Does Mixing Tree Species Affect Water Storage Capacity of the Forest Floor? Laboratory Test of Pine-Oak and Fir-Beech Litter Layers
Abstract
:1. Introduction
2. Materials and Methods
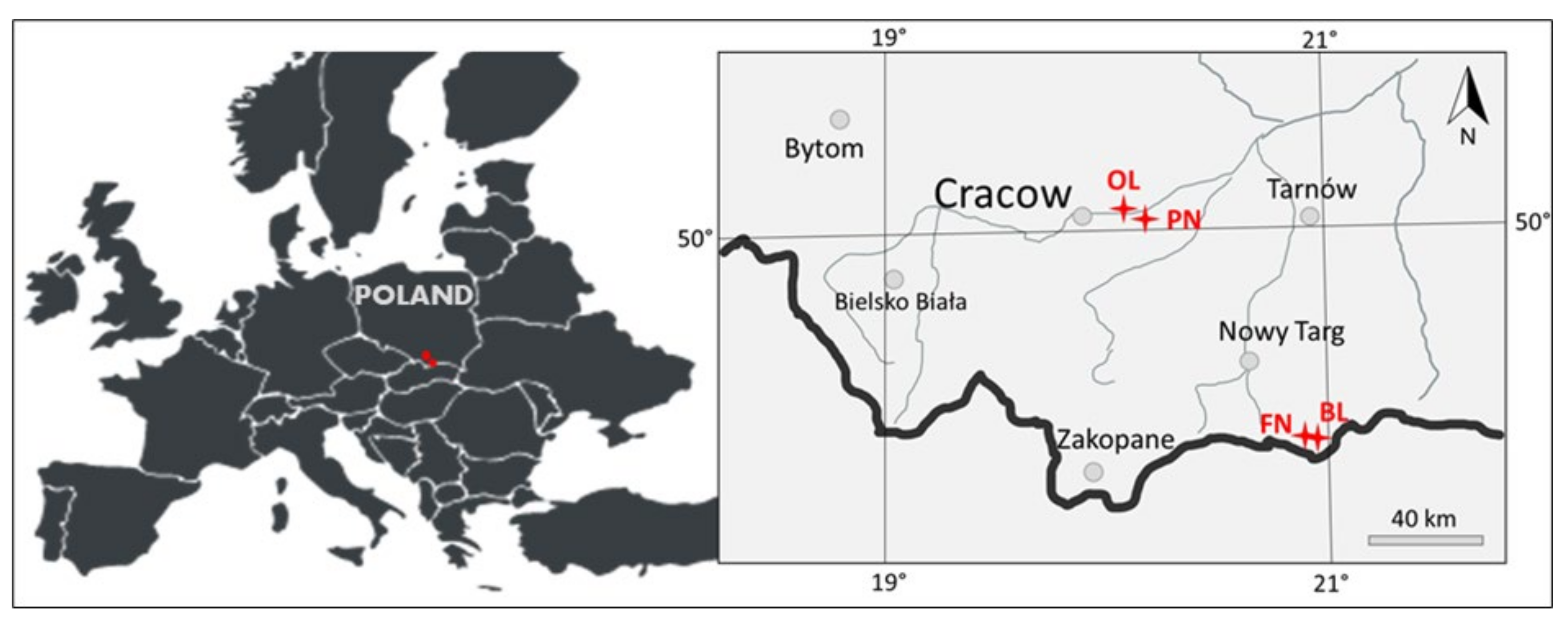
2.1. Study Site
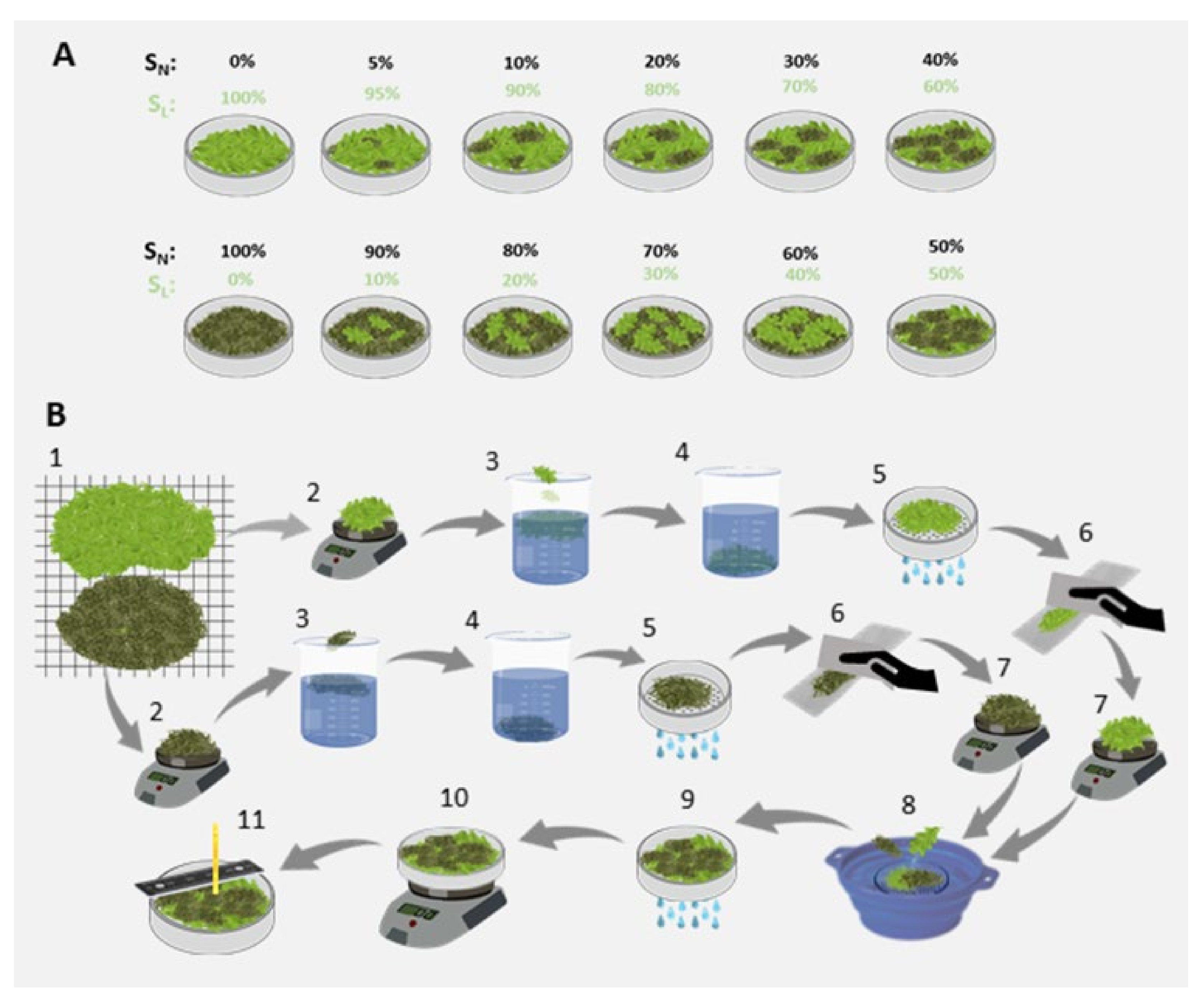
2.2. Laboratory Tests
2.3. Statistical Analysis
3. Results
3.1. Bulk Density and Moisture Content of Leaves and Needles
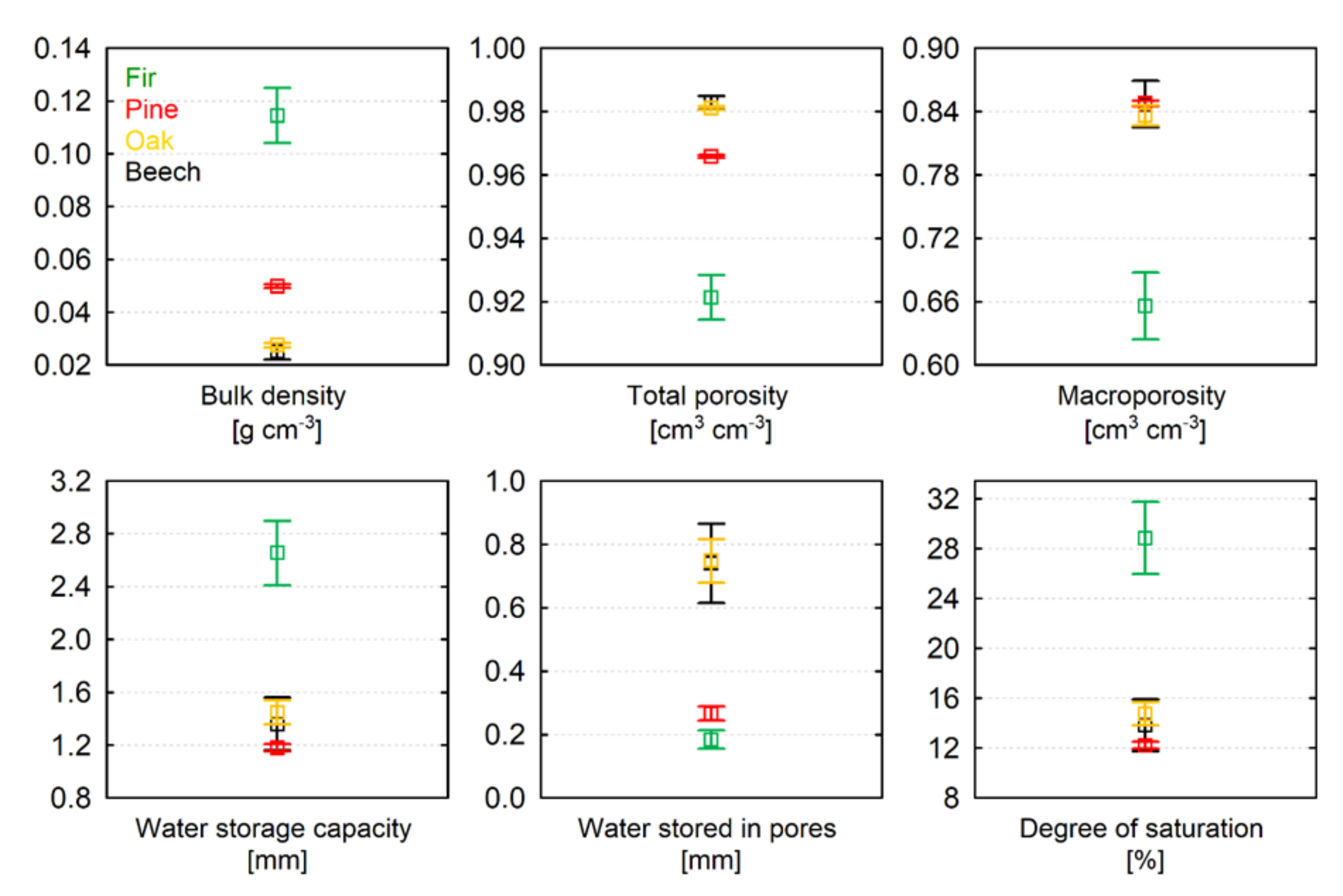
3.2. Hydro-Physical Properties of Pure Litter Layers
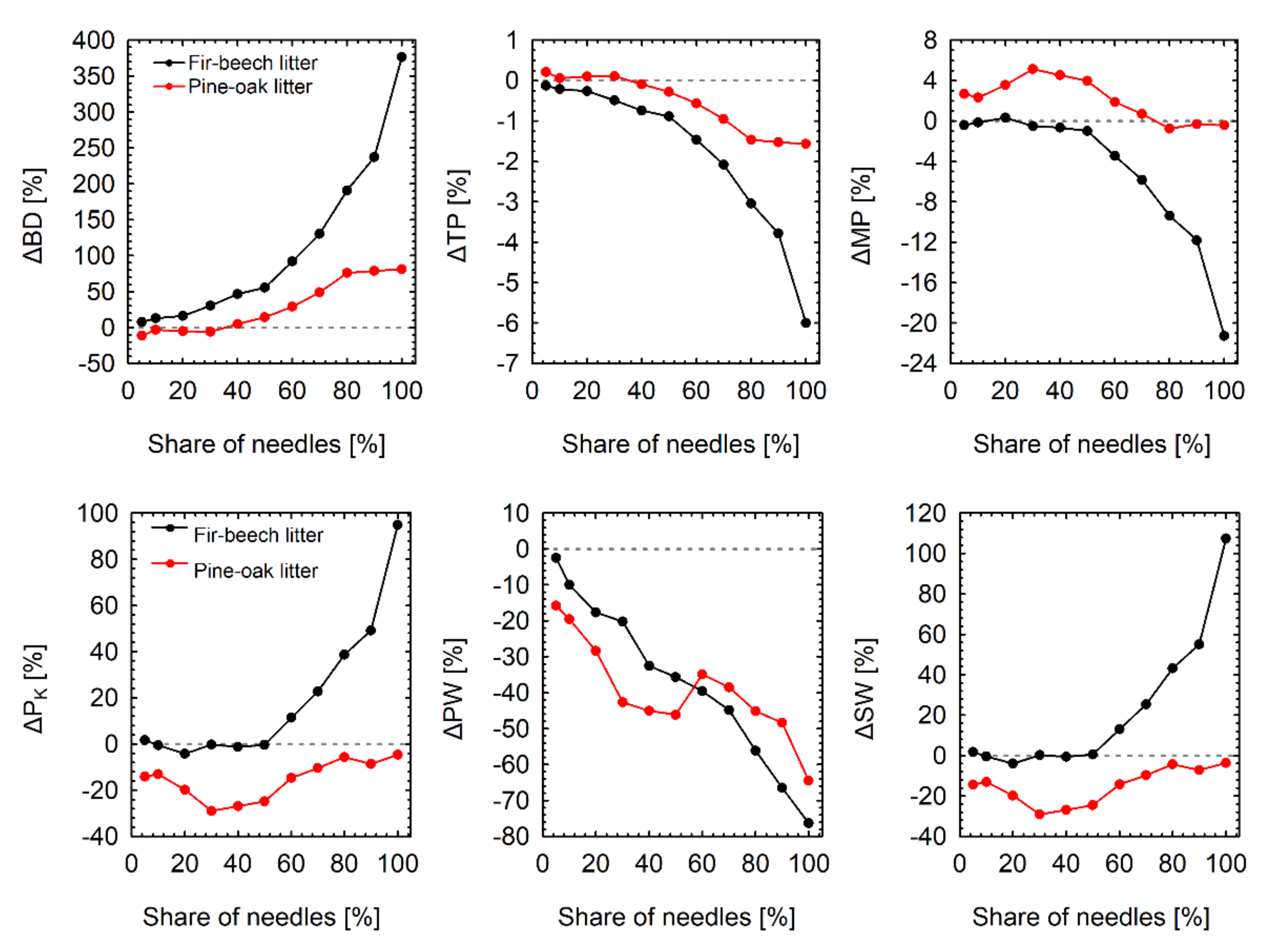
3.3. The Impact of Mixing Tree Species on Hydro-Physical Properties of the Litter Layer
4. Discussion
5. Conclusions
- (1)
- Due to the relatively small size of fir needles, fir litter had the highest bulk density, water storage capacity, degree of saturation, and the lowest total porosity, macroporosity and amount of water stored in pores between organic debris among all the species. The hydro-physical properties of the pine litter layer were closer to the hydro-physical properties of oak and beech litters except for the amount of water stored in pores between organic debris (or on their surface), which was similar to fir litter;
- (2)
- Although the admixture of fir needles increased the bulk density of litter compared to pure beech litter, their influence on total porosity, macroporosity, water storage capacity and the degree of litter saturation was relatively low;
- (3)
- The admixture of pine needles had a slight effect on bulk density and total porosity of litter (compared to pure oak litter), but they increased macroporosity and decreased the water storage capacity and the degree of litter saturation;
- (4)
- Both fir and pine needles decreased the amount of water stored in pores between organic debris (or on its surface), which was probably related to the fact that needles between the leaves in the litter prevent wet leaves from sticking to one another and increase water infiltration through the litter.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilhite, D.A. Introduction: Managing drought risk in a changing climate. Clim. Res. 2016, 70, 99–102. [Google Scholar] [CrossRef] [Green Version]
- O’Gorman, P.A.; Schneider, T. The physical basis for increases in precipitation extremes in simulations of 21st-century climate change. Proc. Natl. Acad. Sci. USA 2009, 106, 14773–14777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, A. Drought under global warming: A review. Wiley Interdiscip. Rev. Clim. Chang. 2010, 2, 45–65. [Google Scholar] [CrossRef] [Green Version]
- Hoegh-Guldberg, O.; Jacob, D.; Bindi, M.; Brown, S.; Camilloni, I.; Diedhiou, A.; Djalante, R.; Ebi, K.; Engelbrecht, F.; Guiot, J.; et al. Impacts of 1.5 °C Global Warming on Natural and Human Systems. In Global Warming of 1.5 C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Portner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Piranji, A., Moufouma-Okia, W., Pean, C., Pidock, R., et al., Eds.; World Meteorological Organization Technical Document: Geneva, Switzerland, 2018; In Press. [Google Scholar]
- Spinoni, J.; Vogt, J.V.; Naumann, G.; Barbosa, P.; Dosio, A. Will drought events become more frequent and severe in Europe? Int. J. Climatol. 2017, 38, 1718–1736. [Google Scholar] [CrossRef] [Green Version]
- Wilhite, D.A. The enigma of drought. In Drought Assessment, Management, and Planning: Theory and Case Studies; Springer: Boston, MA, USA, 1993; pp. 3–15. [Google Scholar]
- Steckel, M.; del Río, M.; Heym, M.; Aldea, J.; Bielak, K.; Brazaitis, G.; Černý, J.; Coll, L.; Collet, C.; Ehbrecht, M.; et al. Species mixing reduces drought susceptibility of Scots pine (Pinus sylvestris L.) and oak (Quercus robur L., Quercus petraea (Matt.) Liebl.)—Site water supply and fertility modify the mixing effect. For. Ecol. Manag. 2020, 461, 117908. [Google Scholar] [CrossRef]
- Gillerot, L.; Forrester, D.I.; Bottero, A.; Rigling, A.; Lévesque, M. Tree Neighbourhood Diversity Has Negligible Effects on Drought Resilience of European Beech, Silver Fir and Norway Spruce. Ecosystems 2020, 24, 20–36. [Google Scholar] [CrossRef]
- Croisé, L.; Lieutier, F.; Cochard, H.; Dreyer, E. Effects of drought stress and high density stem inoculations with Leptographium wingfieldii on hydraulic properties of young Scots pine trees. Tree Physiol. 2001, 21, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Nardini, A.; Gullo, M.A.L.; Trifilò, P.; Salleo, S. The challenge of the Mediterranean climate to plant hydraulics: Responses and adaptations. Environ. Exp. Bot. 2014, 103, 68–79. [Google Scholar] [CrossRef]
- Nardini, A.; Luglio, J. Leaf hydraulic capacity and drought vulnerability: Possible trade-offs and correlations with climate across three major biomes. Funct. Ecol. 2014, 28, 810–818. [Google Scholar] [CrossRef]
- Doffo, G.N.; Monteoliva, S.E.; Rodríguez, M.E.; Luquez, V.M. Physiological responses to alternative flooding and drought stress episodes in two willow (Salix spp.) clones. Can. J. For. Res. 2017, 47, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Meier, I.C.; Leuschner, C. Belowground drought response of European beech: Fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob. Chang. Biol. 2008, 14, 2081–2095. [Google Scholar] [CrossRef]
- Phillips, R.P.; Ibáñez, I.; D’Orangeville, L.; Hanson, P.J.; Ryan, M.G.; McDowell, N.G. A belowground perspective on the drought sensitivity of forests: Towards improved understanding and simulation. For. Ecol. Manag. 2016, 380, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Chitra-Tarak, R.; Ruiz, L.; Dattaraja, H.S.; Kumar, M.S.M.; Riotte, J.; Suresh, H.S.; McMahon, S.M.; Sukumar, R. The roots of the drought: Hydrology and water uptake strategies mediate forest-wide demographic response to precipitation. J. Ecol. 2017, 106, 1495–1507. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, H.; Steckel, M.; Heym, M.; Biber, P.; Ammer, C.; Ehbrecht, M.; Bielak, K.; Bravo, F.; Ordóñez, C.; Collet, C.; et al. Stand growth and structure of mixed-species and monospecific stands of Scots pine (Pinus sylvestris L.) and oak (Q. robur L., Quercus petraea (Matt.) Liebl.) analysed along a productivity gradient through Europe. Eur. J. For. Res. 2020, 139, 349–367. [Google Scholar] [CrossRef] [Green Version]
- Fekete, I.; Berki, I.; Lajtha, K.; Trumbore, S.; Francioso, O.; Gioacchini, P.; Montecchio, D.; Várbíró, G.; Béni, A.; Makádi, M.; et al. How will a drier climate change carbon sequestration in soils of the deciduous forests of Central Europe? Biodegradation 2020, 152, 13–32. [Google Scholar] [CrossRef]
- Osman, K.T. Organic Matter of Forest Soils. In Forest Soils; Springer: Berlin/Heidelberg, Germany, 2013; pp. 63–76. [Google Scholar]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Marin, C.; Bouten, I.; Dekker, S. Forest floor water dynamics and root water uptake in four forest ecosystems in northwest Amazonia. J. Hydrol. 2000, 237, 169–183. [Google Scholar] [CrossRef]
- Gosz, J.R.; Likens, G.E.; Bormann, F.H. Organic matter and nutrient dynamics of the forest and forest floor in the Hubbard Brook forest. Oecologia 1976, 22, 305–320. [Google Scholar] [CrossRef]
- Sayer, E. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 2005, 81, 1–31. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.; Li, H.; Chang, Y.; Wang, H.; Tu, Z.; Liang, H. Simulated erosion using soils from vegetated slopes in the Jiufeng Mountains, China. CATENA 2016, 136, 128–134. [Google Scholar] [CrossRef]
- Xia, L.; Song, X.; Fu, N.; Cui, S.; Li, L.; Li, H.; Li, Y. Effects of forest litter cover on hydrological response of hillslopes in the Loess Plateau of China. CATENA 2019, 181, 104076. [Google Scholar] [CrossRef]
- Helvey, J.D. A summary of rainfall interception by certain conifers of North America. In Proceedings of the International Symposium for Hydrology Professors; Purdue University: Lafayette, IN, USA, 1971; pp. 103–113. [Google Scholar]
- Sun, X.; Wang, G.; Lin, Y.; Liu, L.; Gao, Y. Intercepted rainfall in Abies fabri forest with different-aged stands in southwestern China. Turk. J. Agric. For. 2013, 37, 495–504. [Google Scholar]
- Huber, A.M.; Oyarzun, C.E. Redistribución de las precipitaciones en un bosque siempreverde del sur de Chile. Turrialba 1992, 42, 192–199. [Google Scholar]
- Ilek, A.; Kucza, J.; Szostek, M. The effect of stand species composition on water storage capacity of the organic layers of forest soils. Eur. J. For. Res. 2014, 134, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Acharya, B.S.; Stebler, E.; Zou, C.B. Monitoring litter interception of rainfall using leaf wetness sensor under controlled and field conditions. Hydrol. Process. 2016, 31, 240–249. [Google Scholar] [CrossRef]
- Prescott, C.E.; Vesterdal, L. Effects of British Columbia Tree Species on Forest Floor Chemistry. In Tree Species Effects in Soils: Implications for Global Change; Springer: Berlin/Heidelberg, Germany, 2006; Volume 55, pp. 17–29. [Google Scholar]
- Zagyvai-Kiss, K.A.; Kalicz, P.; Szilágyi, J.; Gribovszki, Z. On the specific water holding capacity of litter for three forest eco-systems in the eastern foothills of the Alps. Agric. For. Meteorol. 2019, 278, 107656. [Google Scholar] [CrossRef]
- Peters, M.; Iverson, L.R.; Matthews, S. Long-term droughtiness and drought tolerance of eastern US forests over five decades. For. Ecol. Manag. 2015, 345, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Fernald, A.; Gallegos, J.; VanLeeuwen, D.; Baker, T.T. Evaluation of litter hydrology in ponderosa pine and mixed conifer stands in northen New Mexico, USA. New Mex. Acad. Sci. 2012, 4, 121–136. [Google Scholar]
- State Forests. National Forest Inventory in Poland. Results for the Period of 2015–2019; Biuro Urządzania Lasu i Geodezji Leśnej: Warsaw, Poland, 2020. [Google Scholar]
- Kucza, J.; Urbaś, J. Water absorption of organic matter taken from horizons of ectohumus of forest soils under Norway spruce stands. EJPAU Forestry 2005, 8, 50–58. [Google Scholar]
- Ilek, A.; Szostek, M.; Kucza, J.; Stanek-Tarkowska, J.; Witek, W. The water absorbability of beech (Fagus sylvatica l.) and fir (Abies alba mill.) organic matter in the forest floor. Ann. For. Res. 2014, 62. [Google Scholar] [CrossRef]
- Ilek, A.; Kucza, J.; Morkisz, K. Hygroscopicity of the bark of selected forest tree species. iForest—Biogeosciences For. 2017, 10, 220–226. [Google Scholar] [CrossRef]
- Eckenwalder, J.E. Conifers of the World: The Complete Reference. Timber Press: Portland, OR, USA, 2009. [Google Scholar]
- Krakau, U.-K.; Liesebach, M.; Aronen, T.; Lelu-Walter, M.-A.; Schneck, V. Scots Pine (Pinus sylvestris L.). In Forest Tree Breeding in Europe; Springer: Berlin/Heidelberg, Germany, 2013; Volume 25, pp. 267–323. [Google Scholar]
- Durrant, T.H.; De Rigo, D.; Caudullo, G. Pinus Sylvestris in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species. Publication Office of the European Union: Luxembourg, 2016; pp. 132–133. [Google Scholar]
- Björse, G.; Bradshaw, R. 2000 years of forest dynamics in southern Sweden: Suggestions for forest management. For. Ecol. Manag. 1998, 104, 15–26. [Google Scholar] [CrossRef]
- Šamonil, P.; Vrška, T. Trends and cyclical changes in natural fir-beech Forests at the north-western edge of the Carpathians. Folia Geobot. Phytotaxon. 2007, 42, 337–361. [Google Scholar] [CrossRef]
- Vrška, T.; Adam, D.; Hort, L.; Kolář, T.; Janík, D. European beech (Fagus sylvatica L.) and silver fir (Abies alba Mill.) rotation in the Carpathians—A developmental cycle or a linear trend induced by man? For. Ecol. Manag. 2009, 258, 347–356. [Google Scholar] [CrossRef]
- Walsh, R.P.D.; Voigt, P.J. Vegetation Litter: An Underestimated Variable in Hydrology and Geomorphology. J. Biogeogr. 1977, 4, 253. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- The Gymnosperm Database. Available online: https://www.conifers.org/ (accessed on 20 October 2021).
- Scartazza, A.; Dibaccio, D.; Bertolotto, P.; Gavrichkova, O.; Matteucci, G. Investigating the European beech (Fagus sylvatica L.) leaf characteristics along the vertical canopy profile: Leaf structure, photosynthetic capacity, light energy dissipation and photoprotection mechanisms. Tree Physiol. 2016, 36, 1060–1076. [Google Scholar] [CrossRef] [Green Version]
- North Carolina Extension Gardener Plant Toolbox. Available online: https://plants.ces.ncsu.edu/ (accessed on 20 October 2021).
- Caudullo, G.; Tinner, W.; de Rigo, D. Picea Abies in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 114–116. [Google Scholar]
- Putuhena, W.M.; Cordery, I. Estimation of interception capacity of the forest floor. J. Hydrol. 1996, 180, 283–299. [Google Scholar] [CrossRef]
- Gerrits, A.M.J. The Role of Interception in the Hydrological Cycle. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2010. [Google Scholar]
- Sato, Y.; Kumagai, T.; Kume, A.; Otsuki, K.; Ogawa, S. Experimental analysis of moisture dynamics of litter layers? The effects of rainfall conditions and leaf shapes. Hydrol. Process. 2004, 18, 3007–3018. [Google Scholar] [CrossRef]
- Guevara-Escobar, A.; Gonzalez-Sosa, E.; Ramos-Salinas, M.; Hernandez-Delgado, G.D. Experimental analysis of drainage and water storage of litter layers. Hydrol. Earth Syst. Sci. 2007, 11, 1703–1716. [Google Scholar] [CrossRef] [Green Version]
- Zema, D.A.; Ii, J.T.V.S.; Plaza-Alvarez, P.A.; Xu, X.; Carra, B.G.; Lucas-Borja, M.E. Effects of stand composition and soil properties on water repellency and hydraulic conductivity in Mediterranean forests. Ecohydrology 2021, 14. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Zhang, Z.; Cui, H.; Lei, Y.; Wang, D.; Sui, J. Water-holding characteristics of litter in different forests at the Lianxiahe watershed. Front. For. China 2006, 1, 413–418. [Google Scholar] [CrossRef]
- Zhang, Z.; Lei, Y.; Su, K.; Wang, G.; Wang, D.; Ma, H. Hydrological characteristics of litter in different forest succession stages at Liuxihe Watershed, southern China. Front. For. China 2009, 4, 317–322. [Google Scholar] [CrossRef]
- Kara, O.; Bolat, I.; Cakıroglu, K.; Senturk, M.E. Litter decomposition and microbial biomass in temperate forests in North-western Turkey. J. Soil Sci. Plant Nutr. 2014, 14, 31–41. [Google Scholar]
- Błońska, E.; Lasota, J.; Januszek, K. Relation between properties of humus horizon and oak participation in a Scots pine stands. Soil Sci. Annu. 2013, 64, 82–87. [Google Scholar] [CrossRef]
- Hart, S.C.; Firestone, M.K.; Paul, E.A. Decomposition and nutrient dynamics of ponderosa pine needles in a Mediterranean-type climate. Can. J. For. Res. 1992, 22, 306–314. [Google Scholar] [CrossRef]
- Chen, S.; Cao, T.; Tanaka, N.; Gao, T.; Zhu, L.; Zou, C. Hydrological properties of litter layers in mixed forests in Mt. Qinling, China. iForest—Biogeosci. For. 2018, 11, 243–250. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilek, A.; Szostek, M.; Mikołajczyk, A.; Rajtar, M. Does Mixing Tree Species Affect Water Storage Capacity of the Forest Floor? Laboratory Test of Pine-Oak and Fir-Beech Litter Layers. Forests 2021, 12, 1674. https://doi.org/10.3390/f12121674
Ilek A, Szostek M, Mikołajczyk A, Rajtar M. Does Mixing Tree Species Affect Water Storage Capacity of the Forest Floor? Laboratory Test of Pine-Oak and Fir-Beech Litter Layers. Forests. 2021; 12(12):1674. https://doi.org/10.3390/f12121674
Chicago/Turabian StyleIlek, Anna, Małgorzata Szostek, Anna Mikołajczyk, and Marta Rajtar. 2021. "Does Mixing Tree Species Affect Water Storage Capacity of the Forest Floor? Laboratory Test of Pine-Oak and Fir-Beech Litter Layers" Forests 12, no. 12: 1674. https://doi.org/10.3390/f12121674
APA StyleIlek, A., Szostek, M., Mikołajczyk, A., & Rajtar, M. (2021). Does Mixing Tree Species Affect Water Storage Capacity of the Forest Floor? Laboratory Test of Pine-Oak and Fir-Beech Litter Layers. Forests, 12(12), 1674. https://doi.org/10.3390/f12121674





