Description of Intra-Annual Changes in Cambial Activity and Differentiation of Secondary Conductive Tissues of Aesculus hippocastanum Trees Affected by the Leaf Miner Cameraria ohridella
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Phenological Observation
2.3. Sampling Methods, Preparation of Slides and Microscopy
2.4. Cambium Activity and Formation of Secondary Conductive Tissues
2.5. Measurements and Statistical Analyses
3. Results
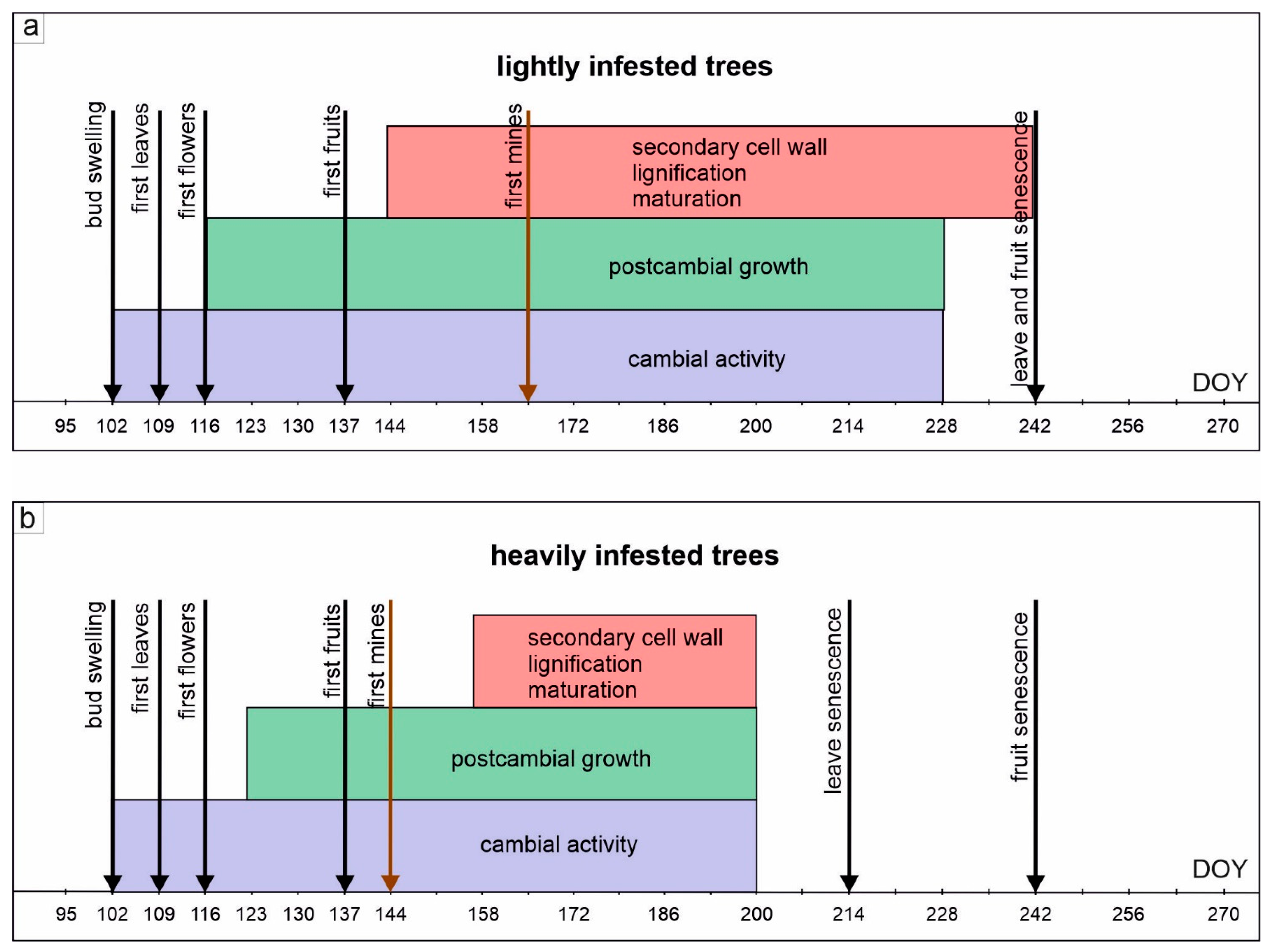
3.1. Phenological Changes and Horse Chestnut Miner Infestation
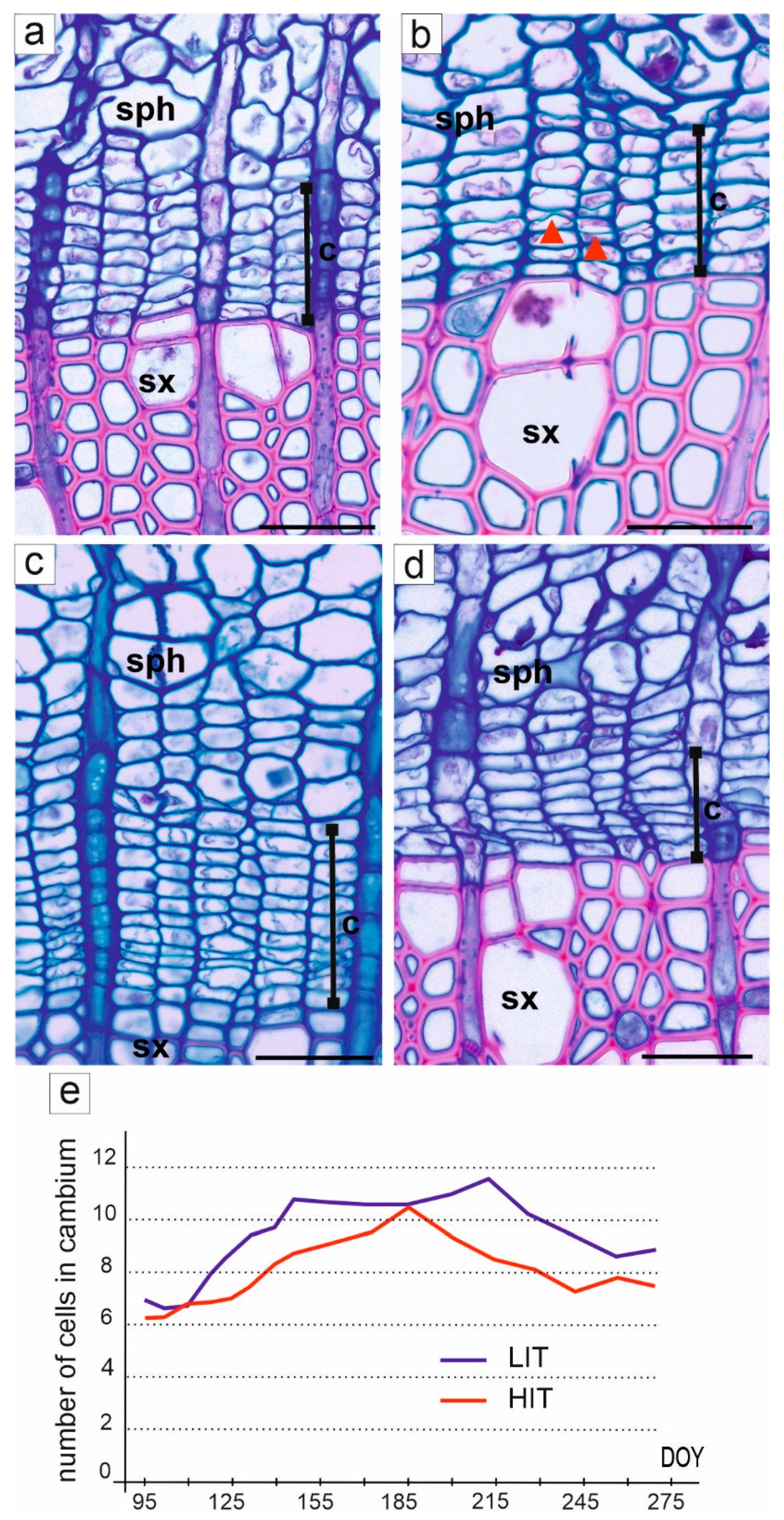
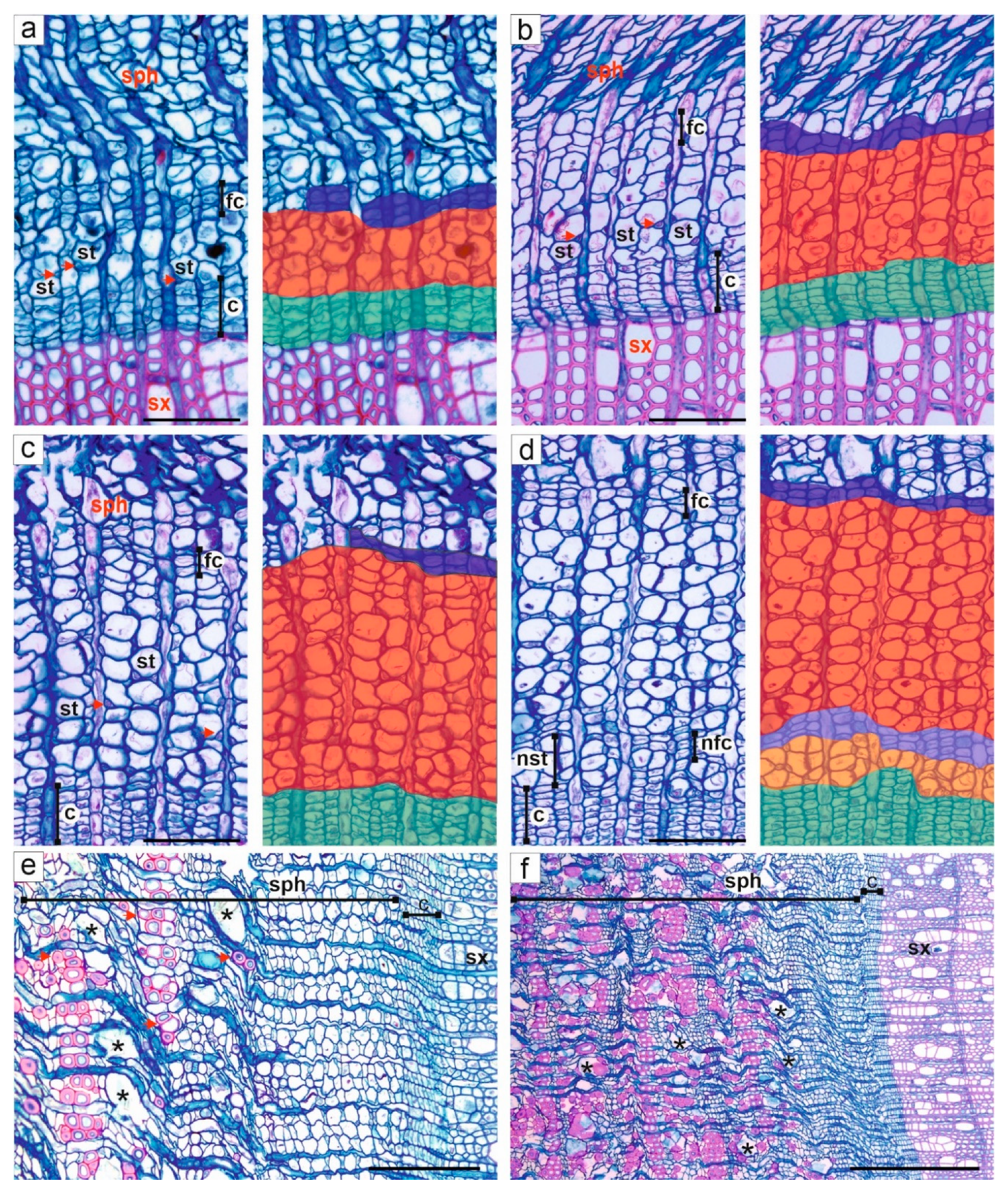
3.2. Activity of Cambium
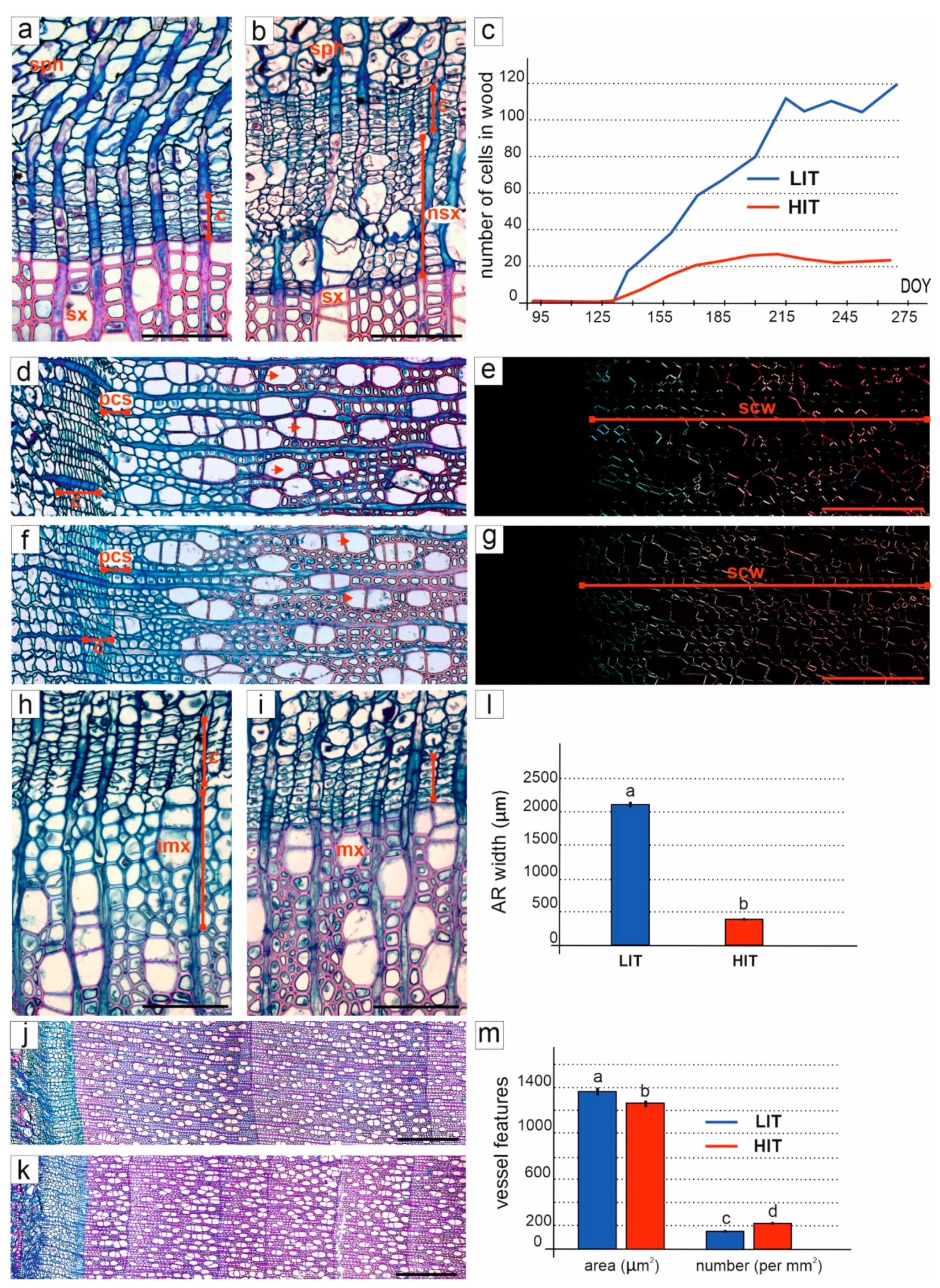
3.3. Formation and Structure of Secondary Xylem
3.4. Formation and Structure of Secondary Phloem
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lack, H.W. The discovery and rediscovery of the horse chestnut. Arnoldia 2002, 61, 15–19. [Google Scholar] [CrossRef]
- Deschka, G.; Dimić, N. Cameraria ohridella sp. n. (Lep., Lithocolletidae) from Macedonia, Yugoslavia. Acta Entomol. Jugosl. 1986, 22, 11–23. [Google Scholar]
- Šefrova, H.; Laštůvka, Z. Dispersal of the horse chestnut leaf miner, Cameraria ohridella Descha & Dimić, 1986, in Europe: Its course, ways and causes (lepidoptera: Gracillariidae). Entomol. Z. Stuttg. 2001, 111, 194–198. [Google Scholar]
- Straw, N.A.; Bellett-Travers, M. Impact and Management of the Horse Chestnut Leaf Miner (Cameraria ohridella). Arboric. J. 2004, 28, 67–83. [Google Scholar] [CrossRef]
- Thalmann, C.; Freise, J.; Heitland, W.; Bacher, S. Effects of Defoliation by Horse Chestnut Leafminer (Cameraria ohridella) on Reproduction in Aesculus hippocastanum. Trees-Struct. Funct. 2003, 17, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Myśkow, E.; Sokołowska, K.; Słupianek, A.; Gil, R. Drukarnia Triada Co gryzie kasztanowiec? Wpływ Szrotówka Kasztanowcowiaczka na Fenologię i Rozwój Drzew. What’s Eating Horse Chestnut? The Influence of Horse—Chestnut Leaf Miner on Tree Phenology and Development; Wydawnictwo i Drukarnia Triada: Wrocław, Poland, 2017; ISBN 978-83-61777-72-4. [Google Scholar]
- Raimondo, F.; Ghirardelli, L.A.; Nardini, A.; Salleo, S. Impact of the Leaf Miner Cameraria ohridella on Photosynthesis, Water Relations and Hydraulics of Aesculus hippocastanum Leaves. Trees 2003, 17, 376–382. [Google Scholar] [CrossRef]
- Percival, G.C.; Barrow, I.; Nosviss, K.; Keary, I.; Pennington, P. The Impact of Horse Chestnut Leaf Miner (Cameraria ohridella Deschka and Dimic; HCLM) on Vitality, Growth and Reproduction of Aesculus hippocastanum L. Urban For. Urban Green. 2011, 10, 11–17. [Google Scholar] [CrossRef]
- Takos, I.; Varsamis, G.; Avtzis, D.; Galatsidas, S.; Merou, T.; Avtzis, N. The Effect of Defoliation by Cameraria ohridella Deschka and Dimic (Lepidoptera: Gracillariidae) on Seed Germination and Seedling Vitality in Aesculus hippocastanum L. For. Ecol. Manag. 2008, 255, 830–835. [Google Scholar] [CrossRef]
- Larson, P.R. The Vascular Cambium; Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 1994; ISBN 978-3-642-78468-2. [Google Scholar]
- Evert, R.F.; Esau, K.; Esau, K. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2006; ISBN 978-0-471-73843-5. [Google Scholar]
- García-Gonzalez, I.; Eckstein, D. Climatic Signal of Earlywood Vessels of Oak on a Maritime Site. Tree Physiol. 2003, 23, 497–504. [Google Scholar] [CrossRef]
- Marion, L.; Gričar, J.; Oven, P. Wood Formation in Urban Norway Maple Trees Studied by the Micro-Coring Method. Dendrochronologia 2007, 25, 97–102. [Google Scholar] [CrossRef]
- Eilmann, B.; Buchmann, N.; Siegwolf, R.; Saurer, M.; Cherubini, P.; Rigling, A. Fast Response of Scots Pine to Improved Water Availability Reflected in Tree-Ring Width and Δ13C. Plant Cell Environ. 2010, 33, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Ursache, R.; Nieminen, K.; Helariutta, Y. Genetic and Hormonal Regulation of Cambial Development. Physiol Plant. 2013, 147, 36–45. [Google Scholar] [CrossRef]
- Eckert, C.; Sharmin, S.; Kogel, A.; Yu, D.; Kins, L.; Strijkstra, G.-J.; Polle, A. What Makes the Wood? Exploring the Molecular Mechanisms of Xylem Acclimation in Hardwoods to an Ever-Changing Environment. Forests 2019, 10, 358. [Google Scholar] [CrossRef] [Green Version]
- Čufar, K.; Prislan, P.; de Luis, M.; Gričar, J. Tree-Ring Variation, Wood Formation and Phenology of Beech (Fagus sylvatica) from a Representative Site in Slovenia, SE Central Europe. Trees 2008, 22, 749–758. [Google Scholar] [CrossRef]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrene, E.; Damesin, C. Comparing the Intra-Annual Wood Formation of Three European Species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as Related to Leaf Phenology and Non-Structural Carbohydrate Dynamics. Tree Physiol. 2012, 32, 1033–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitin, P.; Funada, R. Earlywood Vessels in Ring-Porous Trees Become Functional for Water Transport after Bud Burst and before the Maturation of the Current-Year Leaves. IAWA J. 2016, 37, 315–331. [Google Scholar] [CrossRef]
- Lavrič, M.; Eler, K.; Ferlan, M.; Vodnik, D.; Gričar, J. Chronological Sequence of Leaf Phenology, Xylem and Phloem Formation and Sap Flow of Quercus pubescens from Abandoned Karst Grasslands. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gričar, J.; Krže, L.; Čufar, K. Number of Cells in Xylem, Phloem and Dormant Cambium in Silver Fir (Abies alba), in Trees of Different Vitality. IAWA J. 2009, 30, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Gričar, J.; Čufar, K.; Eler, K.; Gryc, V.; Vavrčík, H.; de Luis, M.; Prislan, P. Transition Dates from Earlywood to Latewood and Early Phloem to Late Phloem in Norway Spruce. Forests 2021, 12, 331. [Google Scholar] [CrossRef]
- Gričar, J. Xylem and Phloem Formation in Sessile Oak from Slovenia in 2007. Wood Res. 2010, 55, 15–22. [Google Scholar]
- Prislan, P.; Gričar, J.; de Luis, M.; Smith, K.T.; Čufar, K. Phenological Variation in Xylem and Phloem Formation in Fagus sylvatica from Two Contrasting Sites. Agric. For. Meteorol. 2013, 180, 142–151. [Google Scholar] [CrossRef]
- Balzano, A.; Čufar, K.; de Micco, V. Xylem and Phloem Formation Dynamics in Quercus ilex L. at a Dry Site in Southern Italy. Forests 2021, 12, 188. [Google Scholar] [CrossRef]
- Chiang, M.-H.; Greb, T. How to Organize Bidirectional Tissue Production? Curr. Opin. Plant Biol. 2019, 51, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wodzicki, T.J. Natural Factors Affecting Wood Structure. Wood Sci. Technol. 2001, 35, 5–26. [Google Scholar] [CrossRef]
- Bräuning, A.; De Ridder, M.; Zafirov, N.; García-González, I.; Petrov Dimitrov, D.; Gärtner, H. Tree-Ring Features: Indicators of Extreme Event Impacts. IAWA J. 2016, 37, 206–231. [Google Scholar] [CrossRef] [Green Version]
- Begum, S.; Kudo, K.; Rahman, M.H.; Nakaba, S.; Yamagishi, Y.; Nabeshima, E.; Nugroho, W.D.; Oribe, Y.; Kitin, P.; Jin, H.-O.; et al. Climate Change and the Regulation of Wood Formation in Trees by Temperature. Trees 2018, 32, 3–15. [Google Scholar] [CrossRef]
- Castagneri, D.; Fonti, P.; von Arx, G.; Carrer, M. How Does Climate Influence Xylem Morphogenesis over the Growing Season? Insights from Long-Term Intra-Ring Anatomy in Picea Abies. Ann. Bot. 2017, mcw274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myśkow, E.; Błaś, M.; Sobik, M.; Godek, M.; Owczarek, P. The Effect of Pollutant Fog Deposition on the Wood Anatomy of Subalpine Norway Spruce. Eur. J. For. Res. 2019, 138, 187–201. [Google Scholar] [CrossRef] [Green Version]
- Büntgen, U.; Frank, D.; Liebhold, A.; Johnson, D.; Carrer, M.; Urbinati, C.; Grabner, M.; Nicolussi, K.; Levanic, T.; Esper, J. Three Centuries of Insect Outbreaks across the European Alps. New Phytol. 2009, 182, 929–941. [Google Scholar] [CrossRef]
- Axelson, J.N.; Bast, A.; Alfaro, R.; Smith, D.J.; Gärtner, H. Variation in Wood Anatomical Structure of Douglas-Fir Defoliated by the Western Spruce Budworm: A Case Study in the Coastal-Transitional Zone of British Columbia, Canada. Trees 2014, 28, 1837–1846. [Google Scholar] [CrossRef]
- Prendin, A.L.; Carrer, M.; Karami, M.; Hollesen, J.; Bjerregaard Pedersen, N.; Pividori, M.; Treier, U.A.; Westergaard-Nielsen, A.; Elberling, B.; Normand, S. Immediate and Carry-over Effects of Insect Outbreaks on Vegetation Growth in West Greenland Assessed from Cells to Satellite. J. Biogeogr 2020, 47, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Bednarz, B.; Scheffler, M. Wpływ żeru szrotówka kasztanowcowiaczka (Cameraria ohridella Deschka & Dimic) na szerokość przyrostów rocznych kasztanowca białego (Aesculus hippocastanum L.). Effect of horse chestnut leaf miner (Cameraria ohridella Deschka and Dimic) outbreak on tree-ring widths of white horse chestnut (Aesculus hippocastanum L.). Sylwan 2008, 7, 53–66. [Google Scholar]
- Jagiełło, R.; Baraniak, E.; Karolewski, P.; Łakomy, P.; Behnke-Borowczyk, J.; Walczak, U.; Giertych, M.J. Ecophysiological Aspects of the Interaction between Cameraria ohridella and Guignardia aesculi on Aesculus hippocastanum. Dendrobiology 2017, 78, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Jagiełło, R.; Walczak, U.; Iszkuło, G.; Karolewski, P.; Baraniak, E.; Giertych, M.J. Impact of Cameraria ohridella on Aesculus hippocastanum Growth and Long-Term Effects of Trunk Injection with Pesticides. Int. J. Pest. Manag. 2019, 65, 33–43. [Google Scholar] [CrossRef]
- IMGW (Institute of Meteorology and Water Management); National Research Institute. Available online: https://www.imgw.pl/index.php/en (accessed on 28 May 2021).
- Gilbert, M.; Svatoš, A.; Lehmann, M.; Bacher, S. Spatial Patterns and Infestation Processes in the Horse Chestnut Leafminer Cameraria ohridella: A Tale of Two Cities. Entomol. Exp. Appl. 2003, 107, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Nardini, A.; Raimondo, F.; Scimone, M.; Salleo, S. Impact of the Leaf Miner Cameraria ohridella on Whole-Plant Photosynthetic Productivity of Aesculus hippocastanum: Insights from a Model. Trees 2004, 18, 714–721. [Google Scholar] [CrossRef]
- Pavan, F.; Barro, P.; Bernardinelli, I.; Gambon, N.; Zandigiacomo, P. Cultural Control of Cameraria ohridella on Horse Chesnut in Urban Areas by Removing Fallen Leaves in Autumn. J. Arboric. 2003, 29, 253–258. [Google Scholar]
- Gričar, J.; Lavrič, M.; Ferlan, M.; Vodnik, D.; Eler, K. Intra-Annual Leaf Phenology, Radial Growth and Structure of Xylem and Phloem in Different Tree Parts of Quercus pubescens. Eur. J. For. Res. 2017, 136, 625–637. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Menardi, R. Trephor: A New Tool for Sampling Microcores from Tree Stems. IAWA J. 2006, 27, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Giagli, K.; Gričar, J.; Vavrčík, H.; Menšík, L.; Gryc, V. The Effects of Drought on Wood Formation in Fagus sylvatica during Two Contrasting Years. IAWA J. 2016, 37, 332–348. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant. Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999; ISBN 978-0-19-508956-1. [Google Scholar]
- Oladi, R.; Pourtahmasi, K.; Eckstein, D.; Bräuning, A. Seasonal Dynamics of Wood Formation in Oriental Beech (Fagus orientalis Lipsky) along an Altitudinal Gradient in the Hyrcanian Forest, Iran. Trees 2011, 25, 425–433. [Google Scholar] [CrossRef]
- Barnett, J.R. Reactivation of the Cambium in Aesculus hippocastanum L.: A Transmission Electron Microscope Study. Ann. Bot. 1992, 70, 169–177. [Google Scholar] [CrossRef]
- Ewers, F.W.; Fisher, J.B. Techniques for Measuring Vessel Lengths and Diameters in Stems of Woody Plants. Am. J. Bot. 1989, 76, 645–656. [Google Scholar] [CrossRef]
- Giordano, R.; Salleo, A.; Salleo, S.; Wanderlingh, F. Flow in Xylem Vessels and Poiseuille’s Law. Can. J. Bot. 1978, 56, 333–338. [Google Scholar] [CrossRef]
- Venturas, M.; López, R.; Gascó, A.; Gil, L. Hydraulic Properties of European Elms: Xylem Safety-Efficiency Tradeoff and Species Distribution in the Iberian Peninsula. Trees 2013, 27, 1691–1701. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1998. [Google Scholar]
- Frankenstein, C.; Eckstein, D.; Schmitt, U. The Onset of Cambium Activity—A Matter of Agreement? Dendrochronologia 2005, 23, 57–62. [Google Scholar] [CrossRef]
- Sundberg, B.; Uggla, C.; Tuominen, H. Cambial growth and auxin gradients. In Cell and Molecular Biology of Wood Formation; Savidge, R.A., Barnett, J.R., Napier, R., Eds.; Experimental biology reviews; BIOS: Oxford, UK, 2000; pp. 169–188. ISBN 978-1-85996-123-0.s. [Google Scholar]
- Fonti, P.; von Arx, G.; García-González, I.; Eilmann, B.; Sass-Klaassen, U.; Gärtner, H.; Eckstein, D. Studying Global Change through Investigation of the Plastic Responses of Xylem Anatomy in Tree Rings. New Phytol. 2010, 185, 42–53. [Google Scholar] [CrossRef]
- Aloni, R. Ecophysiological Implications of Vascular Differentiation and Plant Evolution. Trees 2015, 29, 1–16. [Google Scholar] [CrossRef]
- Schrader, J.; Baba, K.; May, S.T.; Palme, K.; Bennett, M.; Bhalerao, R.P.; Sandberg, G. Polar Auxin Transport in the Wood-Forming Tissues of Hybrid Aspen Is under Simultaneous Control of Developmental and Environmental Signals. Proc. Natl. Acad. Sci. USA 2003, 100, 10096–10101. [Google Scholar] [CrossRef] [Green Version]
- Asshoff, R.; Schweingruber, F.H.; Wermelinger, B. Influence of a Gypsy Moth (Lymantria dispar L.) Outbreak on Radial Growth and Wood Anatomy of Spanish Chestnut (Castanea sativa Mill.) in Ticino (Switzerland). Dendrochronologia 1999, 16–17, 133–145. [Google Scholar]
- Dobbertin, M. Tree Growth as Indicator of Tree Vitality and of Tree Reaction to Environmental Stress: A Review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- De Micco, V.; Zalloni, E.; Balzano, A.; Battipaglia, G. Fire Influence on Pinus halepensis: Wood Responses Close and Far from the Scars. IAWA J. 2013, 34, 446–458. [Google Scholar] [CrossRef] [Green Version]
- Fajstavr, M.; Giagli, K.; Vavrčík, H.; Gryc, V.; Urban, J. The Effect of Stem Girdling on Xylem and Phloem Formation in Scots Pine. Silva. Fenn. 2017, 51. [Google Scholar] [CrossRef] [Green Version]
- Salleo, S.; Nardini, A.; Raimondo, F.; Lo Gullo, M.A.; Pace, F.; Giacomich, P. Effects of Defoliation Caused by the Leaf Miner Cameraria ohridella on Wood Production and Efficiency in Aesculus hippocastanum Growing in North-Eastern Italy. Trees 2003, 17, 367–375. [Google Scholar] [CrossRef]
- Słupianek, A.; Wojtuń, B.; Myśkow, E. Origin, Activity and Environmental Acclimation of Stem Secondary Tissues of the Polar Willow (Salix polaris) in High-Arctic Spitsbergen. Polar Biol. 2019, 42, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Zaccaro, F.D.; Groover, A. Wood and Water: How Trees Modify Wood Development to Cope with Drought. Plants People Planet 2019, 1, 346–355. [Google Scholar] [CrossRef]
- Crawley, M.J. Insect Herbivores and Plant Population Dynamics. Annu. Rev. Entomol. 1989, 34, 531–564. [Google Scholar] [CrossRef]
- Kosola, K.R.; Dickmann, D.I.; Paul, E.A.; Parry, D. Repeated Insect Defoliation Effects on Growth, Nitrogen Acquisition, Carbohydrates, and Root Demography of Poplars. Oecologia 2001, 129, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wiley, E.; Huepenbecker, S.; Casper, B.B.; Helliker, B.R. The Effects of Defoliation on Carbon Allocation: Can Carbon Limitation Reduce Growth in Favour of Storage? Tree Physiol. 2013, 33, 1216–1228. [Google Scholar] [CrossRef] [Green Version]
- Jetton, R.M.; Robison, D.J. Effects of Artificial Defoliation on Growth and Biomass Accumulation in Short-Rotation Sweetgum (Liquidambar styraciflua) in North Carolina. J. Insect Sci. 2014, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rosas, T.; Galiano, L.; Ogaya, R.; Penuelas, J.; Martinez-Vilalta, J. Dynamics of Non-structural Carbohydrates in Three Mediterranean Woody Species Following Long-term Experimental Drought. Front. Plant Sci. 2013, 4, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, K.; Saiki, S.-T.; Yazaki, K.; Ogasa, M.Y.; Shirai, M.; Nakano, T.; Yoshimura, J.; Ishida, A. The Dynamics of Carbon Stored in Xylem Sapwood to Drought-induced Hydraulic Stress in Mature Trees. Sci. Rep. 2016, 4, 24513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, M.R. Phloem: Cell Types, Structure, and Commercial Uses. In Plant Science—Structure, Anatomy and Physiology in Plants Cultured In Vivo and In Vitro; Gonzalez, A., Rodriguez, M., Gören Sağlam, N., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78984-746-8. [Google Scholar]
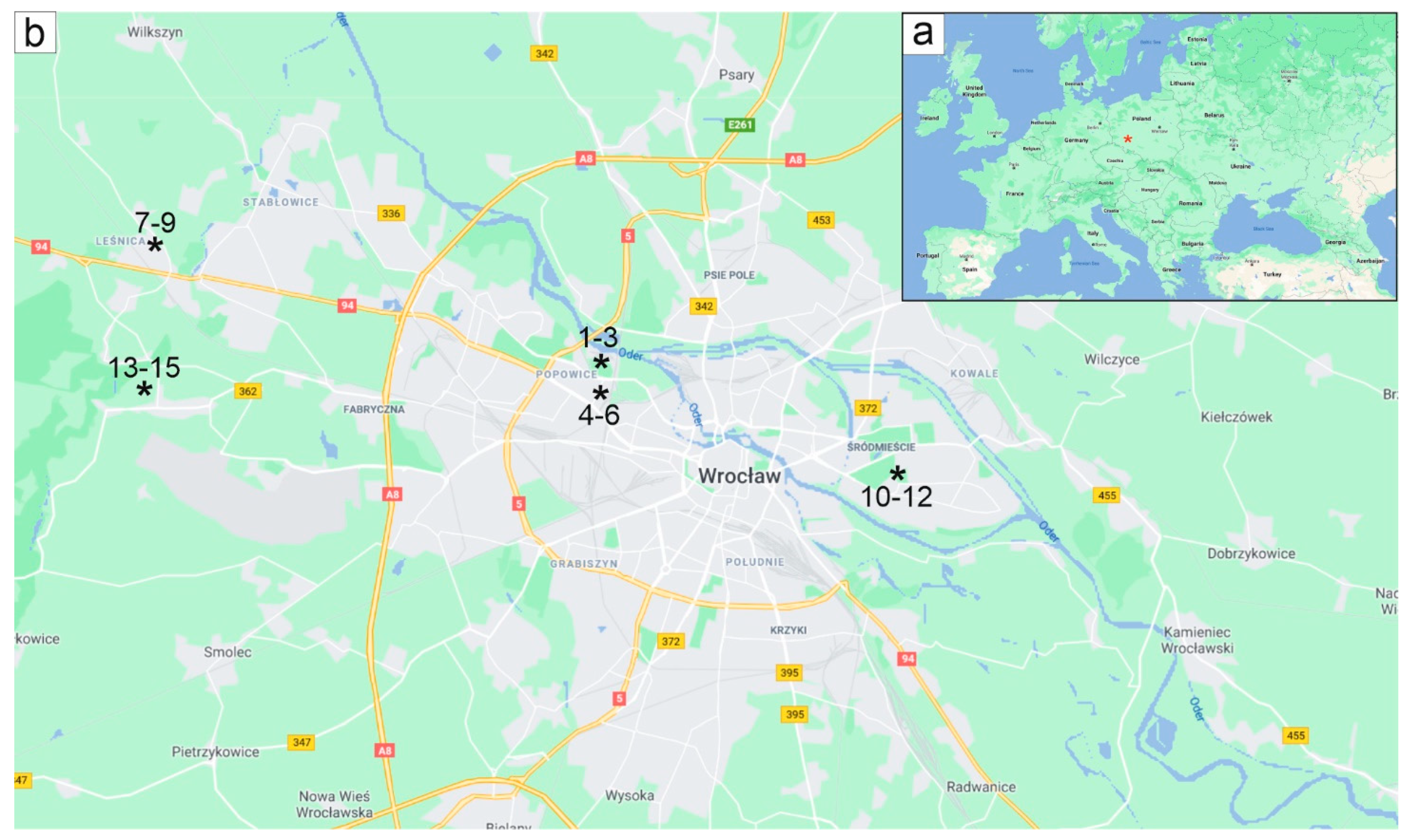

| Year of Sampling | Lightly Infested Trees (LIT) | Heavily Infested Trees (HIT) |
|---|---|---|
| 2014 | trees 1–3 51°07′28.1″ N 16°59′44.8″ E | trees 7–9 51°08′53.4″ N 16°52′12.7″ E trees 10–12 51°06′35.7″ N 17°04′46.8″ E |
| 2015 | trees 4–6 51°07′28.1″ N 16°59′44.8″ E | trees 13–15 51°08′01.4″ N 16°50′50.6″ E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myśkow, E.; Sokołowska, K.; Słupianek, A.; Gryc, V. Description of Intra-Annual Changes in Cambial Activity and Differentiation of Secondary Conductive Tissues of Aesculus hippocastanum Trees Affected by the Leaf Miner Cameraria ohridella. Forests 2021, 12, 1537. https://doi.org/10.3390/f12111537
Myśkow E, Sokołowska K, Słupianek A, Gryc V. Description of Intra-Annual Changes in Cambial Activity and Differentiation of Secondary Conductive Tissues of Aesculus hippocastanum Trees Affected by the Leaf Miner Cameraria ohridella. Forests. 2021; 12(11):1537. https://doi.org/10.3390/f12111537
Chicago/Turabian StyleMyśkow, Elżbieta, Katarzyna Sokołowska, Aleksandra Słupianek, and Vladimir Gryc. 2021. "Description of Intra-Annual Changes in Cambial Activity and Differentiation of Secondary Conductive Tissues of Aesculus hippocastanum Trees Affected by the Leaf Miner Cameraria ohridella" Forests 12, no. 11: 1537. https://doi.org/10.3390/f12111537
APA StyleMyśkow, E., Sokołowska, K., Słupianek, A., & Gryc, V. (2021). Description of Intra-Annual Changes in Cambial Activity and Differentiation of Secondary Conductive Tissues of Aesculus hippocastanum Trees Affected by the Leaf Miner Cameraria ohridella. Forests, 12(11), 1537. https://doi.org/10.3390/f12111537







