Calorific Value and Ash Content of Extracted Birch Bark
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bark Preparation
2.2. Chemicals
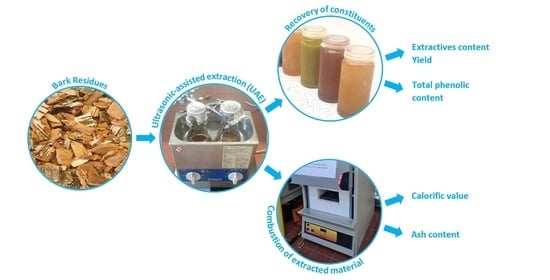
2.3. Ultrasound-Assisted Extraction (UAE)
2.4. Extract Processing
2.5. Yield and Extractives Content
- m1 is the weight of initial bark [g] (dry base) and
- m2 is the weight of extracted bark [g] (dry base).
- c1 is the initial amount of liquid [mL] and
- c2 is the weight of solid components [mg].
2.6. Total Phenolic Content
2.7. Ash Content Determination
- a1 is the weight of the empty melting pot [g],
- a2 is the weight of the melting pot with dried sample [g],
- a3 is the weight of the melting pot with ash [g], and
- Mad is the moisture content of the sample.
2.8. Gross Calorific Value and Energy Content
- is the net calorific value as received [MJ/kg],
- is the net colorific value on a dry basis [MJ/kg],
- is the moisture content as received, and
- 0.02443 is the correction factor of the enthalpy of vaporization at 25 °C.
- is the energy content as received [MWh/m3],
- is the net calorific value as received [MJ/kg], and
- is the bulk density as received [kg/m³].
2.9. Bulk Density
- is the bulk density [kg/m3],
- is the weight of the container and bark [kg]
- is the weight of the container [kg], and
- is the volume of the container [m³]
3. Results and Discussion
3.1. Analysis of Extraction
3.2. Calorific Value and Ash Content of Sample Material
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paletto, A.; Bernardi, S.; Pierattti, E.; Teston, F.; Romagnoli, M. Assessment of environmental impact of biomass power plants to increase the social acceptance of renewable energy technologies. Heliyon 2019, 5, e02070. [Google Scholar] [CrossRef] [Green Version]
- Pieratti, E.; Paletto, A.; Atena, A.; Bernadi, S.; Palm, M.; Pazelt, D.; Romagnoli, M.; Teston, F.; Voglar, G.E.; Grebenc, T.; et al. Environment and climate change impacts of eighteen biomass-based plants in the alpine region: A comparative analysis. J. Clean. Prod. 2020, 242, 118449. [Google Scholar]
- Anerud, E.; Routa, J.; Bergström, D.; Eliasson, L. Fuel quality of stored spruce bark–influence of semi-permeable covering material. Fuel 2020, 279, 118467. [Google Scholar] [CrossRef]
- Routa, J.; Brännström, H.; Laitila, J. Effects of storage on dry matter, energy content and amount of extractives in Norway spruce bark. Biomass Bioenergy 2020, 2, 105821. [Google Scholar] [CrossRef]
- Global Forest Products—Facts and Figures. 2018. Available online: http://www.fao.org/3/ca7415en/ca7415en.pdf (accessed on 27 September 2021).
- Linser, S. Austrian Indicators for Sustainable Forest Management; IUFRO: Vienna, Austria, 2020. [Google Scholar]
- Anerud, E.; Bergström, D.; Routa, J.; Eliasson, L. Fuel quality and dry matter losses of stored wood chips—Influence of cover material. Biomass Bioenergy 2021, 150, 106109. [Google Scholar] [CrossRef]
- Jirjis, R. Effects of particle size and pile height on storage and fuel quality of comminuted Salix viminalis. Biomass Bioenergy 2005, 28, 193–201. [Google Scholar] [CrossRef]
- Routa, J.; Brännström, H.; Hellström, J.; Laitila, J. Influence of storage on the physical and chemical properties of Scots pine bark. BioEnergy Res. 2021, 14, 575–587. [Google Scholar] [CrossRef]
- Dumfort, S. Degradation of biomass during the storage of woodchips. In Proceedings of the 21th European Biomass Conference, Kopenhagen, Denmark, 3–7 June 2013. [Google Scholar]
- Heinek, S.; Huber, M.B.; Larch, C.; Kirchmair, C.; Flörl, K. Biomass conditioning—An investigation of the degradation process of woodchips and forest residues during storage in Western Austria. J. Int. Sci. Publ. Mater. Methods Technol. 2015, 9, 37–44. [Google Scholar]
- Kumaniaev, I.; Navare, K.; Mendes, N.C.; Placet, V.; Van Acker, K.; Samec, J.S.M. Conversion of birch bark to biofuels. Green Chem. 2020, 22, 2255–2263. [Google Scholar] [CrossRef] [Green Version]
- Fengel, D.; Grosser, D. Chemical composition of softwoods and hardwoods—A bibliographical review. Holz Als Roh- Und Werkst. 1975, 33, 32–34. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood Chemistry Ultrastructure Reactions; Verlag Kessel: Remagen, Germany, 2003. [Google Scholar]
- Pásztory, Z.; Moháciné, I.R.; Gorbacheva, G.; Börcsök, Z. The utilization of tree bark. BioResources 2016, 11, 7859–7888. [Google Scholar] [CrossRef]
- Abyshev, A.Z.; Agaev, É.M.; Guseinov, A.B. Studies of the chemical composition of birch bark extracts (Cortex betula) from the Betulaceae family. Pharmceutical Chem. J. 2007, 41, 419–423. [Google Scholar] [CrossRef]
- Routa, J.; Brännström, H.; Anttila, P.; Mäkinen, M.; Jänis, J.; Asikainen, A. Wood Extractives of Finnish Pine, Spruce and Birch–Availablility and Optimal Sources of Compounds: A Literature Review; Natural Resources and Bioeconomy Studies 73; Natural Resource Institute Finland (Luke): Helsinki, Finland, 2017; p. 55. [Google Scholar]
- Hassegawa, M.; Achim, A. Quantification of wood and bark extracts from yellow birch (Betula alleghaniensis Britt.). In Proceedings of the 5th International Scientific Conference on Hardwood Processing 2015, Quebec City, QC, Canada, 15–17 September 2015; pp. 99–106. [Google Scholar]
- Royer, M.; Prado, M.; García-Pérez, M.E.; Diouf, P.N.; Stevanocic, T. Study of nutraceutical, nutricosmetics and cosmeceutical potentials of polyphenolic bark extracts from Canadian forest species. ParmaNutrition 2013, 1, 158–167. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crops Prod. 2018, 12, 316–332. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Kassing, M.; Jenelten, U.; Schenk, J.; Strube, J. A new approach for process development of plant-based extraction processes. Chem. Eng. Technol. 2010, 33, 377–387. [Google Scholar] [CrossRef]
- Wagner, K.; Roth, C.; Willför, S.; Musso, M.; Petutschnigg, A.; Oostingh, G.J.; Schnabel, T. Identification of antimicrobial compounds in different hydrophilic larch bark extracts. BioResources 2019, 14, 5807–5815. [Google Scholar]
- Sillero, L.; Prado, R.; Andrés, M.A.; Labidi, J. Characterisation of bark of six species from mixed Atlantic forest. Ind. Crops Prod. 2019, 137, 276–284. [Google Scholar] [CrossRef]
- Diouf, P.; Stevanovic, T.; Boutin, Y. The effect of extraction process on polyphenol content, triterpene composition and bioactivity of yellow birch (Betula alleghaniensis Britton) extracts. Ind. Crops Prod. 2009, 30, 297–303. [Google Scholar] [CrossRef]
- Liimatainen, J.; Karonen, M.; Sinkkonen, J.; Helander, M.; Salminen, J.-P. Characterization of phenolic compounds from inner bark of Betula pendula. Holzforschung 2012, 66, 171–181. [Google Scholar] [CrossRef]
- Liimatainen, J.; Karonen, M.; Sinkkonen, J.; Helander, M.; Salminen, J.-P. Phenolic compounds of the inner bark of Betula pendula: Seasonal and genetic variation and induction by wounding. J. Chem. Ecol. 2012, 38, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Pietarinen, S.P.; Willför, S.; Ahotupa, M.O.; Hemming, J.E.; Holmbom, B.R. Knotwood and bark extracts: Strong antioxidants from waste materials. J. Wood Sci. 2016, 42, 436–444. [Google Scholar] [CrossRef]
- Laitinen, M.-L.; Julkunen-Tiitto, R.; Yamaji, K.; Heinonen, J.; Rousi, M. Variation in birch bark secondary chemistry between and within clones: Implications for herbivory by hares. Oikos 2004, 104, 316–326. [Google Scholar] [CrossRef]
- Zanetti, M.; Cesprini, E.; Marangon, M.; Szczurek, A.; Tondi, G. Thermal valorization and elemental composition of industrial tannin extracts. Fuel 2021, 289, 119907. [Google Scholar] [CrossRef]
- Dibdiakova, J.; Wang, L.; Li, H. Heating Value and Ash Content of Downy Birch Forest Biomass. Energy Procedia 2017, 105, 1302–1308. [Google Scholar] [CrossRef]
- Kamperidou, V.; Lykidis, C.; Barmpoutis, P. Utilization of wood and bark of fast-growing hardwood species in energy production. J. For. Sci. 2018, 64, 164–170. [Google Scholar]
- Schuster, A.; Ortmayr, N.; Oostingh, G.J.; Stelzhammer, B. Compounds extracted from larch, birch bark, Douglas fir, and alder woods with four different solvents. Effects on five skin-related microbes. Bioresources 2020, 15, 3368–3381. [Google Scholar] [CrossRef]
| Sample | Gross Calorific Value (MJ/kg) | Ash Content (% ± SD) | Yield (% ± SD) |
|---|---|---|---|
| fresh birch bark | 22.21 | 2.12 * ± 0.02 | 17.74 * ± 1.64 |
| extracted birch bark | 20.66 | 2.78 * ± 0.06 | - |
| Sample | Bulk Density (kg/m³ ± SD) | Calculated Energy Content (MWh/m³) |
|---|---|---|
| oven-dried birch bark | 402.45 * ± 13.9 | 2.48 |
| Oven-dried extracted birch bark | 280.15 * ± 7.64 | 1.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruber, L.; Seidl, L.; Zanetti, M.; Schnabel, T. Calorific Value and Ash Content of Extracted Birch Bark. Forests 2021, 12, 1480. https://doi.org/10.3390/f12111480
Gruber L, Seidl L, Zanetti M, Schnabel T. Calorific Value and Ash Content of Extracted Birch Bark. Forests. 2021; 12(11):1480. https://doi.org/10.3390/f12111480
Chicago/Turabian StyleGruber, Lukas, Lukas Seidl, Michela Zanetti, and Thomas Schnabel. 2021. "Calorific Value and Ash Content of Extracted Birch Bark" Forests 12, no. 11: 1480. https://doi.org/10.3390/f12111480
APA StyleGruber, L., Seidl, L., Zanetti, M., & Schnabel, T. (2021). Calorific Value and Ash Content of Extracted Birch Bark. Forests, 12(11), 1480. https://doi.org/10.3390/f12111480