Abstract
Despite recent advances in understanding tree species sensitivities to climate change, ecological knowledge on different species remains scattered across disparate sources, precluding their inclusion in vulnerability assessments. Information on potential sensitivities is needed to identify tree species that require consideration, inform changes to current silvicultural practices and prioritize management actions. A trait-based approach was used to overcome some of the challenges involved in assessing sensitivity, providing a common framework to facilitate data integration and species comparisons. Focusing on 26 abundant tree species from eastern Canada, we developed a series of trait-based indices that capture a species’ ability to cope with three key climate change stressors—increased drought events, shifts in climatically suitable habitat, increased fire intensity and frequency. Ten indices were developed by breaking down species’ response to a stressor into its strategies, mechanisms and traits. Species-specific sensitivities varied across climate stressors but also among the various ways a species can cope with a given stressor. Of the 26 species assessed, Tsuga canadensis (L.) Carrière and Abies balsamea (L.) Mill are classified as the most sensitive species across all indices while Acer rubrum L. and Populus spp. are the least sensitive. Information was found for 95% of the trait-species combinations but the quality of available data varies between indices and species. Notably, some traits related to individual-level sensitivity to drought were poorly documented as well as deciduous species found within the temperate biome. We also discuss how our indices compare with other published indices, using drought sensitivity as an example. Finally, we discuss how the information captured by these indices can be used to inform vulnerability assessments and the development of adaptation measures for species with different management requirements under climate change.
1. Introduction
Climate change is expected to profoundly alter the frequency, duration, and severity of extreme events such as drought and fire [1,2]. In addition, forests will experience altered growing conditions due to rapid increases in temperature and shifting precipitation trends [3,4]. These stressors could result in declining forest health or increasing mortality, and potentially precipitate abrupt shifts in species composition [5,6,7]. To anticipate potential impacts of these stressors on forest ecosystems and develop corresponding adaptation strategies, forestry practitioners are increasingly using climate-based vulnerability assessments [8,9]. These assessments traditionally rely heavily on exposure, i.e., the magnitude of projected environmental change [10,11,12]. In recent years, several authors have pointed out critical gaps in this approach, notably the lack of ecological information on underlying species response to stressors [13,14,15]. Specifically, there is a growing interest in capturing species-specific sensitivity, i.e., the degree to which a given species is likely to be affected by (or respond to) a stressor [16].
How tree species respond to climate change stressors is a complex phenomenon that involves multiple mechanisms operating at different biological, spatial and temporal scales [17,18,19,20]. Species-specific sensitivities cover a wide array of strategies, including species ability to tolerate stress, to avoid damage or to re-establish after impacts. Empirical assessments of species sensitivity requires a series of direct measurements on a large number of individuals across life stages (e.g., from provenance trials in Europe and North America [21,22]). Because this type of assessment is logistically difficult to conduct, knowledge of tree sensitivities is available only for a limited number of commercial tree species (e.g., Picea mariana (Mill.) B.S.P. [23]). Breaking down species’ responses to a stressor into the mechanisms underlying these strategies can overcome these challenges and help capture different aspects of impacts associated with climate change (see Figure 1). Our understanding of these mechanisms has increased considerably in recent decades (for instance, see review in [13]), but their integration into vulnerability assessments remains limited.
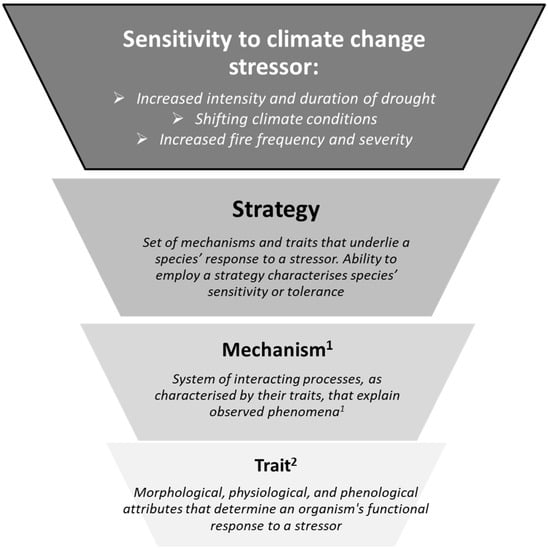
Figure 1.
Conceptual synthesis of the relationship between traits and tree functional response to the three studied climate change stressors. The hierarchy employed here is adapted from [13]. Superscript numbers indicate source of definitions 1 [24], 2 [25].
A trait-based approach can help overcome some of the challenges involved in tree species sensitivity assessment by providing a common framework to facilitate data integration and species comparisons [14,26,27]. Traits are morphological, physiological, and phenological attributes that determine an organism’s functional response to a given environmental filter [25]. Traits are therefore linked to ecological functions and can capture the mechanisms that allow species to persist under a changing climate [18,28,29,30]. Because this information is not species-specific, a trait-based approach provides a common basis to express these mechanisms across many species at once, even those not typically considered together [31,32]. Traits could therefore inform vulnerability by providing a synthetic assessment of species-specific sensitivity [13,33,34].
Using a trait-based framework also provides advantages in the context of adaptation to climate change. Trait-based approaches can guide such decisions and inform practitioners on the mechanisms and strategies underlying species sensitivity to climate change and thus identify species that require special management consideration [35,36]. Furthermore, because it is based on a reproducible and generalizable framework, information can easily be updated as new data become available or as new species are considered.
Such approaches require information from a variety of disciplines. This can be challenging because different fields of research (e.g., tree ecophysiology, ecology) have evolved largely independently, and their information is scattered throughout the scientific literature and elsewhere. The inherent heterogeneity and complexity of the data needed to accurately capture the various strategies and mechanisms underlying tree responses to climate change stressors adds to the challenge of such data integration [37,38]. Synthesising ecological knowledge from various sources into a workable format is therefore an important step towards refining vulnerability assessments [13,21,27].
Building on the frameworks proposed by [13], we show how a trait-based approach can be used to evaluate tree species sensitivity to three key climate change stressors: increased drought events, shifts in climatically suitable habitat, increased fire intensity and frequency. First, for 26 abundant species in eastern Canada, we document and synthesize available data on tree traits that capture species-specific ability to cope with each stressor. We review the quality of this information and identify knowledge and data gaps that can steer future research and data aggregation efforts. Second, we propose a series of trait-based indices that emphasize tree species that could present climate-related sensitivities, as well as identify groups of species with common sensitivities across indices. We discuss how this information can be used by practitioners in vulnerability assessments and evaluate how our indices compare with other published indices. Finally, we discuss how such indices can inform adaptive silvicultural practices in the face of future climate stressors.
2. Materials and Methods
2.1. Trait Documentation
We documented the tree sensitivity of 26 tree species that are among the most abundant in eastern Canada and represent approximately 63% of Canada’s forests total aboveground biomass [39]. Many of these species are commercially important and are considered in forest management planning, while others are important components of the boreal and mixed-temperate forest ecosystems in eastern Canada (see species list in Table 1).

Table 1.
Spearman correlations between the drought indices developed in this paper and other published indices that use various definitions of sensitivity/tolerance (definitions provided below). All indices were standardised to facilitate cross-comparisons (see Table S4). Spearman correlations between indices are indicated at the bottom. * p < 0.1, ** p < 0.05, *** p < 0.01.
Documented traits were selected based on existing frameworks of key mechanisms and traits for each of the three climate change stressors under study [13]: more frequent drought events, shifts in suitable climate conditions, and more frequent and intense fires. These three frameworks are organised around a common conceptual synthesis of the relationship between traits and tree response to climate change impacts (Figure 1). This review identified physiological and ecological traits and related metrics (hereafter called traits) underlying tree response to each stressor (Scheme 1, Scheme 2 and Scheme 3). The development of each of the three frameworks is described in more detail in the Supplementary Materials.
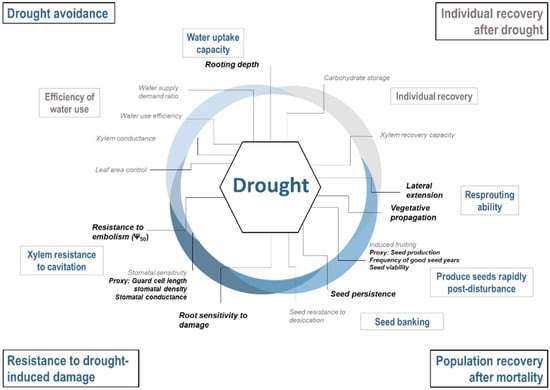
Scheme 1.
Framework of strategies (outer text in black boxes), mechanisms (inner text in grey boxes) and the key traits (inner text) defining species sensitivity to drought. Strategies, mechanisms and traits in grey lack sufficient data to adequately compare sensitivity across species. Traits in bold, black text were used in index development.
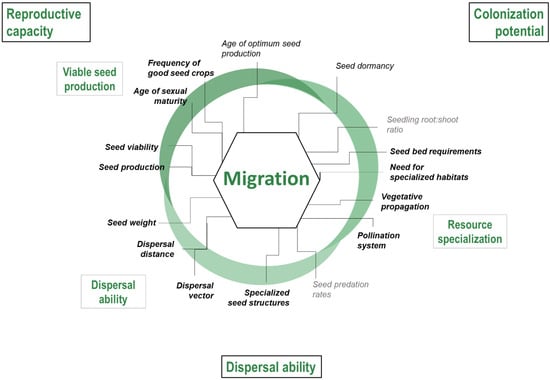
Scheme 2.
Framework of strategies (outer text in black boxes), mechanisms (inner text in grey boxes) and the key traits (inner text) defining ability to track shifts in suitable climate conditions, as defined by capacity for migration. Traits in grey lack sufficient data to adequately compare ability across species. Traits in black text were considered but not chosen for the index. Traits in bold, black text were used in index development.
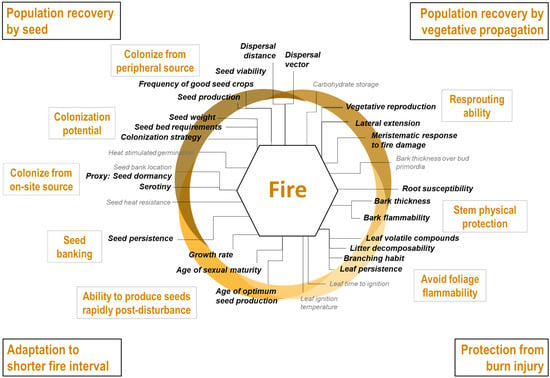
Scheme 3.
Framework of strategies (outer text in black boxes), mechanisms (inner text in grey boxes) and the key traits (inner text) defining tree species sensitivity in more frequent and intense fires. Traits in grey lack sufficient data to adequately compare ability across species. Traits in bold, black text were used in index development.
From each framework, we selected a set of traits that are (1) climate-sensitive, i.e., mechanistically associated with a specific stressor and that characterise differences in species responses (as reviewed by [13]), (2) for which data are available for most species, and (3) without major data comparability issues, i.e., for which available data are comparable among species (selected traits in bold in Scheme 1, Scheme 2 and Scheme 3). We documented the selected traits for our species from an extensive literature review and the TOPIC database [37]. All trait data are available through the TOPIC website (http://cfs.cloud.nrcan.gc.ca/ctn/topic.php).
2.2. Index Development
We developed a series of indices corresponding to the strategies identified in each stressor framework (Scheme 1, Scheme 2 and Scheme 3). Each index (10 in total) ranks the relative ability of the 26 tree species to cope with a stressor based on five classes: low, medium-low, medium, medium-high and high. For the purposes of our classification, species with a low or medium-low score are considered sensitive while tolerant species are those with a medium-high or high score. As our goal was to rank the 26 species studied here, we attributed an index value based on available information from the literature depending if it suggested sensitivity or tolerance (see Section S1 for how values were attributed by strategy). Section S1 and Tables S1–S3 describe index development and species classification.
Drought stress is expected to be an increasingly important factor that could constrain plant growth and survival over the coming century [40,41]. At the level of an individual, a complex set of mechanisms interact during a drought, balancing trade-offs to mitigate water stress, such as carbon gain and water loss [18,42,43,44]. These trade-offs can be summarised within four broad strategies, three of which were used in index development (further detailed in Table S1):
- (1)
- Drought avoidance by maintaining internal water levels: As the water levels drop at the soil surface during drought, continued access to the water and uptake capacity from deeper horizons are crucial to avoid drought (Breda et al. 2006). Shallow rooting species will therefore be the most sensitive.
- (2)
- Resistance to drought-induced damage by maintaining circulation when internal water levels are low: When water access decreases but demand remains constant, mechanisms such as xylem resistance to cavitation become important to avoid mortality. Ψ50 (xylem water potential at which 50% of conductivity is lost) is a widely used metric of xylem resistance [45,46]. Traits that characterize stomatal sensitivity are also important to consider because they underlie species’ ability to limit water loss during prolonged droughts [47].
- (3)
- Population recovery after mortality: the ability of a species to rapidly recolonize an area after drought-induced mortality will influence its persistence in the landscape. Population may recover from several mechanisms including resprouting ability, ability to produce seed rapidly post-disturbance and to store seeds in the seed bank. Traits related to seedling survival are also of importance but was not considered here because of the lack of data on ontological differences in trait expression.
- (4)
- Individual recovery after drought: Several mechanisms influence the ability of individual stems to recover after drought. This includes the capacity to resume hydraulic conductivity after xylem have been embolised and/or produce new conductive tissues [48]. However, questions remain regarding which physiological mechanisms are responsible [49]. Survival may also be influenced by non-structural carbohydrate storage (NSC; [50]), though the exact relationship between NSC and recovery remains an active area of research [51,52,53]. Therefore, we did not develop an index for this strategy.
Large shifts in suitable habitat are projected under climate change by the end of the century and, as a result, several species are expected to fall outside of their suitable climate envelope [3,54]. In such situations, the magnitude and velocity at which climate shifts are expected will require tree species to migrate great distances to keep up [55,56]. Three main strategies drive species range movement and migratory ability: (detailed further in Table S2):
- (1)
- Reproductive capacity: Successful migration is dependent on the reproductive capacity of the source population as well as the time to reach sexual maturity. Propagule pressure, determined by seed production, is an important determinant of the success of recruitment, locally and at their advancing front [57,58,59].
- (2)
- Dispersal ability: Dispersal is the primary mechanism through which species expand their distribution [57,60,61] and species able to disperse seeds over long distances have a higher likelihood of keeping up with rapidly shifting suitable habitats [28,62].
- (3)
- Colonization potential at the advancing front: Once dispersules reach a new site, their ability to germinate, survive, and reproduce will largely determine which species can colonize [63]. Once colonised, species’ ability to tolerate inbreeding and successfully reproduce or propagate in small populations will play a critical role in its migration success [64,65].
Fire frequency and intensity are expected to increase as the climate becomes drier [66,67]. After a fire, a species can persist on the landscape through specific adaptations that protect individual stems or by population recovery after fire [29]. Post-fire tree community composition will depend on intrinsic species characteristics that we organised into four broad strategies (Table S3):
- (1)
- Stem protection from burn injury: Surviving stems are contingent on maintaining intact vascular systems capable of circulating water and sap. Stem physical protection, or the ability to reduce heat transmission through the outer layers to the vascular system (e.g., thick bark), influences whether a stem will survive or not [68]. Additionally, certain leaf traits and crown properties influence flammability and may affect fire intensity and spread [69].
- (2)
- Population recovery by seed, from seed sources on-site or from peripheral areas not affected by the fire. Protecting seeds from fire, i.e., through aerial or soil banks or by protective structures, ensures a direct source of dispersules to re-establish populations [70,71]. When species lack adaptations to protect seeds or when subjected to very intense fires, seeds must originate from the unburnt forest along the edge and beyond, making dispersal ability and seed production crucial mechanisms [72]. Germination requirements also influence post-fire recruitment [73].
- (3)
- Population recovery by vegetative propagation. Resprouting ability is determined mainly by the location of meristematic tissues on the plant and how well protected these tissues are [74]. Like seeds in the soil bank, underground buds may be protected in the soil and consequently have higher survival probabilities, especially in low-intensity fires. The type of vegetative reproduction will also influence the rapidity of population recovery, particularly for species with extensive clonality [75].
- (4)
- Adaptation to shorter fire intervals. Multiple fires in a short time frame may prevent species from re-establishing from seed, even for fire-adapted species [76]. Hence, short fire return intervals favour species that can mature and start to produce seed rapidly after a fire has occurred. Seeds that remain viable for long periods in the soil bank could also provide a source of propagules [70].
2.3. Confidence Scores
We attributed a confidence score to each index score based on the quality and quantity of the information available, i.e., (1) whether the data used was quantitative or ordinal; (2) whether the trait is known to exhibit substantial variation along environmental or temporal gradients (i.e., intraspecific trait variation); (3) the number of independent values available (excluding replicates stemming from circular referencing), (4) the source of the data (primary empirical research, compendium, expert opinion). This information was ranked into five confidence classes (unknown/low, medium-low, medium, medium-sufficient, sufficient), each class receiving a quantitative value (10, 20, 30, 40, 50).
2.4. Defining Species Groups
A principal component analysis (PCA) was conducted on index scores to distinguish groups of species with similar sensitivities. To highlight groups in the PCA, strategy axis scores were ranked from low to high ability and numerical values were attributed to facilitate interpretation (10, 20, 30, 40, 50). Hierarchical clustering was performed on the strategy index scores using a Ward D classification based on Euclidian distances to evaluate whether certain trait values were common to clusters of species. A visual assessment of the plot of within-group sum of squares showed that k = 8 was an appropriate number of clusters.
2.5. Development of Integrated Indices of Drought Sensitivity and Comparison with Other Published Indices
To illustrate different ways species can respond to drought, we showcase two ways of aggregating the information provided by our indices: (a) stem-level only: an integrated index of sensitivity to drought-induced mortality at the stem level that combines the strategies Drought avoidance and Resistance to damage, (b) stem and population levels: an integrated index that combines our three drought strategy indices, covering both stem sensitivity to drought and population recovery after mortality.
To evaluate how our integrated indices compare with other published indices, we surveyed the literature and databases for other indices of drought tolerance/sensitivity and for which data were available for most or all of our 26 species. Six indices were selected from published literature sources and online databases. Spearman rank correlations were used to test the level of association between these indices and their significance as well as between our two integrated indices.
3. Results
Our set of indices outlines species-specific sensitivities to climate change stressors (Figure 2, Figure 3 and Figure 4) and the PCA shows species with common abilities (Figure S1, for group description). Cluster analysis on the PCA scores reveals eight groups of species (Figure S1, Figure 2); the descriptions of the groups are located in Section S2. Of the 26 species assessed, Tsuga canadensis (L.) Carrière and Abies balsamea (L.) Mill are classified as the most sensitive species (i.e., classified as low ability across multiple indices), while Acer rubrum L. and Populus spp. emerge as the least sensitive (i.e., classified as high ability; Figure 2, Figure 3 and Figure 4, Index scores). Tree sensitivity to climate change also varies according to the stressor. For instance, Pinus banksiana Lamb. and Quercus rubra L. possess high drought avoidance and resistance (Figure 2, Index), while early successional deciduous species (Populus spp. and Betula papyrifera Marsh.) possess traits that ensure good reproductive capacity and migration ability (Figure 3, index). With the exception of Pinus spp., many species such as Picea rubens Sarg. and Acer saccharum Marsh. are considered as having a low ability to persist under a more frequent and intense fires (Figure 4, index).
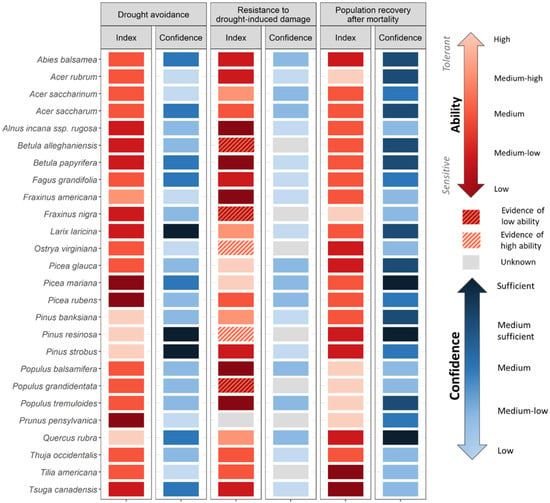
Figure 2.
Tree species ability to cope with drought for three main strategies: Drought avoidance, Resistance to drought-induced damage, Population recovery after drought. Each index ranks the relative ability (from low/sensitive to high/tolerant, reds) of tree species for a specific strategy (see Scheme 1 for which mechanisms/traits were considered). Species for which information was not available were attributed an intermediate value, depending if there was evidence of tolerance or not (diagonal hatched stripes: dark red hatched—evidence of low ability; light red hatched—evidence of high ability). Confidence associated with available data is also presented, as ranked from low to sufficient (blues). Species for which no data was available were considered as Unknown (grey). For more details on definitions and ranking, see Table S1.
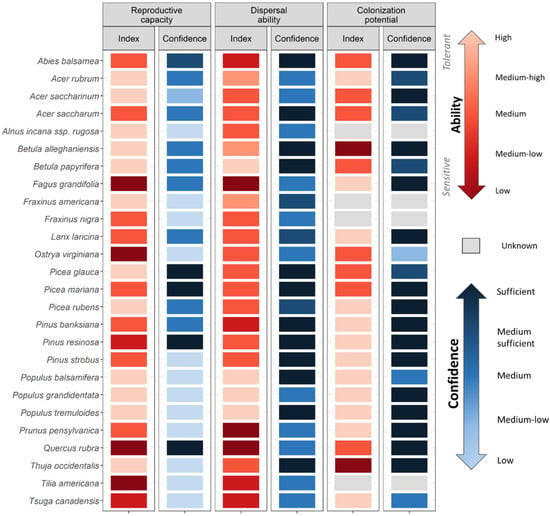
Figure 3.
Tree species ability to track shifts in suitable climate conditions, as considered using three main strategies that influence migration: Reproductive capacity, Dispersal ability and Colonization potential. Each index ranks the relative ability (from low/sensitive to high/tolerant, reds) of tree species for a specific strategy (see Scheme 2 for which mechanisms/traits were considered). Confidence associated with available data is also presented, as ranked from low to sufficient (blues). Species for which no data was available were considered as Unknown (grey). For more details on definitions and ranking, see Table S2.
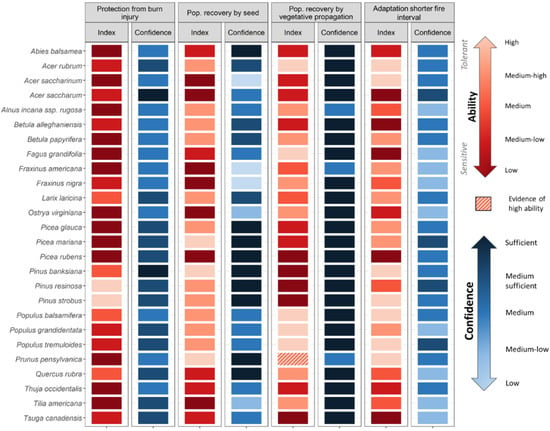
Figure 4.
Tree species ability to persist through more frequent and intense fires, as characterised by four main strategies: Protection from burn injury, Population recovery by seed, Population recovery by vegetative propagation, Adaptation to shorter fire intervals. Each index ranks the relative ability (from low/sensitive to high/tolerant, reds) of tree species for a specific strategy (see Scheme 3 for which mechanisms/traits were considered). Species for which information was not available were attributed an intermediate value, depending if there was evidence of tolerance or not (diagonal hatched stripes: dark red hatched—evidence of low ability; light red hatched—evidence of high ability). Confidence associated with available data is also presented, as ranked from low to sufficient (blues). For more details on definitions and ranking, see Table S3.
We were able to find information for 95% of the trait-species combinations across the 10 indices. The quality of available data varies widely across stressors, strategies and/or mechanisms (Figure 2, Figure 3 and Figure 4, Confidence). Traits related to individual-level sensitivity to drought are generally poorly documented with 62% and 100% of species categorised as low or medium-low confidence for Drought Avoidance and Drought Resistance. By comparison, data coverage for traits related to the ability to track climate and to fire sensitivity was better, with only 23% and 14% of species classified as low to medium-low confidence on average. Among species, conifer species are generally better documented than deciduous ones (average confidence for conifer 37% and 29% for deciduous), reflecting the higher data availability of commercial species (e.g., Picea spp., Pinus spp., and Abies balsamea). Data availability is also generally lower for the temperate biome, with only the most commercially important deciduous species sufficiently documented such as Acer saccharum (9 of 10 indices considered medium confidence or higher), Quercus rubra and Betula alleghaniensis Britt. (both 8 of 10). Species such as Fraxinus spp., Alnus incana subsp. rugosa (Du Roi) R.T. Claussen, Tilia americana L. and Ostrya virginiana (Mill.) K. Koch. are generally poorly documented (at least 6 of 10 indices with low to medium-low confidence).
Our two integrated indices of drought sensitivity (stem-level only and stem and population levels) show some variation in species ranking (Table S4). The largest differences between these two integrated indices arise from Prunus pensylvanica L.f., Populus spp., Fraxinus nigra Marsh. and Acer rubrum, because these species show strong potential for population recovery. Nevertheless, Spearman rank correlations between these two integrated indices are relatively high (r = 0.72, p < 0.01; Table 1). There was substantial variation between these integrated indices and the six indices published in the literature. Our stem-level integrated index was most closely correlated with Niinemets and Valladares [34] (Stem only - r = 0.61, p < 0.01; Table 1) and our stem- and population-level integrated index correlated most closely with Boulet and Huot (Stem and population: r = 0.63, p < 0.01, [77]). Our integrated indices did not correlate well with qualitative indices such as the USDA PLANTS database (Stem only - r = 0.0034, p = 0.99; Stem and population r = −0.061, p = 0.77; [78]). We also observed substantial variation between the published indices: Hightshoe [79] showed the highest correlation with Boulet and Huot (r = 0.65, p < 0.01, [77]), whereas the former showed weaker correlation with USDA NRCS (r= −0.13, p= 0.55. [78]) and the OFGAC database (r = −0.27, p= 0.19; [80]).
4. Discussion
In this paper, we provide a series of trait-based indices on the sensitivities of the most abundant tree species in eastern Canada to three important climate change stressors: increased occurrence of drought, shifts in suitable climate conditions and more frequent and intense fires. Instead of developing a single catch-all index, we provide a set of indices that capture different strategies and mechanisms involved in species sensitivity to the different climate stressors. Each index was developed from a set of traits with a clear conceptual link with each stressor and following a reproducible dataflow. New species can be documented when needed, for instance, when applying this approach to other regions of the world or documenting tree species that are expected to become more abundant in the studied region with climate change (e.g., at their northern limit). Based on current knowledge (as shown in Figure 2, Figure 3 and Figure 4), several key traits lack sufficient information to compare across species and could be added in the future as more data becomes available.
In recent decades, many sensitivity (or inversely, tolerance) indices have been developed using different approaches and objectives. In this paper, we reviewed the various drought sensitivity indices from the published and grey literature. Which species are considered sensitive varied substantially across indices, and largely (Table S4) relate to differences in the definitions employed. For instance, Pinus resinosa Sol. ex. Aiton is classified as highly tolerant to drought by Boulet and Huot [77], intermediate by Hightshoe [79] and Niinemets and Valladares [34], and drought sensitive by USDA NRCS [78] and Matthews et al. [81]. We show that species ranking can vary considerably when using different definitions of drought sensitivity in our own set of indices; we obtained different rankings for some species depending on whether population level recovery mechanisms were included or not. Our stem-level drought sensitivity classification was most comparable to the index proposed by Niinemets and Valladares [34] and which incorporates ecophysiological and morphological data (Table S4). This demonstrates the importance of providing methodology, definitions and data workflow when creating such indices.
The use of a single catch-all index risks missing important response mechanisms by oversimplifying species’ responses to climate change. Breaking down species’ responses to a stressor into mechanisms can capture complementary aspects of sensitivity or tolerance and the relative importance one gives to different strategies can be accounted for in index development. This can facilitate the interpretation of species responses like stem survival and population resilience to stressors. For example, drought-induced mortality can be influenced by drought duration, stand characteristics, soil type and hydrology as well as species characteristics [40,82]. In addition, Populus tremuloides Michx. is generally classified as having low drought tolerance (e.g., [34,81]; Table S4) but can be regarded as drought tolerant if we consider population-level recovery mechanisms. The value of our strategy-based sensitivity indices is that users can combine these strategies into integrated indices that matter for their management objectives, i.e., depending on whether they are more concerned with mature stem mortality or maintaining species presence on a specific landscape. Indeed, the intended goal of this manuscript is to provide a flexible framework to document species sensitivity that could be later supplemented by users. This is the reason the rationale and data workflow behind each index building is made available in Section S1.
In the future, these indices could be refined as new data becomes available, such as, for instance, intraspecific variation in trait values. Many of the traits included in our framework show intraspecific variability that is linked to life stage, and adaptation to local environmental conditions. For example, inclusion of variation in hydraulic traits like resistance to cavitation, especially between life stages or across the geographical distribution, could inform drought indices such as ours [83,84]. Variation in seed production through time would also be informative for an index of migration ability [85], and particularly where temperature imposes constraints on phenology [86,87]. Building upon this variability, our sensitivity indices could be adjusted for different tree populations of the same species exhibiting different phenotypes or to account for variation for species with broad ecological amplitudes (e.g., Acer rubrum). However, low data availability at the intraspecific level currently limits this potential [84,88].
4.1. Limitations of Trait-Based Indices
Index quality is highly dependent on data availability. Some important mechanisms and traits remain insufficiently understood and/or documented to be included in our index (see traits in grey in Scheme 1, Scheme 2 and Scheme 3). Still, identifying clear knowledge gaps helps define a research agenda to address these disparities. For instance, evidence suggests that xylem cavitation resistance using Ψ88 instead of Ψ50 shows a stronger relationship with drought tolerance in angiosperms [89], but not enough data could be found for our species to include it here. With growing interest in considering its importance in drought tolerance [13,45,90], it is likely that these data will become available in the future.
In addition to this uncertainty, differences in trait documentation can impose bias on the quality and interpretability of sensitivity indices. For example, commercial species in Canada (mostly conifers) are generally better documented than species with low economic value (which includes several broadleaved species). While some temperate forests dominated by broadleaved species are expected to be exposed to higher fire activity in the future [91] many traits underlying their response to fire are not systematically documented (e.g., leaf ignition temperature and leaf time to ignition). Breaking the cycle by which only well documented species are included and extensively studied is necessary to build comparable indices between species.
An additional challenge in developing trait-based indices is the quality of trait data itself [92], even in the case of widely documented traits. Several quantitative traits remain documented mainly in qualitative terms which are then aggregated into large classes, severely limiting comparative analyses. For example, the quantitative values associated with “deep” roots in eastern Canada (e.g., Pinus banksiana, P. resinosa and P. strobus L.; 2–5 m) are different than what is considered “deep” in western North America (e.g., Pinus ponderosa, range from 2 to 24 m; [93,94,95,96]). Another example is seed dispersal distance; ideally, this should be documented from seed dispersal kernels but kernels are available for only a handful of species [97]. Dispersal ability is generally inferred from broad classes of dispersal vectors [98]. Recently, efforts have been made to develop standardised measures of seed dispersal distance based on seed traits (e.g., dispeRsal, [99]), but these models still require field validation. Similarly, some categorical traits may show some variation in quantitative values. For instance, we consider serotiny to be a well-documented categorical trait; however, this does not take into account the variation in the proportion of cones exhibiting serotiny, such as shown by Picea mariana [100].
An important step in developing trait-based indices is to identify the extent to which index values capture differences in tree responses. Indices that compare species among each other risk artificially amplifying differences in sensitivity if the species pool is limited to those with similar sensitivities. Although we focused on species common to eastern Canada, our set of 26 tree species does represents a wide range of species’ sensitivity. Similar to our indices, another ecological classification of 11 tree species in Central Europe did not find such redundancies [92], finding instead that certain traits (e.g., Leaf area index, Winter frost sensitivity, and Maximum tree height) were useful to distinguish between various successional groups. Indeed, we found that the drought index most closely related to ours ([34], which assessed drought tolerance of 806 trees and shrubs across 3 continents) had values for our set of 26 species ranging from 1 to 4 out of a scale of 5 (mean value of 2.3 ± 0.69, Table S4), which reflects a substantial part of the range they considered. Likewise, the large differences in migration capacity of our 26 species are exemplified by the range of values for traits underlying reproductive and dispersal abilities. Our selected species show both high variation in viable seed production (22000 seeds—Fagus grandifolia Ehr. to 500 million seeds for Acer rubrum), and dispersal ability (e.g., short-distance dispersal—Quercus rubra (seed weight estimated at 276 seeds/kg) vs. long-distance dispersal—Populus tremuloides (seed weight estimated at 70 million seeds/kg)). Our set of species includes those that are common in boreal forests, where fire is a major force shaping tree adaptations, and temperate forests, where the ecological role of fire is more limited. The functional diversity in sensitivity to increased fire intensity and frequency is further emphasised by incorporating divergent strategies during disturbance and afterwards (e.g. protection from injury for Pinus spp. vs. population recovery for Populus spp.).
4.2. Species Sensitivity to Inform Adaptation Practices
Considering the magnitude of projected climate change, practitioners will likely need to revisit current management approaches and silvicultural practices and assess whether they are suitable under anticipated conditions [101,102]. Sensitivity indices such as those provided here can serve to assist managers in developing climate adaptation strategies by highlighting which species could be favoured—or not—by future climate conditions. As such, trait-based indices could provide some guidance on species that represent potential investment risk or opportunities. From this coarse assessment, more in-depth empirical studies would be needed to assess whether a species effectively tolerates such conditions. Species’ response to a climate stressor depends on site-level factors and is highly affected by synergistic effects of cumulative stressors (e.g., drought and pests; [6,103]), as well as species intrinsic capacity to adapt [17,104]. All components of vulnerability (i.e., exposure, sensitivity and adaptive capacity), as well as the uncertainty related to climate projections [105], should therefore be considered when adapting management approaches. These sensitivity indices can be used in conjunction with such comprehensive assessments to consider which adaptation measures could be employed and under which situations they should be considered (sensu [100]).
Our results suggest that certain species may require less active intervention or may even be likely to be favoured by climate change (e.g., Pinus spp., Populus spp. and Acer rubrum: groups 1 and 2 in Figure 5), while others could require considerable investment to retain in their current location (e.g., Abies balsamea, Acer saccharum and other species of group 8 in Figure 5). The first groups of species may thus represent better options for passive adaptation measures [106], whereas long-term planned and transformational (sensu [107]) actions might be required for certain species, including the assisted migration of those species in the last group [108].
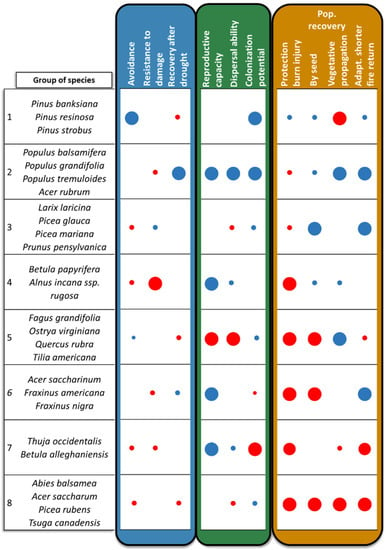
Figure 5.
Groups of species with similar sensitivities or tolerances as defined by the various strategies that characterize species responses to climate change stressors (Drought indices = blue, Migration = green, Fire = orange). Average values within an index are indicated by the red (sensitivity) and blue (tolerance) circles respectively, with larger circles denoting more extreme values. Strategies without circles indicate groups for which species either possess intermediate values or lack a clear trend.
Forest adaptation is also commonly presented as a portfolio of options that aim to resist change, promote resilience of forest ecosystems (i.e., its capacity to recover following a disturbance) or facilitate its transition to alternate state [9,36,109]. Our sensitivity indices can guide species selection under these various scenarios. For example, with drier, more fire prone conditions expected under climate change [6,91], Pinus spp. are the native species most likely to resist change in eastern Canada because they possess traits that favour drought avoidance and protection from burn injury (group 1 in Figure 5). In contrast, despite relatively low resistance of individuals, Populus spp. and Acer rubrum have the highest potential of population recovery following drought and fire as well as the highest capacity to migrate. Stands comprised of these species may therefore be more resilient to change than resistant to it. Other species groups might represent different combinations of resistance or resilience options depending on the stressor considered. For example, the group comprised of Larix laricina (Du Roi) K. Koch, Picea spp. and Prunus pensylvanica (group 3 in Figure 5) has a low sensitivity to drought damage but might be resilient to fire because of their high ability to re-establish from seed and their adaptation to shorter fire return.
Species that are sensitive at stem and the population levels could be candidates for the transition option (e.g., Acer saccharum and other species in group 8). Transition can be achieved by replacing local seed sources with provenances of the same species that are better adapted to future conditions or with more resistant or resilient species depending on the management objectives [17,102]. Similarly, species that are transitioned out of one site might become translocated species in another [110]. For example, the climatic conditions suitable for Acer saccharum are predicted to shift northward beyond its current range and at a rate that will exceed its migration capacity [54,56]. Because this species has a low resistance to both drought and fire and possesses a low potential for population recovery, assisted range expansion of this species northward might be a viable option [102]. However, translocating tree populations is expensive and may have mixed success especially for species whose colonisation potential is limited due to specialised requirements, such as those found in groups 6 and 7 (Figure 5). For these species, assisted migration should be combined with targeted interventions to ensure seedling establishment. An example of such measures includes seedbed preparation by scarification to favour regeneration of Betula alleghaniensis [111].
Our results also highlight that certain species that are likely to cope well under climate change, i.e., those with a high recovery potential and migration capacity (species from group 2 and 4; Figure 5) are currently undesired species from an economic point of view. Their presence may affect competition dynamics with species of higher commercial value (e.g., Pinus spp. and Picea spp.), especially if these species are sensitive to climate change (e.g., Acer saccharum, Betula alleghaniensis). Conversely, evidence suggests that some potentially climate-resilient species may favour the successful establishment and migration of other more commercially important species by promoting changes in stand characteristics. For instance, in sites dominated by conifers that are currently limiting the establishment of commercial hardwood species [112,113], Populus spp. and Acer rubrum could act as catalysers of change by improving soil nutrient status and seedling establishment conditions [114]. New management approaches – and possibly new market opportunities—will be needed for resilient species found in sites where other commercially valuable species are found.
5. Conclusions
In this paper, we evaluated the sensitivity of 26 tree species to stressors associated with climate change and provided a set of trait-based indices that are reproducible and can be adjusted to the specific context of the user and as new data becomes available. Overall, species like Populus spp., Pinus spp. and Acer rubrum could be favoured under climate change, while others like Abies balsamea and Acer saccharum could face considerable challenges in persisting under increasing prevalence of climate-related disturbances. While a trait-based approach provides an important perspective in addressing species sensitivities, the interpretability of such indices is still limited by data availability and quality, as well as by which species are considered and the breadth of sensitivities or tolerances they represent. Additionally, such an approach does not provide insight as to which stressor—drought, fire or ability to track suitable conditions as well as others not considered here (e.g., pests, disease)—will be most important for forest health (on their own or synergistically), and could result in ecological surprises that are difficult to anticipate [115]. Despite these challenges, these indices could nevertheless help adaptation decision making, by improving models based on climate change exposure, identifying species that may require specific management consideration or rethinking species that may become economically non-viable in some regions. Despite a new offering in the toolbox, this paper underscores the difficult task of managing species in a changing climate [100,116,117]. These issues outline how knowledge gaps are still important roadblocks towards the development of robust indicators and a better understanding of the thresholds at which species are sensitive. This highlights the continued need for foundational research into species biology and the invaluable field data collections that are necessary for synthesis, comparisons between species, and modelling.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/11/9/989/s1. Table S1: Index development of species’ sensitivity to drought for three main strategies, their component mechanisms and traits. Table S2: Index development of species’ ability to track shifting climate conditions (i.e. migration ability), their component mechanisms and traits. Table S3: Index development of species’ ability to persist in more frequent and intense fires, their component mechanisms and traits. Table S4: Comparison of the integrated drought sensitivity indices developed in this paper with six published drought tolerance indices. Figure S1. Principal component analysis showing overlapping sensitivities for the 26 tree species.
Author Contributions
Conceptualisation, I.A., L.B.-M.; methodology, I.A., L.B.-M.; formal analysis, L.B.-M.; writing—original draft, L.B.-M., I.A.; writing—review and editing, S.R.-T., P.N., F.D., L.B.-M., I.A.; supervision: I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Forest Change Initiative (Canadian Forest Service, Natural Resources Canada).
Acknowledgments
Thank you to Françoise Cardou and Hedi Kebli for early work in developing the Drought and Migration indices. We also thank Françoise Cardou for revising the text and graphics. Thank you to Kevin Good, Sandrine Gautier-Éthier, Kellina Higgins and Margot Downey for surveying the literature on traits. This work was nourished by stimulating discussions from the Tree Traits and Climate Change workshop held in Mont St-Hilaire in April 2013 and with several end users (Carine Annecou [Agence forestière des Bois Francs] and Éric Domaine).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lemprière, T.C.; Bernier, P.; Carroll, A.; Flannigan, M.; Gilsenan, R.; McKenney, D.; Hogg, E.; Pedlar, J.; Blain, D. The Importance of Forest Sector Adaptation to Climate Change; Information Report NOR-X-416E; Canadian Forest Service, Natural Resources Canada: Québec, QC, Canada, 2008; 78p.
- Reyer, C.P.O.; Leuzinger, S.; Rammig, A.; Wolf, A.; Bartholomeus, R.P.; Bonfante, A.; de Lorenzi, F.; Dury, M.; Gloning, P.; Abou Jaoudé, R.; et al. A plant’s perspective of extremes: Terrestrial plant responses to changing climatic variability. Glob. Chang. Biol. 2013, 19, 75–89. [Google Scholar] [CrossRef] [PubMed]
- McKenney, D.W.; Pedlar, J.H.; Rood, R.B.; Price, D. Revisiting projected shifts in the climate envelopes of North American trees using updated general circulation models. Glob. Chang. Biol. 2011, 17, 2720–2730. [Google Scholar] [CrossRef]
- Zhang, X.; Flato, G.; Kirchmeier-Young, M.; Vincent, L.; Wan, H.; Wang, X.; Rong, R.; Fyfe, J.; Li, G.; Kharin, V. Changes in Temperature and Precipitation across Canada; Canada’s Changing Climate Report; Bush, E., Lemmen, D.S., Eds.; Government of Canada: Ottawa, ON, Canada, 2019; pp. 112–193. Available online: https://changingclimate.ca/CCCR2019/ (accessed on 5 September 2019).
- Bouchard, M.; Aquilué, N.; Périé, C.; Lambert, M.-C. Tree species persistence under warming conditions: A key driver of forest response to climate change. Ecol. Manag. 2019, 442, 96–104. [Google Scholar] [CrossRef]
- Boucher, D.; Boulanger, Y.; Aubin, I.; Bernier, P.Y.; Beaudoin, A.; Guindon, L.; Gauthier, S. Current and projected cumulative impacts of fire, drought, and insects on timber volumes across Canada. Ecol. Appl. 2018, 28, 1245–1259. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef]
- Edwards, J.; Pearce, C.; Ogden, A.; Williamson, T. Climate Change and Sustainable Forest Management in Canada: A Guidebook for Assessing Vulnerability and Mainstreaming Adaptation into Decision Making; Canadian Council of Forest Ministers: Ottawa, ON, Canada, 2015; p. 160. Available online: https://cfs.nrcan.gc.ca/publications/download-pdf/35956 (accessed on 5 September 2019).
- Swanston, C.W.; Janowiak, M.K.; Brandt, L.A.; Butler, P.R.; Handler, S.D.; Shannon, P.D.; Lewis, A.D.; Hall, K.; Fahey, R.T.; Scott, L. Forest Adaptation Resources: Climate Change Tools and Approaches for Land Managers; General Technical Reports; US Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2016; p. 161. [CrossRef]
- Nadeau, C.P.; Fuller, A.K.; Rosenblatt, D.L. Climate-smart management of biodiversity. Ecosphere 2015, 6, 1–17. [Google Scholar] [CrossRef]
- Rogers, B.M.; Jantz, P.; Goetz, S.J. Vulnerability of eastern US tree species to climate change. Glob. Chang. Biol. 2017, 23, 3302–3320. [Google Scholar] [CrossRef] [PubMed]
- Zolkos, S.G.; Jantz, P.; Cormier, T.; Iverson, L.R.; McKenney, D.W.; Goetz, S.J. Projected Tree Species Redistribution Under Climate Change: Implications for Ecosystem Vulnerability Across Protected Areas in the Eastern United States. Ecosystems 2015, 18, 202–220. [Google Scholar] [CrossRef]
- Aubin, I.; Munson, A.D.; Cardou, F.; Burton, P.J.; Isabel, N.; Pedlar, J.H.; Paquette, A.; Taylor, A.R.; Delagrange, S.; Kebli, H.; et al. Traits to stay, traits to move: A review of functional traits to assess sensitivity and adaptive capacity of temperate and boreal trees to climate change. Environ. Rev. 2016, 24, 164–186. [Google Scholar] [CrossRef]
- Stahl, U.; Reu, B.; Wirth, C. Predicting species’range limits from functional traits for the tree flora of North America. Proc. Natl. Acad. Sci. USA 2014, 111, 13739–13744. [Google Scholar] [CrossRef]
- Urban, M.C.; Bocedi, G.; Hendry, A.P.; Mihoub, J.B.; Pe’er, G.; Singer, A.; Bridle, J.R.; Crozier, L.G.; De Meester, L.; Godsoe, W.; et al. Improving the forecast for biodiversity under climate change. Science 2016, 353, aad8466. [Google Scholar] [CrossRef] [PubMed]
- Glick, P.; Stein, B.; Edelson, N. Scanning the Conservation Horizon: A Guide to Climate Change Vulnerability Assessment; National Wildlife Federation: Washington, DC, USA, 2011; p. 168. Available online: https://www.fs.usda.gov/treesearch/pubs/37406 (accessed on 5 September 2019).
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L. Spatial and temporal variation in plant hydraulic traits and their relevance for climate change impacts on vegetation. New Phytol. 2015, 205, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.E.; Urban, M.C.; Tewksbury, J.; Gilchrist, G.W.; Holt, R.D. A framework for community interactions under climate change. Trends Ecol. Evol. 2010, 25, 325–331. [Google Scholar] [CrossRef]
- Helmuth, B.; Kingsolver, J.G.; Carrington, E. BIOPHYSICS, PHYSIOLOGICAL ECOLOGY, AND CLIMATE CHANGE: Does Mechanism Matter? Annu. Rev. Physiol. 2005, 67, 177–201. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M.; Holland, V.; Brüggemann, W. Functional traits and adaptive capacity of European forests to climate change. Environ. Exp. Bot. 2015, 111, 91–113. [Google Scholar] [CrossRef]
- Alberto, F.J.; Aitken, S.N.; Alía, R.; González-Martínez, S.C.; Hänninen, H.; Kremer, A.; Lefèvre, F.; Lenormand, T.; Yeaman, S.; Whetten, R.; et al. Potential for evolutionary responses to climate change—Evidence from tree populations. Glob. Chang. Biol. 2013, 19, 1645–1661. [Google Scholar] [CrossRef]
- Lamhamedi, M.S.; Bernier, P.Y. Ecophysiology and field performance of black spruce (Picea mariana): A review. Ann. Sci. 1994, 51, 529–551. [Google Scholar] [CrossRef]
- Craver, C.; Tabery, J. Mechanisms in Science. In The Stanford Encyclopedia of Philosophy; Zalta, E.N., Ed.; Metaphysics Research Lab, Stanford University: Stanford, CA, USA, 2019; Available online: https://plato.stanford.edu/entries/science-mechanisms/ (accessed on 7 September 2019).
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Shipley, B. Comparative Plant Ecology as a Tool for Integrating Across Scales. Ann. Bot. 2007, 99, 965–966. [Google Scholar] [CrossRef]
- Willis, S.G.; Foden, W.; Baker, D.J.; Belle, E.; Burgess, N.D.; Carr, J.A.; Doswald, N.; Garcia, R.A.; Hartley, A.; Hof, C.; et al. Integrating climate change vulnerability assessments from species distribution models and trait-based approaches. Biol. Conserv. 2015, 190, 167–178. [Google Scholar] [CrossRef]
- Angert, A.L.; Crozier, L.G.; Rissler, L.J.; Gilman, S.E.; Tewksbury, J.J.; Chunco, A.J. Do species’traits predict recent shifts at expanding range edges? Ecol. Lett. 2011, 14, 677–689. [Google Scholar] [CrossRef]
- Pausas, J.G.; Verdú, M. Plant Persistence Traits in Fire-Prone Ecosystems of the Mediterranean Basin: A Phylogenetic Approach. Oikos 2005, 109, 196–202. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Anderegg, W.R.L.; Paine, C.E.T.; Hoffmann, W.A.; Kartzinel, T.; Rabin, S.S.; Sheil, D.; Franco, A.C.; Pacala, S.W. Convergence of bark investment according to fire and climate structures ecosystem vulnerability to future change. Ecol. Lett. 2017, 20, 307–316. [Google Scholar] [CrossRef]
- Garnier, E.; Navas, M.-L.; Grigulis, K. Plant Functional Diversity: Organism Traits, Community Structure, and Ecosystem Properties; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Lavorel, S.; McIntyre, S.; Landsberg, J.; Forbes, T.D.A. Plant functional classifications: From general groups to specific groups based on response to disturbance. Trends Ecol. Evol. 1997, 12, 474–478. [Google Scholar] [CrossRef]
- Foden, W.B.; Butchart, S.H.M.; Stuart, S.N.; Vié, J.-C.; Akçakaya, H.R.; Angulo, A.; DeVantier, L.M.; Gutsche, A.; Turak, E.; Cao, L.; et al. Identifying the World’s Most Climate Change Vulnerable Species: A Systematic Trait-Based Assessment of all Birds, Amphibians and Corals. PLoS ONE 2013, 8, e65427. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Valladares, F. Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecol. Monogr. 2006, 76, 521–547. [Google Scholar] [CrossRef]
- Butt, N.; Gallagher, R. Using species traits to guide conservation actions under climate change. Clim. Chang. 2018, 151, 317–332. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate change and forests of the future: Managing in the face of uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Aubin, I.; Cardou, F.; Boisvert-Marsh, L.; Garnier, E.; Strukelj, M.; Munson, A.D. Managing data locally to answer questions globally: The role of collaborative science in ecology. J. Veg. Sci. 2020, 31, 509–517. [Google Scholar] [CrossRef]
- Hicks, C.C.; Fitzsimmons, C.; Polunin, N.V.C. Interdisciplinarity in the environmental sciences: Barriers and frontiers. Env. Conserv. 2010, 37, 464–477. [Google Scholar] [CrossRef]
- Beaudoin, A.; Bernier, P.Y.; Guindon, L.; Villemaire, P.; Guo, X.J.; Stinson, G.; Bergeron, T.; Magnussen, S.; Hall, R.J. Mapping attributes of Canada’s forests at moderate resolution through kNN and MODIS imagery. Can. J. Res. 2014, 44, 521–532. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Clark, J.S.; Iverson, L.; Woodall, C.W.; Allen, C.D.; Bell, D.M.; Bragg, D.C.; D'Amato, A.W.; Davis, F.W.; Hersh, M.H.; Ibanez, I.; et al. The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Glob. Chang. Biol. 2016, 22, 2329–2352. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; OsÓRio, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How Plants Cope with Water Stress in the Field? Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Hartmann, H.; Ziegler, W.; Trumbore, S. Lethal drought leads to reduction in nonstructural carbohydrates in Norway spruce tree roots but not in the canopy. Funct. Ecol. 2013, 27, 413–427. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Johnson, D.M.; Lachenbruch, B.; McCulloh, K.A.; Woodruff, D.R. Xylem hydraulic safety margins in woody plants: Coordination of stomatal control of xylem tension with hydraulic capacitance. Funct. Ecol. 2009, 23, 922–930. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Klein, T.; Jansen, S.; Choat, B.; Sack, L. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc. Natl. Acad. Sci. USA 2016, 113, 13098. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M. Stomatal protection against hydraulic failure: A comparison of coexisting ferns and angiosperms. New Phytol. 2004, 162, 663–670. [Google Scholar] [CrossRef]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef]
- Johnson, D.M.; McCulloh, K.A.; Woodruff, D.R.; Meinzer, F.C. Hydraulic safety margins and embolism reversal in stems and leaves: Why are conifers and angiosperms so different? Plant Sci. 2012, 195, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Yakir, D.; Buchmann, N.; Grünzweig, J.M. Towards an advanced assessment of the hydrological vulnerability of forests to climate change-induced drought. New Phytol. 2014, 201, 712–716. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Chang. 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural Carbon in Woody Plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef]
- McDowell, N.G.; Beerling, D.J.; Breshears, D.D.; Fisher, R.A.; Raffa, K.F.; Stitt, M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 2011, 26, 523–532. [Google Scholar] [CrossRef]
- Tague, C.L.; McDowell, N.G.; Allen, C.D. An Integrated Model of Environmental Effects on Growth, Carbohydrate Balance, and Mortality of Pinus ponderosa Forests in the Southern Rocky Mountains. PLoS ONE 2013, 8, e80286. [Google Scholar] [CrossRef]
- Périé, C.; de Blois, S. Dominant forest tree species are potentially vulnerable to climate change over large portions of their range even at high latitudes. PeerJ 2016, 4, e2218. [Google Scholar] [CrossRef]
- Aubin, I.; Boisvert-Marsh, L.; Kebli, H.; McKenney, D.; Pedlar, J.; Lawrence, K.; Hogg, E.H.; Boulanger, Y.; Gauthier, S.; Ste-Marie, C. Tree vulnerability to climate change: Improving exposure-based assessments using traits as indicators of sensitivity. Ecosphere 2018, 9, e02108. [Google Scholar] [CrossRef]
- Boisvert-Marsh, L.; Périé, C.; de Blois, S. Shifting with climate? Evidence for recent changes in tree species distribution at high latitudes. Ecosphere 2014, 5, 1–33. [Google Scholar] [CrossRef]
- Clark, J.S. Why trees migrate so fast: Confronting theory with dispersal biology and the Paleorecord. Am. Nat. 1998, 152, 204–224. [Google Scholar] [CrossRef]
- Hampe, A. Plants on the move: The role of seed dispersal and initial population establishment for climate-driven range expansions. Acta Oecol. 2011, 37, 666–673. [Google Scholar] [CrossRef]
- Nathan, R.; Horvitz, N.; He, Y.; Kuparinen, A.; Schurr, F.M.; Katul, G.G. Spread of North American wind-dispersed trees in future environments. Ecol. Lett. 2011, 14, 211–219. [Google Scholar] [CrossRef]
- Broennimann, O.; Thuiller, W.; Hughes, G.; Midgley, G.F.; Alkemade, J.M.R.; Guisan, A. Do geographic distribution, niche property and life form explain plants’vulnerability to global change? Glob. Chang. Biol. 2006, 12, 1079–1093. [Google Scholar] [CrossRef]
- Corlett, R.T.; Westcott, D.A. Will plant movements keep up with climate change? Trends Ecol. Evol. 2013, 28, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.; Morales-Castilla, I.; Caplat, P.; Early, R. Usefulness of Species Traits in Predicting Range Shifts. Trends Ecol. Evol. 2016, 31, 190–203. [Google Scholar] [CrossRef]
- Godoy, O.; Valladares, F.; Castro-Díez, P. The relative importance for plant invasiveness of trait means, and their plasticity and integration in a multivariate framework. New Phytol. 2012, 195, 912–922. [Google Scholar] [CrossRef]
- Pannell, J.R.; Barrett, S.C.H. Baker’s law revisited: Reproductive assurance in a metapopulation. Evolution 1998, 52, 657–668. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Boulanger, Y.; Gauthier, S.; Burton, P.J. A refinement of models projecting future Canadian fire regimes using homogeneous fire regime zones. Can. J. Res. 2014, 44, 365–376. [Google Scholar] [CrossRef]
- Girardin, M.P.; Ali, A.A.; Carcaillet, C.; Gauthier, S.; Hély, C.; Le Goff, H.; Terrier, A.; Bergeron, Y. Fire in managed forests of eastern Canada: Risks and options. Ecol. Manag. 2013, 294, 238–249. [Google Scholar] [CrossRef]
- van Mantgem, P.; Schwartz, M. Bark heat resistance of small trees in Californian mixed conifer forests: Testing some model assumptions. Ecol. Manag. 2003, 178, 341–352. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E.; Schwilk, D.W. Flammability as an ecological and evolutionary driver. J. Ecol. 2017, 105, 289–297. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Buma, B.; Brown, C.D.; Donato, D.C.; Fontaine, J.B.; Johnstone, J.F. The Impacts of Changing Disturbance Regimes on Serotinous Plant Populations and Communities. BioScience 2013, 63, 866–876. [Google Scholar] [CrossRef]
- Greene, D.F.; Zasada, J.C.; Sirois, L.; Kneeshaw, D.; Morin, H.; Charron, I.; Simard, M.J. A review of the regeneration dynamics of North American boreal forest tree species. Can. J. Res. 1999, 29, 824–839. [Google Scholar] [CrossRef]
- Zasada, J.C.; Norum, R.A.; Veldhuizen, R.M.V.; Teutsch, C.E. Artificial regeneration of trees and tall shrubs in experimentally burned upland black spruce/feather moss stands in Alaska. Can. J. Res. 1983, 13, 903–913. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, J.J. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef]
- Keeley, J.E.; Keeley, M.B.; Bond, W.J. Stem demography and post-fire recruitment of a resprouting serotinous conifer. J. Veg. Sci. 1999, 10, 69–76. [Google Scholar] [CrossRef]
- Boulet, B.; Huot, M. Le Guide Sylvicole Du Québec: Les Fondements Biologiques De La Sylviculture; Publications du Québec: Québec, QC, Canada, 2013; Volume 1, p. 1044. [Google Scholar]
- USDA NRCS. The PLANTS Database; National Plant Data Team: Greensboro, NC, USA, 2009. Available online: http://plants.usda.gov (accessed on 19 September 2019).
- Hightshoe, G.L. Native Trees, Shrubs, and Vines for Urban and Rural America: A Planting Design Manual for Environmental Designers; Van Nostrand Reinhold: New York, NY, USA, 1988. [Google Scholar]
- Ottawa Forests and Greenspace Committee. OFGAC Native Trees and Shrubs Database. Available online: https://ofnc.ca/programs/fletcher-wildlife-garden/flora-and-fauna-at-the-fwg/trees-and-shrubs-for-wildlife-gardens (accessed on 12 September 2019).
- Matthews, S.N.; Iverson, L.R.; Prasad, A.M.; Peters, M.P.; Rodewald, P.G. Modifying climate change habitat models using tree species-specific assessments of model uncertainty and life history-factors. Ecol. Manag. 2011, 262, 1460–1472. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.; Anderegg, W.R.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Flint, A.; Huang, C.-y.; Flint, L.; Berry, J.A.; Davis, F.W.; Sperry, J.S.; Field, C.B. Tree mortality predicted from drought-induced vascular damage. Nat. Geosci. 2015, 8, 367–371. [Google Scholar] [CrossRef]
- Wesołowski, T.; Rowiński, P.; Maziarz, M. Interannual variation in tree seed production in a primeval temperate forest: Does masting prevail? Eur. J. Res. 2015, 134, 99–112. [Google Scholar] [CrossRef]
- Morin, X.; Augspurger, C.; Chuine, I. Process-based modeling of species’ distributions: What limits temperate tree species’ range boundaries? Ecology 2007, 88, 2280–2291. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.F.; Bergeron, Y.; Lalonde, D.; Mauffette, Y. The potential effects of sexual reproduction and seedling recruitment on the maintenance of red maple (Acer rubrum L.) populations at the northern limit of the species range. J. Biogeogr. 2002, 29, 365–373. [Google Scholar] [CrossRef]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database—Enhanced coverage and open access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef] [PubMed]
- Urli, M.; Porté, A.J.; Cochard, H.; Guengant, Y.; Burlett, R.; Delzon, S. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol. 2013, 33, 672–683. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Flannigan, M.D.; Krawchuk, M.A.; de Groot, W.J.; Wotton, B.M.; Gowman, L.M. Implications of changing climate for global wildland fire. Int. J. Wildland Fire 2009, 18, 483–507. [Google Scholar] [CrossRef]
- Leuschner, C.; Meier, I.C. The ecology of Central European tree species: Trait spectra, functional trade-offs, and ecological classification of adult trees. Perspect. Plant Ecol. Evol. Syst. 2018, 33, 89–103. [Google Scholar] [CrossRef]
- Canadell, J.; Jackson, R.B.; Ehleringer, J.B.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. Maximum rooting depth of vegetation types at the global scale. Oecologia 1996, 108, 583–595. [Google Scholar] [CrossRef]
- Farrar, J.L. Trees in Canada; Fitzhenry & Whiteside Ltd. and Canadian Forest Service: Ottawa, ON, Canada, 1995.
- Minore, D. Comparative Autecological Characteristics of Northwestern Tree Species: A Literature Review. General Technical Reports PNW-GTR-087; Department of Agriculture, Forest Service, Pacific Northwest Forest Station: Portland, OR, USA, 1979; p. 72. [Google Scholar]
- Stone, E.L.; Kalisz, P.J. On the maximum extent of tree roots. Ecol. Manag. 1991, 46, 59–102. [Google Scholar] [CrossRef]
- Bullock, J.M.; Mallada González, L.; Tamme, R.; Götzenberger, L.; White, S.M.; Pärtel, M.; Hooftman, D.A.P. A synthesis of empirical plant dispersal kernels. J. Ecol. 2017, 105, 6–19. [Google Scholar] [CrossRef]
- Vittoz, P.; Engler, R. Seed dispersal distances: A typology based on dispersal modes and plant traits. Bot. Helv. 2007, 117, 109–124. [Google Scholar] [CrossRef]
- Tamme, R.; Götzenberger, L.; Zobel, M.; Bullock, J.M.; Hooftman, D.A.P.; Kaasik, A.; Pärtel, M. Predicting species’ maximum dispersal distances from simple plant traits. Ecology 2014, 95, 505–513. [Google Scholar] [CrossRef]
- Gauthier, S.; Bernier, P.; Burton, P.J.; Edwards, J.; Isaac, K.; Isabel, N.; Jayen, K.; Le Goff, H.; Nelson, E.A. Climate change vulnerability and adaptation in the managed Canadian boreal forest. Environ. Rev. 2014, 22, 256–285. [Google Scholar] [CrossRef]
- Messier, C.; Bauhus, J.; Doyon, F.; Maure, F.; Sousa-Silva, R.; Nolet, P.; Mina, M.; Aquilué, N.; Fortin, M.-J.; Puettmann, K. The functional complex network approach to foster forest resilience to global changes. For. Ecosyst. 2019, 6, 21. [Google Scholar] [CrossRef]
- Park, A.; Puettmann, K.; Wilson, E.; Messier, C.; Kames, S.; Dhar, A. Can Boreal and Temperate Forest Management be Adapted to the Uncertainties of 21st Century Climate Change? Crit. Rev. Plant Sci. 2014, 33, 251–285. [Google Scholar] [CrossRef]
- Hogg, E.H.; Brandt, J.P.; Kochtubajda, B. Growth and dieback of aspen forests in northwestern Alberta, Canada, in relation to climate and insects. Can. J. Res. 2002, 32, 823–832. [Google Scholar] [CrossRef]
- Wade, A.A.; Hand, B.K.; Kovach, R.P.; Luikart, G.; Whited, D.C.; Muhlfeld, C.C. Accounting for adaptive capacity and uncertainty in assessments of species’ climate-change vulnerability. Conserv. Biol. 2017, 31, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Belote, R.T.; Carroll, C.; Martinuzzi, S.; Michalak, J.; Williams, J.W.; Williamson, M.A.; Aplet, G.H. Assessing agreement among alternative climate change projections to inform conservation recommendations in the contiguous United States. Sci. Rep. 2018, 8, 9441. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.D.; Fahrig, L.; Henein, K.; Merriam, G. Connectivity is a vital element of landscape structure. Oikos 1993, 68, 571–573. [Google Scholar] [CrossRef]
- Fischer, A.P. Adapting and coping with climate change in temperate forests. Glob. Env. Chang. 2019, 54, 160–171. [Google Scholar] [CrossRef]
- Pedlar, J.H.; McKenney, D.W.; Aubin, I.; Beardmore, T.; Beaulieu, J.; Iverson, L.; O’Neill, G.A.; Winder, R.S.; Ste-Marie, C. Placing Forestry in the Assisted Migration Debate. BioScience 2012, 62, 835–842. [Google Scholar] [CrossRef]
- Nagel, L.M.; Palik, B.J.; Battaglia, M.A.; D’Amato, A.W.; Guldin, J.M.; Swanston, C.W.; Janowiak, M.K.; Powers, M.P.; Joyce, L.A.; Millar, C.I.; et al. Adaptive Silviculture for Climate Change: A National Experiment in Manager-Scientist Partnerships to Apply an Adaptation Framework. J. For. 2017, 115, 167–178. [Google Scholar] [CrossRef]
- Morelli, T.L.; Daly, C.; Dobrowski, S.Z.; Dulen, D.M.; Ebersole, J.L.; Jackson, S.T.; Lundquist, J.D.; Millar, C.I.; Maher, S.P.; Monahan, W.B.; et al. Managing Climate Change Refugia for Climate Adaptation. PLoS ONE 2016, 11, e0159909. [Google Scholar] [CrossRef]
- Prévost, M.; Raymond, P.; Lussier, J.-M. Regeneration dynamics after patch cutting and scarification in yellow birch—Conifer stands. Can. J. Res. 2010, 40, 357–369. [Google Scholar] [CrossRef]
- Brice, M.-H.; Vissault, S.; Vieira, W.; Gravel, D.; Legendre, P.; Fortin, M.-J. Moderate disturbances accelerate forest transition dynamics under climate change in the temperate–boreal ecotone of eastern North America. Glob. Chang. Biol. 2020, 26, 4418–4435. [Google Scholar] [CrossRef]
- Lafleur, B.; Paré, D.; Munson, A.D.; Bergeron, Y. Response of northeastern North American forests to climate change: Will soil conditions constrain tree species migration? Env. Rev. 2010, 18, 279–289. [Google Scholar] [CrossRef]
- Boisvert-Marsh, L.; de Blois, S. Unravelling potential northward migration pathways for tree species under climate change. J. Biogeogr. 2020. in review. [Google Scholar]
- Lindenmayer, D.B.; Likens, G.E.; Krebs, C.J.; Hobbs, R.J. Improved probability of detection of ecological “surprises”. Proc. Natl. Acad. Sci. USA 2010, 107, 21957. [Google Scholar] [CrossRef]
- Janowiak, M.K.; Swanston, C.W.; Nagel, L.M.; Brandt, L.A.; Butler, P.R.; Handler, S.D.; Shannon, P.D.; Iverson, L.R.; Matthews, S.N.; Prasad, A.; et al. A Practical Approach for Translating Climate Change Adaptation Principles into Forest Management Actions. J. For. 2014, 112, 424–433. [Google Scholar] [CrossRef]
- Urban, M.C. Climate-tracking species are not invasive. Nat. Clim. Chang. 2020, 10, 382–384. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).