The Effectiveness of Soil Extracts from Selangor Peat Swamp and Pristine Forest Soils on the Growth of Green Microalgae sp.
Abstract
1. Introduction
2. Results
2.1. Soil Properties Analysis
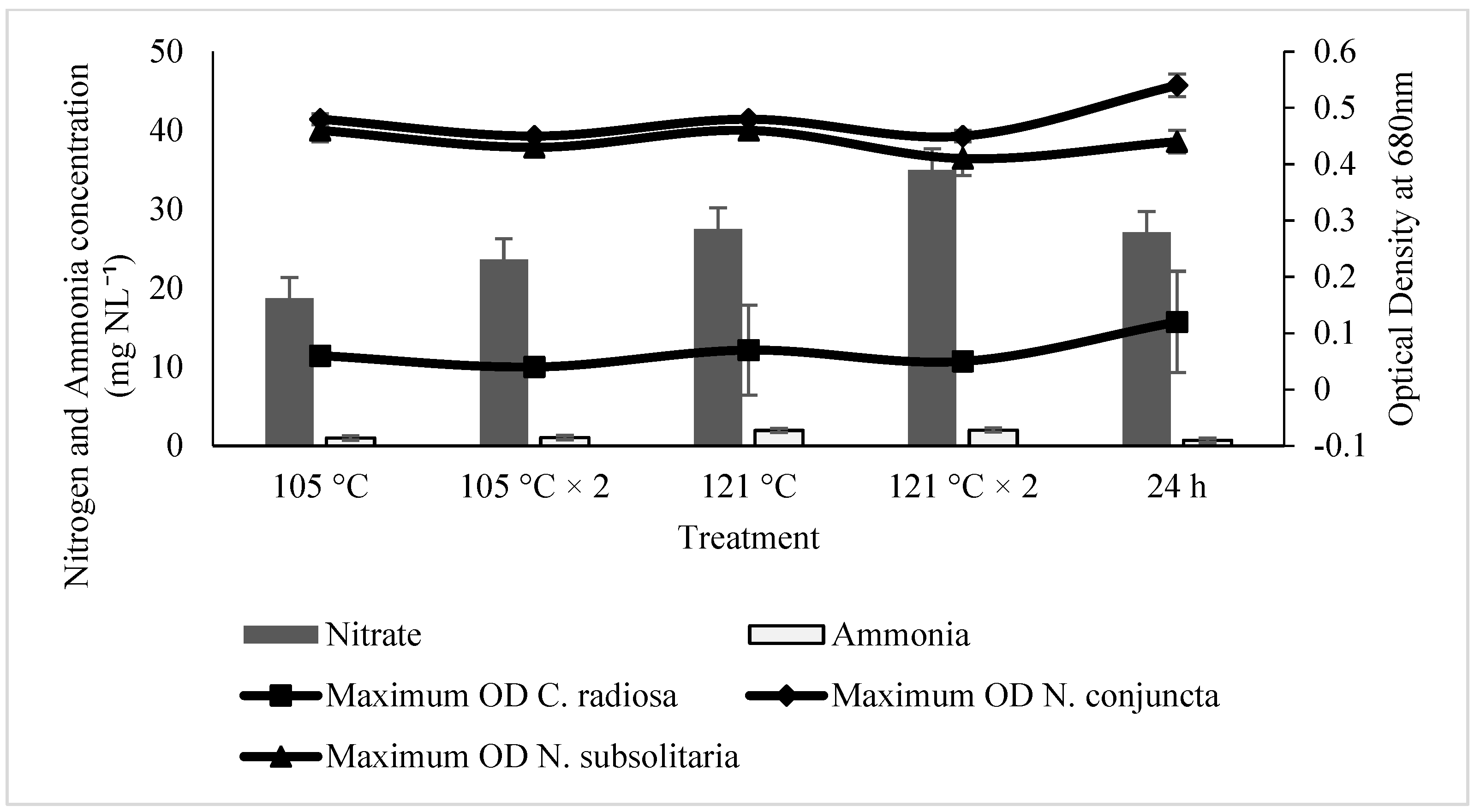
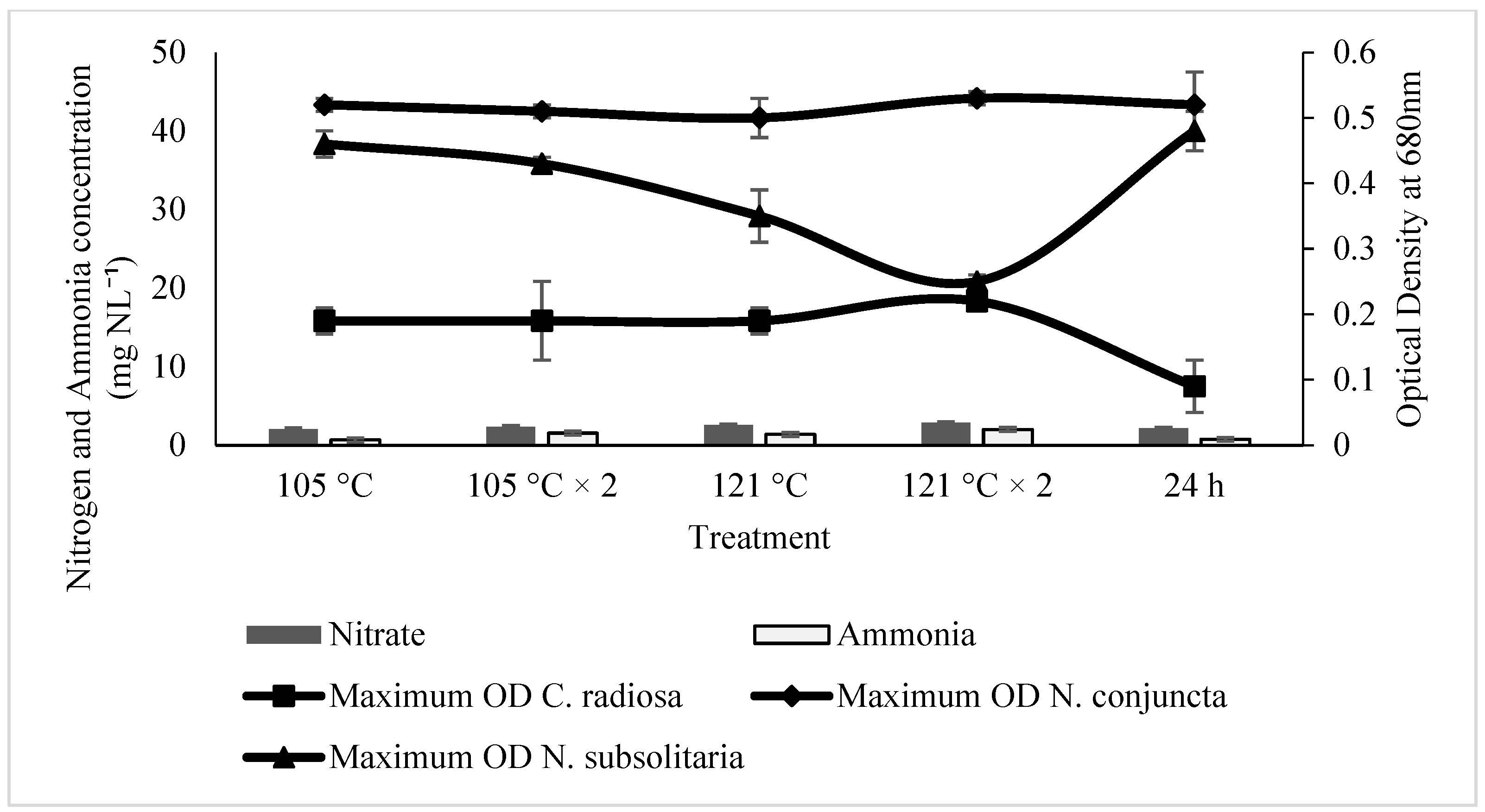
2.2. Effect of Ammonia and Nitrate on Microalgae Growth
2.3. The Influences of SE for the Microalgal Growth
2.4. Evaluation of Specific Growth Rate (SGR, µ) and Division Rate (k) for Selected Microalgae in Modified SE
3. Discussion
4. Materials and Methods
4.1. Collection and Preparation of Sample
4.2. Soil Extraction
4.3. Microalgae
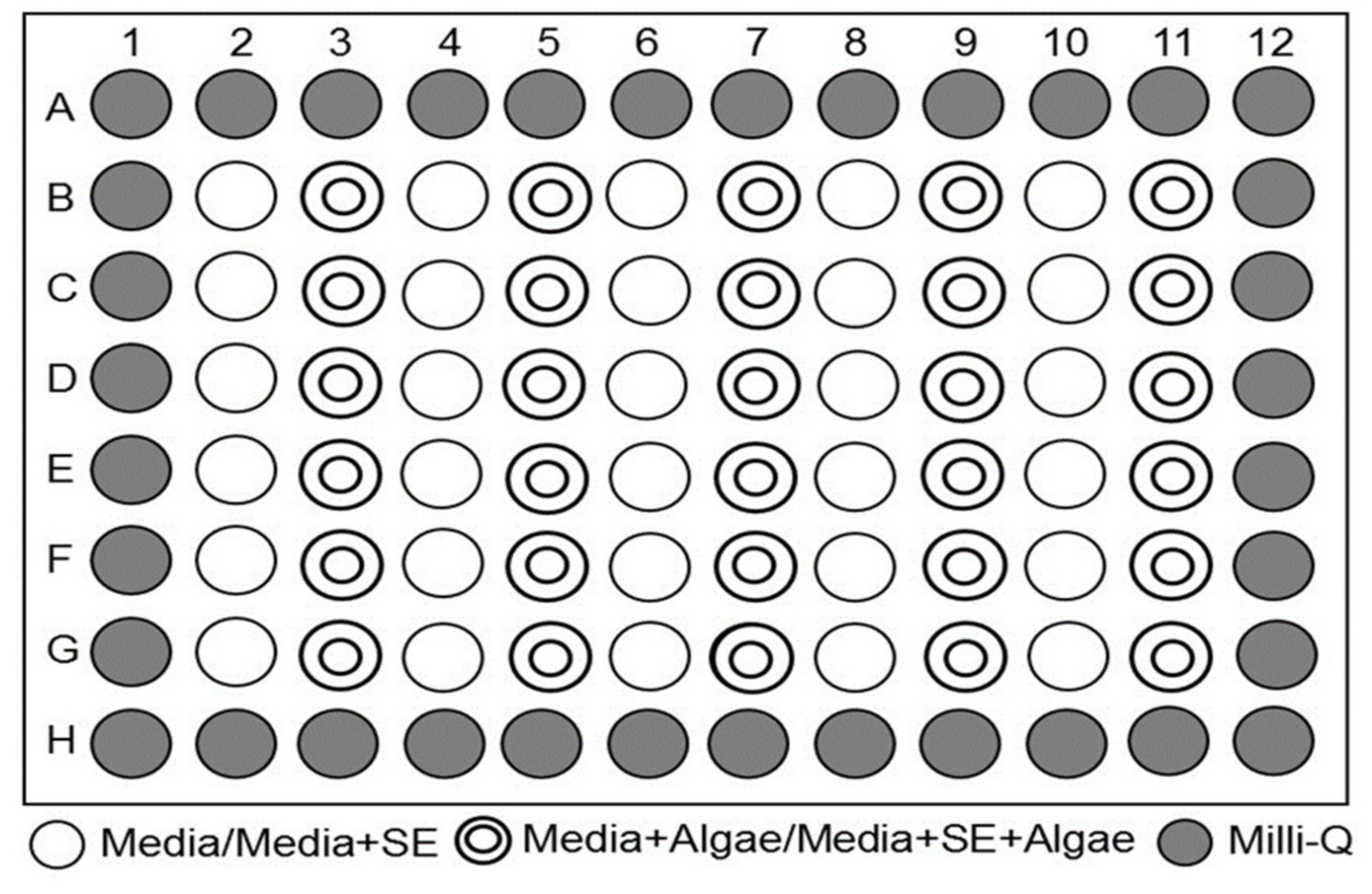
4.4. Technique of Microplate Incubation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sonnekus, M.J. Effects of Salinity on the Growth and Lipid Production of Ten Species of Microalgae from the Swartkops Saltworks: A Biodiesel Perspective. Ph.D. Thesis, Nelson Mandela Metropolitan University, Port Elizabeth, South Africa, 2010. [Google Scholar]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, and fermenters. Prog. Ind. Microbiol. 1999, 35, 313–321. [Google Scholar]
- Medipally, S.R.; Yusoff, F.M.; Banerjee, S.; Shariff, M. Microalgae as sustainable renewable energy feedstock for biofuel production. BioMed. Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Metting, F.B. Biodiversity and application of microalgae. J. Ind. Microbiol. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mobin, S.; Alam, F. Some promising microalgal species for commercial applications: A review. Energy Procedia 2017, 110, 510–517. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Util. 2012, 3, 89–100. [Google Scholar]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Schenk, P.; Thomas-Hall, S.; Stephens, E.; Marx, U.; Mussgnug, J.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High efficiency microalgae for biodiesel production. Bioenergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Colla, L.M.; Reinehr, C.O.; Reichert, C.; Costa, J.A.V. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour. Technol. 2007, 98, 1489–1493. [Google Scholar] [CrossRef]
- Enright, C.T.; Newkirk, G.F.; Craigie, J.S.; Castell, J.D. Growth of juvenile Ostrea edulis L. fed Chaetoceros gracilis Schütt of varied chemical composition. J. Exp. Mar. Biol. Ecol. 1986, 96, 15–26. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Guedes, A.C.; Malcata, F.X. Nutritional value and uses of microalgae in aquaculture. Aquaculture 2012, 10, 59–78. [Google Scholar]
- Cai, T.; Ge, X.; Park, S.Y.; Li, Y. Comparison of Synechocystis sp. PCC6803 and Nannochloropsis salina for lipid production using artificial seawater and nutrients from anaerobic digestion effluent. Bioresour. Technol. 2013, 144, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hende, S.; Vervaeren, H.; Saveyn, H.; Maes, G.; Boon, N. Microalgal bacterial floc properties are improved by a balanced inorganic/organic carbon ratio. Biotechnol. Bioeng. 2011, 108, 549–558. [Google Scholar] [CrossRef]
- Karthikeyan, S. A critical review: Microalgae as a renewable source for biofuel production. Int. J. Eng. Res. Technol. 2012, 1, 1–6. [Google Scholar]
- Andersen, R.A.; Kawachi, M. Microalgae isolation techniques. Algal Cult. Tech. 2005, 83–100. [Google Scholar]
- Kurano, N.; Ikemoto, H.; Miyashita, H.; Hasegawa, T.; Hata, H.; Miyachi, S. Fixation and utilization of carbon dioxide by microalgal photosynthesis. Energy Convers. Manag. 1995, 36, 689–692. [Google Scholar] [CrossRef]
- Velichkova, K.; Sirakov, I.; Stoyanova, S. Biomass production and wastewater treatment from aquaculture with Chlorella vulgaris under different carbon sources. Sci. Bulletin. Ser. F. Biotechnol. 2014, 18, 83–88. [Google Scholar]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Doig III, M.T.; Martin, D.F. The effect of naturally occurring organic substances on the growth of a red tide organism. Water Res. 1974, 8, 601–606. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Muller-Feuga, A. The role of microalgae in aquaculture: Situation and trends. J. Appl. Phycol. 2000, 12, 527–534. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, S.K.; Sharma, G.; Julka, J.M.; Kumar, A.; Sharma, S.; Stadler, F.J. Impact of heavy metals and nanoparticles on aquatic biota. Environ. Chem. Lett. 2018, 16, 919–946. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Morris, P. Method of Environmental Impact Assessment; Taylor and Francis Science: Abingdon, UK, 2009. [Google Scholar]
- Ichikogu, V.I. The dynamics of soil physical properties and exchangeable cations in secondary forest regenerating from degraded abandoned rubber plantation (Hevea brasiliensis) in orogun area of southern Nigeria. Ethiop. J. Environ. Stud. Manag. 2011, 4, 117–126. [Google Scholar] [CrossRef][Green Version]
- Amlin, G.; Suratman, M.N.; Isa, N.N.M. Soil chemical analysis of secondary forest 30 years after logging activities at Krau Wildlife Reserve, Pahang, Malaysia. APCBEE Procedia 2014, 9, 75–81. [Google Scholar] [CrossRef][Green Version]
- Heryati, Y.; Abdu, A.; Mahat, M.N.; Abdul-Hamid, H.; Jusop, S.; Majid, N.M.; Heriansyah, I.; Ajang, L.; Ahmad, K. Comparing the fertility of soils under Khaya ivorensis plantation and regenerated dengraded secondary forest. Am. J. Appl. Sci. 2011, 8, 472–480. [Google Scholar] [CrossRef]
- Abdu, A.; Karam, D.S.; Shamshuddin, J.; Majid, N.M.; Radziah, O.; Hazandy, A.H.; Zahari, I. Proposing a suitable soil quality index for natural, secondary and rehabilitated tropical forests in Malaysia. Afr. J. Biotechnol. 2012, 11, 3297–3309. [Google Scholar]
- FAO/WHO (Codex Alimentarius Commission). Food Additives and Contaminants. Joint FAO/WHO Food Standards Program, ALINORM 01/12A; FAO/WHO: Rome, Italy, 2001; pp. 1–289. [Google Scholar]
- Xu, D.M.; Fu, R.B.; Liu, H.Q.; Guo, X.P. Current knowledge from heavy metal pollution in Chinese smelter contaminated soils, health risk implications and associated remediation progress in recent decades: A critical review. J. Clean. Prod. 2021, 286, 124989. [Google Scholar] [CrossRef]
- Suess, H.E.; Urey, H.C. Abundances of the elements. Rev. Mod. Phys. 1956, 28, 53. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Venkateswaran, V.; Senthilkumar, M.; Anandham, R.; Selvakumar, G.; Kim, K.; Kang, Y.; Sa, T. Potential microbiological approaches for the remediation of heavy metal-contaminated soils. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Singapore, 2017; pp. 341–366. [Google Scholar]
- Singh, R.; Ahirwar, N.K.; Tiwari, J.; Pathak, J. Review on sources and effect of heavy metal in soil: Its bioremediation. Int. J. Res. Appl. Nat. Soc. Sci. 2018, 2018, 1–22. [Google Scholar]
- Osmani, M.; Bani, A.; Hoxha, B. Heavy Metals and Ni Phytoextractionin in the Metallurgical Area Soils in Elbasan. Albanian J. Agric. Sci. 2015, 14, 414. [Google Scholar]
- Zhang, W.; Liu, M.; Li, C. Soil heavy metal contamination assessment in the Hun-Taizi River watershed, China. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Addis, W.; Abebaw, A. Determination of heavy metal concentration in soils used for cultivation of Allium sativum L. (garlic) in East Gojjam Zone, Amhara Region, Ethiopia. Cogent Chem. 2017, 3, 1419422. [Google Scholar] [CrossRef]
- Lenart, A.; Wolny-Koładka, K. The effect of heavy metal concentration and soil pH on the abundance of selected microbial groups within ArcelorMittal Poland steelworks in Cracow. Bull. Environ. Contam. Toxicol. 2013, 90, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Harter, R.D. Effect of soil pH on adsorption of lead, copper, zinc, and nickel. Soil Sci. Soc. Am. J. 1983, 47, 47–51. [Google Scholar] [CrossRef]
- Wang, A.S.; Angle, J.S.; Chaney, R.L.; Delorme, T.A.; Reeves, R.D. Soil pH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant Soil 2006, 281, 325–337. [Google Scholar] [CrossRef]
- Yi, L.; Hong, Y.; Wang, D.; Zhu, Y. Determination of free heavy metal ion concentrations in soils around a cadmium rich zinc deposit. Geochem. J. 2007, 41, 235–240. [Google Scholar] [CrossRef]
- Ogundele, D.T.; Adio, A.A.; Oludele, O.E. Heavy metal concentrations in plants and soil along heavy traffic roads in North Central Nigeria. J. Environ. Anal. Toxicol. 2015, 5, 1. [Google Scholar]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Ewers, U. Standards, guidelines and legislative regulations concerning metals and their compounds. In Metals and Their Compounds in the Environment: Occurrence; Merian, E., Ed.; Analysis and Biological Relevance; VCH: Weinheim, Germany, 1991; pp. 458–468. [Google Scholar]
- Sharma, S.; Arunachalam, K.; Arunachalam, A. Soil Characteristics in the Disturbed and Undisturbed Plots of a Sal (Shorea robusta) Forest in the Shiwaliks of Indian Himalayan Region. Indian J. Hill Farming 2017, 30, 176–180. [Google Scholar]
- Hussain, F.; Eom, H.; Ali Toor, U.; Lee, C.S.; Oh, S.E. Rapid assessment of heavy metal-induced toxicity in water using micro-algal bioassay based on photosynthetic oxygen evolution. Environ. Eng. Res. 2020, 26, 200391. [Google Scholar] [CrossRef]
- Pan, X.; Chang, F.; Wang, S. Toxic effects of mercury on PSI and PSII activities, membrane potential and transthylakoid proton gradient in Microsorium pteropus. J. Photochem. Photobiol. B Biol. 2013, 127, 1–7. [Google Scholar]
- Singh, R.; Dubey, G.; Singh, V.P.; Srivastava, P.K.; Kumar, S.; Prasad, S.M. High light intensity augments mercury toxicity in cyanobacterium Nostoc muscorum. Biol. Trace Elem. Res. 2012, 149, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Książyk, M.; Asztemborska, M.; Stęborowski, R.; Bystrzejewska-Piotrowska, G. Toxic effect of silver and platinum nanoparticles toward the freshwater microalga Pseudokirchneriella subcapitata. Bull. Environ. Contam. Toxicol. 2015, 94, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Gallego-Urrea, J.A.; Jurkschat, K.; Crossley, A.; Hassellöv, M.; Taylor, C.; Loureiro, S. Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci. Total. Environ. 2014, 466, 232–241. [Google Scholar] [CrossRef]
- Lukavský, J.F.; Cepák, V. Toxicity of metals, Al, Cd, Co, Cr, Cu, Fe, Ni, Pb and Zn on microalgae, using microplate bioassay 1: Chlorella kessleri, Scenedesmus quadricauda, sc. subspicatus and Raphidocelis subcapitata (Selenastrum capricornutum). Algol. Stud. Arch. Für Hydrobiol. Suppl. Vol. 2003, 110, 127–141. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, B.; Zhang, J.; Yang, C.; Wang, J.; Song, F.; Li, S. Absorption and speciation of arsenic by microalgae under arsenic-copper Co-exposure. Ecotoxicol. Environ. Saf. 2021, 213, 112024. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Beardall, J.; Raven, J.A. (Eds.) The Physiology of Microalgae; Springer International Publishing: Cham, Switzerland, 2016; Volume 6. [Google Scholar]
- Sanz-Luque, E.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Understanding nitrate assimilation and its regulation in microalgae. Front. Plant Sci. 2015, 6, 899. [Google Scholar] [CrossRef]
- Kolzau, S.; Wiedner, C.; Rücker, J.; Köhler, J.; Köhler, A.; Dolman, A.M. Seasonal patterns of nitrogen and phosphorus limitation in four German lakes and the predictability of limitation status from ambient nutrient concentrations. PLoS ONE 2014, 9, e96065. [Google Scholar] [CrossRef] [PubMed]
- Podevin, M.; De Francisci, D.; Holdt, S.L.; Angelidaki, I. Effect of nitrogen source and acclimatization on specific growth rates of microalgae determined by a high-throughput in vivo microplate autofluorescence method. J. Appl. Phycol. 2015, 27, 1415–1423. [Google Scholar] [CrossRef]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. High productivity cultivation of a heat-resistant microalga Chlorella sorokiniana for biofuel production. Bioresour. Technol. 2013, 131, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.F.; Chen, P.C.; Lee, C.M. The effects of nitrogen sources and temperature on cell growth and lipid accumulation of microalgae. Int. Biodeterior. Biodegrad. 2013, 85, 506–510. [Google Scholar] [CrossRef]
- Chen, M.; Tang, H.; Ma, H.; Holland, T.C.; Ng, K.S.; Salley, S.O. Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour. Technol. 2011, 102, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.S.; Rodrigues, M.S.; Converti, A.; Sato, S.; Carvalho, J.C.M. A new approach to ammonium sulphate feeding for fed-batch Arthrospira (Spirulina) platensis cultivation in tubular photobioreactor. Biotechnol. Prog. 2010, 26, 1271–1277. [Google Scholar] [CrossRef]
- Lourenco, S.O.; Barbarino, E.; Mancini-Filho, J.; Schinke, K.P.; Aidar, E. Effects of different nitrogen sources on the growth and biochemical profile of 10 marine microalgae in batch culture: An evaluation for aquaculture. Phycologia 2002, 41, 158–168. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- Roopnarain, A.; Sym, S.; Gray, V.M. Effect of nitrogenous resource on growth, biochemical composition and ultrastructure of I sochrysis galbana (I sochrysidales, H aptophyta). Phycol. Res. 2015, 63, 43–50. [Google Scholar] [CrossRef]
- Ruangsomboon, S. Effects of different media and nitrogen sources and levels on growth and lipid of green microalga Botryococcus braunii KMITL and its biodiesel properties based on fatty acid composition. Bioresour. Technol. 2015, 191, 377–384. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Ren, L.; Wang, P.; Wang, C.; Chen, J.; Hou, J.; Qian, J. Algal growth and utilization of phosphorus studied by combined mono-culture and co-culture experiments. Environ. Pollut. 2017, 220, 274–285. [Google Scholar] [CrossRef]
- Berns, A.E.; Philipp, H.; Narres, H.D.; Burauel, P.; Vereecken, H.; Tappe, W. Effect of gamma-sterilization and autoclaving on soil organic matter structure as studied by solid state NMR, UV and fluorescence spectroscopy. Eur. J. Soil Sci. 2008, 59, 540–550. [Google Scholar] [CrossRef]
- Daliry, S.; Hallajisani, A.; Mohammadi, R.J.; Nouri, H.; Golzary, A. Investigation of optimal condition for Chlorella vulgaris microalgae growth. Glob. J. Environ. Sci. Manag. 2007, 3, 217–230. [Google Scholar]
- Metsoviti, M.N.; Katsoulas, N.; Karapanagiotidis, I.T.; Papapolymerou, G. Effect of nitrogen concentration, two-stage and prolonged cultivation on growth rate, lipid and protein content of Chlorella vulgaris. J. Chem. Technol. Biotechnol. 2019, 94, 1466–1473. [Google Scholar] [CrossRef]
- Lian, J.; Wijffels, R.H.; Smidt, H.; Sipkema, D. The effect of the algal microbiome on industrial production of microalgae. Microb. Biotechnol. 2018, 11, 806–818. [Google Scholar] [CrossRef]
- Marjakangas, J.M.; Chen, C.Y.; Lakaniemi, A.M.; Puhakka, J.A.; Whang, L.M.; Chang, J.S. Simultaneous nutrient removal and lipid production with Chlorella vulgaris on sterilized and non-sterilized anaerobically pretreated piggery wastewater. Biochem. Eng. J. 2015, 103, 177–184. [Google Scholar] [CrossRef]
- Warcup, J.H. Chemical and biological aspects of soil sterilization. Soils Fertil. 1957, 20, 1–5. [Google Scholar]
- Powlson, D.S.; Jenkinson, D.S. Effects of biocidal treatments on metabolism in soil. 2. gamma-irradiation, autoclaving, air-drying and fumigation. Soil Biol. Biochem. 1976, 8, 179–188. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Yap, Y.J.; Ling, T.C.; Chang, J.S. Effects of water culture medium, cultivation systems and growth modes for microalgae cultivation: A review. J. Taiwan Inst. Chem. Eng. 2018, 91, 332–344. [Google Scholar] [CrossRef]
- Kawasaki, N.; Kushairi, M.R.M.; Nagao, N. Release of nitrogen and phosphorus from aquaculture farms to Selangor River, Malaysia. Int. J. Environ. Sci. Dev. 2016, 7, 113. [Google Scholar] [CrossRef]
- Yaacob, N.S.; Ahmad, M.F.; Kawasaki, N.; Maniyam, M.N.; Abdullah, H.; Hashim, E.F.; Sjahrir, F.; Wan Mohd Zamri, W.M.; Komatsu, K.; Kuwahara, V.S. Kinetics Growth and Recovery of Valuable Nutrients from Selangor Peat Swamp and Pristine Forest Soils Using Different Extraction Methods as Potential Microalgae Growth Enhancers. Molecules 2021, 26, 653. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, K.; Ahmad, M.F.; Yaacob, N.S.; Ikram, W.M.; Maniyam, M.N.; Abdullah, H.; Katayama, T.; Komatsu, K.; Kuwahara, V.S. Enhancement of targeted microalgae species growth using aquaculture sludge extracts. Heliyon 2020, 6, e04556. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.R.; Raju, N.S. Correlation of heavy metal contamination with soil properties of industrial areas of Mysore, Karnataka, India by cluster analysis. Int. Res. J. Environ. Sci. 2013, 2, 22–27. [Google Scholar]
- Reay, D.S.; Nedwell, D.B.; Priddle, J.; Ellis-Evans, J.C. Temperature dependence of inorganic nitrogen uptake: Reduced affinity for nitrate at suboptimal temperatures in both algae and bacteria. Appl. Environ. Microbiol. 1999, 65, 2577. [Google Scholar] [CrossRef]




| Soil Sample | Raja Musa | Ayer Hitam |
|---|---|---|
| pH | 3.36 ± 0.00 | 5.81 ± 0.02 |
| Sand (%) | 42.48 ± 0.00 | 33.69 ± 0.00 |
| Silt (%) | 50.95 ± 0.00 | 58.3 ± 0.00 |
| Clay (%) | 6.52 ± 0.04 | 8.01 ± 0.00 |
| Soil Location | Raja Musa | Ayer Hitam | ||
|---|---|---|---|---|
| Soil | Extract | Soil | Extract | |
| Total Organic Carbon (%) | 5.03 ± 0.02 | 5.23 ± 0.07 | ||
| Nitrogen (%) | 0.28 ± 0.00 | 0.00812 ± 0.00 | 0.35 ± 0.00 | 0.00084 ± 0.00 |
| Phosphorus (%) | 1.07 ± 0.00 | 0.001786 ± 0.00 | 1.49 ± 0.00 | 0.000423 ± 0.00 |
| Potassium (mg/l) | 3.66 ± 0.15 | 9.66 ± 0.00 | 886.77 ± 30.67 | 6.09 ± 0.00 |
| Trace Metals (mg/kg) | ||||
| Iron | 832.84 ± 3.74 | - | 1905.48 ± 68.42 | - |
| Arsenic | ND < 1 | ND < 1 | 86.79 ± 4.98 | ND < 10 |
| Cadmium | ND < 0.002 | ND < 0.002 | ND < 0.5 | ND < 0.002 |
| Chromium | ND < 2 | ND < 2 | 35.84 ± 1.16 | ND < 10 |
| Plumbum/Lead | 9.15 ± 0.68 | ND < 0.01 | 506.16 ± 33.54 | ND < 0.01 |
| Copper | 0.70 ± 0.05 | ND < 0.01 | 84.41 ± 61.44 | 0.01 ± 0.00 |
| Nickel | ND < 0.01 | 0.02 ± 0.00 | ND < 0.5 | 0.01 ± 0.00 |
| Zinc | 4.91 ± 0.06 | 0.19 ± 0.00 | 121.83 ± 33.44 | 0.07 ± 0.00 |
| Mercury | ND < 1 | ND < 1 | ND < 0.5 | ND < 0.5 |
| Silver | ND < 10 | ND < 10 | ND < 0.5 | ND < 0.5 |
| SE Type | Microalgae | Control | Modified SE | ||||
|---|---|---|---|---|---|---|---|
| Media with 105 °C | Media with 105 °C twice | Media with 121 °C | Media with 121 °C twice | Media with 24 h | |||
| RMFR SE | C. radiosa | 0.16 ± 0.00 a | 0.06 ± 0.00 a | 0.04 ± 0.00 b | 0.07 ± 0.08 a | 0.05 ± 0.01 b | 0.12 ± 0.09 a |
| N. conjuncta | 0.62 ± 0.00 a | 0.48 ± 0.01 a | 0.45 ± 0.00 a | 0.48 ± 0.00 a | 0.45 ± 0.01 a | 0.54 ± 0.02 a | |
| N. subsolitaria | 0.59 ± 0.00 a | 0.46 ± 0.02 a | 0.43 ± 0.00 a | 0.46 ± 0.00 a | 0.41 ± 0.03 a | 0.44 ± 0.02 a | |
| AHFR SE | C. radiosa | 0.63 ± 0.02 a | 0.19 ± 0.02 b | 0.19 ± 0.06 b | 0.19 ± 0.02 b | 0.22 ± 0.01 b | 0.09 ± 0.04 b |
| N. conjuncta | 0.28 ± 0.04 a | 0.52 ± 0.01 a | 0.51 ± 0.01 a | 0.50 ± 0.03 a | 0.53 ± 0.01 a | 0.52 ± 0.05 a | |
| N. subsolitarias | 0.27 ± 0.02 a | 0.46 ± 0.02 a | 0.43 ± 0.01 a | 0.35 ± 0.04 a | 0.25 ± 0.01 a | 0.48 ± 0.03 a | |
| SE Type | Microalgae | Control | Modified SE | ||||
|---|---|---|---|---|---|---|---|
| Media with 105 °C | Media with 105 °C twice | Media with 121 °C | Media with 121 °C twice | Media with 24 h | |||
| RM SE | C. radiosa | 0.13 ± 0.01 a | 0.00 ± 0.00 a | 0.01 ± 0.02 a | 0.06 ± 0.00 a | 0.01 ± 0.01 a | 0.07 ± 0.01 a |
| N. conjuncta | 0.18 ± 0.00 a | 0.15 ± 0.01 a | 0.15 ± 0.00 a | 0.15 ± 0.01 a | 0.02 ± 0.00 a | 0.18 ± 0.00 a | |
| N. subsolitaria | 0.16 ± 0.02 a | 0.13 ± 0.01 a | 0.13 ± 0.00 a | 0.14 ± 0.00 a | 0.13 ± 0.01 a | 0.14 ± 0.00 a | |
| AH SE | C. radiosa | 0.11 ± 0.01 a | 0.01 ± 0.01 a | 0.04 ± 0.00 a | 0.06 ± 0.00 a | 0.07 ± 0.00 a | 0.01 ± 0.01 a |
| N. conjuncta | 0.06 ± 0.00 a | 0.16 ± 0.00 a | 0.15 ± 0.00 a | 0.14 ± 0.00 a | 0.16 ± 0.00 a | 0.15 ± 0.01 a | |
| N. subsolitaria | 0.09 ± 0.00 a | 0.13 ± 0.00 a | 0.13 ± 0.00 a | 0.09 ± 0.00 a | 0.10 ± 0.00 a | 0.15 ± 0.00 a | |
| Site | Gps |
|---|---|
| Raja Musa Forest Reserve (RM) | (3°26′45.2” N 101°19′20.9” E) |
| Ayer Hitam Forest Reserve (AH) | (3°00′’27.7” N 101°38′46.9” E) |
| Parameters | Unit | Analysis Method |
|---|---|---|
| Particle Size Distribution (PSD) | ||
| Sand | % | USDA/NRCS Soil Taxonomy |
| Silt | % | USDA/NRCS Soil Taxonomy |
| Clay | % | USDA/NRCS Soil Taxonomy |
| pH | - | APHA 4500-H B |
| Nitrogen (N) | % | MS 417L PART 3: 1994 |
| Phosphorus (P) | % | MS 678: PART 8 |
| Potassium (K) | % | (MS 417: PART 3: 1994): MS 678: PART VI to IX: 1980 (APHA 3500 K)) |
| Total organic carbon | % | MS 678: PART 3A: 1980 |
| Arsenic (As) | mg/kg | USEPA 1311 (APHA 3111-B) |
| Iron (Fe) | mg/kg | USEPA 1311 (APHA 3111-B) |
| Cadmium (Cd) | mg/kg | USEPA 1311 (APHA 3111-B) |
| Chromium (Cr) | mg/kg | USEPA 1311 (APHA 3111-B) |
| Lead (Pb) | mg/kg | USEPA 1311 (APHA 3111-B) |
| Copper (Cu) | mg/kg | USEPA 1311 (APHA 3111-B) |
| Nickel (Ni) | mg/kg | USEPA 1311 (APHA 3111-B) |
| Zinc (Zn) | mg/kg | USEPA 1311 (APHA 3111-B) |
| Mercury (Hg) | mg/kg | USEPA 1311 (APHA 3111-B) |
| Silver (Ag) | mg/kg | USEPA 1311 (APHA 3111-B) |
| Extraction Methods | Procedure |
|---|---|
| Natural Extraction 24 H (NE-24 H) | Soil extracted at room temperature for 24 h |
| Autoclave 105 °C (105 °C) | Soil extracted at 105 °C autoclave, 1 h |
| Autoclave 121 °C (121 °C) | Soil extracted at 121 °C autoclave, 1 h |
| Autoclave 105 °C twice (105 °C × 2) | Soil extracted at 105 °C autoclave for 1 h, and after cooled (~30 min), soil extracted at 105 °C autoclave, 1 h |
| Autoclave 121 °C twice (×2) (121 °C × 2) | Soil extracted at 121 °C autoclave for 1 h, and after cooled (~30 min), soil extracted at 121 °C autoclave, 1 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaacob, N.S.; Ahmad, M.F.; Sivam, A.; Hashim, E.F.; Maniyam, M.N.; Sjahrir, F.; Dzulkafli, N.F.; Wan Mohd Zamri, W.M.I.; Komatsu, K.; Kuwahara, V.S.; et al. The Effectiveness of Soil Extracts from Selangor Peat Swamp and Pristine Forest Soils on the Growth of Green Microalgae sp. Forests 2022, 13, 79. https://doi.org/10.3390/f13010079
Yaacob NS, Ahmad MF, Sivam A, Hashim EF, Maniyam MN, Sjahrir F, Dzulkafli NF, Wan Mohd Zamri WMI, Komatsu K, Kuwahara VS, et al. The Effectiveness of Soil Extracts from Selangor Peat Swamp and Pristine Forest Soils on the Growth of Green Microalgae sp. Forests. 2022; 13(1):79. https://doi.org/10.3390/f13010079
Chicago/Turabian StyleYaacob, Nor Suhaila, Mohd Fadzli Ahmad, Ashvini Sivam, Emi Fazlina Hashim, Maegala Nallapan Maniyam, Fridelina Sjahrir, Noor Fazreen Dzulkafli, Wan Muhammad Ikram Wan Mohd Zamri, Kazuhiro Komatsu, Victor S. Kuwahara, and et al. 2022. "The Effectiveness of Soil Extracts from Selangor Peat Swamp and Pristine Forest Soils on the Growth of Green Microalgae sp." Forests 13, no. 1: 79. https://doi.org/10.3390/f13010079
APA StyleYaacob, N. S., Ahmad, M. F., Sivam, A., Hashim, E. F., Maniyam, M. N., Sjahrir, F., Dzulkafli, N. F., Wan Mohd Zamri, W. M. I., Komatsu, K., Kuwahara, V. S., & Abdullah, H. (2022). The Effectiveness of Soil Extracts from Selangor Peat Swamp and Pristine Forest Soils on the Growth of Green Microalgae sp. Forests, 13(1), 79. https://doi.org/10.3390/f13010079








