Assessment of Various Land Uses in Marginal Land. J. Sci. Eng. Technol. 2018, 6, 201–207. [Google Scholar]
- Tesfaye, M.A.; Gardi, O.; Anbessa, T.B.; Blaser, J. Aboveground biomass, growth and yield for some selected introduced tree species, namely Cupressus lusitanica, Eucalyptus saligna, and Pinus patula in Central Highlands of Ethiopia. J. Ecol. Environ. 2020, 44, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- Rahman, S.A.; Sunderland, T.; Kshatriya, M.; Roshetko, J.M.; Pagella, T.; Healey, J.R. Towards productive landscapes: Trade-offs in tree-cover and income across a matrix of smallholder agricultural land-use systems. Land Use Policy 2016, 58, 152–164. [Google Scholar] [CrossRef] [Green Version]
- Nuthan, D.; Chandrashekara Reddy, K.M.; Sunil Kumar, P.; Vajranabhaiah, S.N.; Yogeesha, T.D. Cultivation of Melia dubia on Farm Lands in Kanakapura Taluk, Ramanagara District of Karnataka-a Success Story; University of Agricultural Sciences: Bangalore, India, 2009; 33p. [Google Scholar]
- QGIS. Development Team Geographic Information System. Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org2017 (accessed on 26 October 2021).
- Thien, S.J. A flow diagram for teaching texture by feel analysis. J. Agron. Educ. 1979, 8, 54–55. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff method for determining soil organic matter and a proposed modifica-tion of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Subbiah, B.V.; Asija, G.L. A rapid procedure for the estimation of available nitrogen in soils. Curr. Sci. 1934, 25, 59–60. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicar-Bonate; Circular/United States Department of Agriculture: Washington, DC, USA, 1954.
- Richards, L.A. Diagnosis and Improvement of Saline and Sodic Soils; Richards, L.A., Ed.; United States Department of Agriculture: Washington, DC, USA, 1954; 160p.
- Kassambara, A. ggcorrplot: Visualization of a Correlation Matrix Using “ggplot2”. R Package Version 0.1.3, 2019. Available online: https://CRAN.R-project.org/package=ggcorrplot (accessed on 4 June 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Inkscape Inkscape 1.0 (4035a4fb49, 2020-05-01). Open Source Scalable Vector Graphics Editor 2020. Available online: https://inkscape.org/ (accessed on 26 October 2021).
- Thomas, S.C.; Martin, A.R. Carbon content of tree tissues: A synthesis. Forests 2012, 3, 332–352. [Google Scholar] [CrossRef] [Green Version]
- Mani, S.; Parthasarathy, N. Above-ground biomass estimation in ten tropical dry evergreen forest sites of peninsular India. Biomass Bioenergy 2007, 31, 284–290. [Google Scholar] [CrossRef]
- Naveenkumar, J.; Arunkumar, K.S.; Sundarapandian, S. Biomass and carbon stocks of a tropical dry forest of the Javadi Hills, Eastern Ghats, India. Carbon Manag. 2017, 8, 351–361. [Google Scholar] [CrossRef]
- Kothandaraman, S.; Dar, J.A.; Sundarapandian, S.; Dayanandan, S.; Khan, M.L. Ecosystem-level carbon storage and its links to diversity, structural and environmental drivers in tropical forests of Western Ghats, India. Sci. Rep. 2020, 10, 13444. [Google Scholar] [CrossRef] [PubMed]
- Karmacharya, S.B.; Singh, K.P. Biomass and net production of teak plantations in a dry tropical region in India. For. Ecol. Manage. 1992, 55, 233–247. [Google Scholar] [CrossRef]
- Buvaneswaran, C.; George, M.; Perez, D.; Kanninen, M. Biomass of Teak Plantations in Tamil Nadu, India and Costa Rica Com-pared. J. Trop. For. Sci. 2006, 18, 195–197. [Google Scholar]
- Kanime, N.; Kaushal, R.; Tewari, S.K.; Raverkar, K.P.; Chaturvedi, S.; Chaturvedi, O.P. Biomass production and carbon sequestra-tion in different tree-based systems of Central Himalayan Tarai region. For. Trees Livelihoods 2013, 22, 38–50. [Google Scholar] [CrossRef]
- Arora, G.; Chaturvedi, S.; Kaushal, R.; Nain, A.; Tewari, S.; Alam, N.M.; Chaturvedi, O.P. Growth, biomass, carbon stocks, and sequestration in an age series of Populus deltoids plantations in Tarai region of central Himalaya. Turkish J. Agric. For. 2014, 38, 550–560. [Google Scholar] [CrossRef]
- Swamy, S.L.; Puri, S.; Singh, A.K. Growth, biomass, carbon storage and nutrient distribution in Gmelina arborea Roxb. stands on red lateritic soils in central India. Bioresour. Technol. 2003, 90, 109–126. [Google Scholar] [CrossRef]
- Swamy, S.L.; Mishra, A.; Puri, S. Biomass production and root distribution of Gmelina arborea under an agrisilviculture system in subhumid tropics of Central India. New For. 2003, 26, 167–186. [Google Scholar] [CrossRef]
- Kumar, B.M.; George, S.J.; Jamaludheen, V.; Suresh, T.K. Comparison of biomass production, tree allometry and nutrient use efficiency of multipurpose trees grown in woodlot and silvopastoral experiments in Kerala, India. For. Ecol. Manag. 1998, 112, 145–163. [Google Scholar] [CrossRef]
- Tyagi, K.; Sharma, S.D.; Tyagi, P. Development of biomass and productivity in an age series of Dalbergia sissoo plantations in sodic lands of Uttar Pradesh. Ann. For. 2009, 17, 219–233. [Google Scholar]
- Rana, B.S.; Rao, O.P.; Singh, B.P. Biomass production in 7 year old plantations of Casuarina equisetifolia on sodic soil. Trop. Ecol. 2001, 42, 207–212. [Google Scholar]
- Rajendran, K.; Devaraj, P. Biomass and nutrient distribution and their return of Casuarina equisetifolia inoculated with biofertiliz-ers in farm land. Biomass Bioenergy 2004, 26, 235–249. [Google Scholar] [CrossRef]
- Frederick, D.J.; Madgwick, H.A.I.; Jurgensen, M.F.; Oliver, G.R. Dry matter content and nutrient distribution in an age series of Eucalyptus regnans plantations in New Zealand. New Zeal. J. For. Sci. 1985, 15, 158–179. [Google Scholar]
- Frederick, D.J.; Madgwick, H.A.I.; Oliver, G.R.; Jurgensen, M.F. Dry matter and nutrient content of 8-year-old Eucalyptus saligna growing at Taheke forest. New Zeal. J. For. Sci. 1985, 15, 251–254. [Google Scholar]
- Wang, D.; Bormann, F.H.; Lugo, A.E.; Bowden, R.D. Comparison of nutrient-use efficiency and biomass production in five tropical tree taxa. For. Ecol. Manag. 1991, 46, 1–21. [Google Scholar] [CrossRef]
- Fuwape, J.A.; Akindele, S.O. Biomass yield and energy value od some fast-growing multipurpose trees in Nigeria. Biomass Bioenergy 1997, 12, 101–106. [Google Scholar] [CrossRef]
- Paula, R.R.; Reis, G.G.; Reis, M.G.F.; Neto, S.N.O.; Leite, H.G.; Melido, R.C.N.; Lopes, H.N.S.; Souza, F.C. Eucalypt growth in monoculture and silvopastoral systems with varied tree initial densities and spatial arrangements. Agrofor. Syst. 2013, 87, 1295–1307. [Google Scholar] [CrossRef]
- Acuña, E.; Cancino, J.; Rubilar, R.; Sandoval, S. Aboveground biomass growth and yield of first rotation cutting cycle of Acacia and Eucalyptus short rotation dendroenergy crops. Rev. Árvore 2017, 41, e410608. [Google Scholar] [CrossRef]
- Turner, B.L.; Brenes-Arguedas, T.; Condit, R. Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature 2018, 555, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Soong, J.L.; Janssens, I.A.; Grau, O.; Margalef, O.; Stahl, C.; Van Langenhove, L.; Urbina, I.; Chave, J.; Dourdain, A.; Ferry, B.; et al. Soil properties explain tree growth and mortality, but not biomass, across phosphorus-depleted tropical forests. Sci. Rep. 2020, 10, 2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Clare, S.; Mack, M.C.; Brooks, M. A direct test of nitrogen and phosphorus limitation to net primary productivity in a lowland tropical wet forest. Ecology 2013, 94, 1540–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Parameters | Estimate | SE | t Statistic | p-Value |
|---|---|---|---|---|
| Intercept | 4.52 | 0.32 | 14.27 | <0.001 |
| Age | 1.45 | 0.09 | 16.28 | <0.001 |
| Stand density | 0.54 | 0.29 | 1.84 | 0.06 |
| Age: Stand density | −0.07 | 0.09 | −0.83 | 0.40 |
| Age: Irrigation (irrigated) | 0.06 | 0.04 | 1.67 | 0.09 |
| Age: CWD | 0.08 | 0.03 | 2.62 | <0.01 |
| Age: Nsoil | 0.03 | 0.02 | 1.62 | 0.10 |
| Age: Psoil | 0.05 | 0.02 | 2.89 | <0.01 |
| Age: CWD: Irrigation (irrigated) | −0.05 | 0.03 | −1.37 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Röll, A.; Ramesha, M.N.; Link, R.M.; Hertel, D.; Schuldt, B.; Patil, S.L.; Hölscher, D. Water Availability Controls the Biomass Increment of Melia dubia in South India. Forests 2021, 12, 1675. https://doi.org/10.3390/f12121675
Röll A, Ramesha MN, Link RM, Hertel D, Schuldt B, Patil SL, Hölscher D. Water Availability Controls the Biomass Increment of Melia dubia in South India. Forests. 2021; 12(12):1675. https://doi.org/10.3390/f12121675
Chicago/Turabian StyleRöll, Alexander, Mundre N. Ramesha, Roman M. Link, Dietrich Hertel, Bernhard Schuldt, Shekhargouda L. Patil, and Dirk Hölscher. 2021. "Water Availability Controls the Biomass Increment of Melia dubia in South India" Forests 12, no. 12: 1675. https://doi.org/10.3390/f12121675






