Abstract
Climate change is leading to increased drought intensity and fire frequency, creating early-successional landscapes with novel disturbance–recovery dynamics. In the Klamath Mountains of northwestern California and southwestern Oregon, early-successional interactions between nitrogen (N)-fixing shrubs (Ceanothus spp.) and long-lived conifers (Douglas-fir) are especially important determinants of forest development. We sampled post-fire vegetation and soil biogeochemistry in 57 plots along gradients of time since fire (7–28 years) and climatic water deficit (aridity). We found that Ceanothus biomass increased, and Douglas-fir biomass decreased with increasing aridity. High aridity and Ceanothus biomass interacted with lower soil C:N more than either factor alone. Ceanothus biomass was initially high after fire and declined with time, suggesting a large initial pulse of N-fixation that could enhance N availability for establishing Douglas-fir. We conclude that future increases in aridity and wildfire frequency will likely limit post-fire Douglas-fir establishment, though Ceanothus may ameliorate some of these impacts through benefits to microclimate and soils. Results from this study contribute to our understanding of the effects of climate change and wildfires on interspecific interactions and forest dynamics. Management seeking to accelerate forest recovery after high-severity fire should emphasize early-successional conifer establishment while maintaining N-fixing shrubs to enhance soil fertility.
1. Introduction
Disturbance–recovery dynamics shape the structure and function of forests and are highly sensitive to climate change [1,2]. Harsh abiotic post-disturbance conditions can drastically slow rates of community-level succession and delay recovery [3]. Climate change impacts early-successional dynamics through altered disturbance regimes and a potentially harsher post-disturbance abiotic environment [4]. Combined with exacerbated drought conditions, increased frequency and severity of wildfires can suppress tree regeneration both from slower tree recruitment and growth rates, and enhanced competition due to post-disturbance shrub sprouting [2,5,6]. Empirical evidence shows that climate change strongly influences post-fire recovery dynamics on multiple scales [7]. At broader scales, altered fire regimes due to climate change can influence landscape-level post-fire recovery trajectories [8]. At fine scales, plant functional types (e.g., shrubs, conifers) might be differentially impacted by climate change, leading to novel assemblage patterns [1]. To further our understanding of climatic effects on disturbance–recovery dynamics, it is important to focus on the current community-level interactions of shrubs and conifers during the early-successional period after wildfire disturbances.
The Klamath region of northwestern California and southwestern Oregon is among the most diverse forest regions of North America, and has undergone substantial shifts in vegetation community composition and structure since the early Holocene [9,10]. It is estimated that with warmer climates, increased drought, and more frequent and severe fires, by the end of the century, almost one-third of the Klamath forest setting will be at risk of minimal conifer establishment after fire, potentially enabling transitions from a conifer forest to a shrub-dominated chaparral ecosystem [11]. Substantial conversion is likely to occur over the coming decades even without additional climate change [11,12]. Shrub-dominated ecosystems that establish soon after high-severity fires may be prone to re-burning, at least within the first couple of decades following fire [13]. Repeat burning could lead to a feedback that sustains shrub cover, which could ultimately limit tree establishment and growth [14,15,16]. Increased drought can further limit post-fire conifer regeneration as conifer seedling growth rates are reduced in drier conditions [17].
Ceanothus (L.) is a shrub genus that is typically among the most abundant, if not the dominant genus, following high-severity fires in the Klamath region. Its dormant seed banks can persist in the soil for decades [18], enhancing its competitive advantage after severe fires remove overstory shade [19]. The high abundance and rapid growth rate of Ceanothus, along with several re-sprouting broadleaf tree and shrub species (e.g., Lithocarpus densiflorus, (Hook. & Arn.) Rehder; Quercus chrysolepis, Liebm.) may limit the window of regeneration for coniferous trees such as Douglas-fir (Pseudotsuga menziesii, (Mirb.) Franco), whose post-fire establishment depends on wind-blown seeds from surviving trees [11,14]. Conifer growth rates typically increase when they overtop nearby shrubs [20]. This indicates the competitive disadvantage of relatively shade-intolerant tree species (e.g., Douglas-fir) under the initial rapid growth of Ceanothus [11,14]. Furthermore, the over-crowding effect of Ceanothus might slow the growth of Douglas-fir seedlings due to space limitations [21]. Early-successional interactions between Ceanothus and Douglas-fir are crucial for determining the future vegetation constituents of the Klamath forests, along with energy exchange, water balance, and biogeochemical cycling.
High-severity wildfires can reduce soil carbon (C) and nitrogen (N) stocks in the Klamath region up to 50 and 25%, respectively [22]. Given that Douglas-fir growth in the Klamath region is often N limited, wildfire-induced losses of soil fertility could intensify N limitation of forest growth [23]. Whether this occurs, however, depends strongly on potential C and N resupply by early-successional shrubs, especially by symbiotic N-fixing Ceanothus species [24]. Ceanothus species vary widely in reported rates of N-fixation [23,25,26,27,28,29,30]. N-fixation by Ceanothus integerrimus in the Klamath region depends on interspecific competition and aridity, which interact to affect N availability for the growth of regenerating Douglas-fir [30]. Shrubs might also help to ameliorate warm, dry microclimatic conditions or foster conifer growth through the enhanced production of soil organic matter [28,29] and mycorrhizal connections that facilitate the acquisition of soil nutrients [31]. In some cases, however, Ceanothus may not directly enrich soil N or enhance the foliar N levels of regenerating conifers [25], especially where adequate soil N causes downregulation of N-fixation [32]. In these cases, shrub–conifer interactions may be more strongly driven by competition for light [20,33] or soil moisture [14,20,21,34]. Shrubs are expected to compete strongly for soil moisture, especially under climate change-induced drought conditions, which might reduce the establishment and growth rates of regenerating Douglas-fir.
The net outcome of competition for space, light, and soil moisture, and facilitation will determine the nature of the interaction between Ceanothus and Douglas-fir. If Ceanothus has an overall facilitative effect on Douglas-fir through reducing heat stress and increasing soil N availability from biological N-fixation, we would expect Ceanothus to buffer some of the negative effects of aridity on Douglas-fir. In contrast, if the nature of the interaction between Ceanothus and Douglas-fir is mainly negative due to interspecific competition for light and soil moisture, then the presumed effects of aridity slowing conifer forest recovery after severe fire would be exacerbated. Finally, the balance between competitive and facilitative interactions may slowly change through time as N-rich shrubs reach peak biomass and increasingly recycle N to soil, coincident with increasing conifer growth and N-demands from seedling to sapling stages [35].
Here, we aim to add to our knowledge regarding early-successional patterns of interspecific interactions between Ceanothus and Douglas-fir along gradients of time since fire and soil moisture availability (climatic water deficit). We specifically address the following questions in the context of the hypothesized relationships illustrated in Figure 1: (1) How do Ceanothus and Douglas-fir live aboveground biomass (referred to as biomass from hereon) vary along gradients of time since fire and climatic water deficit? (2) How do soil resources (soil C, N, and C:N) vary along gradients of time since fire and climatic water deficit? (3) Does the abundance of Ceanothus increase soil N levels, and is this influenced by climatic water deficit? (4) Does competition from Ceanothus affect early-successional Douglas-fir biomass?
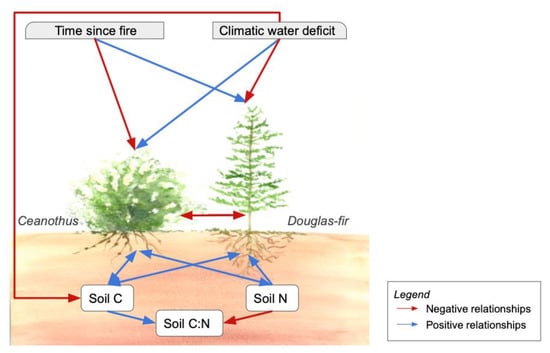
Figure 1.
Schematic diagram of relationships between Ceanothus spp. and Douglas-fir. Line color specifies positive (blue) and negative (red) relationships. Double headed arrows show reciprocal relationships.
2. Materials and Methods
2.1. Study Sites and Data
The Klamath region of northwestern California and southwestern Oregon has steep topographic and meso-climatic gradients, with a mean annual precipitation range of 1100 to 2000 mm yr−1 [34] and a mean annual temperature of 10.2 °C [36] (Available online: https://www.worldclim.org/ (Accessed on: 16 January 2021)). Due to a Mediterranean climate, most precipitation falls between October and May. Diverse patterns of climate, topography, and parent material lead to complex vegetation patterns in the Klamath region. Plant species diversity generally increases from coasts to inner regions with coniferous forests and woodlands dominating at all elevations [10]. Mixed conifer forests of the area commonly include Douglas-fir, ponderosa pine (Pinus ponderosa, Douglas ex P. Lawson & C. Lawson), Jeffrey pine (Pinus jeffreyi, Balf.), sugar pine (Pinus lambertiana, Douglas), white fir (Abies concolor, (Gordon & Glend.) Lindl. ex Hildebr), and incense cedar (Calocedrus decurrens, (Torr.) Florin) [10]. Douglas-fir is the dominant conifer throughout much of the region, with white fir becoming more abundant at higher elevations and ponderosa pine becoming dominant in the driest sites [21]. Abundant broadleaf tree species include tanoak (Lithocarpus densiflorus, (Hook. & Arn.) Rehder), madrone (Arbutus menziesii, Pursh), chinkapin (Chrysolepis chrysophylla, (Douglas ex Hook.) Hjelmq.), and canyon live oak (Quercus chrysolepis, Liebm.). Common shrubs consist of Ceanothus spp. (buckbrush, snow brush, deer brush) and Arctostaphylos spp. (hairy manzanita and kinnikinnick) [11,14]. Ceanothus and other shrub species are typically most abundant during the first few decades following high-severity fire [11].
2.2. Field Methods
Field methods and plot selection are described in detail in [11]. To assess the effects of time since fire and soil moisture availability, post-fire sites were sampled across a matrix of time since fire and climatic water deficit (Figure 2 and Figure S1).
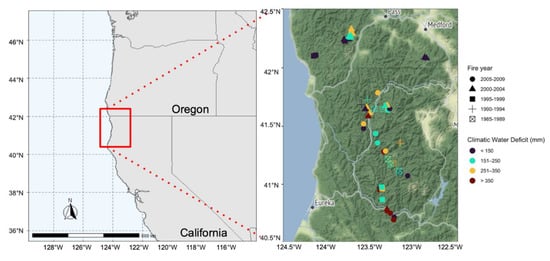
Figure 2.
Sample plot locations, with points coded by their climatic water deficit and fire year bin. The geographic area shown in the second figure corresponds to the red rectangle in the first figure.
Climatic water deficit (an index of site aridity) was calculated following [37], using 30-year normals (1981–2010) of monthly precipitation and mean, maximum, and minimum monthly temperatures at 800-m resolution [38], and 10-m resolution data for topographic variables and soil water-holding capacity [39]. The calculated climatic water deficit data for our sample plots ranged from 78 to 470 mm, with higher values indicating greater aridity. Candidate sampling sites were limited to areas that burned at high severity between 1985–2009. High-severity fires were determined by a regionally calibrated RdNBR threshold >574, which was calibrated to represent a 75% reduction in both canopy cover and basal area based on an analysis of 25 fires in the Klamath Mountains and Sierra Nevada [40]. Sites that have been burned within 30 years prior to the most recent fire and sites that had any type of post-fire management (including salvage logging and planting seedlings) were excluded to avoid any confounding effects [11]. Plot selection was stratified by sampling plots in each cell of a 5-by-4 matrix of fire years (1985–1989, 1990–1994, 1995–1999, 2000–2004, 2005–2009) and climatic water deficit (<150, 151–250, 251–350, >350 mm). Plots within each criteria combination were randomly selected and a matrix of the plots are provided in the supplementary materials in [11]. The 57 plots experienced fires between 1987 and 2008 and represented nine fire years and 22 fires in total (sampling was conducted at seven to 28 years since fire). Forty-four of the 57 plots had no live trees that survived the fires. Each plot was 450 m2 (15 × 30 m), and all plots were sampled during the summer of 2015. The study design provided randomly selected sample plots that were well-distributed along climatic water deficit and time since fire gradients with no significant correlation between the two variables (Figure S1). Our analysis of additional geographic and topographic variables showed that about half of the variation in climatic water deficit across the sample plots was explained by broadscale gradients of latitude and elevation (Figure S2).
We sampled plant community composition, plant foliar nutrient concentrations, and soil properties. Woody vegetation was sampled in three size classes: overstory (>15 cm diameter at breast height (dbh)), small trees (1.5–15.0 cm dbh), and the shrub/sapling layer (all shrub species and trees species <1.5 cm dbh). All woody plant species were sampled. The dbh for all live and standing dead stems was recorded for the overstory and small-tree classes. In the shrub/sapling layer, height and crown diameter were measured for each individual shrub or sapling. Crown diameter was measured along one axis per individual plant, selected to represent the average crown diameter. The live aboveground biomass of trees and shrubs was estimated using genus- or species-specific allometries when possible (all large trees, 67% of small tree stems, 94% of all shrubs). For the less-common species, for which species-specific equations were not available, a common equation was applied for species or groups of species with similar growth form (for details on the allometric calculations see [11]).
To measure foliar C and N concentrations, live foliage was sampled for 3–5 individuals per plot including the dominant conifer species, the dominant broadleaf tree species, the dominant shrub species, and two additional species representing the next most abundant woody species in the plot. For each broadleaf tree and shrub species, 2–4 leaves (without woody material) were collected. For coniferous species, we collected 2–4 shoots (including the needles for the current year’s growth) per individual plant. For all species, we only collected sun-exposed foliage. The sampling yielded foliar nutrient concentration data for both Ceanothus and Douglas-fir in only 17 of our 57 plots, limiting our ability to rigorously compare how leaf stoichiometry of our study species varied with climate.
Three samples of mineral soil were taken in each plot to a depth of 10 cm. We acknowledge that plants in arid sites may root deeper than our sampling depth, however, we focused on upper mineral soil because wildfire effects are typically greatest in shallow depths [41], and surface litter and organic horizons were extremely rare in these intensely burned sites. This focus on shallow soil precludes development of detailed biogeochemical budgets, but allowed us to distribute sampling efforts toward extensive coverage of plots that varied widely in aridity and time since fire. All plant and soil samples were air-dried in paper bags before being shipped to the University of Virginia for chemical analyses. Plant and soil samples were then oven-dried, ground, and analyzed for C and N using a Carlo Erba Elemental Analyzer (CE Instruments, Ltd., Wigam, UK).
2.3. Analyses
We used a series of linear bivariate regressions (“lm” function, [42]) to examine the effects of time since fire and climatic water deficit individually on each of the following response variables: Ceanothus biomass, Douglas-fir biomass, and soil C, N, and C:N. We also used multiple regressions to address how time since fire and climatic water deficit influence Ceanothus and Douglas-fir biomass. Of the 57 plots, Ceanothus spp. were present in 53, Douglas-fir was present in 44, and both species were present in 40 plots. Therefore, regression models included different numbers of plots depending on the response and explanatory variables used. Additionally, we removed one plot from our Douglas-fir biomass and one plot from our Ceanothus biomass analyses due to biomass values greater than two standard deviations from the mean. Douglas-fir was chosen as the main conifer species of the analyses as it was the most abundant tree species in our study sites, covering 94.54% of the regenerating conifer biomass.
To assess Ceanothus effects on soil N, we used soil N as the response variable in a linear regression with the explanatory variable of Ceanothus biomass. Since both soil C:N ratio and Ceanothus biomass were significantly related to climatic water deficit, we also conducted a multiple regression with the independent variables being Ceanothus biomass, climatic water deficit, and their interaction. We recognize the need for a more fine-grained approach in understanding the true effect of the difference in various Ceanothus growth forms. As we lose statistical power when Ceanothus is separated into individual species, we focused our analyses at the genus level.
To assess interactions between Ceanothus and Douglas-fir, we evaluated Douglas-fir biomass with the explanatory variable of Ceanothus biomass with linear and asymptotic regressions (“SSasymp’’ function, [42]). In addition to direct interactions with Ceanothus, factors such as seed availability and dispersal, damage from deer browsing, and competition from other species such as hardwoods might also influence the natural regeneration of Douglas-fir, but we did not have data to test such effects [21]. Since Douglas-fir biomass and Ceanothus biomass were both significantly related to climatic water deficit, we also conducted a multiple regression with Ceanothus biomass, climatic water deficit, and their interaction as the independent variables. Model selection was assessed with an Akaike Information Criterion (AIC) score for each relationship and the goodness of fit of the models was evaluated based on the R2 values.
3. Results
3.1. Ceanothus and Douglas-Fir Biomass
Ceanothus biomass per plot was high within the first 10–15 years after fire, but it significantly decreased with time since fire (Figure 3a and Figure S3; p = 0.01, R2 = 0.12), and significantly increased with climatic water deficit (Table S2; Figure 3b; p = 0.04, R2 = 0.08). Douglas-fir biomass was highly variable among plots including near-zero values in at least one plot sampled at each time since fire, which resulted in the lack of a significant linear relationship with time since fire (p = 0.69). However, Douglas-fir biomass was negatively related to climatic water deficit (Figure 3c,d; p = 0.02, R2 = 0.13). Multiple regression with both time since fire and climatic water deficit as independent variables also supported these relationships with Ceanothus biomass (p = 0.01 and p = 0.04, R2 = 0.19). The multiple regression with Douglas-fir biomass as the response variable preserved the negative association with climatic water deficit (p = 0.02, R2 = 0.14).
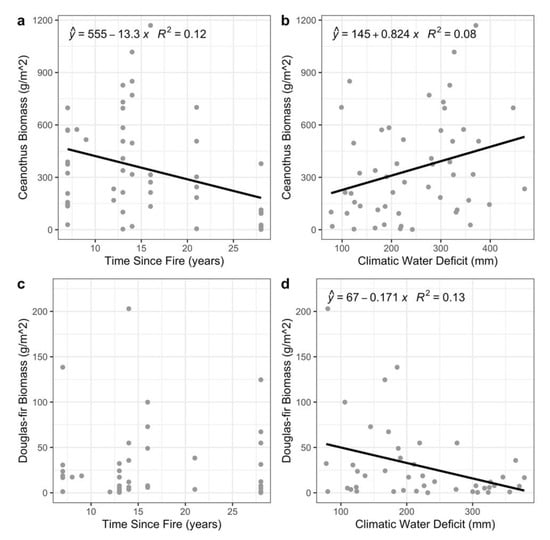
Figure 3.
Linear regressions of Ceanothus spp. and Douglas-fir biomass with time since fire (a,c), and climatic water deficit (b,d). Black regression lines demonstrate significant relationships (p < 0.05).
3.2. Soil Resources (C and N)
There were no significant relationships between soil biogeochemical variables (soil C, N, and C:N) and time since fire (Figure 4a–c). Soil N values did not vary significantly with climatic water deficit (Figure 4e). However, soil C and C:N ratio both decreased significantly with climatic water deficit (Figure 4d,f; p = 0.02 and 0.002).
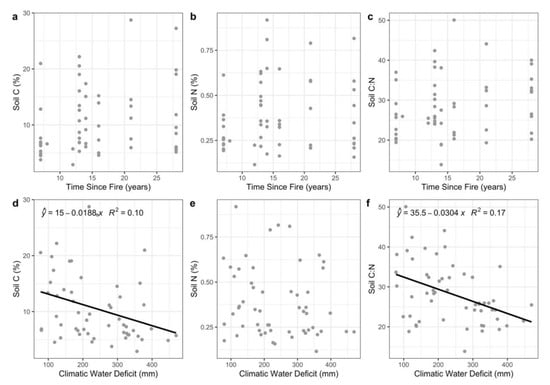
Figure 4.
Relationships of soil C, N, and C:N with time since fire (a-c) and climatic water deficit (d-f). Lines in (d) and (f) denote significant linear regressions (p < 0.05).
3.3. Ceanothus Effects on Soil Nitrogen Availability and Concomitant Effects on Douglas-Fir
Four Ceanothus (C.) species (C. cordulatus, C. integerrimus, C. sanguineus, and C. velutinus) had high average foliar nitrogen concentrations (2.39%), which were outside the 95% CI of the average of all other species present (1.28%, p < 0.001) (Figure 5). The relationship between Ceanothus biomass and soil N was not significant (Figure 6a). There was, however, a significant negative relationship between Ceanothus biomass and soil C:N (Figure 6b; p < 0.001, R2 = 0.26), and the relationship remained significant after an interaction between Ceanothus and climatic water deficit was considered (p = 0.002, R2 = 0.43). The model including the interaction between Ceanothus and climatic water deficit was more parsimonious than the model of Ceanothus as the sole driver of soil C:N (ΔAIC = 9.18), and the model of climatic water deficit as the sole driver of soil C:N (ΔAIC = 11.63).
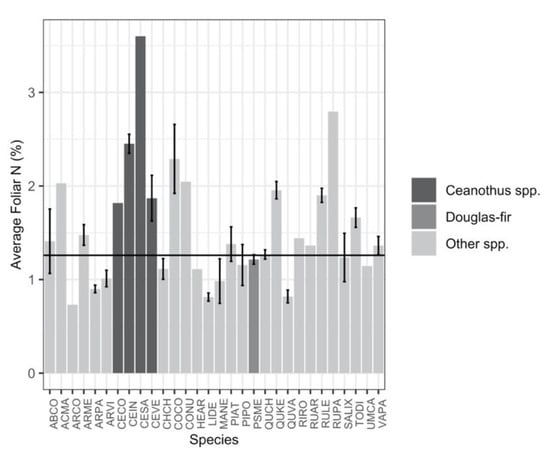
Figure 5.
Plot-level average foliar nitrogen concentrations (%) for all species, with Ceanothus spp. and Douglas-fir marked with different colors. The black line shows the average of all species other than Ceanothus spp. Error bars represent standard errors of the means. Species that do not have error bars indicate sampling from a single plot (n = 1). Species abbreviations and corresponding Latin names used on the x-axis can be found in Table S1.
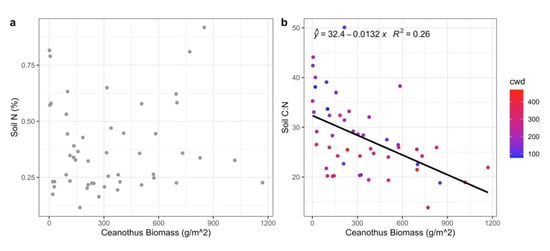
Figure 6.
Linear regression of (a) Soil N (%) with Ceanothus spp. biomass, (b) soil C:N and Ceanothus spp. biomass with climatic water deficit (cwd). Line in (b) denotes a significant regression (p < 0.001), and the relationship remains significant when an interaction with climatic water deficit is considered (p = 0.002, R2 = 0.43, not shown).
3.4. Interactions between Ceanothus and Douglas-Fir
Douglas-fir biomass was asymptotically regressed against Ceanothus biomass. Douglas-fir biomass decreased significantly with greater Ceanothus biomass, reaching an asymptote of 11.56 g/m2 (Figure 7a; p < 0.001, R2 = 0.27). The tendency for Douglas-fir biomass to decrease with increasing Ceanothus biomass remained significant even after considering the underlying relationship of Ceanothus biomass (i.e., increasing) with climatic water deficit (Figure 7; βCeanothus biomass = −0.17, p = 0.01, R2 = 0.31).
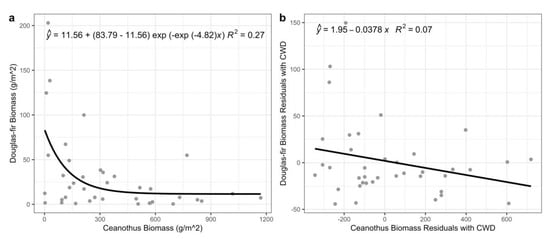
Figure 7.
Asymptotic decay regression for Douglas-fir biomass against Ceanothus biomass (a). Linear regression between Douglas-fir biomass and Ceanothus biomass residuals with climatic water deficit (b).
4. Discussion
Across the Klamath Mountains, we found that aridity (measured as climatic water deficit) was a key factor structuring plant competition and soil biogeochemistry over the first 28 years following high-severity wildfire. More arid sites displayed greater biomass of N-fixing Ceanothus shrubs, lower biomass of Douglas-fir trees, and lower C:N in surface mineral soil. Increasing Ceanothus biomass at more arid sites was associated with an asymptotic decline in Douglas-fir biomass, which reached low yet stable levels when Ceanothus biomass was ca. >550 g/m2. Thus, it seems that rather than completely excluding Douglas-fir from a site, high Ceanothus biomass and cover would more likely reduce Douglas-fir height and diameter growth in the initial decades after fire [43]. With time, the turnover of Ceanothus biomass with high foliar N will eventually return N to the soil to support growth of Douglas-fir, with N supply (mineralization) aided by the lower soil C:N in areas where Ceanothus was abundant [44]. Overall, the proportion of variance explained by our regressions was relatively low (R2 < 0.45), which reflects both the enormous physiographic variability of the region and our sampling scheme, in which plots span nearly the full range climatic water deficit of values that support forest cover in the region. Compared to previous work on facilitative and competitive interactions in similar tree-shrub systems [20,33,35,43], our sampling across a range of physiography and landscape history necessarily led to more variable results than might be expected when focusing on adjacent paired plots that differ only in time since fire. Although there was less control of individual environmental factors than a simpler experiment, this field study captured greater representation of the diversity of landscape conditions across the Klamath region. Understanding this underlying heterogeneity will be essential in generating expectations for post-fire management planning.
4.1. Post-Fire Succession
We did not detect the expected trend of increasing Douglas-fir biomass with time since fire [11,45]. Note that our evaluation of Douglas-fir biomass per plot was influenced by both the density of Douglas-fir juveniles and their growth rates, each of which may be driven by a different suite of factors. Knobcone pine (Pinus attenuata) and white fir (Abies concolor) were sometimes abundant, and ponderosa pine (Pinus ponderosa) was occasionally present in some of the plots, and total conifer biomass increased nonlinearly with time since fire [11]. The lack of statistical significance of Douglas-fir biomass along the gradient of time since fire might also reflect the relatively short time extent of the chronosequence (maximum of 28 years) [46]. A longer time interval may be needed for significant trends to become apparent (e.g., as more of the suppressed individuals emerge above the competing vegetation and their growth rates increase).
Ceanothus biomass was also greatest in the plots sampled at the shortest interval since fire (seven years), and its biomass decreased with time since fire but increased with increasing aridity. In contrast, Douglas-fir was more abundant in more mesic plots (i.e., lower climatic water deficit) (Figure 3d). These species-level responses to site aridity followed our expectations and are supported by the findings of previous literature [11,47,48]. Hotter and drier climatic conditions will likely continue to favor the growth of more drought-tolerant shrub species such as Ceanothus, while limiting the growth of conifers such as Douglas-fir [5,30,49,50,51,52].
4.2. Soil Biogeochemistry along Time since Fire and Climatic Water Deficit Gradients
We expected soil C and N to increase with time since fire [27,28,53,54] due to increased C inputs from plant production, and redistribution of deep soil N or an input from potential fixed N by Ceanothus to surface soil. Lack of observed change in soil C and N (Figure 4) may reflect that most C and N fixed after fire remain in accreting biomass in recovering forests [55], or that effects of individual wildfires on soil C and N may be small [56] and confounded with changes in soil bulk density [57]. Although we limited our sampling to sites that burned at high severity 7–28 years before sampling with no record of previous fire for at least 30 years prior to the most recent fire, some of the soil properties may still have been undergoing gradual responses to past fire or other disturbances. Our sampling across a broad range of sites also encompassed wide landscape variation in topography, parent material, soil profile development, pre-fire forest composition, and structural properties, and past fire history, all of which can influence soil C and N in these forests [58,59,60,61,62]. The ability to detect temporal changes in soil C and N would also be improved by pre-fire soil data, though such data are rare, and are usually limited to few sites.
We did observe patterns in soil biogeochemistry that were related to climatic water deficit. This suggests that broader climatic factors play an important role in biogeochemistry across these sites. Our results support the previously found pattern of low soil organic C at arid sites [63]. Low soil C:N at arid sites is also broadly consistent with the global trend [64] and may be reinforced in our study by inputs of low C:N Ceanothus tissue to surface soil (detailed in Section 4.3). Climate effects on soil biogeochemistry may also operate through more frequent fires as a “press” disturbance (referring to repeated burning) at more arid sites, causing small yet cumulative changes in C and N inputs to soil over longer time scales [41,56,65]. Such long-term effects observable across climatic gradients at the landscape scale may be difficult to detect in space-for-time studies of individual chronosequences and in heterogeneous landscapes such as the Klamath.
4.3. Ceanothus, Climatic Water Deficit, and Implications for the Cycling of Nitrogen in the Klamath
Greater climatic aridity in post-fire vegetation of the Klamath Mountains led to greater Ceanothus biomass (Figure 3b). This may not result in greater stand-level N-fixation, however, due to declines in individual plant N-fixation in arid sites with high Ceanothus biomass [30]. Regardless of the source of N in Ceanothus, the combination of high biomass and N-rich tissues can cause greater N cycling through Ceanothus at arid sites (Figure 3; Figure 5).This greater recycling of N through Ceanothus may be especially important relative to N demands by Douglas-fir, which are lower at arid sites due to slow growth and generally low conifer density.
To disentangle the effects of climatic aridity and Ceanothus on soil biogeochemistry, we used a multiple regression including the effect of climatic water deficit. The results confirm that high climatic aridity combined with abundant Ceanothus biomass reduced soil C:N ratio more than expected by the effects of climatic water deficit alone (Figure 6b) [44]. Low soil C:N values generally increase N mineralization and availability in soil [66] including across age-classes of nearby forests of the western Oregon Cascades where Ceanothus can also be among the dominant shrubs following wildfire [58]. Furthermore, Ceanothus is likely contributing fixed N at arid sites, suggested by lower soil C:N. Due to extremely rocky soils at our sites and the absence of pre-fire data, we could not accurately quantify N budgets of how fire and N-fixation may have influenced total soil C and N stocks with depth [29,44], nor how soil N stocks may have influenced early-successional community dynamics [67]. Even without detailed N budgets, our finding that soil N concentrations and Ceanothus biomass were unrelated is qualitatively consistent with prior evidence from these forests that Ceanothus’ N-fixation may not fully replace N losses in high-severity stand-replacing fires that occur within this region of historically mixed-severity fire regimes [30].
4.4. Interactions between Ceanothus and Douglas-Fir
In this study, we have shown that Ceanothus and Douglas-fir are in early-successional competition. Our finding that Douglas-fir biomass decreased toward an asymptote of ~12 g/m2 with increasing Ceanothus biomass suggests that high coverage of Ceanothus is unlikely to completely prevent Douglas-fir seedlings from becoming established, but it may substantially reduce the growth and/or survival of those seedlings that do establish (Figure 7a). Site aridity can partially explain these relationships, given the significant decrease in Douglas-fir biomass (Figure 3d) and increase in Ceanothus biomass (Figure 3b) with climatic water deficit. The negative relationship between Douglas-fir biomass and Ceanothus biomass still holds, even when accounting for the effects of aridity (p = 0.01), indicating that high abundance of Ceanothus negatively effects Douglas-fir (e.g., competition for light, moisture, or both. Douglas-fir and other conifers in these forests eventually grow taller than Ceanothus and outcompete it for light, though competition can remain intense between Douglas-fir and taller broadleaf tree species [20].
Integrating the effect of climatic aridity on the N cycle with our understanding of interspecific interactions between Ceanothus and Douglas-fir can shed light on forest recovery in these ecosystems in a warmer, drier future. Future increases in aridity and wildfire in this region are likely to increase N cycling through Ceanothus biomass and lower long-term soil C:N. These processes will increase soil N availability in arid sites more than expected in the absence of N-fixing Ceanothus, which is needed to sustain long-term forest recovery in these harsh environments. Longer term studies in other arid conifer forests show that tree growth can recover from early-successional competition when N-fixing shrubs eventually senesce and enhance soil fertility [20,35,68]. Additionally, the competitive interaction between Ceanothus and Douglas-fir in early succession can transition to facilitation through cooler air and surface temperatures, increased surface soil moisture, and protection against excessive radiation [31,33,43].
5. Conclusions
We combined observations from the growth and biomass development of Douglas-fir and Ceanothus following high-severity fire with data on foliage and soil C and N dynamics and compared these across a broad range of climatic aridity. Building on relevant work on facilitation and competition in tree-shrub systems [20,33,35,43], we gained new insights into the interactions between these species and their potential influence on post-fire vegetation development in a warming climate. Ceanothus initially flourished, then rapidly declined in biomass within two decades as it became overtopped by taller Douglas-fir and other conifer or broadleaf tree species. Despite strong competitive dominance of Ceanothus in the initial decade after fire, we anticipate that Ceanothus may also provide a facilitative effect via recycling of N from litterfall and ultimately upon senescence, with previously fixed N becoming available to growing Douglas-fir. Furthermore, the strong dominance of Ceanothus on drier sites implies that projected increases in aridity due to climate change will likely favor greater future abundance of Ceanothus during early-successional stages following high-severity fire in the Klamath region. The degree to which increased abundance of Ceanothus following fire in a warmer, drier environment limits initial post-fire establishment by Douglas-fir via resource competition or enhances the survival of those seedlings that do become established through increased N-fixation and microclimatic amelioration remains unknown. By solidifying our understanding of how disturbance–recovery dynamics are strongly shaped by climate change-induced conditions, this study implies a need to refine existing hypotheses to account for more than simplistic competitive interactions.
Our findings have implications for post-fire management, where facilitating the recovery of conifer forests following severe fire is a high priority. The relatively high Douglas-fir biomass in plots with low climatic water deficit suggests a high payoff for directing efforts toward planting conifer seedlings in moist sites that have a low density of naturally-established seedlings due to a lack of seed sources. In contrast, planting seedlings in dry sites may be less successful, as illustrated by our findings that Douglas-fir biomass tended to decrease with climatic aridity alone and with Ceanothus biomass, which tended to be greater at drier sites. Limited conifer recruitment and growth at arid sites may not lead to a high N demand, but the building of soil organic matter by rapidly growing Ceanothus may ultimately benefit soil fertility and moisture retention at arid sites [69]. Additional efforts such as reducing shrub cover may be needed to enhance long-term growth and survival of conifer seedlings planted at drier sites, while recognizing that reducing shrub cover still does not ensure conifer seedling survival. Understanding post-disturbance early-successional spatio-temporal interactions among species can provide crucial knowledge of the structural and functional responses of plant communities to climate change.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/f12111567/s1, Figure S1: Climatic water deficit and time since fire, Figure S2: Elevation and latitude, Figure S3: Foliar biomass regressions, Table S1: Species’ Latin names, and Table S2: Regression models.
Author Contributions
Conceptualization, D.C. and H.E.E.; Methodology, D.C. and H.E.E.; Software, D.C.; Validation, H.E.E.; Formal analysis, D.C. and H.E.E.; Investigation, D.C. and H.E.E.; Resources, D.C., H.E.E., A.J.T., K.J.A.-T. and J.R.T.; Data curation, H.E.E., A.J.T., K.J.A.-T. and J.R.T.; Writing—original draft preparation, D.C.; Writing—review and editing, D.C., H.E.E., A.J.T., K.J.A.-T., J.R.T. and S.S.P.; Visualization, D.C. and K.J.A.-T.; Supervision, H.E.E.; Project administration, H.E.E.; Funding acquisition, H.E.E., A.J.T., K.J.A.-T. and J.R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation grant #DEB-1353301.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Chris So, Matthew Davis, Eleanor Pearson, Lily Clarke, Charles Maxwell, Kim Hack, Dunbar Carpenter, Rob Pabst, Jeff Shatford, Tom Spies, Carl Skinner, Dan Blessing, Ken Wearstler, Todd Drake, and Patricia Hochhalter for their field assistance and feedback. We also thank Bella Reyes, Mary McCabe, Christopher Fernandez, Olivia Christopher, Kate Aldrich, Ruth Cumberland, Thomas Blair, Anna Rollosson, and Maria Wang for their help with sample preparation, Luca Morreale for assistance with climatic water deficit calculations, and Sarah K. Ortiz and Rebecca E. Carden for feedback on data visualization.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
References
- Anderson-Teixeira, K.J.; Miller, A.D.; Mohan, J.E.; Hudiburg, T.W.; Duval, B.D.; DeLucia, E.H. Altered dynamics of forest recovery under a changing climate. Glob. Chang. Biol. 2013, 19, 2001–2021. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368. [Google Scholar] [CrossRef]
- Anderson, K.J. Temporal patterns in rates of community change during succession. Am. Nat. 2007, 169, 780–793. [Google Scholar] [CrossRef]
- Westerling, A.L.; Turner, M.G.; Smithwick, E.A.; Romme, W.H.; Ryan, M.G. Continued warming could transform Greater Yellowstone fire regimes by mid-21st century. Proc. Natl. Acad. Sci. USA 2011, 208, 13165–13170. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.T.; Dobrowski, S.Z.; Higuera, P.E.; Holden, Z.A.; Veblen, T.T.; Rother, M.T.; Parks, S.A.; Sala, A.; Maneta, M.P. Wildfires and climate change push low-elevation forests across a critical climate threshold. Proc. Natl. Acad. Sci. USA 2019, 116, 6193–6198. [Google Scholar] [CrossRef] [Green Version]
- Stevens-Rumann, C.S.; Morgan, P. Tree regeneration following wildfires in the western U.S.: A review. Fire Ecol. 2019, 15, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kenny, S.A.; Bennett, A.F.; Clarke, M.F.; Morgan, J.W. Time-since-fire and climate interact to affect the structural recovery of an Australian semi-arid plant community. Austral Ecol. 2018, 43, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Tepley, A.J.; Thomann, E.; Veblen, T.T.; Perry, G.L.W.; Holz, A.; Partisis, J.; Kitzberger, T.; Anderson-Teixeira, K.J. Influences of fire-vegetation feedbacks and post-fire recovery rates on forest landscape vulnerability to altered fire regimes. J. Ecol. 2018, 106, 1925–1940. [Google Scholar] [CrossRef] [Green Version]
- Briles, C.; Whitlock, C.; Bartlein, P. Postglacial vegetation, fire, and climate history of the Siskiyou Mountains, Oregon, USA. Quat. Res. 2005, 64, 44–56. [Google Scholar] [CrossRef]
- Skinner, C.N.; Taylor, A.H.; Agee, J.K.; Briles, C.E.; Whitlock, C.L. Klamath Mountains Bioregion. In Fire in California’s Ecosystems; Van Wagtendonk, J.W., Sugihara, N.G., Stephens, S.L., Thode, A.E., Shaffer, K.E., Fites-Kaufman, J.A., Eds.; University of California Press: Oakland, CA, USA, 2006; pp. 173–196. [Google Scholar]
- Tepley, A.J.; Thompson, J.R.; Epstein, H.E.; Anderson-Teixeira, K.J. Vulnerability to forest loss through altered post-fire recovery dynamics in a warming climate in the Klamath Mountains. Glob. Chang. Biol. 2017, 23, 4117–4132. [Google Scholar] [CrossRef] [PubMed]
- Serra-Diaz, J.M.; Maxwell, C.; Lucash, M.S.; Scheller, R.M.; Laflower, D.M.; Miller, A.D.; Tepley, A.J.; Epstein, H.E.; Anderson-Teixeira, K.J.; Thompson, J.R. Disequilibrium of fire-prone forests sets the stage for a rapid decline in conifer dominance during the 21st century. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.R.; Spies, T.A.; Ganio, L.M. Reburn severity in managed and unmanaged vegetation in a large wildfire. Proc. Natl. Acad. Sci. USA 2007, 104, 10743–10748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wagtendonk, J.W.; Sugihara, N.G.; Stephens, S.L.; Thode, A.E.; Shaffer, K.E.; Fites-Kaufman, J.A.; Agee, J.K. Fire in California’s Ecosystems; University of California Press: Oakland, CA, USA, 2006. [Google Scholar]
- Odion, D.C.; Moritz, M.A.; DellaSala, D.A. Alternative community states maintained by fire in the Klamath Mountains, USA. J. Ecol. 2009, 98, 96–105. [Google Scholar] [CrossRef]
- Miller, A.D.; Thompson, J.R.; Tepley, A.J.; Anderson-Teixeira, K.J. Alternative stable equilibria and critical thresholds created by fire regimes and plant responses in a fire-prone community. Ecography 2018, 42, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Parra, A.; Moreno, J.M. Drought differentially affects the post-fire dynamics of seeders and resprouters in a Mediterranean shrubland. Sci. Total Environ. 2018, 626, 1219–1229. [Google Scholar] [CrossRef]
- Knapp, E.E.; Weatherspoon, C.P.; Skinner, C.N. Shrub seed banks in mixed conifer forests of Northern California and the role of fire in regulating abundance. Fire Ecol. 2012, 8, 32–48. [Google Scholar] [CrossRef]
- Peterson, C.E.; Hazard, J.W. Regional variation in growth response of coastal Douglas-fir to nitrogen fertilizer in the Pacific Northwest. For. Sci. 1990, 36, 625–640. [Google Scholar]
- Oakley, B.B.; North, M.P.; Franklin, J.F. Facilitative and competitive effects of N-fixing shrub on white fir saplings. For. Ecol. Manag. 2006, 223, 100–107. [Google Scholar] [CrossRef]
- Strothmann, R.O.; Roy, D.F. Regeneration of Douglas-Fir in the Klamath Mountain Region, California and Oregon; U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1984.
- Bormann, B.T.; Homann, P.S.; Darbyshire, R.L.; Morisette, B.A. Intense forest wildfire sharply reduces mineral soil C and N: The first direct evidence. Can. J. For. Res. 2008, 38, 2771–2783. [Google Scholar] [CrossRef]
- Oakley, B.B.; North, M.P.; Franklin, J.F. The effects of fire on soil nitrogen associated with patches of the actinorhizal shrub Ceanothus cordulatus. Plant Soil 2003, 254, 35–46. [Google Scholar] [CrossRef]
- Oakley, B.B.; North, M.P.; Franklin, J.F.; Hedlund, B.P.; Staley, J.T. Diversity and Distribution of Frankia Strains Symbiotic with Ceanothus in California. Appl. Environ. Microbiol. 2004, 70, 6444–6452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavitovski, J.; Newton, M. Ecological importance of snowbrush Ceanothus velutinus in the Oregon Cascades. Ecology 1968, 49, 1113–1145. [Google Scholar]
- Youngberg, C.T.; Wollum, A.G. Nitrogen accretion in developing Ceanothus velutinus stands. Soil Sci. Soc. Am. J. 1976, 40, 109–112. [Google Scholar] [CrossRef]
- Binkley, D.; Cromack, K.; Fredriksen, R.L. Nitrogen accretion and availability in some snowbrush ecosystems. For. Sci. 1982, 28, 720–724. [Google Scholar]
- Johnson, D.W. Soil properties beneath Ceanothus and Pine Stands in the eastern Sierra Nevada. Soil Sci. Soc. Am. J. 1995, 59, 918–924. [Google Scholar] [CrossRef]
- Johnson, D.W.; Murphy, J.F.; Susfalk, R.B.; Caldwell, T.G.; Miller, W.W.; Walker, R.F.; Powers, R.F. The effects of wildfire, salvage logging, and post-fire N-fixation on the nutrient budgets of a Sierran forest. For. Ecol. Manag. 2005, 220, 155–165. [Google Scholar] [CrossRef]
- Yelenik, S.; Perakis, S.; Hibbs, D. Regional constraints to biological nitrogen fixation in post-fire forest communities. Ecology 2013, 9, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Irvine, D.R.; Hibbs, D.E.; Shatford, J.P.A. The relative importance of biotic and abiotic controls on young conifer growth after fire in the Klamath-Siskiyou Region. Northwest Sci. 2009, 83, 334–347. [Google Scholar] [CrossRef]
- Rodriguez-Barrueco, C.; Mackintosh, A.H.; Bond, G. Some effects of combined nitrogen on the nodule symbioses of Casuarina and Ceanothus. Plant Soil 1970, 33, 129–139. [Google Scholar] [CrossRef]
- Zavitovski, J.; Newton, M.; El-Hassan, B. Effects of snowbrush on growth of some conifers. J. For. 1969, 67, 242–246. [Google Scholar]
- Lopez-Ortiz, M.J.; Marcey, T.; Lucash, M.S.; Hibbs, D.; Shatford, P.A.; Thompson, J.R. Post-fire management affects species composition but not Douglas- fir regeneration in the Klamath Mountains. For. Ecol. Manag. 2019, 432, 1030–1040. [Google Scholar] [CrossRef]
- Zhang, J.; Oliver, W.W.; Busse, M.D. Growth and development of ponderosa pine on sites of contrasting productivities: Relative importance of stand density and shrub competition effects. Can. J. For. Res. 2006, 36, 2426–2438. [Google Scholar] [CrossRef]
- Hijmans, R.J.; van Etten, J. Raster: Geographic Data Analysis and Modeling, R Package Version 3.3-13. 2014.
- Lutz, J.A.; Van Wagtendonk, J.W.; Franklin, J.F. Climatic water deficit, tree species ranges, and climate change in Yosemite National Park. J. Biogeogr. 2010, 37, 936–950. [Google Scholar] [CrossRef]
- PRISM Climate Group, Oregon State University. Available online: http://prism.oregonstate.edu (accessed on 18 March 2015).
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Soil Survey Geographic (SSURGO). Available online: https://data.nal.usda.gov/dataset/soil-survey-geographic-database-ssurgo (accessed on 25 May 2015).
- Miller, J.D.; Knapp, E.E.; Key, C.H.; Skinner, C.N.; Isbell, C.J.; Creasy, R.M.; Sherlock, J.W. Calibration and validation of the relative differenced Normalized Burn Ratio (RdNBR) to three measures of fire severity in the Sierra Nevda and Klamath Mountains, CA, USA. Remote Sens. Environ. 2009, 113, 645–656. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2006. [Google Scholar]
- Erickson, H.E.; Harrington, C.A. Conifer-Ceanothus interactions influence tree growth before and after shrub removal in a forest plantation in the western Cascade Mountains, USA. For. Ecol. Manag. 2006, 229, 183–194. [Google Scholar] [CrossRef]
- Johnson, D.W.; Walker, R.F.; McNulty, M.; Rau, B.M.; Miller, W.W. The long-term effects of wildfire and post-fire vegetation on Sierra Nevada forest soils. Forests 2012, 3, 398–416. [Google Scholar] [CrossRef] [Green Version]
- Swanson, M.E.; Franklin, J.F.; Beschta, R.L.; Crisafulli, C.M.; DellaSala, D.A.; Hutto, R.L.; Lindenmaver, D.B.; Swanson, F.J. The forgotten stage of forest succession: Early-successional ecosystems on forest sites. Front. Ecol. Environ. 2011, 9, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Walker, L.R.; Wardle, D.A.; Bardgett, R.D.; Clarkson, B.D. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 2010, 98, 725–736. [Google Scholar] [CrossRef]
- Conard, S.G.; Jaramillo, A.E.; Cormack, K., Jr.; Rose, S. The Role of the Genus Ceanothus in Western Forest Ecosystems; U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1985.
- Montwé, D.; Spiecker, H.; Hamann, A. Five decades of growth in a genetic field trial of Douglas-fir reveal trade-offs between productivity and drought-tolerance. Tree Genet. Genomes 2015, 11, 29. [Google Scholar] [CrossRef]
- Laflower, D.M.; Hurteau, M.D.; Koch, G.W.; North, M.P.; Hungate, B.A. Climate-driven changes in forest succession and the influence of management on forest carbon dynamics in the Puget Lowlands of Washington State, USA. For. Ecol. Manag. 2016, 362, 194–204. [Google Scholar] [CrossRef] [Green Version]
- McNabb, D.H.; Cromack, K., Jr. Dinitrogen N-fixation by a mature Ceanothus velutinus (Dougl.) stand in the western Oregon Cascades. Can. J. Microbiol. 1983, 29, 1014–1021. [Google Scholar] [CrossRef]
- Rother, M.T.; Veblen, T.T. Limited conifer regeneration following wildfires in dry ponderosa pine forests of the Colorado Front Range. Ecosphere 2016, 7, e01594. [Google Scholar] [CrossRef]
- Adams, M.A.; Turnbull, T.L.; Sprent, J.I.; Buchmann, N. Legumes are different: Leaf nitrogen, photosynthesis, and water use efficiency. Proc. Natl. Acad. Sci. USA 2016, 113, 4098–4103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köster, K.; Köster, E.; Orumaa, A.; Parro, K.; Jōgiste, K.; Berninger, F.; Pumpanen, J.; Metslaid, M. How time since forest fire affects stand structure, soil physical-chemical properties and soil CO2 efflux in hemiboreal Scots Pine forest fire chronosequence? Forests 2016, 7, 201. [Google Scholar] [CrossRef] [Green Version]
- Marion, G.M.; Black, C.H. Potentially available nitrogen and phosphorus along a chaparral fire cycle chronosequence. Soil Sci. Soc. Am. J. 1988, 52, 1155–1162. [Google Scholar] [CrossRef]
- Page-Dumrose, D.; Jurgensen, M.F.; Harvey, A.E. Fire and fire-suppression impacts on forest-soil carbon. In The Potential of U.S. Forest Soils to Sequester Carbon and Mitigate the Greenhouse Effect; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Pellegrini, A.F.A.; Jackson, R.B. The long and short of it: A review of the timescales of how fire affects soils using the pulse-press framework. Adv. Ecol. Res. 2020, 62, 147–171. [Google Scholar]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Fire effects on temperate forest soil C and N storage. Ecol. Appl. 2011, 21, 1189–1201. [Google Scholar] [CrossRef] [Green Version]
- Perakis, S.S.; Tepley, A.J.; Compton, J.E. Disturbance and topography shape nitrogen availability and δ15 N over long-term forest succession. Ecosystems 2015, 18, 573–588. [Google Scholar] [CrossRef]
- Cross, A.; Perakis, S.S. Tree species and soil nutrient profiles in old-growth forests of the Oregon Coast Range. Can. J. For. Res. 2011, 41, 195–210. [Google Scholar] [CrossRef]
- Homann, P.S.; Bormann, B.T.; Darbyshire, R.L.; Morrissette, B.A. Forest soil carbon and nitrogen losses associated with wildfire and prescribed fire. Soil Sci. Soc. Am. J. 2011, 75, 1926–1934. [Google Scholar] [CrossRef]
- Borchers, J.G.; Perry, D.A. The influence of soil texture and aggregation on carbon and nitrogen dynamics in southwest Oregon forests and clearcuts. Can. J. For. Res. 1992, 22, 298–305. [Google Scholar] [CrossRef]
- Morford, S.L.; Houlton, B.Z.; Dahlgren, R.A. Geochemical and tectonic uplift controls on rock nitrogen inputs across terrestrial ecosystems. Glob. Biogeochem. Cycles 2016, 30, 333–349. [Google Scholar] [CrossRef] [Green Version]
- Post, W.M.; Emanuel, W.R.; Zinke, P.J.; Stangenberger, A.G. Soil carbon pools and world life zones. Nature 1982, 298, 156–159. [Google Scholar] [CrossRef]
- Post, W.M.; Pastor, J.; Zinke, P.J.; Stangenberger, A.G. Global patterns of soil nitrogen storage. Nature 1985, 317, 613–616. [Google Scholar] [CrossRef]
- McLauchlan, K.K.; Higuera, P.E.; Gavin, D.G.; Perakis, S.S.; Mack, M.C.; Alexander, H.; Battles, J.; Biondi, F.; Buma, B.; Colombaroli, D.; et al. Reconstructing disturbances and their biogeochemical consequences over multiple timescales. BioScience 2014, 64, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Waksman, S.A.; Tenney, F.G. The composition of natural organic materials and their decomposition in the soil. Soil Sci. 1927, 24, 257–384. [Google Scholar]
- Erickson, H.E.; Soto, P.; Johnson, D.W.; Roath, B.; Hunsaker, C. Effects of vegetation patches on soil nutrient pools and fluxes within a mixed-conifer forest. For. Sci. 2005, 51, 211–220. [Google Scholar]
- Busse, M.D.; Cochran, P.H.; Barrett, J.W. Changes in ponderosa pine productivity following removal of understory vegetation. Soil Sci. Soc. Am. J. 1996, 60, 1614–1621. [Google Scholar] [CrossRef] [Green Version]
- Binkley, D. How nitrogen-fixing trees change soil carbon. In Tree Species Effects on Soils: Implications for Global Change; Springer: Dordrecht, The Netherlands, 2005; pp. 155–164. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).