Phenology of Oenocarpus mapora H. Karst in Low-Terrace and High-Terrace Forests of the Madre de Dios Region, Peru
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Studied
2.2. Study Area
2.3. Selection of Individuals
2.4. Reproductive Phenology, Rainfall and Temperature
2.5. Biometric Characteristics of the Fruits
2.6. Data Analysis
3. Results
3.1. Plant Height and Mature Green Leaves
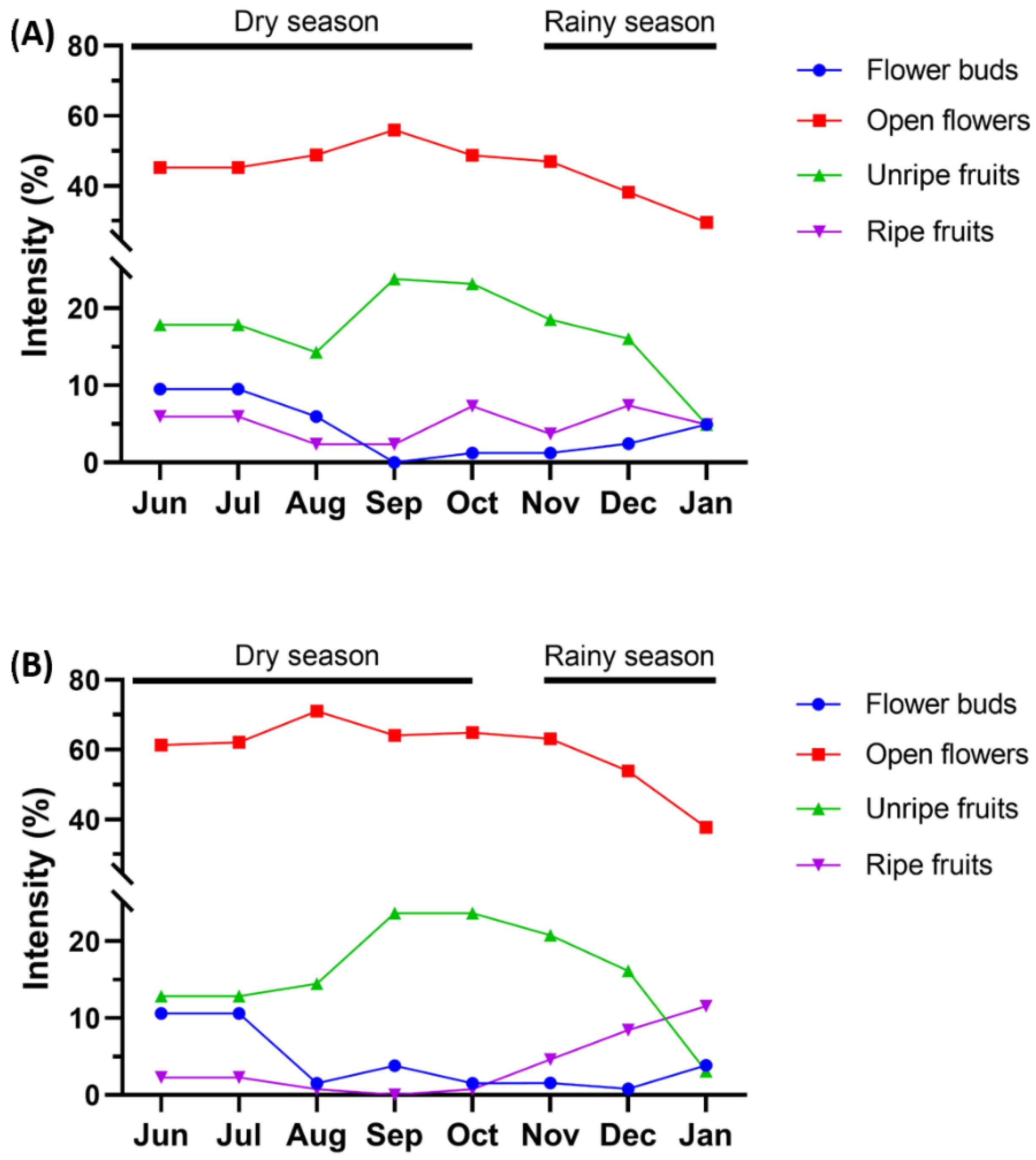
3.2. Flowering and Fructification
4. Discussion
4.1. Plant Height and Mature Green Leaves
4.2. Flowering and Fructification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morellato, L.P.C.; Talora, D.C.; Takahasi, A.; Bencke, C.C.; Romera, E.C.; Zipparro, V.B. Phenology of Atlantic Rain Forest Trees: A Comparative Study1. Biotropica 2000, 32, 811–823. [Google Scholar] [CrossRef]
- Morellato, L.P.C.; Alberton, B.; Alvarado, S.T.; Borges, B.; Buisson, E.; Camargo, M.G.G.; Cancian, L.F.; Carstensen, D.W.; Escobar, D.F.E.; Leite, P.T.P.; et al. Linking Plant Phenology to Conservation Biology. Biol. Conserv. 2016, 195, 60–72. [Google Scholar] [CrossRef]
- Nadia, T.d.L.; Morellato, L.P.C.; Machado, I.C. Reproductive Phenology of a Northeast Brazilian Mangrove Community: Environmental and Biotic Constraints. Flora 2012, 207, 682–692. [Google Scholar] [CrossRef]
- Morellato, L.P.C.; Camargo, M.G.G.; Gressler, E. A Review of Plant Phenology in South and Central America. In Phenology: An Integrative Environmental Science, 2nd ed.; Schwartz, M., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 91–113. ISBN 978-94-007-6924-3. [Google Scholar]
- Sun, C.; Kaplin, B.A.; Kristensen, K.A.; Munyaligoga, V.; Mvukiyumwami, J.; Kajondo, K.K.; Moermond, T.C. Tree Phenology in a Tropical Montane Forest in Rwanda. Biotropica 1996, 28, 668–681. [Google Scholar] [CrossRef]
- Villasana, A.R.A.; Suárez, A. Estudio Fenológico de Dieciseis Especies Forestales Presentes en la Reserva Forestal IMATACA, Edo. Bolívar-Venezuela; Universidad de los Andes: Mérida, Venezuela, 2017. [Google Scholar]
- Henderson, A.; Salazar, A. The Palms of the Amazon; Oxford University Press: Oxford, UK; New York, NY, USA, 1995; ISBN 978-0-19-508311-8. [Google Scholar]
- Henderson, A. Bactris (Palmae). Flora Neotrop. 2000, 79, 1–181. [Google Scholar]
- Balslev, H.; Macía, M.; Navarrete, H. Cosecha de Palmas en el Noroeste de Suramérica: Bases Científicas Para Su Manejo y Conservación; Pontificia Universidad Católica del Ecuador: Ambato, Ecuador, 2015; ISBN 978-9978-77-230-0. [Google Scholar]
- Dirección Regional de Salud Madre de Dios. Dirección de Epidemiología. Análisis de Situación de Salud 2016. Available online: http://dge.gob.pe/portal/Asis/indreg/asis_madrededios.pdf (accessed on 16 June 2021).
- Phillips, O.; Martinez, R.; Vargas, P.; Monteagudo, A.; Zans, M.E.; Galiano Sánchez, W.; Cruz, A.; Timana, M.; Yli-Halla, M.; Rose, S. Efficient Plot-Based Floristic Assessment of Tropical Forests. J. Trop. Ecol. 2003, 19, 629–645. [Google Scholar] [CrossRef]
- Balslev, H.; Laumark, P.; Pedersen, D.; Grández, C. Tropical Rainforest Palm Communities in Madre de Dios in Amazonian Peru. Rev. Peru. Biol. 2016, 23, 3–12. [Google Scholar] [CrossRef][Green Version]
- Sosnowska, J.; Walanus, A.; Balslev, H. Asháninka Palm Management and Domestication in the Peruvian Amazon. Hum. Ecol. Interdiscip. J. 2015, 43, 451–466. [Google Scholar] [CrossRef]
- Balslev, H.; Grandez, C.; Paniagua Zambrana, N.Y.; Møller, A.L.; Hansen, S.L. Palmas (Arecaceae) Útiles En Los Alrededores de Iquitos, Amazonía Peruana. Rev. Peru. Biol. 2008, 15, 121–132. [Google Scholar] [CrossRef]
- Genini, J.; Galetti, M.; Morellato, L.P.C. Fruiting Phenology of Palms and Trees in an Atlantic Rainforest Land-Bridge Island. Flora-Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 131–145. [Google Scholar] [CrossRef]
- Balick, M.J. Systematics and Economic Botany of the Oenocarpus-Jessenia (Palmae) Complex. Adv. Econ. Bot. 1986, 3, 1–140. [Google Scholar]
- Moura, E.F.; de Oliveira, M.S.P. Genetic Diversity in a Germplasm Bank of Oenocarpus Mapora (Arecaceae). Genet. Mol. Res. 2012, 11, 4008–4018. [Google Scholar] [CrossRef]
- Smith, N. Oenocarpus mapora. In Palms and People in the Amazon; Smith, N., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 421–428. ISBN 978-3-319-05509-1. [Google Scholar]
- Pitman, N.C.A. An Overview of the Los Amigos Watershed, Madre de Dios, Southeastern Peru; (Unpublished Report); Amazon Conservation Association: Washington, DC, USA, 2010. [Google Scholar]
- Terborgh, J.; Andresen, E. The Composition of Amazonian Forests: Patterns at Local and Regional Scales. J. Trop. Ecol. 1998, 14, 645–664. [Google Scholar] [CrossRef]
- Ureta, A.M.; Martinez, P.; Tupayachi, R.; Zuniga, A. Fenología de palmeras arborescentes nativas de Madre De Dios–Perú. Intropica 2014, 9, 60–74. [Google Scholar] [CrossRef]
- Holdridge, L.; Instituto Interamericano de Ciencias Agrícolas (IICA); Jiménez Saa, H. Ecología Basada en Zonas de Vida; Instituto Interamericano de Cooperación para la Agricultura: San José, Costa Rica, 1978; ISBN 978-92-9039-131-9. [Google Scholar]
- Dumont, J.F.; Deza, E.; Garcia, F. Morphostructural Provinces and Neotectonics in the Amazonian Lowlands of Peru. J. South Am. Earth Sci. 1991, 4, 373–381. [Google Scholar] [CrossRef]
- Foster, R.B.; Hernandez, N.C.; Kakudidi, E.K.; Burnham, R.J. Rapid Assessment of Tropical Plant Communities Using Variable Transects: An Informal and Practical Guide; Field Museum of Chicago: Chicago, IL, USA, 1998. [Google Scholar]
- World Meteorological Organization (WMO). Guidelines for Plant Phenological Observations; WCDMP-No. 70; WMO: Geneva, Switzerland, 2009. [Google Scholar]
- Gillespie, M.A.K.; Baggesen, N.; Cooper, E.J. High Arctic Flowering Phenology and Plant–Pollinator Interactions in Response to Delayed Snow Melt and Simulated Warming. Environ. Res. Lett. 2016, 11, 115006. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F. New Robust Weighted Averaging- and Model-Based Methods for Assessing Trait–Environment Relationships. Methods Ecol. Evol. 2019, 10, 1962–1971. [Google Scholar] [CrossRef]
- Harper, J.L.; Ltd, H.P. Population Biology of Plants; Academic Press: Cambridge, MA, USA, 1977; ISBN 978-0-12-325850-2. [Google Scholar]
- Chazdon, R.L. Patterns of Growth and Reproduction of Geonoma Congesta, a Clustered Understory Palm. Biotropica 1992, 24, 43–51. [Google Scholar] [CrossRef]
- Otero-Arnaiz, A.; Oyama, K. Reproductive Phenology, Seed-Set and Pollination in Chamaedorea Alternans, an Understorey Dioecious Palm in a Rain Forest in Mexico. J. Trop. Ecol. 2001, 17, 745–754. [Google Scholar] [CrossRef]
- Da Silva, P.A.D.; Scariot, A. Phenology, Biometric Parameters and Productivity of Fruits of the Palm Butia Capitata (Mart.) Beccari in the Brazilian Cerrado in the North of the State of Minas Gerais. Acta Bot. Bras. 2013, 27, 580–589. [Google Scholar] [CrossRef][Green Version]
- Coomes, D.A.; Allen, R.B. Effects of Size, Competition and Altitude on Tree Growth. J. Ecol. 2007, 95, 1084–1097. [Google Scholar] [CrossRef]
- Gray, R.E.J.; Ewers, R.M. Monitoring Forest Phenology in a Changing World. Forests 2021, 12, 297. [Google Scholar] [CrossRef]
- Rojas-Robles, R.; Stiles, F.G. Analysis of a Supra-Annual Cycle: Reproductive Phenology of the Palm Oenocarpus Bataua in a Forest of the Colombian Andes. J. Trop. Ecol. 2009, 25, 41–51. [Google Scholar] [CrossRef]
- Cifuentes, L.; Moreno, F.; Arango, D.A. Reproductive Phenology and Fruit Productivity of Oenocarpus Bataua (Mart.) in Flooded Forests in the Chocó Biogeographic Region, Colombia. Biota Neotrop. 2010, 10, 101–109. [Google Scholar] [CrossRef]
- Cabrera, W.H.; Wallace, R. Patrones fenológicos de ocho especies de palmeras en un bosque amazónico de Bolivia. Rev. Bol. Ecol. Cons. Amb. 2007, 21, 1–18. [Google Scholar]
- Nord, E.A.; Lynch, J.P. Plant Phenology: A Critical Controller of Soil Resource Acquisition. J. Exp. Bot. 2009, 60, 1927–1937. [Google Scholar] [CrossRef]
- Kiltie, R.A. Distribution of Palm Fruits on a Rain Forest Floor: Why White-Lipped Peccaries Forage near Objects. Biotropica 1981, 13, 141–145. [Google Scholar] [CrossRef]
- Abbott, D.L.; Bull, V.; Bishop, S.N. Effect of Summer Temperature on Flower Initiation; Report of the Long Ashton Research Station; University of Bristol: Bristol, UK, 1974; Volume 1973, pp. 31–32. [Google Scholar]
- Tromp, J. Flower-Bud Formation and Shoot Growth in Apple as Affected by Temperature. Sci. Hortic. 1976, 5, 331–338. [Google Scholar] [CrossRef]
- Ichimura, K.; Suto, K. Environmental Factors Controlling Flower Opening and Closing in a Portulaca Hybrid. Ann. Bot. 1998, 82, 67–70. [Google Scholar] [CrossRef]
- Ashwell, I.Y. Mountain Weather and Climate, Roger, G. Barry, Methuen, London, 1981, No. of Pages; 313. Rice: £17.50. J. Climatol. 1982, 2, 304. [Google Scholar] [CrossRef]
- Kimball, S.; Gremer, J.R.; Angert, A.L.; Huxman, T.E.; Venable, D.L. Fitness and Physiology in a Variable Environment. Oecologia 2012, 169, 319–329. [Google Scholar] [CrossRef] [PubMed]
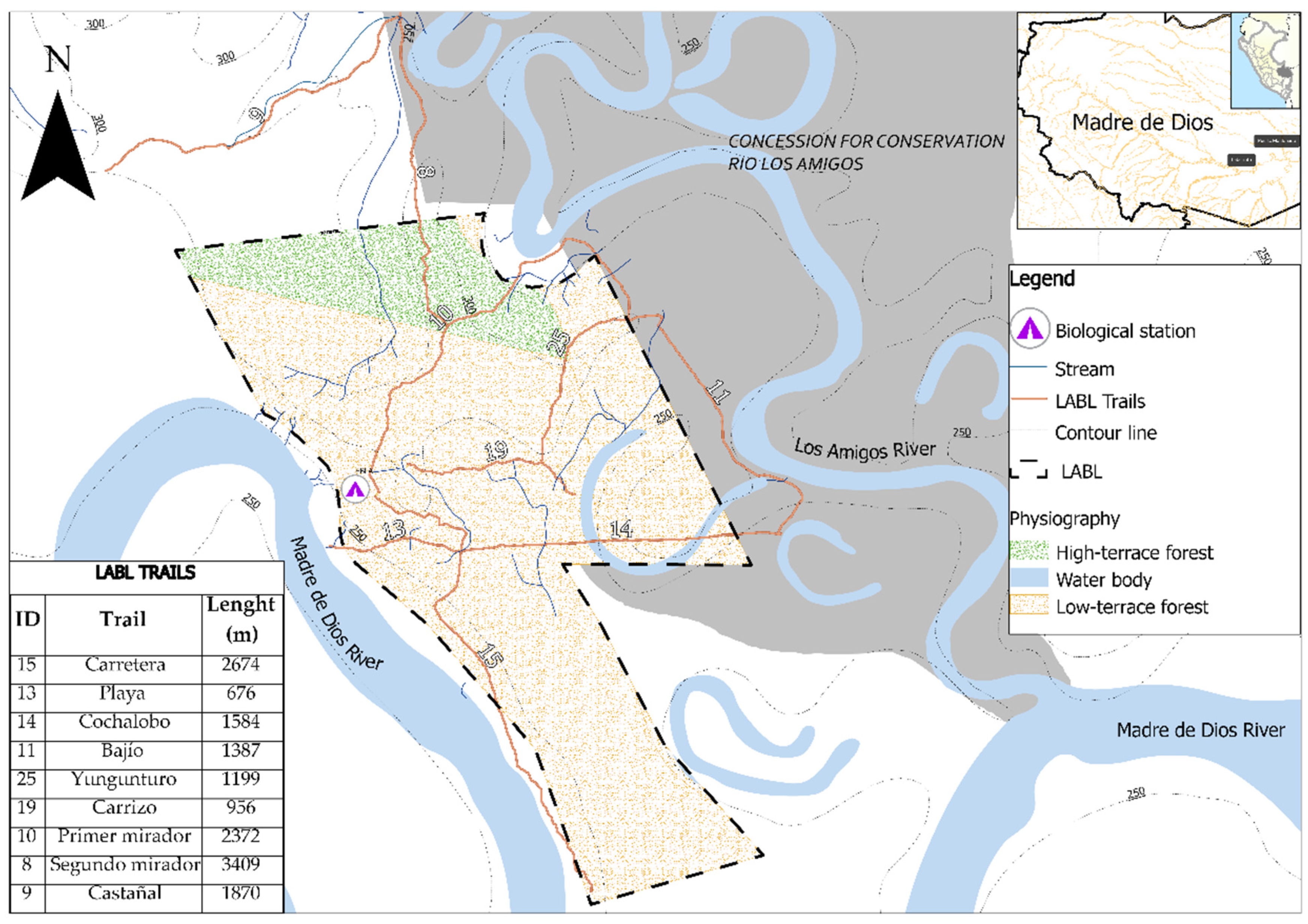


| Trail | Habitat | Length (m) | Altitude (m asl) | Longitude | Latitude |
|---|---|---|---|---|---|
| Carretera | Low-terrace forest | 2674 | 250–300 | −70.094397 | −12.576631 |
| Playa | Low-terrace forest | 676 | 250–270 | −70.088417 | −12.570647 |
| Cochalobo | Low-terrace forest | 1584 | 250–275 | −70.098553 | −12.570858 |
| Bajío | Low-terrace forest | 1387 | 250–286 | −70.083242 | −12.565974 |
| Yungunturo | High-terrace forest | 1199 | 285–300 | −70.089977 | −12.562769 |
| Carrizo | High-terrace forest | 956 | 268–300 | −70.094028 | −12.567466 |
| Primer mirador | High-terrace forest | 2372 | 250–300 | −70.094459 | −12.561381 |
| Segundo mirador | High-terrace forest | 3409 | 264–300 | −70.099235 | −12.547318 |
| Castañal | High-terrace forest | 1870 | 270–300 | −70.104610 | −12.551184 |
| Parameter | Low-Terrace Forest (n = 84) | High-Terrace Forest (n = 132) |
|---|---|---|
| Height (m) | 10.52 ± 2.93 | 11.95 ± 1.89 * |
| Number of mature green leaves per individual | 8.07 ± 1.40 | 7.84 ± 1.35 * |
| Number of bunches per individual | 1.55 ± 0.67 | 1.42 ± 0.60 * |
| Habitat | Mature Green Leaves | Bunches | Flower Buds | Open Flowers | Unripe Fruits | Ripe Fruits |
|---|---|---|---|---|---|---|
| Low-terrace forest | ||||||
| Mean date | 222 (10 August 2019) | 223 (11 August 2019) | 182 (1 July 2019) | 231 (19 August 2019) | 244 (1 September 2019) | 226 (14 August 2019) |
| High-terrace forest | ||||||
| Mean date | 222 (10 August 2019) | 224 (12 August 2019) | 184 (3 July 2019) | 231 (19 August 2019) | 254 (11 September 2019) | 185 (4 July 2019) |
| Variables | Habitat | Temperature | Rainfall | Number of Days of Precipitation | ||
|---|---|---|---|---|---|---|
| Average | Maximum | Minimum | ||||
| Height | Low-terrace forest High-terrace forest | 0.526 −0.761 * | 0.451 −0.638 | 0.801 * −0.896 ** | 0.801 * −0.896 ** | 0.743 * −0.722 * |
| Number of Mature Green Leaves | Low-terrace forest High-terrace forest | −0.552 0.401 | −0.552 0.250 | −0.430 0.451 | −0.381 0.375 | −0.173 0.315 |
| Number of Bunches | Low-terrace forest High-terrace forest | 0.132 −0.073 | 0.419 0.146 | −0.180 −0.073 | −0.036 0.146 | −0.277 0.012 |
| Flower Buds | Low-terrace forest High-terrace forest | −0.790 * −0.181 | −0.946 ** −0.361 | −0.443 −0.253 | −0.515 −0.422 | −0.090 −0.194 |
| Open Flowers | Low-terrace forest High-terrace forest | 0.144 −0.048 | 0.419 0.262 | −0.563 −0.619 | −0.443 −0.500 | −0.789 * −0.766 * |
| Unripe Fruits | Low-terrace forest High-terrace forest | 0.407 0.482 | 0.539 0.711 * | −0.132 0.024 | 0.036 0.241 | −0.283 −0.182 |
| Ripe Fruits | Low-terrace forest High-terrace forest | −0.277 0.108 | −0.337 −0.193 | 0.337 0.687 | 0.410 0.615 | 0.612 0.861 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Best, I.; Rengifo, H.; Velarde, E.; Loja, J.F.; Portugal, A.; Rengifo, P.; Aguilar, L.; Ramos-Escudero, F.; Muñoz, A.M. Phenology of Oenocarpus mapora H. Karst in Low-Terrace and High-Terrace Forests of the Madre de Dios Region, Peru. Forests 2021, 12, 1424. https://doi.org/10.3390/f12101424
Best I, Rengifo H, Velarde E, Loja JF, Portugal A, Rengifo P, Aguilar L, Ramos-Escudero F, Muñoz AM. Phenology of Oenocarpus mapora H. Karst in Low-Terrace and High-Terrace Forests of the Madre de Dios Region, Peru. Forests. 2021; 12(10):1424. https://doi.org/10.3390/f12101424
Chicago/Turabian StyleBest, Ivan, Helmut Rengifo, Ernesto Velarde, Juan Francisco Loja, Alan Portugal, Piero Rengifo, Luis Aguilar, Fernando Ramos-Escudero, and Ana María Muñoz. 2021. "Phenology of Oenocarpus mapora H. Karst in Low-Terrace and High-Terrace Forests of the Madre de Dios Region, Peru" Forests 12, no. 10: 1424. https://doi.org/10.3390/f12101424
APA StyleBest, I., Rengifo, H., Velarde, E., Loja, J. F., Portugal, A., Rengifo, P., Aguilar, L., Ramos-Escudero, F., & Muñoz, A. M. (2021). Phenology of Oenocarpus mapora H. Karst in Low-Terrace and High-Terrace Forests of the Madre de Dios Region, Peru. Forests, 12(10), 1424. https://doi.org/10.3390/f12101424









