Opposing Ecological Strategies Together Promote Biomass Carbon Storage in Homegardens Agroforestry of Southern Bangladesh
Abstract
:1. Introduction
2. Materials and Methods
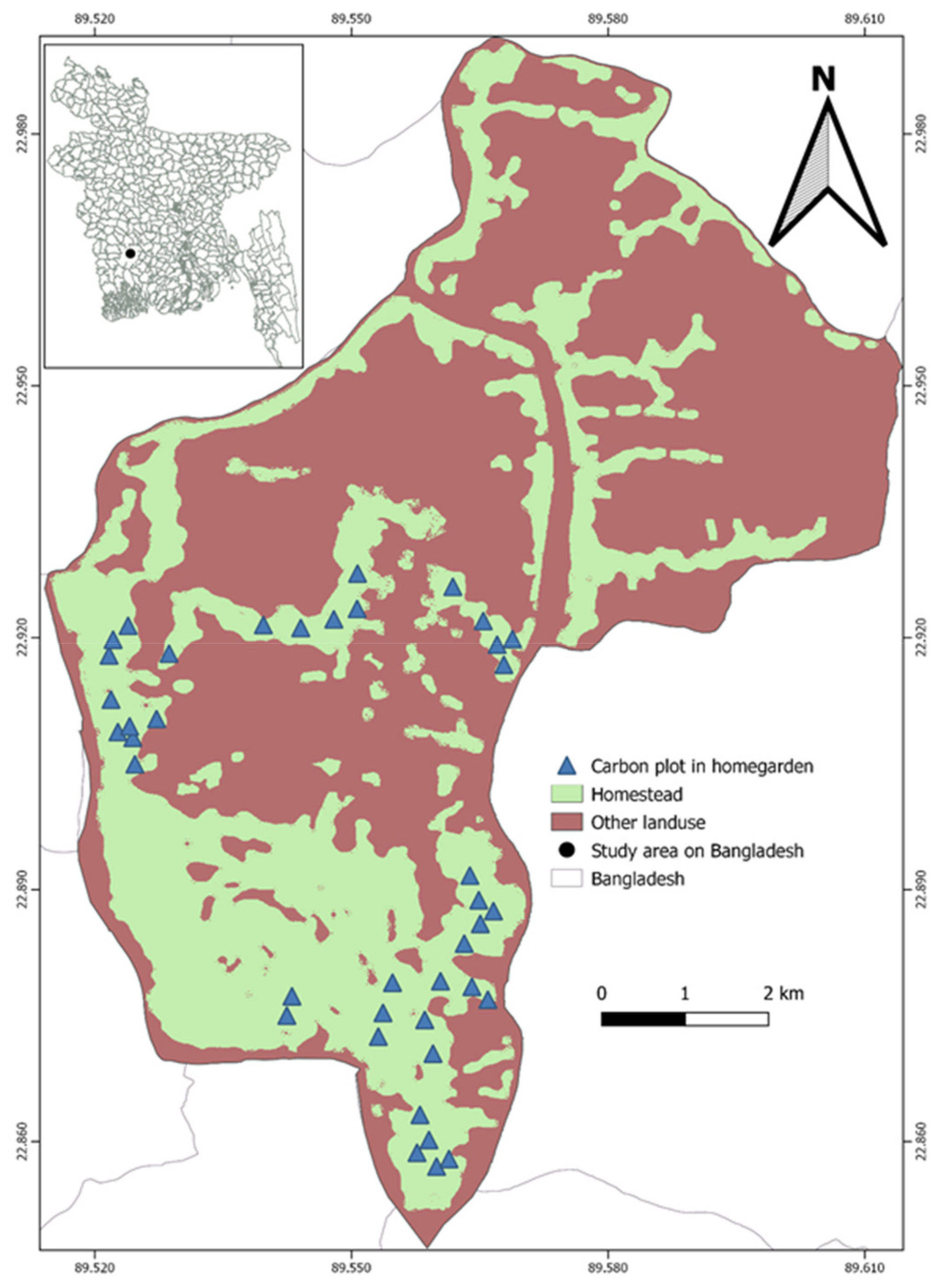
2.1. Study Area
2.2. Field Inventory
2.3. Data Analysis
Biomass Carbon
2.4. Functional Traits and Diversity Metrics
2.5. Statistiscal Analysis
3. Results
3.1. Bivariate Relationship between Different Predictors and Carbon Storage
3.2. Underlying Mechanism of Relationship between Soil Nutrient, Biodiversity and Carbon Storage
4. Discussion
4.1. Relative Importance of Biodiversity Components to Maintain Aboveground Carbon
4.2. Underlying Theory of Aboveground Carbon Storage in Homegardens
4.3. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavanaugh, K.C.; Gosnell, J.S.; Davis, S.L.; Ahumada, J.; Boundja, P.; Clark, D.B.; Mugerwa, B.; Jansen, P.; O’Brien, T.G.; Rovero, F.; et al. Carbon storage in tropical forests correlates with taxonomic diversity and functional dominance on a global scale. Glob. Ecol. Biogeogr. 2014, 23, 563–573. [Google Scholar] [CrossRef]
- Mensah, S.; Veldtman, R.; Assogbadjo, A.E.; Kakaï, R.G.; Seifert, T. Tree species diversity promotes aboveground carbon storage through functional diversity and functional dominance. Ecol. Evol. 2016, 6, 7546–7557. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Benito, P.; Gómez-Aparicio, L.; Paquette, A.; Messier, C.; Kattge, J.; Zavala, M.A. Diversity increases carbon storage and tree productivity in Spanish forests. Glob. Ecol. Biogeogr. 2013, 23, 311–322. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Wright, J.P.; Cadotte, M.W.; Carroll, I.T.; Hector, A.; Srivastava, D.S.; Loreau, M.; Weis, J.J. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl. Acad. Sci. USA 2007, 104, 18123–18128. [Google Scholar] [CrossRef] [Green Version]
- Van der Sande, M.T.; Peña-Claros, M.; Ascarrunz, N.; Arets, E.J.M.M.; Licona, J.C.; Toledo, M.; Poorter, L. Abiotic and biotic drivers of biomass change in a Neotropical forest. J. Ecol. 2017, 105, 1223–1234. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Bao, W.; Bongers, F.; Chen, B.; Chen, G.; Guo, K.; Jiang, M.; Lai, J.; Lin, D.; Liu, C.; et al. Drivers of tree carbon storage in subtropical forests. Sci. Total. Environ. 2018, 654, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Mattsson, E. Individual tree size inequality enhances aboveground biomass in homegarden agroforestry systems in the dry zone of Sri Lanka. Sci. Total. Environ. 2017, 575, 6–11. [Google Scholar] [CrossRef]
- Ali, A.; Mattsson, E. Wood density is a sustainability indicator for the management of dry zone homegarden agroforests: Evidences from biodiversity–ecosystem function relationships. Ecol. Indic. 2019, 105, 474–482. [Google Scholar] [CrossRef]
- Takimoto, A.; Nair, P.R.; Nair, V.D. Carbon stock and sequestration potential of traditional and improved agroforestry systems in the West African Sahel. Agric. Ecosyst. Environ. 2008, 125, 159–166. [Google Scholar] [CrossRef]
- Kabir, E.; Webb, E.L. Household and homegarden characteristics in southwestern Bangladesh. Agrofor. Syst. 2008, 75, 129–145. [Google Scholar] [CrossRef]
- Kumar, B.M.; Nair, P.K.R. The enigma of tropical homegardens. Agrofor. Syst. 2004, 61–62, 135–152. [Google Scholar]
- Birhane, E.; Ahmed, S.; Hailemariam, M.; Negash, M.; Rannestad, M.M.; Norgrove, L. Carbon stock and woody species diversity in homegarden agroforestry along an elevation gradient in southern Ethiopia. Agrofor. Syst. 2020, 94, 1099–1110. [Google Scholar] [CrossRef]
- Mattsson, E.; Ostwald, M.; Nissanka, S.P.; Marambe, B. Homegardens as a Multi-functional Land-Use Strategy in Sri Lanka with Focus on Carbon Sequestration. Ambio 2013, 42, 892–902. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Chen, H.Y.; You, W.-H.; Yan, E.-R. Multiple abiotic and biotic drivers of aboveground biomass shift with forest stratum. For. Ecol. Manag. 2019, 436, 1–10. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In SSSA Book Series; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; ISBN 978-0-89118-866-7. [Google Scholar]
- Craswell, E.T.; Lefroy, R.D.B. The role and function of organic matter in tropical soils. In Managing Organic Matter in Tropical Soils: Scope and Limitations; Martius, C., Tiessen, H., Vlek, P.L.G., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 7–18. ISBN 978-90-481-5947-5. [Google Scholar]
- Grigal, D.F.; Vance, E.D. Influence of soil organic matter on forest productivity. N. Z. J. For. Sci. 2000, 30, 169–205. [Google Scholar]
- Turnbull, L.A.; Levine, J.M.; Loreau, M.; Hector, A. Coexistence, niches and biodiversity effects on ecosystem functioning. Ecol. Lett. 2012, 16, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Mensah, S.; Salako, V.K.; Assogbadjo, A.E.; Kakaï, R.G. Differential Responses of Taxonomic, Structural, and Functional Diversity to Local-Scale Environmental Variation in Afromontane Forests in South Africa. Trop. Conserv. Sci. 2018, 11. [Google Scholar] [CrossRef]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The Influence of Functional Diversity and Composition on Ecosystem Processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef] [Green Version]
- Conti, G.; Díaz, S. Plant functional diversity and carbon storage - an empirical test in semi-arid forest ecosystems. J. Ecol. 2012, 101, 18–28. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Ruiz-Jaen, M.C.; Potvin, C. Can we predict carbon stocks in tropical ecosystems from tree diversity? Comparing species and functional diversity in a plantation and a natural forest. New Phytol. 2010, 189, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Bongers, L.; Bongers, F. Architecture Of 54 Moist-Forest Tree Species: Traits, Trade-Offs, And Functional Groups. Ecology 2006, 87, 1289–1301. [Google Scholar] [CrossRef]
- Potapov, P.; Siddiqui, B.N.; Iqbal, Z.; Aziz, T.; Zzaman, B.; Islam, A.; Pickens, A.; Talero, Y.; Tyukavina, A.; Turubanova, S.; et al. Comprehensive monitoring of Bangladesh tree cover inside and outside of forests, 2000–2014. Environ. Res. Lett. 2017, 12, 104015. [Google Scholar] [CrossRef]
- Thomas, N.M.; Baltezar, P.; Lagomasino, D.; Lee, S.K.; Fatoyinbo, T.; Green, J.; Rahman, M. Extent and canopy height maps of Trees outside Forest (ToF) for Bangladesh. AGUFM 2018, 2018, B31I-2599. [Google Scholar]
- Bardhan, S.; Jose, S.; Biswas, S.; Kabir, K.; Rogers, W. Homegarden agroforestry systems: An intermediary for biodiversity conservation in Bangladesh. Agrofor. Syst. 2012, 85, 29–34. [Google Scholar] [CrossRef]
- Kabir, E.; Webb, E.L. Floristics and structure of southwestern Bangladesh homegardens. Int. J. Biodivers. Sci. Manag. 2008, 4, 54–64. [Google Scholar] [CrossRef]
- Rahman, M.; Kabir, E.; Akon, A.J.U.; Ando, K. High carbon stocks in roadside plantations under participatory management in Bangladesh. Glob. Ecol. Conserv. 2015, 3, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.; Dey, A.; Rahman, M. Effect of Tree Diversity on Soil Organic Carbon Content in the Homegarden Agroforestry System of North-Eastern Bangladesh. Small-Scale For. 2014, 14, 91–101. [Google Scholar] [CrossRef]
- Statistic Division, Ministry of Planning, Bangladesh Secretariat. BBS Statistical Year Book of Bangladesh.Bangladesh Bureau of Statistics (BSS); The Government of the People’s Republic of Bangladesh: Dhaka, Bangladesh, 2012.
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Yakub, M.; Omar Ali, M.; Bhattacharjee, D.K. Strength Properties of Some Bangladesh Timber Species; Govt. of the People’s Republic of Bangladesh, Forest Research Institute: Chittagong, Bangladesh, 1972.
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Data from: Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Pearson, T.; Walker, S.M.; Brown, S. Sourcebook for Land Use, Land-Use Change and Forestry Projects; BioCarbon Fund, Winrock International: Washington, DC, USA, 2005; p. 64. [Google Scholar]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Finegan, B.; Peña-Claros, M.; Oliveira, A.; Ascarrunz, N.; Bret-Harte, M.S.; Carreño-Rocabado, G.; Casanoves, F.; Díaz, S.; Velepucha, P.E.; Fernandez, F.; et al. Does functional trait diversity predict above-ground biomass and productivity of tropical forests? Testing three alternative hypotheses. J. Ecol. 2014, 103, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.; Grimshaw, H.; Parkinson, J.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Hoboken, NJ, USA, 1974. [Google Scholar]
- Rahman, M.; Zimmer, M.; Ahmed, I.; Donato, D.; Kanzaki, M.; Xu, M. Co-benefits of protecting mangroves for biodiversity conservation and carbon storage. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Grace, J.B.; Anderson, T.M.; Seabloom, E.; Borer, E.; Adler, P.B.; Harpole, W.S.; Hautier, Y.; Hillebrand, H.; Lind, E.; Pärtel, M.; et al. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nat. Cell Biol. 2016, 529, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; van der Sande, M.; Thompson, J.; Arets, E.; Alarcón, A.; Álvarez-Sánchez, J.; Ascarrunz, N.; Balvanera, P.; Barajasguzman, G.; Boit, A.; et al. Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 2015, 24, 1314–1328. [Google Scholar] [CrossRef]
- Team, R.C. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Rosseel, Y. Lavaan:anRpackageforstructuralequationmodeling and more. Version 0.5-12 (BETA). J. Stat. Softw. 2012, 48, 1–36. [Google Scholar]
- Grace, J.B.; Bollen, K.A. Interpreting the Results from Multiple Regression and Structural Equation Models. Bull. Ecol. Soc. Am. 2005, 86, 283–295. [Google Scholar] [CrossRef]
- Hooper, D.; Coughlan, J.; Mullen, M. Structural Equation Modelling: Guidelines for Determining Model Fit. J. Bus. Res. Methods 2008, 6, 53–60. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems: Data exploration. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.-R.; Chen, H.Y.H.; Chang, S.X.; Zhao, Y.-T.; Yang, X.-D.; Xu, M.-S. Stand structural diversity rather than species diversity enhances aboveground carbon storage in secondary subtropical forests in Eastern China. Biogeosciences 2016, 13, 4627–4635. [Google Scholar] [CrossRef] [Green Version]
- Aponte, C.; Kasel, S.; Nitschke, C.R.; Tanase, M.A.; Vickers, H.; Parker, L.; Fedrigo, M.; Kohout, M.; Ruiz-Benito, P.; Zavala, M.A.; et al. Structural diversity underpins carbon storage in Australian temperate forests. Glob. Ecol. Biogeogr. 2020, 29, 789–802. [Google Scholar] [CrossRef]
- Marquis, R.J.; Ricklefs, R.E.; Abdala-Roberts, L. Testing the low latitude/high defense hypothesis for broad-leaved tree species. Oecologia 2012, 169, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Hofhansl, F.; Chacón-Madrigal, E.; Fuchslueger, L.; Jenking, D.; Morera-Beita, A.; Plutzar, C.; Silla, F.; Andersen, K.M.; Buchs, D.M.; Dullinger, S.; et al. Climatic and edaphic controls over tropical forest diversity and vegetation carbon storage. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, S.; Hodgson, J.; Thompson, K.; Cabido, M.; Cornelissen, J.; Jalili, A.; Montserrat-Martí, G.; Grime, J.; Zarrinkamar, F.; Asri, Y.; et al. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Yan, E.-R.; Chang, S.X.; Cheng, J.-Y.; Liu, X.-Y. Community-weighted mean of leaf traits and divergence of wood traits predict aboveground biomass in secondary subtropical forests. Sci. Total. Environ. 2017, 574, 654–662. [Google Scholar] [CrossRef] [PubMed]


| Predictors | Path Ways | Response | Std. Effect | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Soil nutrient | Direct | Aboveground carbon | 0.120 | −0.100 | 0.330 | 0.310 |
| Soil nutrient | Indirect effect via species richness | Aboveground carbon | 0.040 | −0.060 | 0.130 | 0.440 |
| Soil nutrient | Indirect effect via FC MCH | Aboveground carbon | −0.020 | −0.100 | 0.060 | 0.650 |
| Soil nutrient | Indirect effect via DBH variation | Aboveground carbon | 0.010 | −0.160 | 0.180 | 0.900 |
| Soil nutrient | Indirect effect via Functional diversity WD | Aboveground carbon | −0.010 | −0.050 | 0.030 | 0.560 |
| Soil nutrient | Indirect effect via species richness and FC MCH | Aboveground carbon | −0.020 | −0.060 | 0.030 | 0.450 |
| Soil nutrient | Indirect effect via species richness and DBH variation | Aboveground carbon | 0.020 | −0.030 | 0.060 | 0.480 |
| Soil nutrient | Indirect effect via species richness and functional diversity WD | Aboveground carbon | 0.000 | −0.020 | 0.010 | 0.560 |
| Soil nutrient | Sum of indirect effect | Aboveground carbon | 0.010 | −0.200 | 0.220 | 0.910 |
| Soil nutrient | Total effect | Aboveground carbon | 0.130 | −0.170 | 0.420 | 0.400 |
| Soil nutrient | Direct | Species richness | 0.130 | −0.180 | 0.440 | 0.410 |
| Soil nutrient | Direct | FC MCH | −0.060 | −0.340 | 0.210 | 0.640 |
| Soil nutrient | Direct | DBH variation | 0.020 | −0.280 | 0.320 | 0.900 |
| Soil nutrient | Direct | Functional diversity WD | 0.120 | −0.180 | 0.410 | 0.440 |
| Species richness | Direct | Aboveground carbon | 0.300 | 0.030 | 0.560 | 0.030 |
| Species richness | Indirect effect via FC MCH | Aboveground carbon | −0.140 | −0.270 | 0.000 | 0.060 |
| Species richness | Indirect effect via DBH variation | Aboveground carbon | 0.140 | −0.050 | 0.310 | 0.150 |
| Species richness | Indirect effect via functional diversity WD | Aboveground carbon | −0.040 | −0.120 | 0.050 | 0.400 |
| Species richness | Sum of indirect effect | Aboveground carbon | −0.040 | −0.280 | 0.200 | 0.750 |
| Species richness | Total effect | Aboveground carbon | 0.260 | −0.030 | 0.550 | 0.080 |
| Species richness | Direct | FC MCH | −0.470 | −0.740 | −0.200 | 0.000 |
| Species richness | Direct | DBH variation | 0.230 | −0.070 | 0.540 | 0.130 |
| Species richness | Direct | Functional diversity WD | 0.330 | 0.040 | 0.620 | 0.030 |
| FC MCH | Direct | Aboveground carbon | 0.300 | 0.040 | 0.530 | 0.020 |
| DBH variation | Direct | Aboveground carbon | 0.580 | 0.340 | 0.790 | 0.000 |
| Functional diversity WD | Direct | Aboveground carbon | −0.110 | −0.340 | 0.120 | 0.360 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Kundu, G.K.; Kabir, M.E.; Ahmed, H.; Xu, M. Opposing Ecological Strategies Together Promote Biomass Carbon Storage in Homegardens Agroforestry of Southern Bangladesh. Forests 2021, 12, 1669. https://doi.org/10.3390/f12121669
Rahman MM, Kundu GK, Kabir ME, Ahmed H, Xu M. Opposing Ecological Strategies Together Promote Biomass Carbon Storage in Homegardens Agroforestry of Southern Bangladesh. Forests. 2021; 12(12):1669. https://doi.org/10.3390/f12121669
Chicago/Turabian StyleRahman, Md Mizanur, Gauranga Kumar Kundu, Md Enamul Kabir, Heera Ahmed, and Ming Xu. 2021. "Opposing Ecological Strategies Together Promote Biomass Carbon Storage in Homegardens Agroforestry of Southern Bangladesh" Forests 12, no. 12: 1669. https://doi.org/10.3390/f12121669
APA StyleRahman, M. M., Kundu, G. K., Kabir, M. E., Ahmed, H., & Xu, M. (2021). Opposing Ecological Strategies Together Promote Biomass Carbon Storage in Homegardens Agroforestry of Southern Bangladesh. Forests, 12(12), 1669. https://doi.org/10.3390/f12121669







