Tree Mortality following Thinning and Prescribed Burning in Central Oregon, U.S.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Treatments
- (1)
- Fifty (50) UMZ (low density stand): Thinned from below to 50% of the upper management zone (UMZ) for the dominant plant association based on stand density index (SDI) values for ponderosa pine. Stand density was reduced by removing trees from the subdominant crown classes to improve residual tree growth and tree vigor. Thinning was followed by mastication and prescribed burning;
- (2)
- Seventy-five (75) UMZ (medium density stand): Thinned from below to 75% of the UMZ followed by mastication and prescribed burning;
- (3)
- Seventy-five (75) UMZ Gap: A regeneration cut applied to 75% of the UMZ followed by mastication and prescribed burning. Small gaps in the canopy (~0.1 ha) were created by augmenting existing gaps or creating new gaps;
- (4)
- One hundred (100) UMZ (high density stand): Thinned from below to 100% of the UMZ followed by mastication and prescribed burning;
- (5)
- Untreated control (high density stand): No manipulation.
2.3. Data Collection
2.4. Analyses
3. Results and Discussion
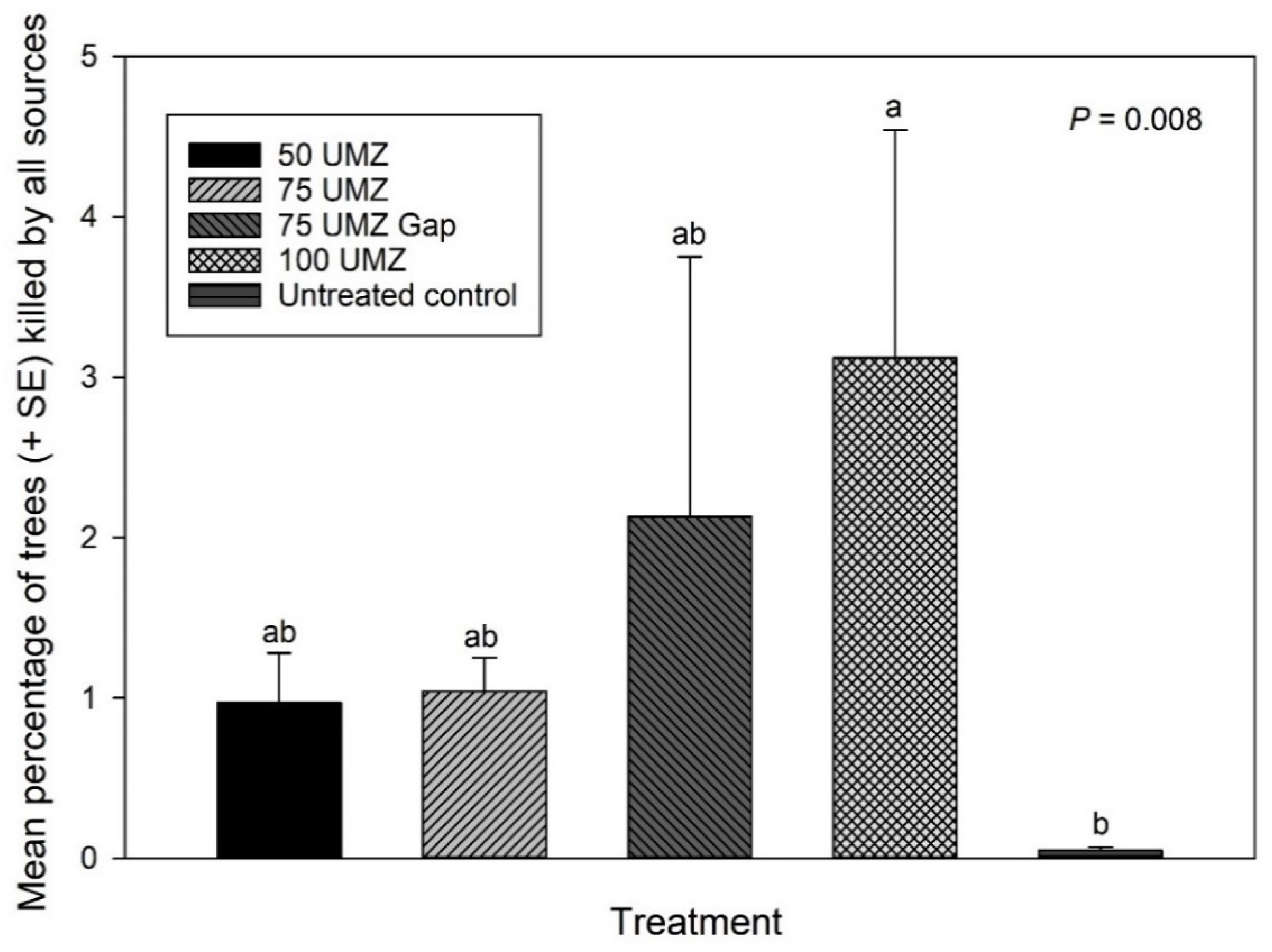
3.1. Overall Tree Mortality
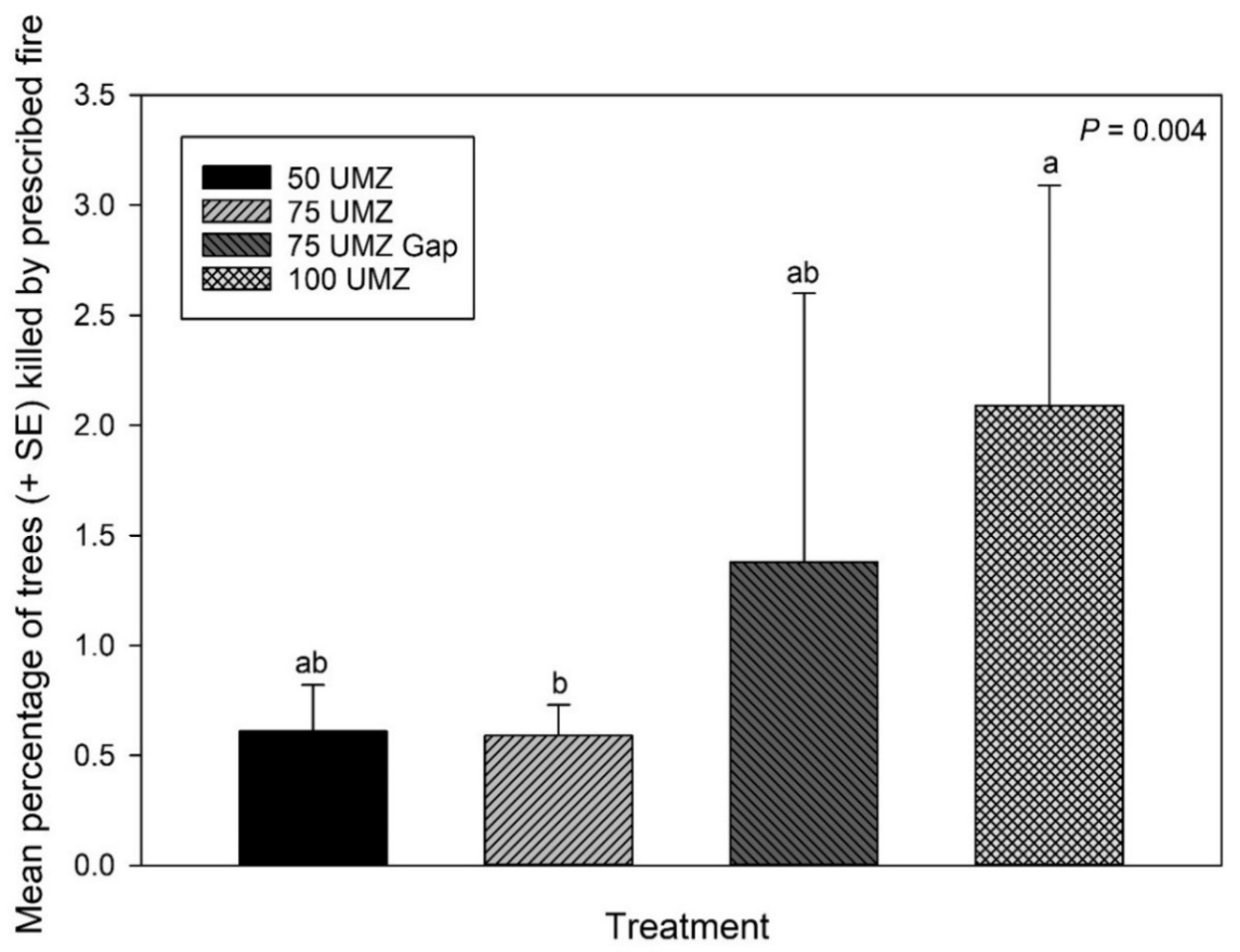
3.2. Prescribed Fire-Caused Tree Mortality
3.3. Bark Beetle-Caused Tree Mortality
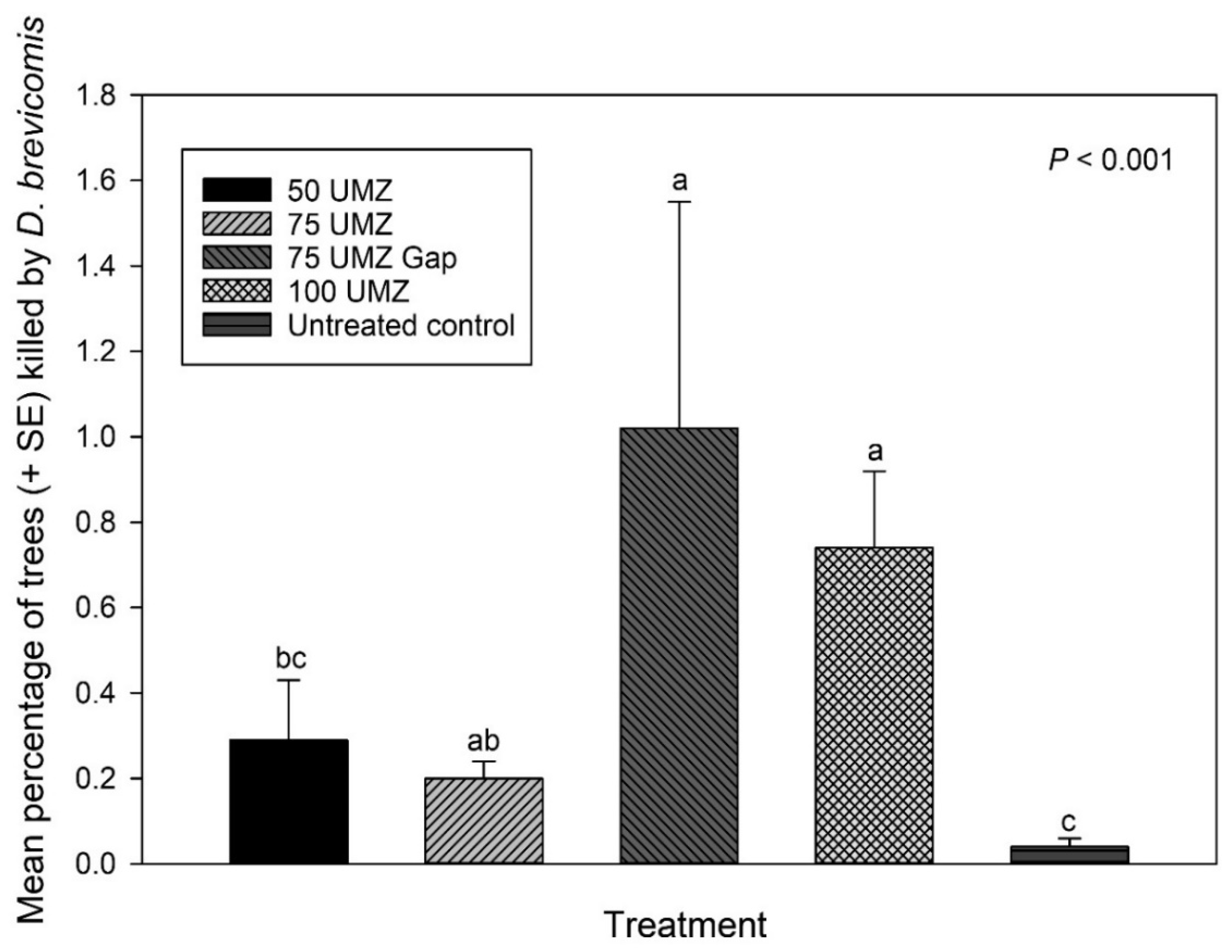
3.4. Western Pine Beetle
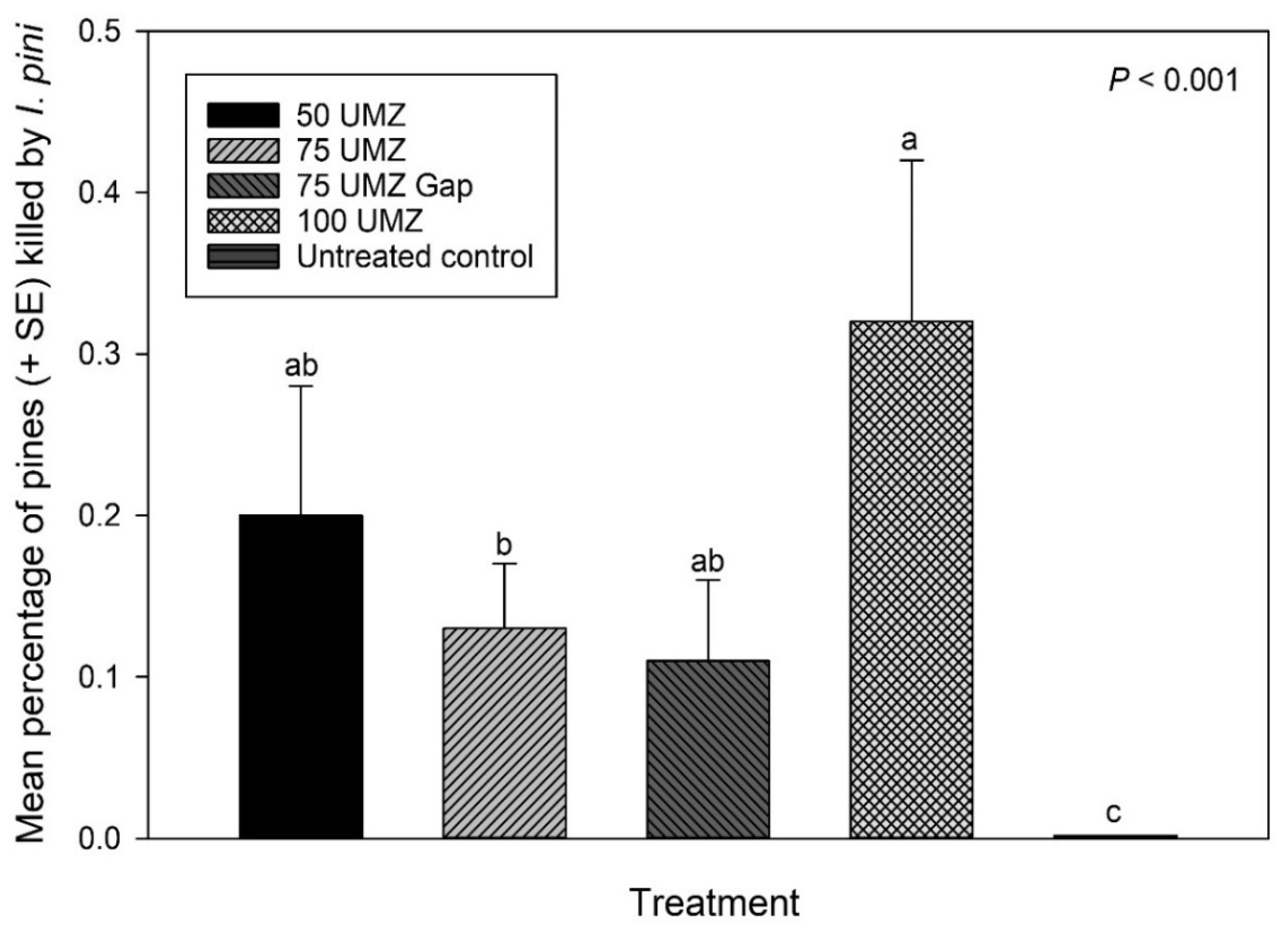
3.5. Pine Engraver
3.6. Fir Engraver
3.7. Red Turpentine Beetle
3.8. Stand Density
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achim, A.; Moreau, G.; Coops, N.C.; Axelson, J.N.; Barrette, J.; Bédard, S.; Byrne, K.E.; Caspersen, J.; Dick, A.R.; D’Orangeville, L.; et al. The changing culture of silviculture. For. Int. J. For. Res. 2021, cpab047. [Google Scholar] [CrossRef]
- Agee, J.K. Fire Ecology of Pacific Northwest. Forests; Island Press: Washington, DC, USA, 1993; p. 505. [Google Scholar]
- Stine, P.; Hessburg, P.; Spies, T.; Kramer, M.; Fettig, C.J.; Hansen, A.; Lehmkuhl, J.; O’Hara, K.; Polivka, K.; Singleton, P.; et al. The Ecology and Management of Moist Mixed-Conifer Forests in Eastern Oregon and Washington: A Synthesis of the Relevant Biophysical Science and Implications for Future Land Management; USDA Forest Service Gen. Tech. Rep. PNW-GTR-897; Pacific Northwest Research Station: Portland, OR, USA, 2014; p. 254.
- Vose, J.M.; Peterson, D.L.; Domke, G.M.; Fettig, C.J.; Joyce, L.A.; Keane, R.E.; Luce, C.H.; Prestemon, J.P.; Band, L.E.; Clark, J.S.; et al. Forests. In Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment, Volume II; Reidmiller, D.R., Avery, C.W., Easterling, D.R., Kunkel, K.E., Lewis, K.L.M., Maycock, T.K., Stewart, B.C., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2018; pp. 223–258. [Google Scholar]
- Flannigan, M.D.; Amiro, B.D.; Logan, K.A.; Stocks, B.J.; Wotton, M. Forest fires and climate. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 847–859. [Google Scholar] [CrossRef]
- Agee, J.K.; Skinner, C.N. Basic principles of forest fuel reduction treatments. For. Ecol. Manag. 2005, 211, 83–96. [Google Scholar] [CrossRef]
- Stephens, S.L.; Moghaddas, J.J.; Edminster, C.; Fiedler, C.E.; Haase, S.; Harrington, M.; Keeley, J.E.; Knapp, E.E.; McIver, J.D.; Metlen, K.; et al. Fire treatment effects on vegetation structure, fuels, and potential fire severity in western U.S. forests. Ecol. Appl. 2009, 19, 305–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, S.L.; McIver, J.D.; Boerner, R.E.J.; Fettig, C.J.; Fontaine, J.B.; Hartsough, B.R.; Kennedy, P.L.; Schwilk, D.W. Effects of forest fuel-reduction treatments in the United States. Bioscience 2012, 62, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, M.W.; Skinner, C.N.; Hamilton, T.A. Probability of tree survival after wildfire in an interior pine forest of northern California: Effects of thinning and prescribed fire. For. Ecol. Manag. 2007, 247, 200–208. [Google Scholar] [CrossRef]
- McIver, J.; Stephens, S.; Agee, J.; Barbour, J.; Boerner, R.; Edminster, C.; Erickson, K.; Farris, K.; Fettig, C.; Fiedler, C.; et al. Ecological effects of alternative fuel reduction treatments: Highlights of the national Fire and Fire Surrogate study (FFS). Int. J. Wildland Fire 2013, 22, 63–82. [Google Scholar] [CrossRef]
- Crotteau, J.S.; Keyes, C.R.; Hood, S.M.; Affleck, D.L.R.; Sala, A. Fuel dynamics after a bark beetle outbreak impacts experimental fuel treatments. Fire Ecol. 2018, 14, 13. [Google Scholar] [CrossRef]
- Furniss, R.L.; Carolin, V.M. Western Forest Insects; USDA Forest Service Misc. Pub. 1339; Washington Office: Washington, DC, USA, 1977; p. 654.
- Fettig, C.J.; Mortenson, L.A.; Bulaon, B.M.; Foulk, P.B. Tree mortality following drought in the central and southern Sierra Nevada, California, U.S. For. Ecol. Manag. 2019, 432, 164–178. [Google Scholar] [CrossRef]
- Morris, J.L.; Cottrell, S.; Fettig, C.J.; DeRose, R.J.; Mattor, K.W.; Carter, V.A.; Clear, J.; Clement, J.; Hansen, W.D.; Hicke, J.A.; et al. Bark beetles as agents of change in social-ecological systems. Front. Ecol. Environ. 2018, 16, S34–S43. [Google Scholar] [CrossRef]
- Thistle, H.W.; Peterson, H.G.; Allwine, G.; Lamb, B.K.; Strand, T.; Holsten, E.H.; Shea, P.J. Surrogate pheromone plumes in three forest trunk spaces: Composite statistics and case studies. For. Sci. 2004, 50, 610–625. [Google Scholar]
- Seybold, S.J.; Huber, D.P.W.; Lee, J.C.; Graves, A.D.; Bohlmann, J. Pine monoterpenes and pine bark beetles: A marriage of convenience for defense and chemical communication. Phytochem. Rev. 2006, 5, 143–178. [Google Scholar] [CrossRef]
- Fettig, C.J.; McMillin, J.D.; Anhold, J.A.; Hamud, S.M.; Borys, R.R.; Dabney, C.P.; Seybold, S.J. The effects of mechanical fuel reduction treatments on the activity of bark beetles (Coleoptera: Scolytidae) infesting ponderosa pine. For. Ecol. Manag. 2006, 230, 55–68. [Google Scholar] [CrossRef]
- Fettig, C.J.; Hood, S.M.; Runyon, J.B.; Stalling, C.M. Bark beetle and fire interactions in western coniferous forests: Research findings. Fire Manag. Today 2021, 79, 14–23. [Google Scholar]
- Davis, R.S.; Hood, S.; Bentz, B.J. Fire-injured ponderosa pine provide a pulsed resource for bark beetles. Can. J. For. Res. 2012, 42, 2022–2036. [Google Scholar] [CrossRef] [Green Version]
- Youngblood, A. Study Plan: Forest Dynamics after Thinning and Fuel Reduction in Dry Forests; USDA Forest Service, Pacific Northwest Research Station: La Grande, OR, USA, 2009; p. 58.
- Sherman, L.M.; Anderson, P.D. Forest Dynamics after Thinning and Fuel Reduction in Pringle Falls Experimental Forest—Establishment and Early Observations of the Lookout Mountain Unit Study; USDA Forest Service Gen. Tech. Rep. PNW–GTR–XX; Pacific Northwest Research Station: Portland, OR, USA, 2021; in press.
- Fettig, C.J.; Borys, R.R.; McKelvey, S.R.; Dabney, C.P. Blacks Mountain Experimental Forest: Bark beetle responses to differences in forest structure and the application of prescribed fire in interior ponderosa pine. Can. J. For. Res. 2008, 38, 924–935. [Google Scholar] [CrossRef]
- Fettig, C.J.; Borys, R.R.; Dabney, C.P. Effects of fire and fire surrogate treatments on bark beetle-caused tree mortality in the Southern Cascades, California. For. Sci. 2010, 56, 60–73. [Google Scholar]
- Fettig, C.J. Native bark beetles and wood borers in Mediterranean forests of California. In Insects and Diseases of Mediterranean Forest Systems; Lieutier, F., Paine, T.D., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 499–528. [Google Scholar]
- Harrison, X.A. A comparison of observation-level random effect and beta-binomial models for modelling overdispersion in binomial data in ecology and evolution. Peer J. 2015, 3, e1114. [Google Scholar] [CrossRef] [Green Version]
- Douma, J.C.; Weedon, J.T. Analysing continuous proportions in ecology and evolution: A practical introduction to beta and Dirichlet regression. Methods Ecol. Evol. 2019, 10, 1412–1430. [Google Scholar] [CrossRef] [Green Version]
- Zuur, A.; Leno, E.; Walker, N.; Saveliev, A.; Smith, G. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; p. 574. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://R-project.org/ (accessed on 28 December 2020).
- Hood, S.M. Mitigating Old Tree Mortality in Long-Unburned, Fire-Dependent Forests: A Synthesis; USDA Forest Service Gen. Tech. Rep. RMRS–GTR–238; Rocky Mountain Research Station: Fort Collins, CO, USA, 2010; p. 71.
- Hood, S.; Varner, M.; van Mantgem, P.; Cansler, C.A. Fire and tree death: Understanding and improving modeling of fire-induced tree mortality. Environ. Res. Lett. 2018, 13, 113004. [Google Scholar] [CrossRef]
- Fettig, C.J.; McKelvey, S.R. Resiliency of an interior ponderosa pine forest to bark beetle infestations following fuel-reduction and forest-restoration treatments. Forests 2014, 5, 153–176. [Google Scholar] [CrossRef] [Green Version]
- Westlind, D.J.; Kerns, B.K. Repeated fall prescribed fire in previously thinned Pinus ponderosa increases growth and resistance to other disturbances. For. Ecol. Manag. 2021, 480, 118645. [Google Scholar] [CrossRef]
- Hood, S.; Sala, A.; Heyerdahl, E.K.; Boutin, M. Low-severity fire increases tree defense against bark beetle attacks. Ecology 2015, 96, 1846–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.M.; Keen, F.P. Biology and Control of the Western Pine Beetle; USDA Forest Service Misc. Pub. 800; Washington Office: Washington, DC, USA, 1960; p. 381.
- Fettig, C.J.; McKelvey, S.R.; Cluck, D.R.; Smith, S.L.; Otrosina, W.J. Effects of prescribed fire and season of burn on direct and indirect levels of tree mortality in ponderosa and Jeffrey pine forests in California, USA. For. Ecol. Manag. 2010, 260, 207–218. [Google Scholar] [CrossRef]
- Perrakis, D.D.B.; Agee, J.K. Seasonal fire effects on mixed-conifer forest structure and ponderosa pine resin properties. Can. J. For. Res. 2006, 36, 238–254. [Google Scholar] [CrossRef]
- Thomas, T.L.; Agee, J.K. Prescribed fire effects on mixed conifer forest structure at Crater Lake, Oregon. Can. J. For. Res. 1986, 16, 1082–1087. [Google Scholar] [CrossRef]
- Westlind, D.J.; Kelsey, R.G. Predicting post-fire attack of red turpentine or western pine beetle on ponderosa pine and its impact on mortality probability in Pacific Northwest forests. For. Ecol. Manag. 2019, 434, 181–192. [Google Scholar] [CrossRef]
- Kolb, T.E.; Agee, J.K.; Fulé, P.Z.; McDowell, N.G.; Pearson, K.; Sala, A.; Waring, R.H. Perpetuating old growth ponderosa pine. For. Ecol. Manag. 2007, 249, 141–157. [Google Scholar] [CrossRef]
- Berryman, A.A.; Ferrell, G.T. The fir engraver beetle in western states. In Dynamics of Forest Insect Populations: Patterns, Causes, Implications; Berryman, A.A., Ed.; Plenum Press: New York, NY, USA, 1988; pp. 556–577. [Google Scholar]
- Schwilk, D.W.; Knapp, E.E.; Ferrenberg, S.M.; Keeley, J.E.; Caprio, A.C. Tree mortality from fire and bark beetles following early and late season prescribed fires in a Sierra Nevada mixed-conifer forest. For. Ecol. Manag. 2006, 232, 36–45. [Google Scholar] [CrossRef]
- Filip, G.M.; Maffei, H.; Chadwick, K.L. Forest health decline in a central Oregon mixed-conifer forest revisited after wildfire: A 25-year case study. West. J. Appl. For. 2007, 22, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Fettig, C.J.; Klepzig, K.D.; Billings, R.F.; Munson, A.S.; Nebeker, T.E.; Negrón, J.F.; Nowak, J.T. The effectiveness of vegetation management practices for prevention and control of bark beetle outbreaks in coniferous forests of the western and southern United States. For. Ecol. Manag. 2007, 238, 24–53. [Google Scholar] [CrossRef]
- Geiszler, D.R.; Gara, R.I.; Gallucci, V.F. Modeling dynamics of mountain pine beetle aggregation in a lodgepole pine stand. Oecologia 1980, 46, 244–253. [Google Scholar] [CrossRef]
- Christiansen, E.; Waring, R.H.; Berryman, A.A. Resistance of conifers to bark beetle attack: Searching for general relationships. For. Ecol. Manag. 1987, 22, 89–106. [Google Scholar] [CrossRef]
- Swezy, D.M.; Agee, J.K. Prescribed-fire effects on fine-root and tree mortality in old-growth ponderosa pine. Can. J. For. Res. 1991, 21, 626–634. [Google Scholar] [CrossRef]
- Ryan, K.C.; Knapp, E.E.; Varner, J.M. Prescribed fire in North American forests and woodlands: History, current practice, and challenges. Front. Ecol. Environ. 2013, 11, e15–e24. [Google Scholar] [CrossRef]
- Vose, J.M.; Peterson, D.L.; Fettig, C.J.; Halofsky, J.E.; Hiers, J.K.; Keane, R.E.; Loehman, R.; Stambaugh, M. Fire and forests in the 21st century: Managing resilience under changing climates and fire regimes in US Forests. In Past, Present, and Future Fire Ecology and Management across US Forested Ecosystems; Collins, B., Greenberg, C.H., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 465–502. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fettig, C.J.; Mortenson, L.A.; Audley, J.P. Tree Mortality following Thinning and Prescribed Burning in Central Oregon, U.S. Forests 2021, 12, 1677. https://doi.org/10.3390/f12121677
Fettig CJ, Mortenson LA, Audley JP. Tree Mortality following Thinning and Prescribed Burning in Central Oregon, U.S. Forests. 2021; 12(12):1677. https://doi.org/10.3390/f12121677
Chicago/Turabian StyleFettig, Christopher J., Leif A. Mortenson, and Jackson P. Audley. 2021. "Tree Mortality following Thinning and Prescribed Burning in Central Oregon, U.S." Forests 12, no. 12: 1677. https://doi.org/10.3390/f12121677
APA StyleFettig, C. J., Mortenson, L. A., & Audley, J. P. (2021). Tree Mortality following Thinning and Prescribed Burning in Central Oregon, U.S. Forests, 12(12), 1677. https://doi.org/10.3390/f12121677



