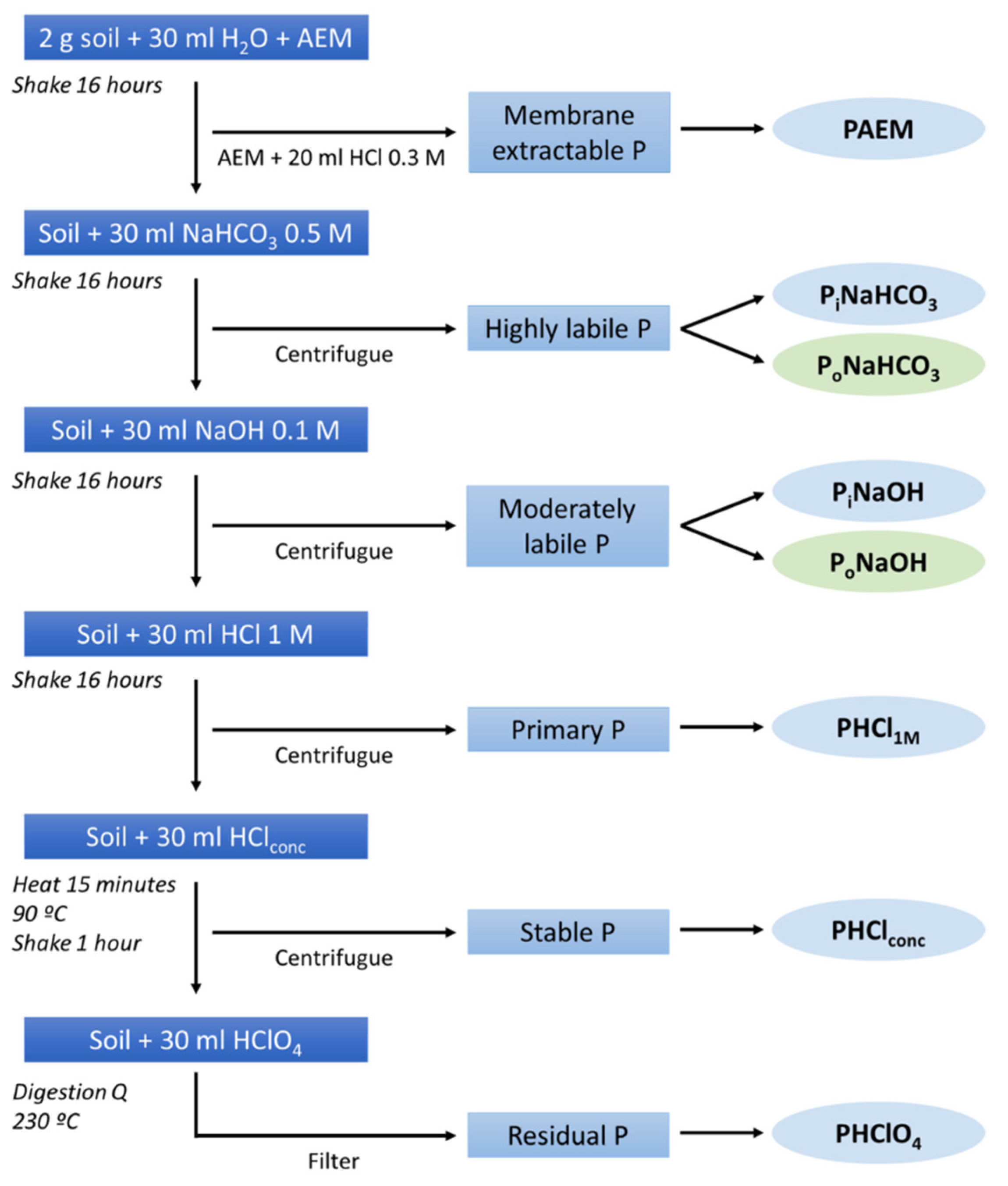
4.1. Soil Phosphorus Status and Availability
Membrane extractable P was the lowest fraction in the studied soils. As expected, we found positive correlations between membrane extractable P and highly labile inorganic P since this fraction includes easily interchangeable P compounds and simulates the action of plant roots in dissolving P minerals [
39,
40]. Thus, these results indicated that the highly labile inorganic P fraction in the studied soils could act as a source of available P in the short term. However, and unlike other studies (such as [
16]), we did not find significant correlations between the most available P and highly labile organic P, even when this fraction is considered a very active fraction of soil organic P [
58]. Organic P concentration calculated as the sum of PoNaHCO
3 and PoNaOH, represented around 12% of the total P, following previous results in other studies in inceptisols or in Mediterranean forest soils [
42,
59]. The most refractory forms of P (PHClconc and PHClO
4) were positively correlated with the highly labile organic P fraction, corroborating that the most stable and residual fractions may even be sources of available P in the long term [
60].
Primary and stable fractions were the predominant forms of P in the soils studied as observed in other calcareous soils [
1,
61], among others). Primary P was just correlated with the moderately labile inorganic P and with total P, indicating that in these calcareous soils total P is mostly formed by the P included in Ca minerals. The relationship between primary and moderately labile inorganic P deserves an in-depth study to understand its significance in P dynamics in limestone soils with high carbonates content. Few studies have indicated that 1M HCl extraction removes not only Ca-P but also Fe and Al-related P forms [
62,
63,
64], which could explain this correlation. In fact, Fe, Al, and Mn are sometimes more significant in controlling P solubility in calcareous soils than free lime [
65]. However, this possible Ca-P miss quantification by sequential chemical extraction methods is soil-specific (depending on soil properties) and several studies have shown that in calcareous soils the Ca-P and organic P fractions extracted with sequential methods are comparable and consistent with those extracted using other novel methods such as synchrotron X-ray absorption near-edge structure (XANES) spectroscopy (59,63], among others). Total P concentration in the studied soils was low, but in the range found on forest soils [
1,
17,
27] and was also alike to that found in limestone soils of northwestern Spain [
61] and in inceptisol-type soils [
42].
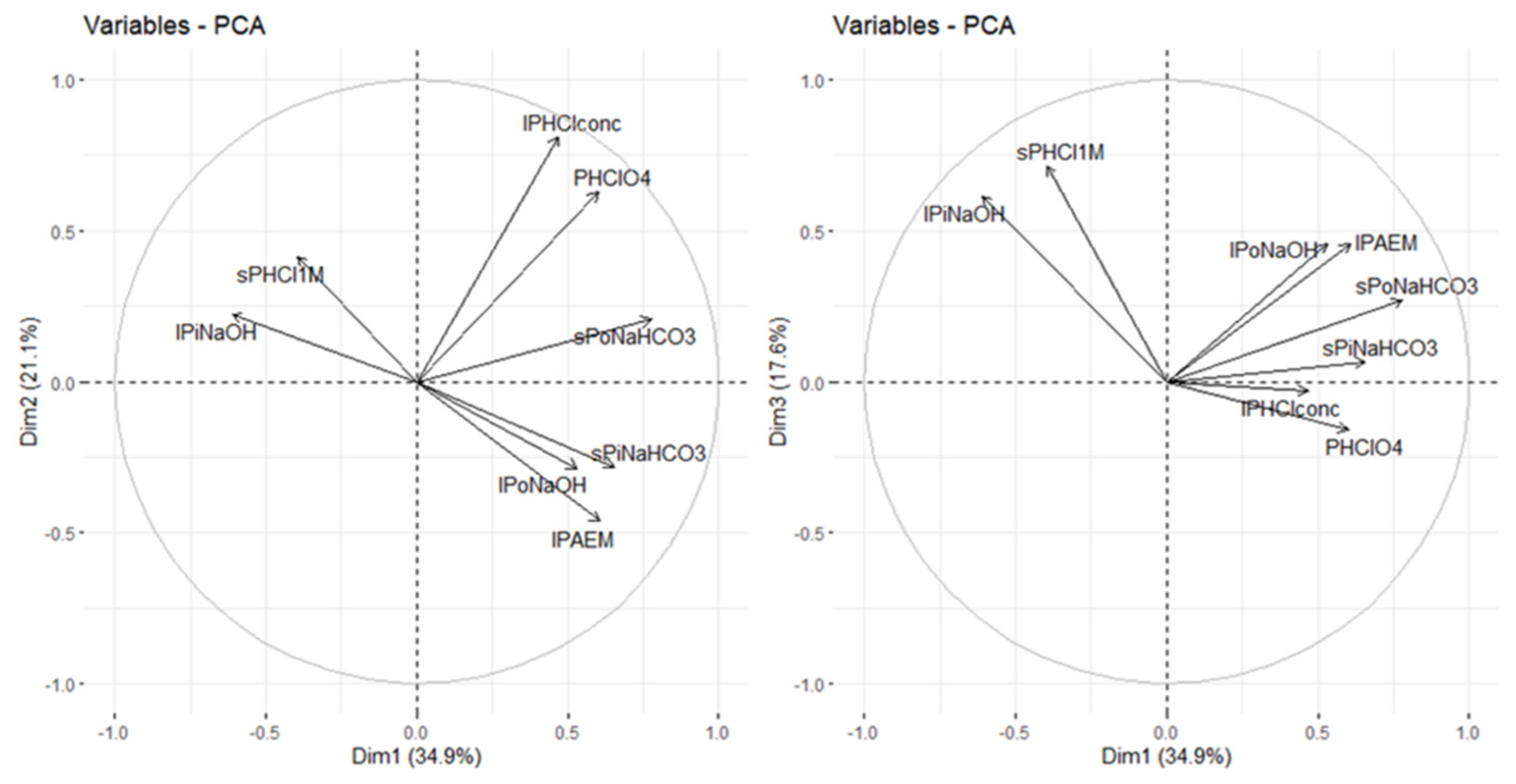
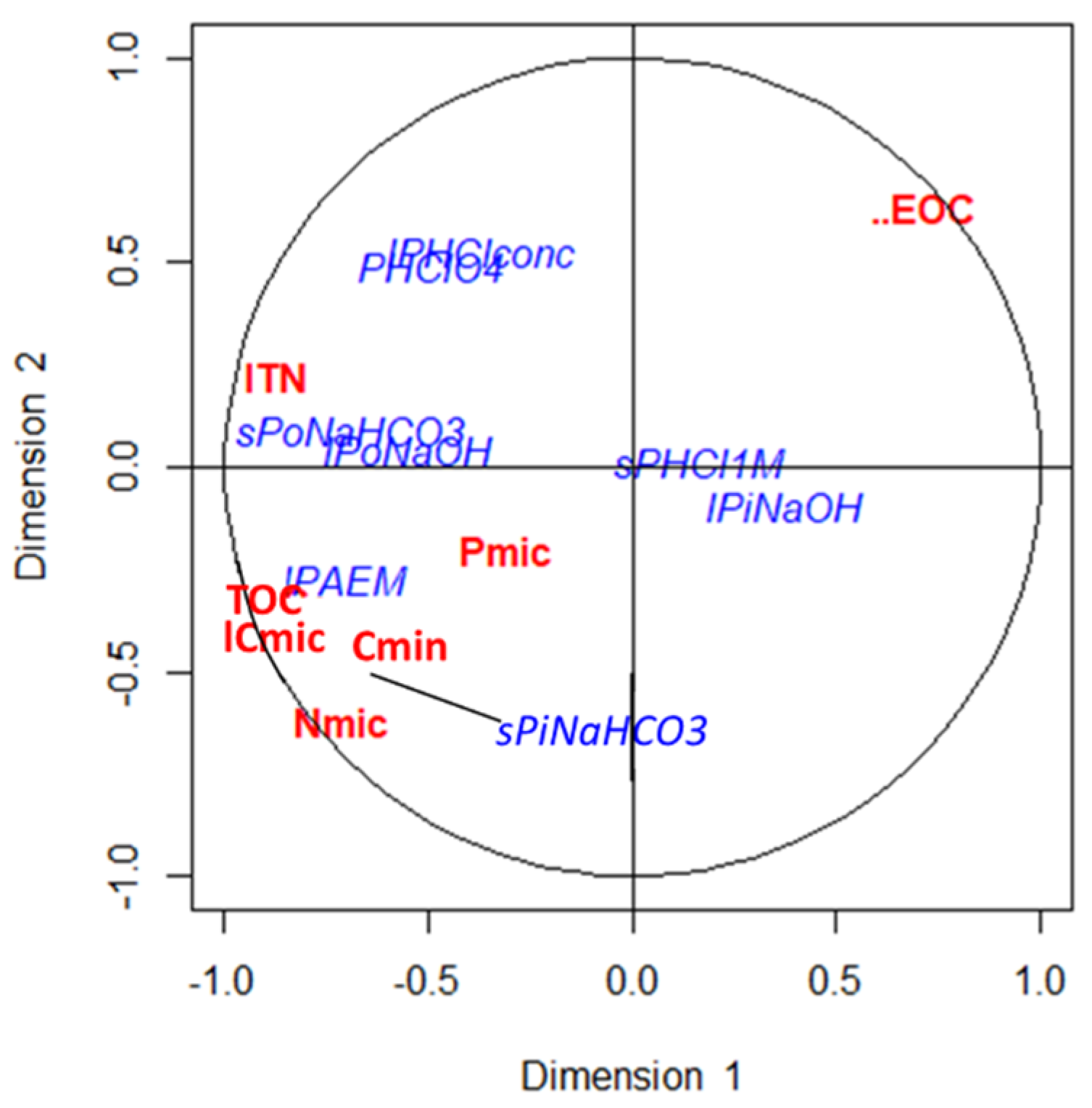
The principal component analysis allowed us to differentiate three pools of P according to their lability (see, for example, [
60,
66]). A first pool related to the first axis of the PCA corresponding to both organic and inorganic labile fractions, that is, the short-term available P fractions. The second PCA was associated with the P forms extracted in the last steps of fractionation, which are the most stable forms and available in the very long term. Finally, in the third axis, the medium-term-available forms appeared, which represent a greater proportion of the total P in these soils and are mainly inorganic forms of P extracted with NaOH and 1M HCl.
4.2. Relationship between P Fractions and Soil Parameters
Specific characteristics of each soil determine the solubility and bioavailability of nutrients, and soil properties affect differently to the diverse soil P forms ([
17,
59], among others). Therefore, soil processes involved in the availability and stability of soil P will vary in soils with unlike characteristics and in different ecosystems. Our results showed that the organic fractions and the P available forms might be affected by organic matter and microbiological activity, while the P fractions linked to Ca seem to be influenced by the composition of the soil, mainly by its sand and carbonates content.
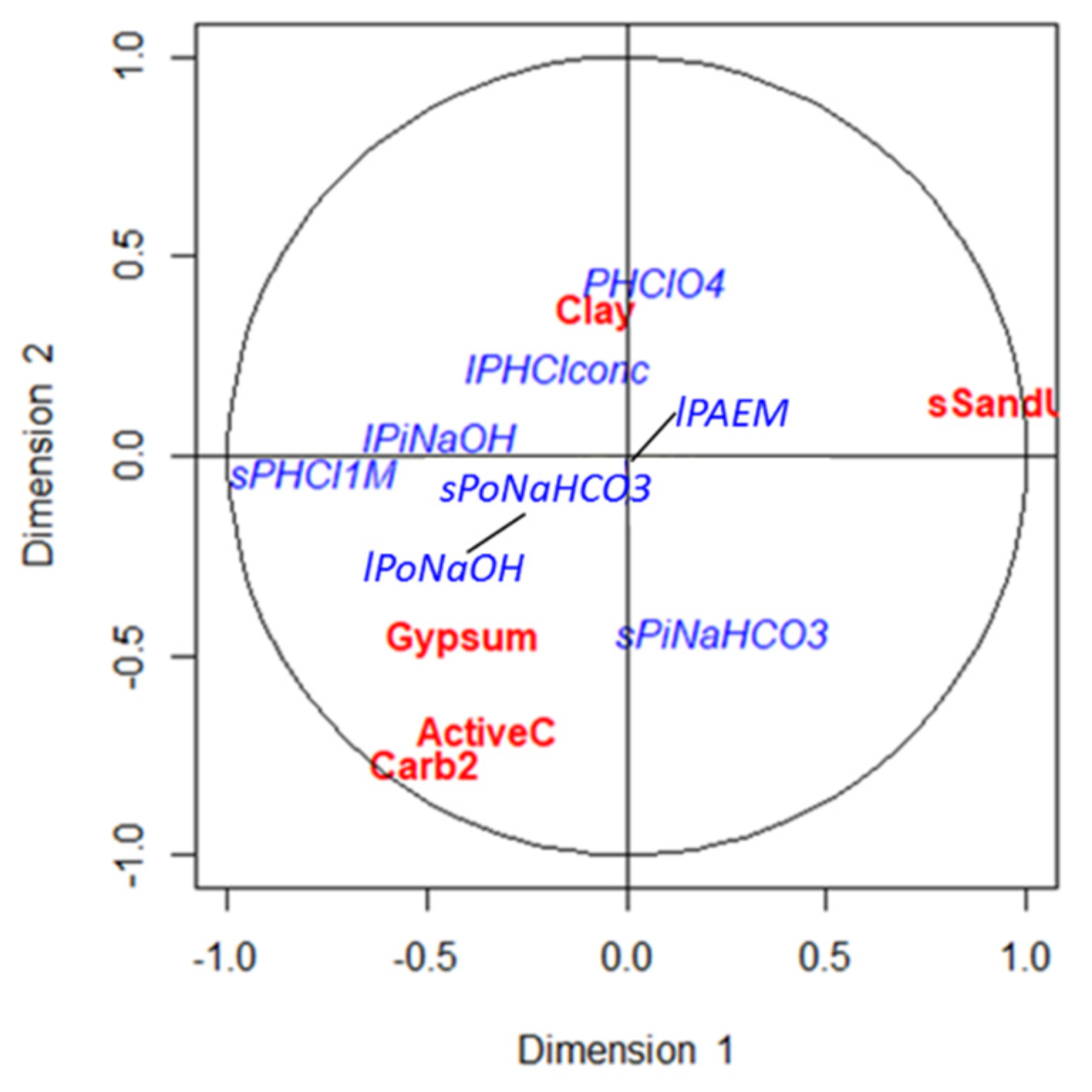
The highly significant correlations of the parameters related to the composition of the soil were established with the most stable fractions of P. Although important, the direct effect of texture on the distribution of P pools in forest soils has been little studied [
17,
67]. Strong negative correlations were found between sand content and primary P, a fraction that represents the total P content in the studied soils. The negative influence of sand content on primary P and total P concentrations has been previously demonstrated in soils from very different climatic areas around the world [
17,
35]. This influence could be interpreted as the reduction of surfaces to which phosphorus can bind, such as the surfaces of the clay–humic complex and, in the specific case of limestone soils, the lower content of active limestone, a material with the size of silt and clay which shows great chemical activity in the soil and is often a better predictor of P behavior than the soil total lime content [
65].
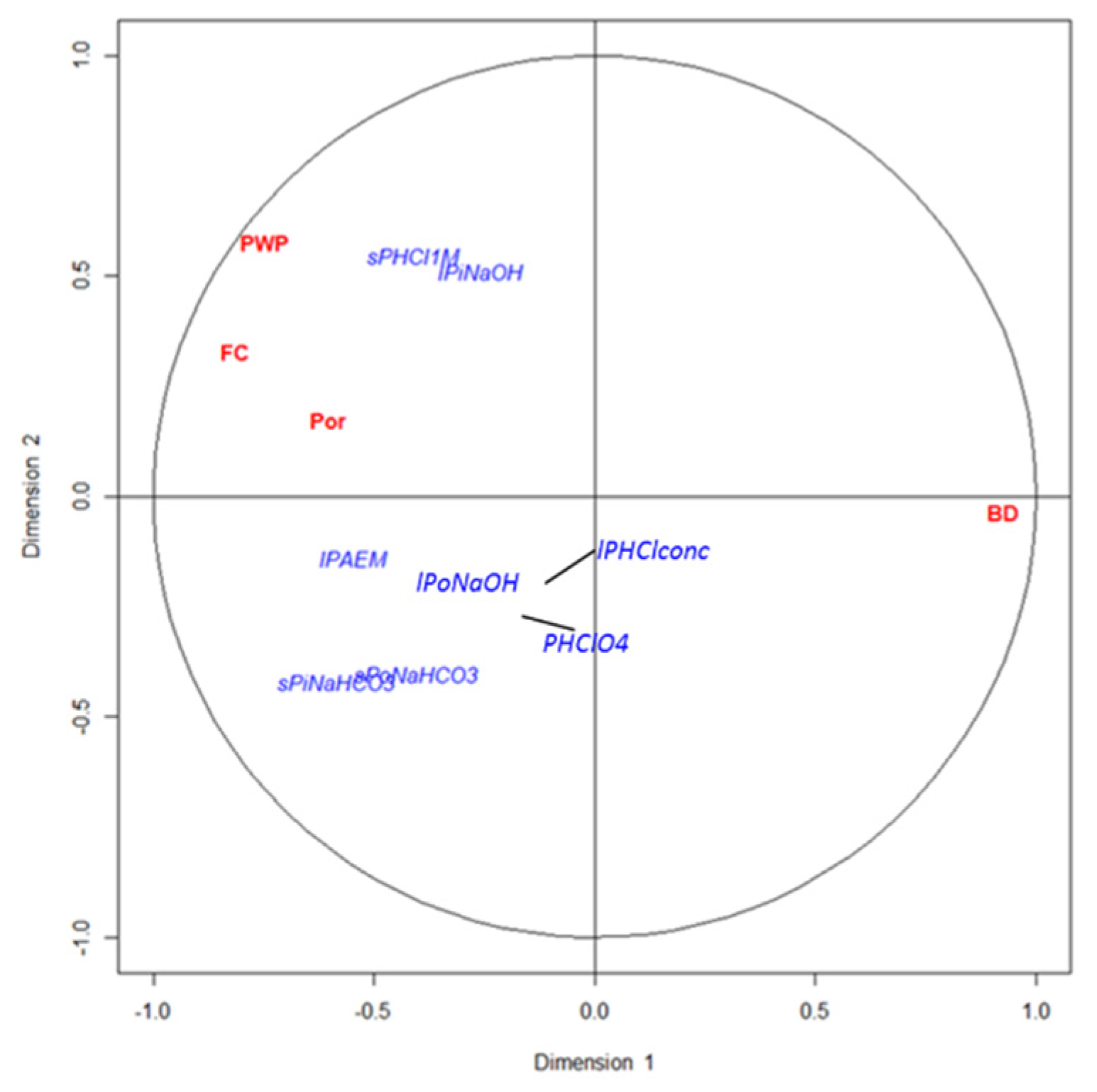
Soil aeration and water retention capacity are important factors affecting P dynamics because they play important roles in the mineralization and immobilization of this element [
11]. However, the significance of these factors in the P cycle remains uncertain. In the present study the effect of soil aeration in P fractions has been considered through bulk density and porosity. Our results indicated that labile P fractions were reduced with increased soil bulk density, i.e., with reduced soil aeration. Lower soil aeration might imply lower soil microbial activities and subsequently reduced mineralization processes. However, contradictory results have been reported in this matter, with higher mineralization rates seen both in good aeration conditions and in bad conditions [
10]. The reason why bulk density greatly affects the soil P available forms may be, on the one hand, to the content of organic matter, since the higher the organic matter, the lower the bulk density. On the other hand, it may be due to aspects related to the textural class.
As expected, parameters related to soil water retention were positively correlated with the available and labile P, since soil moisture is essential for soil microorganisms’ survival and for phosphatases production [
11,
68], thus increasing the labile P fractions. However, whether the soil has more or less water depends not only on its retention capacity but also, and more importantly, on the amount of water that reaches the soil, that is, on rainfall. In this study we did not consider climatic or physiographic variables, we cannot determine either the availability of water or the actual aeration level of the soils studied. Therefore, that the studied soils show high field capacity does not imply that they have enough water for the microorganisms to carry out their activities and consequently, increase the amount of labile P in the soil. In fact, the studied plots are under a Mediterranean climate where annual precipitation is low (less than 500 mm) and the summer drought is intense and long-lasting. Consequently, in the studied plots as in other forest systems, the scarcity of precipitation and low soil moisture, together with a low water retention capacity, can greatly reduce the enzymatic activities in the soil, and so reducing the mineralization of P [
45,
68].
Phosphorus mineralization rates as well as its solubility, fixation, and concentration of its different pools are influenced by soil pH ([
14,
15], among others). However, unlike previous studies, our results did not show a strong effect of pH on P pools, which is probably due to the low pH variation among the studied soils (range 8.0–8.9).
The different phosphorus fractions, mainly labile P, were positively related to cation exchange capacity and the amount of exchangeable potassium, which could be explained by the strong relationship of these parameters with the quantity and quality of soil organic matter. The short-term availability of P to vegetation is strongly influenced by biochemical processes that affect organic matter, while its long-term status is generally determined by geochemical transformations [
26,
27]. In addition, primary P was negatively related to assimilable iron and manganese since when carbonates or active carbonates content in the soil increase, the amount of assimilable Fe and Mn decreases, while primary P bounded in Ca-minerals increases [
66].
As expected, the most labile fractions of P both inorganic (PAEM and PiNaHCO
3) and organic P (PoNaHCO
3) were highly correlated with soil parameters related to organic matter and enzymatic activity. This indicates that in the soils studied the biological cycle of P has great importance in the availability of this element. The availability of P from organic forms may be due to two possible causes: (1) The first is the general mineralization of soil organic matter which is regulated by the energy demand of microorganisms [
69]. Results from CCA and Spearman’s correlations completed with the group of organic matter-related parameters showed the relevance of this biological P mineralization in the studied soils, following previous results [
45]. (2) The second possible cause of the release of P from organic forms could be the specific release of P due to the action of particular enzymes involved (acid phosphatase and, especially in the studied soils, alkaline phosphatase [
70]) which is governed by the specific need for available P. In general, it is considered that the lower the availability of P in the soil, the higher the activity of phosphatase (either acid or alkaline, depending on the soil pH), since this enzyme is released when available P levels are limiting. In the calcareous soils studied, alkaline phosphatase did not show any correlation with assimilable forms of phosphorus. Moreover, it neither showed high relevance in the first canonical axis of the CCA performed with the group of enzyme activity-related parameters. The absence of importance in the CCA and of correlation between the available P forms and alkaline phosphatase could explain that in these calcareous soils the concentration of assimilable P, although low, may be sufficient to satisfy the demand of the vegetation and soil microorganisms, unlike what was found in acid soils in northern Spain [
46]. The rest of the enzymes considered in this study (without phosphatase activity) showed highly significant correlations with the labile forms of P, both in the Spearman correlations and in the CCA. The fluorescein diacetate hydrolysis reaction (FDA), which showed a higher weight in the corresponding canonical dimension, reflects all the hydrolytic activity in the soil [
71] and is commonly used as an indicator of the general microbial activity in the soil [
72]. Furthermore, all the enzymes were related to each other and to the microbiological parameters Cmic, Cmin, Nmic. Microbial populations, besides participating in the mineralization and solubilization of soil P, are also a reserve of organic P that is released when microorganisms die [
3,
9]. Our results showed that the microbial biomass of C, N and P were significantly correlated with the most labile phosphorus and the microbial biomass C also correlated with organic P. The microbial biomass P in our study represented 2.7% of the total P. The positive correlation found between microbial biomass P and membrane extractable P is consonant with previous studies [
58,
59] and with the fact that microbial biomass regulates soil P availability through the release of its P content. Therefore, all these results might indicate that the biological mineralization of P in these soils plays a more important role in P liberation than the biochemical mineralization.
Although in the studied soils, organic forms of P represented a low percentage of total P compared to other studies [
17,
73], a strong influence of total soil carbon (TOC) and easily oxidizable carbon (EOC) on the distribution of P could be expected. Following previous studies, we found a positive correlation between organic P, available P and highly labile inorganic P with soil organic C ([
59,
67], among others), since these soluble and labile forms of P come from the mineralization of organic matter [
21]. The positive relationship between inorganic P and easily oxidizable carbon may be because the organic matter could act as a source of labile inorganic P in the studied soils [
74]. In addition, CCA results showed that the first canonical factor was highly correlated with TOC and the second canonical factor with EOC, indicating that these two parameters might be good predictors of labile P.
4.3. Relationship between Forest Productivity, Soil P Fractions and Soil Parameters
Using globally the large set of soil parameters considered in this work (including the different forms of soil P) we explained around 67% of the variance of the site index calculated by [
25] for the
P. halepensis stands studied. Our results indicated that site index in the studied plots was conditioned by parameters related to soil texture and carbonate content as well as to the primary P, which is the P pool that mostly represents the total P in these plots. Soil water retention-related parameters also explained much of the site index variance in the studied plots. These results are in line with those obtained by other authors for
P. halepensis since this species growth (associated with its site index and productivity) is mainly driven by soil water availability considering both its supply and its retention in the soil [
23,
24]. Surprisingly, climatic variables included in the model which were related to water supply did not influence the site index as much as have been expected. This may be since in these plots, soil composition parameters, mainly the high presence of Ca, largely modulates trees growth. However, the inclusion of climatic variables did considerably improve site index variance explanation. In addition, soil chemical fertility related to the supply of macronutrients was also important in explaining site index. This may be due to high availability of macronutrients as K and Mg could favor resistance to drought since trees with a good supply of these nutrients reduce water loss through transpiration [
24,
75]. The availability of micronutrients did not affect the forest productivity of these plots while in agricultural soils this factor is one of the most important for crops’ growth [
76]. Generally, organic matter decomposition affects the productivity of ecosystems, particularly in nutrient-poor forests such as Mediterranean forests [
77]. However, the parameters related to organic matter did not seem to influence the site index of the studied plots. This could be due to the release of nutrients from plant litter depends not only on the activity of the microorganisms but also and mainly, on the litter quality and on environmental factors. In our case, the quality of the litter is the same in all the plots: same species of
Pinus with the same chemical composition in needles, same strategies for the conservation of nutrients in their tissues, and same decomposition rates [
78]. Furthermore, the plots are in areas with very similar environmental conditions so environmental factors would not make great differences in the nutrient release process between plots either.