Abstract
In the context of global warming and increasing wildfire occurrence, this study aims to examine, for the first time, the changes in multi-level biodiversity and key soil features related to soil functioning in a burned Mediterranean beech forest. Two years after the 2017 wildfire, changes between burned and unburned plots of beech forest were analyzed for plant communities (vascular plant and cover, bryophytes diversity, structural, chorological, and ecological variables) and soil features (main chemical properties, microbial biomass and activity, bacterial community composition, and diversity), through a synchronic study. Fire-induced changes in the micro-environmental conditions triggered a secondary succession process with colonization by many native pioneer plant species. Indeed, higher frequency (e.g., Scrophularia vernalis L., Rubus hirtus Waldst. and Kit. group, and Funaria hygrometrica Hedw.) or coverage (e.g., Verbascum thapsus L. subsp. thapsus and Digitalis micrantha Roth ex Schweigg.) of the species was observed in the burned plots, whereas the typical forest species showed a reduction in frequency, but not in cover, except for Fagus sylvatica subsp. sylvatica. Overall, an increase in plant species and family richness was found in the burned plots, mainly in the herbaceous and bryophyte layers, compared to the unburned plots. Burned plots showed an increase in therophytes, chamaephytes, cosmopolites, steno-Mediterranean and Atlantic species, and a decrease in geophytes and Eurasiatic plants. Significant differences were found in burned vs. control soils for 10 phyla, 40 classes, 79 orders, 145 families, 342 genera, and 499 species of bacteria, with about 50% of each taxon over-represented and 50% under-represented in burned than in control. Changes in bacterial richness within several families (reduction in Acidobacteriaceae, Solibacteraceae, Rhodospirillaceae, and Sinobacteraceae; increase in Micrococcaceae, Comamonadaceae, Oxalobacteraceae, Pseudomonadaceae, Hymenobacteraceae, Sphingomonadaceae, Cytophagaceae, Nocardioidaceae, Opitutaceae, Solirubrobacteraceae, and Bacillaceae) in burned soil were related to fire-induced chemical changes of soil (pH, electrical conductivity, and cation exchange capacity). No evident effect of the wildfire was found on organic C content, microbial biomass (total microbial carbon and fungal mycelium) and activity, and microbial indexes (fungal percentage of microbial C, metabolic quotient, and quotient of mineralization), suggesting that soil functions remained unchanged in the burned area. Therefore, we hypothesize that, without an additional disturbance event, a re-establishment of beech forest can be expected but with an unpredictable time of post-fire succession.
1. Introduction
Forest fires represent a complex global disturbance associated with factors of different origins, such as climate, human activities, vegetation types and conditions [1]. Climate changes affect fire regimes in many regions [2,3,4], not only in fire-prone systems but also in forest ecosystems that have not historically experienced fires [5]. Climate projections for northern America suggest that these extreme disturbance events will increase during the 21st century [6]. The year 2017 is remembered for its exceptionally high number of wildfire and the surface area affected by fires, worldwide. In Europe, despite the efforts by national and regional wildfire administrations to prevent and fight wildfires, in 2017 wildfires burned about one million hectares of natural lands [7]. Although anthropogenic ignition is found to be dominant in most worldwide regions [8], it is widely known that variation in the floristic composition, structure, connectivity, and fuel moisture conditions of forest vegetation determine the fire intensity and impact on the ecosystem biodiversity and functioning [9,10,11,12,13].
Variation in the fire regime could influence the recruitment of canopy trees and overall stand dynamic processes [14]. Resulting trajectories of natural vegetation changes can be strongly variable, depending on the extent of forest loss, the nature of the surrounding vegetation, ongoing disturbances within the regenerating system, other human disturbances, and environmental factors, such as rainfall and temperature [15].
In addition to causing damage to the canopy, trunk, and root system of trees [16], fire can affect physical, chemical, and biological characteristics of soil and related processes [17,18]. Fire can adversely affect the most sensitive component of soil, i.e., soil microbial community [19] involved in many soil functions and ecosystem services [20]. In forest ecosystems, the microbial community is considered as a driver of the plant community structure and dynamics [21], thanks to its key role in the C and N fluxes and control of the various plant pathogens [22,23]. Since the microbial community responds quickly to environmental changes and stress [24], its alterations can be used as useful indicators of the effects of stress or disturbance factors, such as fire, on soil [25]. Depending on its intensity, fire could cause both changes in microbial community diversity and alterations in microbial biomass and activity [26,27,28]. Bacteria tend to be less affected by fire than fungi [29], as has been observed from a few days up to two years after the fire [27]. This may be due to bacterial lower sensitivity to temperature and pH increase [29] and higher rate of recolonization of heated soil [30].
Vegetation is highly fire-prone in the Mediterranean climate ecosystems where the precipitation exceeds potential evapotranspiration during the rainy season, resulting in plant growth, sufficient to produce contiguous fuels which are highly flammable during the summer drought [31]. Fire has become one of the dominant drivers of vegetation patterns of these areas. Under the pressure of this ancient disturbance factor, native species communities have evolved morphological, anatomical, and physiological adaptive traits to tolerate periodic fires [32,33]. In forests dominated by species that lack specific fire-adaptive traits, the resilience of the whole ecosystem is questionable, especially if fire occurs as a novel disturbance. This is the case with montane Mediterranean beech forests, mesophilous formations normally spread between about 900 and 1800 m a.s.l., which have been affected by fires in recent years [34]. These plant communities play a key role in the conservation of European biodiversity (being included in Annex 1, Habitats Directive 92/43/EEC) and some of them show the characteristics of old-growth forests. The more ancient beech forests recorded in 12 European countries were recognized by the UNESCO (United Nations Educational, Scientific and Cultural Organization) World Heritage in a transnational environment named “Primeval forests of the Carpathian beech and other parts of Europe”). Their conservation value is even greater in southern Europe, where beech forests are ecologically and biogeographically older than those in central and northern Europe [35]. Here, some beech forests are located at a short distance from the sea, in a Mediterranean climate, which limits their ability to tolerate water and thermal stress, as well as fire disturbance. This makes these plant communities very vulnerable and potentially subjected to dynamic processes that could determine their replacement with more thermo-xerophilous formations. Fagus sylvatica L. subsp. sylvatica (Fagaceae), the dominant Eurasiatic taxon of these stands, lacks typical fire-adaptive traits: its thin bark cannot protect vital tissue (e.g., vascular cambium) from lethal heat during the fire and its sprouting capacity declines with tree age [36,37]. Mature beeches are thus considered highly susceptible to fire [38]. However, some recent short- [34,39] and mid-term [40] studies on post-fire regeneration of beech forest of the southern Alps and the central Apennines highlighted that beech forests can potentially regenerate naturally after fire events. As for other tree species, processes in post-fire beech regeneration are related to burn severity [41,42] and episodic forest fires seem not to represent a major threat to the resilience of beech populations under current climatic changes [40]. Nonetheless, no research has exhaustively analyzed the effect of fire on the vascular and bryophytic flora of the beech forests. Little research has been performed to understand the effect of fire on the soil bacterial community [18], none of which was conducted in a beech forest, despite the essential role of bacteria in post-fire ecosystem recovery processes. Moreover, no study has so far investigated the change in the relationship between multi-level biodiversity and soil features after a wildfire in beech forests. The removal or reduction of the vegetation cover due to fire can lead to decreased water retention, faster runoff, soil erosion, and nutrient leaching which influence dramatically secondary succession [43]. The processes of post-fire recovery in these plant communities could be also dramatically altered due to short- or mid-term modification of other ecological variables (e.g., light availability) or processes (e.g., competition) which regulate plant presence and growth. Understanding the post-fire responses of biodiversity is a crucial issue in planning forest management actions of the burned area in the mid- and long-term during post-fire succession.
The overall objective of this study was to improve the knowledge of this forest type in relation to fire disturbances. We hypothesize a great shift in the plant and bacterial richness and diversity, as well as in soil features, due to changes in the micro-environmental conditions in forest affected by the fire.
Specific goals of this research were (a) to analyze the short-term (two years after disturbance) impact of wildfire on the vascular plant, bryophyte, and soil bacterial diversity; (b) to evaluate the effect of fire on soil chemical properties and microbial metabolism; (c) to investigate the relationships between biodiversity and functioning in plant/soil system to evaluate its resilience; and (d) to provide useful insights for conservative management of the Mediterranean beech forests. Plant compositional change after the fire was evaluated by assessing vascular plant and bryophyte diversity and cover, as well as through the analysis of structural (life-forms), chorological (chorotypes), and ecological (Ellenberg Indicator Values) features of the plant communities. Soil response to the fire was evaluated by analyzing changes in the main chemical properties (pH, organic matter content, cation exchange capacity, and electrical conductivity), as well as in microbial biomass, activity, and bacterial diversity. This study can be used as a reference for the post-fire management plans of Fagus sylvatica forests, especially since an increase in the wildfire frequency at higher elevations is expected under the current climate change scenarios.
2. Materials and Methods
2.1. Study Area and Wildfire Event
The research was conducted in the Lattari Mountains (Campania region, southern Italy), the Mesozoic carbonatic mountain range of Sorrento Peninsula which separates the gulfs of Naples and Salerno in the Tyrrhenian Sea (Figure 1A). According to the Bioclimatic map of Europe [44], this territory has a Pluvioseasonal Oceanic Mediterranean bioclimate with a distribution of precipitation mainly in the autumn–winter period and with minimum peaks in summer (248 mm and 37 mm, respectively). Annual average temperatures vary from 6 °C in winter to 21 °C in summer (data calculated by daily data recorded in the period 2008–2019 by Multi-risk Civil Protection Functional Centre of Campania Region, Agerola station, http://centrofunzionale.regione.campania.it/#/pages/dashboard).
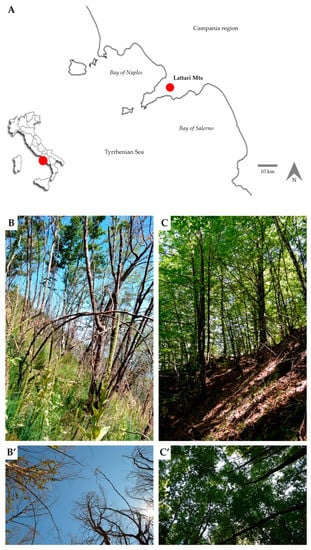
Figure 1.
Location in southern Italy and Campania region (red circles) of the study area (A). Burned (B) and control (C) plots with related tree canopies (B’,C’) (photos by A. Stinca).
The study sites were located just NW of Mt Sant’Angelo a Tre Pizzi (Pimonte municipality, 40°38′51.19″ and 14°30′21.16″, 1176–1384 m a.s.l.), the highest peak of the Lattari Mountains with its 1444 m a.s.l. summit, about 2.5 km from the sea as the crow flies. They fall within the Site of Community Importance “Dorsale dei Monti Lattari” (SCI code IT8030008, Habitats Directive 92/43/EEC), the Monti Lattari Regional Park, and the Important Plant Area “Monti Lattari” (code IPA CAMP 6 [45]). These sites show a lithostratigraphic unit composed by dolomitic limestones and limestones with pyroclastic sediments from the main Campania eruptive centers [46]. Their soils are a Molli-Eutrisilic Andosols and can be ascribed to the great land system of “high mountain” [47].
The vegetation of the area is characterized by an old-growth beech forest left to natural evolution for over half a century. In fact, in the past it was managed for wood production and for the conservation of snow in the so-called “neviere” (i.e., holes dug in the ground where snow accumulated and compacted in winter and it was then covered by leaves to get ice in summer). This vegetation type can be attributed to the habitat “Apennine beech forests with Taxus and Ilex” (code 9210*, Habitats Directive 92/43/EEC), widespread in the Italian Peninsula and Sicily in the supratemperate bioclimatic plan and rarely in the upper mesotemperate [48]. Moreover, from a syntaxonomical viewpoint, the plant community was ascribed by Cancellieri et al. [49] to the association Anemono apenninae-Fagetum sylvaticae (Gentile 1970) Brullo 1983, which represents the southern Italian thermophilous beech forests [50]. To increase the naturalistic value of this stand is the presence of the locus classicus (named “Sorgente Acqua Santa”) of the Lonicera stabiana Guss. and Pasq. (Caprifoliaceae) [51], a very rare Italian endemic species known only in a few localities in the Lattari Mountains [52], as well as other rare vascular plants (e.g., Aquilegia champagnatii Moraldo, E. Nardi and la Valva, Erica terminalis Salisb., and Pinguicula hirtiflora Ten.) [53,54].
Between summer and autumn of 2017, many sequential wildfire events affected a large area of the Lattari Mountains. The first of these fires started on 12 July on the southern slope of Mt Faito (https://www.ilmattino.it/napoli/cronaca/fiamme_faito_bloccati_soccorsi_non_arrivano-2558030.html), a mountain peak close to the study area. In the last days of August, a fire also affected the area of Mt Sant’Angelo a Tre Pizzi (https://www.stabiachannel.it/Cronaca/funivia-e-fiamme-le-due-facce-del-faito-ancora-roghi-sul-monte-a-fuoco-il-molare-66140.html), leaving a mosaic of burned and unburned areas (A. Stinca, personal observations). Its extreme spread and impacts probably resulted from the complex interplay among the local environmental conditions (e.g., low fuel moisture content, complex geomorphology, very strong and variable winds, and landscape connectivity) and poor initial attack to fire because many fire-fighters were engaged in a disastrous wildfire that was simultaneously affecting the nearby Mt Vesuvius [55]. The fire was extinguished after 48–72 h using fresh and salt-water, sprayed with air vehicles, as well as through mechanical actions by firefighters on the ground (A. Stinca, personal observations).
2.2. Field Work and Laboratory Measurements
2.2.1. Sampling Design
On 12 August 2019, two years after the wildfire event, 5 disturbed sites with a canopy gap at least 20 m in diameter (hereafter referred to as “burned”, Figure 1B,B’) and 5 undisturbed sites under the closed canopy (hereafter referred to as “control”, Figure 1C,C’) were randomly selected. No evidence of post-fire management and silvicultural measures was observed in the selected sites. These were characterized by homogeneous biophysical conditions for the local factors (e.g., altitude, exposure, slope, and microclimate). At the center of each site, we delimited a permanent circular plot (r = 5 m) within which we sampled vegetation, soil, and detected site conditions. The thermo-pluviometric trend in the study area from a fire event up to sampling time is reported in Figure 2A. Physical site characteristics, including slope (°), exposure, elevation (m a.s.l.), and photosynthetic photon flux density (PPFD, λ = 400–700 nm) were recorded for each plot (Table S1). PPFD (µmol m−2 s−1) was measured using a LI-250A light meter as a quantum sensor (Li-Cor Inc. Environmental, Lincoln, NE, USA), located at 2 (PPFD2), 1 (PPFD1), 0.5 (PPFD0.5), and 0 m (PPFD0) above the ground for 3 randomly selected locations within the burned and control plots (Figure 2B). Instantaneous measurements averaged at every 15 s, were taken during a bright, sunny day in August 2019.
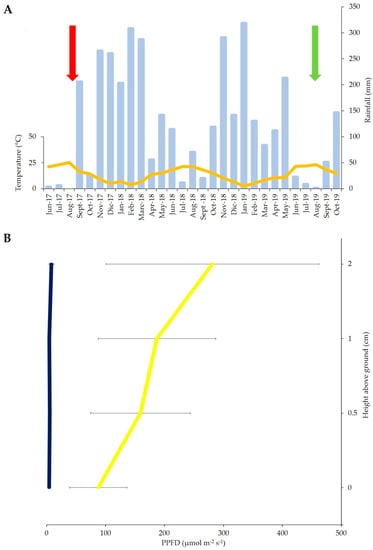
Figure 2.
(A) Values of mean monthly temperatures (°C, orange curve) and total monthly rainfall (mm, light blue bars) of the study area during the 2017–2019 period calculated by daily data recorded by Multi-risk Civil Protection Functional Centre of Campania Region, Agerola station (red and green arrows indicate the time of wildfire event and field samplings, respectively). (B) Average values ± standard deviations of photosynthetic photon flux density (PPFD) in control closed-canopy (blue) and burned canopy gap (yellow) plots.
2.2.2. Vegetation Sampling and Analyses
Vegetation was sampled in terms of coverage of the different layers and all individual plant species. Vascular species cover was visually assessed based on the Braun-Blanquet [56] scale (i.e., (r) rare; (+) <1%; (1) 1%–5%; (2a) 6%–15%; (2b) 16%–25%; (3) 26%–50%; (4) 51%–75%; and (5) 76%–100%), a method widely used in vegetation studies worldwide. Collected specimens were conserved in Herbarium Stinca. The taxonomic identification was carried out using standard floras [57,58,59,60,61,62,63,64,65], while nomenclature and taxa delimitation followed the checklist of Italian vascular flora [66]. Bryophytes were visually estimated as total coverage (%) and identified at the species level based on standard floras [67,68,69,70], while nomenclature followed the recent checklist of bryophytes of Europe, Macaronesia, and Cyprus [71]. To investigate the plant diversity changes in the burned and control plots, we analyzed species composition in terms of richness and cover. Changes in the structural, chorological, and ecological features of the plant community were evaluated using life-forms [62,63,64], chorotypes [62,63,64,66], and Ellenberg Indicator Values (EIVs [72]), respectively. EIVs are considered as an appropriate useful tool to relate observed variation in plant communities to variations in environmental factors after a disturbance or over the time [73,74,75,76].
2.2.3. Soil Sampling and Analyses
To assess the effects of disturbance on chemical and microbial properties of the soil, 3 soil samples were randomly collected from each plot and immediately mixed to obtain a representative and homogeneous sample [77]. All samples (about 1 kg each) were collected from the topsoil layer (depth 0–15 cm), after removing the litter layer. Samples were packed in polyethylene bags, placed in a thermal bag with ice, transferred to the laboratory on the sampling day and sieved through 2 mm sieve to remove the coarse fragments (stones, roots, etc.). Further, the samples were divided into three aliquots. The first was air-dried to constant weight for determination of pH, electrical conductivity (EC), cation exchange capacity (CEC), and total organic carbon (Corg) content. The second aliquot was stored at 4 °C for the assessment of water content (to express microbial variables to dry weight), total microbial biomass carbon (Cmic), and fungal mycelium (FM) content, and microbial activity (respiration and indexes of microbial metabolism). The third aliquot was stored at −20 °C for the metagenomic analysis. Water content was determined gravimetrically on 5 g of soil [78]. The pH (on 10 g of soil) and EC (on 20 g of soil) were detected on a water suspension of air-dried soil (1:2.5 and 1:2 soil:water, respectively) using calibrated electrodes [77]. CEC was measured on 2 g of soil, after soil treatment with barium chloride and triethanolamine solution at pH 8.2 [77]. Corg was assayed on 0.25 g of soil by wet digestion in 0.1667 M of K2Cr2O7 [79,80]. Cmic was assayed by chloroform fumigation-extraction methods [81]; both fumigated and not fumigated soil samples (5 g of soil each) were extracted with 0.5 M K2SO4 (1:4 ratio). Organic carbon concentration in each extract was determined by wet oxidation with a 0.33 N K2Cr2O4 solution followed by back titration with 0.033 N Fe(II)SO4 solution. Cmic was calculated from the differences between organic carbon of fumigated and non-fumigated samples using the equation of Vance et al. [81]. This measure also provided data on the extractable organic C (Cext) that was obtained from non-fumigated samples. FM was determined with the membrane filter [82] from 1 g of soil. The length of hyphae was determined with the intersection method [83]. The mass of mycelia was evaluated from the average values of cross-section (9.3 µm2), density (1.1 g mL−1), and dry mass of the hyphae (15% of the wet mass) [84]. Soil respiration was evaluated by alkali NaOH trap of CO2 evolved from fresh soil samples (5 g) during incubation of 15 days in standard condition (20 °C, 55% of water-holding capacity, in the dark), according to ISO 16072 [85]. A pre-incubation step at 20 °C for about 3 days was performed to diminish the initial CO2 flush by soil microbial community following sampling and sieving disturbance [86]. Each soil sample was incubated in 500-mL glass jar, containing 10 mL NaOH solution (0.1 M) at the bottom, at room temperature (about 25 °C) and darkness. After 1, 4, 7, 10, and 15 days of incubation, the jar was opened and the excess of NaOH solution was titrated with 0.1 M HCl solution, after adding 2 mL of 0.75 M BaCl2 and 2 drops of phenolphthalein indicator. After every time point, new NaOH solution was added and the jar with the sample was incubated again. Respiration (R; mg CO2-C g−1 d.w. d−1) was calculated as a daily average of the C-CO2 evolved during the 15-days incubation. Moreover, the mineralizable C (g CO2-C kg−1 d.w.), i.e., the maximum amount of CO2-C evolved, was calculated fitting cumulated CO2-C evolved from samples against incubation time, using a first-order pool kinetics model [87], with the following Equation (1):
where “C” is the cumulated mineralized C (g C-CO2 kg−1 d.w.); “t” is the incubation time (days); “C0” (g CO2-C kg−1 d.w.) is the asymptotic maximum quantity of CO2-C produced; and “k” is the mineralization rate constant (days−1).
Several microbial indexes were calculated: (i) the Cfung%Cmic, derived from Cmic and fungal C mycelium data, the last being calculated, according to D’Ascoli et al. [88], based on mean values reported for C/N ratio and N content [89,90]; (ii) the quotient of mineralization (qM; CO2-C % Corg) derived from the asymptotic maximum quantity of CO2-C (C0 of the Equation (1)) and organic carbon [87]; and (iii) the metabolic quotient (qCO2; mg CO2-C g−1 Cmic d−1), calculated from respiration and Cmic [91].
2.2.4. Metagenomic Analysis
Metagenomic analysis was carried out on all the soil samples collected. DNA isolation, samples quality control, 16S Metagenomic Library Preparation, Next Generation Sequencing and bioinformatics analysis were performed by Genomix4Life S.r.l. (Baronissi, Salerno, Italy). Microbial DNA was extracted from 0.25 g of soil sample using a Danagene Microbiome Soil DNA kit (Danagen-Bioted S.L., Badalona, Spain) following the manufacturer’s instructions. Final yield and quality of extracted DNA were determined by using a NanoDrop One spectrophotometer (Thermo Scientific, Waltham, MA, USA) and a Qubit Fluorometer 4.0 (Invitrogen Co., Carlsbad, CA, USA). 16S amplification was performed using the universal primers forward U341F 5′-CCTACGGGNGGCWGCAG-3′ and reverse U785R 5′-GACTACHVGGGTATCTAATCC-3 [92], which target the hypervariable V3 and V4 region of the 16S rRNA gene. Each PCR reaction was assembled according to Metagenomic Sequencing Library Preparation (Illumina, San Diego, CA, USA). Libraries were quantified using a Qubit fluorometer 4.0 (Invitrogen Co., Carlsbad, CA, USA) and pooled to an equimolar amount of each index-tagged sample to a final concentration of 4 nM, including the Phix Control Library (Illumina; expected 30%). Pooled samples were subject to cluster generation and sequenced on the MiSeq platform (Illumina, San Diego, CA, USA) in a 2 × 250 paired-end format.
2.3. Bioinformatics and Statistics Analysis
For each variable and each experimental condition (burned and control), mean and standard deviation of 5 field replicates were calculated. Vegetation (richness, life-forms, chorotypes, and EIVs) and PPFD differences between burned and control areas were assessed by t-test. Species abundance scores were transformed in percent cover data, using the midpoint of the cover range for each category [93,94] as follows: (r) and (+) 0.5%; (1) 3%; (2a) 10%; (2b) 20%; (3) 37.5%; (4) 62.5%; and (5) 87.5%. Vascular plant diversity was assessed with the Shannon–Wiener diversity index (H’ [95]) using the percent cover data instead of the individual numbers, with the following Equation (2):
where “pi” is the relative proportion (%i/%tot) of a single species (i.e., obtained by the division between the cover of an individual species [%i] and the total coverage of all species found in each plot [%tot]; and “k” is the number of the species found in each plot.
Regarding soil data, a normality test (Kolmogorov–Smirnov) was applied to data sets before parametric tests; the data were transformed by log10 when not normally distributed [96]. The significance of differences between burned and control was assessed by t-test.
Multivariate analysis using the SYN-TAX software (version 2000; Podani, 2001 [97]) was applied to plant and soil data matrices to test the degree of similarity between burned and control plots. For plant data, the sampling cover scale was transformed into the ordinal scale according to Van der Maarel [98] and the obtained matrix was processed by Hierarchical Classification, using UPGMA (Unweighted Pair Group Method with Arithmetic mean) as the agglomeration criterion and Similarity ratio as the dissimilarity index, and Principal Component Analysis with the application of the Euclidean biplot to the graphical exploration of the distances among species and communities. To investigate the significance of overall differences in plant diversity between burned and control plots, we applied, to the matrix containing richness and cover data, the Permutational Multivariate Analysis Of Variance (PERMANOVA) using the “adonis” function of the “vegan” package in R (version 3.7; R Core Team, 2018) at 999 permutations.
For soil data, a matrix of bacterial family abundance values and soil properties was created and processed by Principal Component Analysis applying the Euclidean biplot to the graphical exploration of the distances among variables and samples.
Regarding bacterial data, bioinformatic processing of raw sequences was performed using the GAIA platform suite (version 2.0; Paytuví et al., 2019 [99]). After trimming, Burrows-Wheeler Alignment (BWA) tool (version 0.7.13; Li and Durbin, 2010 [100]) was used to map the 436,791 high-quality reads/pairs against custom-made databases created by Sequentia Biotech including National Center for Biotechnology Information (NCBI) sequences [101]. Reads were clustered into Operational Taxonomic Units (OTUs) using an in-house Lowest Common Ancestor (LCA) algorithm. Minimum identity thresholds were applied to classify reads into strains, species, genus, family, order, class, phylum, and domain levels. OTU distribution among samples was used to calculate rarefaction curves. Alpha and beta diversities were calculated using Phyloseq (version 3.11; Mcmurdie et al., 2013 [102]). Dissimilarities between pairs of samples were estimated using the Bray–Curtis dissimilarity index. PERMANOVA was performed using the “adonis” function of the “vegan” package in R (version 3.7; R Core Team, 2018) at 999 permutations. The relative abundances of OUTs were expressed in terms of percentage of reads while differential abundance analysis was performed by DESeq2 package (version 3.11; Love et al., 2014 [103]) using negative binomial generalized linear models.
3. Results
3.1. Fire Effects on Vascular Plants and Bryophyte Diversity
The wildfire that occurred in 2017 in the study area destroyed the tree canopy with a consequent greater penetration of light along the vertical profile of the vegetation. In fact, PPFD (µmol m−2 s−1) within the disturbed plots was significantly (t-test, p < 0.05) higher than the control plots at different heights along the profile (PPFD2: 281.37 ± 180.21 vs. 8.46 ± 4.22; PPFD1: 187.21 ± 99.60 vs. 4.92 ± 1.71; PPFD0.5: 159.26 ± 84.48 vs. 5.71 ± 2.22; and PPFD0: 87.95 ± 48.46 vs. 4.68 ± 1.80).
Vegetation sampling carried out two years after the fire event showed overall 80 vascular plant species (including five tree species at seedlings stage) (Table S2). The cluster analysis, based on the matrix of phytosociological sampling (80 species × 10 plots), clearly showed the separation of two well-defined main groups. One group corresponded to all the surveys carried out in the sites burned by the 2017 fire and the other to the control areas (Figure 3A). These results were also confirmed by PERMANOVA (F = 6.51, R2 = 0.45, p = 0.017). Based on the obtained clusters, the variation in the average number of species and the average percentage cover was analyzed for each group (Figure 3B–D).
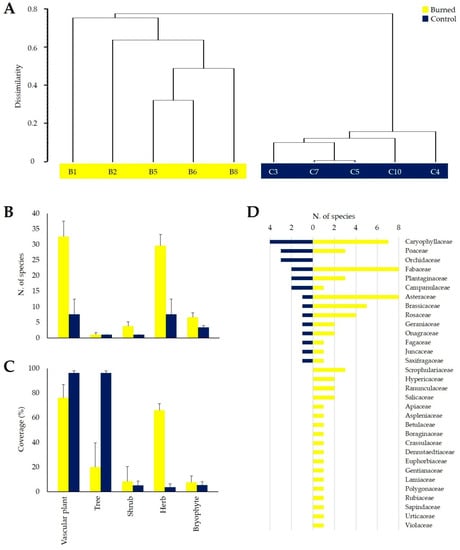
Figure 3.
Dendrogram of cluster analysis (A) based on vascular plants coverage data using the average linkage (UPGMA) as the agglomeration criterion and the Similarity ratio as dissimilarity index. Comparison between burned and control areas in terms of species richness (B) and coverage (C) in total vascular plant community (Vascular plant), tree layer (Tree), shrub layer (Shrub), herbaceous layer (Herb), and bryophyte layer (Bryophyte) (mean + standard deviation, and raw data are available in Table S2). Number of plant species within each plant family in burned and control plots (D) (total values).
The burned plots, compared to control plots, showed a greater floristic richness of total vascular plant species (32.6 ± 4.9 vs. 7.6 ± 4.9 species; p < 0.01) as a consequence of colonization of new taxa mainly into the herbaceous layer (29.6 ± 3.6 vs. 7.6 ± 4.9; p < 0.01) (Figure 3B and Table S2). Moreover, a significant but slight increase in plant species in the shrub (3.8 ± 1.3 vs. 1.0 ± 0.0; p < 0.01) and bryophyte (6.6 ± 1.5 vs. 3.4 ± 0.5; p < 0.01) layers was also observed in the burned plots (Figure 3B). Shannon–Wiener diversity index values, calculated for the total vascular flora, also showed significant differences between burned and unburned areas (0.94 ± 0.11 vs. 0.11 ± 0.09; p < 0.01).
Regarding the vascular species cover, as expected, lower values were observed in burned plots compared to unburned ones considering both total (76.0 ± 10.8 vs. 96.2 ± 1.6; p = 0.01) and tree layer (20.0 ± 19.7 vs. 96.2 ± 1.6; p < 0.01) average coverage (Figure 3C). In contrast, the herbaceous layer of the burned plots highlighted a great increase in coverage (66.0 ± 5.5 vs. 3.8 ± 2.7; p < 0.01) (Figure 3C), in accordance with the richness data.
From a taxonomic point of view, in addition to the total number of species, a greater number of families of vascular plants were observed in the burned plots compared to the control ones (31 vs. 14), with a great richness increase for Asteraceae (8 vs. 1 species) and Fabaceae (8 vs. 2) (Figure 3D). On the contrary, in wildfire plots, species belonging to Orchidaceae were not found (0 vs. 3) (Figure 3D).
Two years after the occurrence of the wildfire in the beech forest, structural significant differences (p < 0.05) were observed between burned and control plots with an increase in therophytes and chamaephytes, as well as a reduction in geophytes in burned plots (Figure 4A and Table S3).
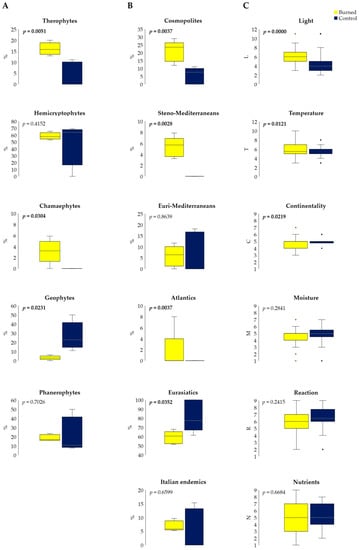
Figure 4.
Variations of life-forms (A), chorological types (B), and Ellenberg Indicator Values (C) in burned and control plots (total values; rectangles define 25th and 75th percentiles, horizontal lines show median values, whiskers indicate extreme values, t-test p < 0.05, and raw data are available in Table S3).
Post-fire plant colonization produced a significant (p < 0.05) increase in cosmopolites, steno-Mediterranean and Atlantic species, but also a decrease in Eurasiatic plants (Figure 4B and Table S3). Fire disturbance did not produce the invasion of alien plants, which were absent also in the control sites.
Regarding the ecological features of the found vascular plants, in burned plots there was an increase of species with greater needs for light and high temperatures, as well as a reduction of plants typical of the continental climates (Figure 4C and Table S3). In contrast, changes in the specific composition did not induce significant shifts in the EIVs referring to edaphic conditions: moisture, pH reaction, and nutrient availability (Figure 4C).
Tree regeneration in terms of coverage of seedlings and suckers was not significantly different between burned and control areas. However, in the canopy gaps, Fagus sylvatica L. subsp. sylvatica and Acer opalus Mill. subsp. obtusatum (Waldst. and Kit. ex Willd.) Gams showed a greater production of seedlings (5.4 ± 8.3 vs. 1.5 ± 1.4) and suckers (6.1 ± 13.4 vs. 0.0 ± 0.0), respectively. Additionally, the total number of tree seedling species in burned plots was higher than in the unburned equivalent (5 vs. 1, Table S2).
The ordination of the sampled burned and control plots, with a data matrix of 35 species (species with frequency 1 and 2 were excluded) × 10 plots, according to the first and second principal components is depicted in Figure 5. Clustered samples of burned and control plots are highlighted along the first axis which accounted for 47.79% of the total plant community variation, with unburned plots having negative scores whereas burned plots having positive scores. However, burned plots showed a separation according to the second axis, which accounted for 16.01% of the remaining variation and reflecting their more marked heterogeneity of environmental site conditions (Table S2) with respect to the homogeneity of the unburned plots.
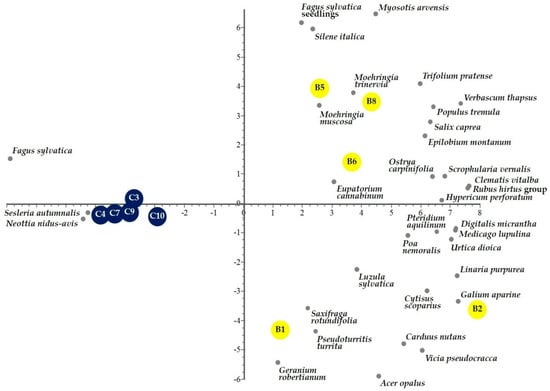
Figure 5.
Biplot of the Principal Component Analysis of burned (yellow circles) and control (blue circles) plots with the indication of vascular plant species (grey circles) in the first two principal components space.
In addition, the graphical representation of species variables (Figure 5) in the same ordination space showed Fagus sylvatica subsp. sylvatica, Sesleria autumnalis (Scop.) F.W.Schultz, and Neottia nidus-avis (L.) Rich., in the negative score characterized by control plots. On the other hand, positive axis 1 scores were associated with a high number of species with some reaching the highest variance values such as Geranium robertianum L. (10.3%), Verbascum thapsus L. subsp. thapsus (9.7%), Digitalis micrantha Roth ex Schweigg. (5.4%), Cytisus scoparius (L.) Link subsp. scoparius (5.3%), and Trifolium pratense L. subsp. pratense (4.7%).
At species level, the wildfire produced an increase in the frequency and/or coverage in many taxa that generally grow in the surrounding study area in open environments and/or at lower altitudes, while the species typical of the beech forest showed a reduction in frequency but not in cover except for Fagus sylvatica subsp. sylvatica (Figure 6A). More specifically, the vascular plants Scrophularia vernalis L. (+5), Rubus hirtus Waldst. and Kit. group (+5), Clematis vitalba L. (+5), Neottia nidus-avis (−3), and Sesleria autumnalis (−3) (Figure 6A) showed the greatest changes in frequency. It is interesting to note that the mentioned species that increased in frequency were found only in the burned areas, while the others were found only in the control areas, and they were completely absent after the fire. Likewise, Verbascum thapsus subsp. thapsus and Digitalis micrantha showed a significant increase in coverage (p < 0.05), in addition to Fagus sylvatica subsp. sylvatica, which decreased in its coverage after the fire (Figure 6B).
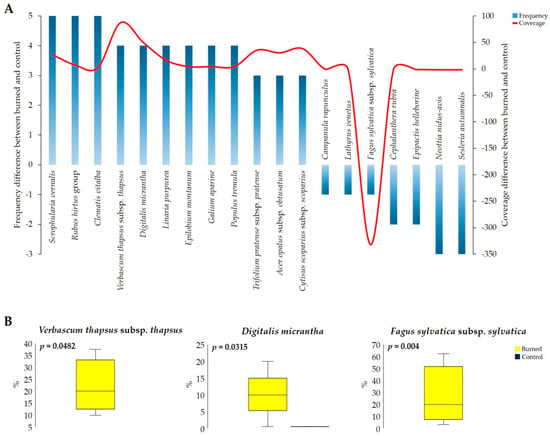
Figure 6.
(A) Main vascular plant species that showed an increase or a reduction in frequency and coverage after the wildfire (total values; the original cover categories for individual species were converted to percent cover using the midpoint of the cover range for each category). (B) Variation in coverage of Verbascum thapsus subsp. thapsus, Digitalis micrantha, and Fagus sylvatica subsp. sylvatica in burned and control plots (total values; rectangles define 25th and 75th percentiles, horizontal lines show median values, whiskers indicate extreme values, t-test p < 0.05, and raw data are available in Table S3).
Bryophytes, at the species level, have been recorded in each burned and unburned plot only as frequency. A total of 13 species belonging to 9 families were identified. Ditrichaceae, Fossombroniaceae, Funariaceae, and Lunulariaceae were found exclusively in burned plots, while Bartramiaceae only in the control plots. Brachytheciaceae, Pottiaceae, Bryaceae, and Fissidentaceae were sampled in both burned and unburned sites (Figure 7A). Changes in the frequency of each species were evident in Figure 7B with the acrocarpous Funaria hygrometrica Hedw. (+5), Lunularia cruciata (L.) Dumort. ex Lindb. subsp. cruciata (+4), as well as the pleurocarpous Homalothecium sericeum (Hedw.) Schimp. (−3), and Rhynchostegium confertum (Dicks.) Schimp. (−5) showing the greatest changes after the fire.
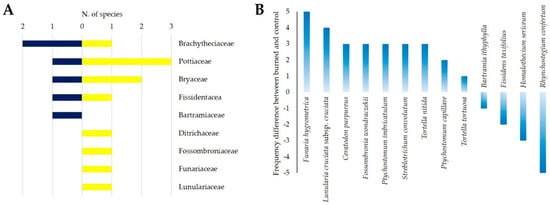
Figure 7.
(A) Number of bryophyte species within each family in burned and control plots (total values). (B) Frequency changes of bryophytes species after the wildfire.
3.2. Fire Effect on Soil Chemical and Microbial Properties
Surface soil (0–15 cm) of unburned beech forest analyzed in this study was found to be slightly acidic (6.0 ± 0.2), and had average values of Corg, CEC and EC of 94.0 ± 31.2 g kg−1 d.w., 37.0 ± 3.3 cmol kg−1, and 0.30 ± 0.06 dS m−1, respectively. Two years after the wildfire, significant differences between burned and unburned soils were observed for pH and EC, both showed an increase in burned soil (Table 1 and Table S1). CEC marginally decreased after the fire (p = 0.054). On the contrary, no effect of wildfire was found for content in Corg, Cext and easily mineralizable C (Table 1). Similarly, total microbial biomass, fungal mycelium, and microbial activity (i.e., soil respiration) showed no significant difference between burned and control soils (Table 1 and Table S1). Moreover, burned soils did not show alterations in the relative abundance of the fungal component of the total microbial community (Cfung%Cmic), percentage of total organic C that is easily mineralizable (quotient of mineralization, qM), and metabolic status of the microbial community (metabolic quotient, qCO2), although, at the time of sampling, the drought conditions (Figure 2A) could have increased in the burned soil due to reduced tree cover (Figure 3C).

Table 1.
Mean values (± standard deviations) of soil pH, electrical conductivity (EC), cation exchange capacity (CEC), organic carbon (Corg), extractable organic C (Cext), mineralizable C content, microbial biomass (Cmic), fungal mycelium (FM), fungal percentage of Cmic (Cfung%Cmic), respiration, quotient of mineralization (qM), and metabolic quotient (qCO2), in the burned and control plots. Significant (p < 0.05) differences between treatments (determined by t-test), for each variable are indicated by different letters in superscripts.
3.3. Fire Effect on Soil Bacterial Diversity
By metagenomic analysis, 436,791 high quality reads/pairs were obtained from 10 soil samples. Sequence numbers ranged from 28,999 to 58,168 reads per sample, with the number of OTUs ranging from 742 to 801. Rarefaction curves tended towards asymptote (Figure S1) indicating that the sequencing effort was sufficient, and the sequences obtained were representative of the bacterial communities in these soils. PERMANOVA further revealed that bacterial communities in the burned soil were significantly different from those in the unburned soil (F = 4.76, R2 = 0.37, p = 0.007). The identified bacterial sequencing reads belong to 36 phyla, 96 classes, 204 orders, 354 families, 707 genera, and 1012 species (Figure S2 and Table S4). The most abundant phylum was Proteobacteria (average 37.7%), followed by Acidobacteria (13.6%), Actinobacteria (11.0%), Bacteroidetes (10.1%), Planctomycetes (6.4%), Verrucomicrobia (5.4%), Firmicutes (3.4%), Gemmatimonadetes (2.7%), and Chloroflexi (1.2%). Significantly different taxa abundance in burned vs. control soils was observed for 10 phyla, 40 classes, 79 orders, 145 families, 342 genera, and 499 species, with about 50% of each taxon over-represented and 50% under-represented in burned than in control plots (Figure S2).
As presented in the Volcano plot in Figure 8A, over-represented phyla in burned soil were Euryarchaeota, Rhodothermaeota, Tenericutes, unknown Archaea(d), and Actinobacteria, while those under-represented were Candidatus Tectomicrobia, Elusimicrobia, Chlamydiae, Acidobacteria, and Synergistetes.
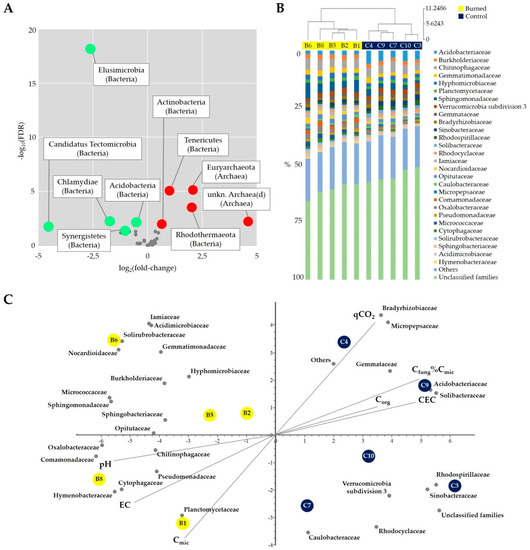
Figure 8.
(A) Volcano plot representing the relationship between the fold-change (on the X-axis) and the significance of the differential abundance test (Y-axis) for each phylum operational taxonomic units (OTU) (grey circles represent the OTUs that are not significantly differentially represented, while red and green circles are the OTUs that are significantly over- and under-represented in burned soil, respectively). (B) Cluster analysis and family level composition of each sample (dendrogram is based on family OTU percentage abundance, using Euclidean distance; only families accounting for at least 1% of sequences are included; all other families are grouped into “Others”). (C) Biplot of the Principal Component Analysis of burned (yellow circles) and control (blue circles) plots with the indication of bacterial families (grey circles) and soil variable vectors (grey lines) in the first two principal components space.
A clear separation of bacterial communities was found between burned and control soil as showed by cluster analysis (Figure 8B) and Principal Coordinates Analysis (PCoA) on Bray–Curtis dissimilarity index (data not shown) applied to bacterial families. The separation of burned and control soil was also evidenced by the Principal Component Analysis (Figure 8C), which was applied to a matrix of combined data concerning soil samples (bacterial families with chemical and microbial properties).
Burned and control plots were clearly clustered along the first axis which accounted for 49.52% of the variation of the composition of bacterial communities and soil microbial and chemical characteristics. Burned plots had negative scores together with higher pH and EC values, whereas unburned plots were neatly distributed with positive scores with higher values of relative abundance in fungal mycelium vs. total microbial biomass (Cfung%Cmic), Corg, CEC. On the other hand, separation according to Cmic and qCO2 was evident on the second axis which accounted for 15.42% of the remaining variation.
The graphical representation of bacterial families in the same component space showed several families (e.g., Micrococcaceae, Sphingomonadaceae, Sphingobacteriaceae, Opitutaceae, Chitinophagaceae, Oxalobacteraceae, and Comamonadaceae) in the negative score characterized by burned plots. On the other hand, other families (e.g., Caulobacteraceae, Verrucomicrobia subdivision 3, Sinobacteraceae, Rhodospirillaceae, Solibacteraceae, Acidobacteriaceae, Gemmatacea, and Micropepsaceae) were distributed along the positive scores of axis 1 corresponding to unburned plots. Finally, the positive score of unburned plots showed a clear dominance of unclassified families and others (i.e., the families with less 1% of sequences) (Figure 8C).
Burned soil showed a significant relative frequency reduction of different OTUs within the families Acidobacteriaceae, Solibacteraceae, Rhodospirillaceae and Sinobacteraceae, as well as an increase in Micrococcaceae, Comamonadaceae, Oxalobacteraceae, Pseudomonadaceae, Hymenobacteraceae, Sphingomonadaceae, Cytophagaceae, Nocardioidaceae, Opitutaceae, Solirubrobacteraceae, and Bacillaceae (Figure 9). However, Shannon index calculated for the bacterial families did not significantly differ between burned and control soils (4.66 ± 0.05 vs. 4.68 ± 0.07). Interestingly, certain species were exclusively found in the burned soils (e.g., Arthrobacter spp., Bacillus spp., Bradyrhizobium spp., Curvibacter spp., Frankia sp., Massilia spp., Pedobacter spp., and Ramlibacter spp.), whereas others (e.g., Bradyrhizobium japonicum (Kirchner) Jordan, Burkholderia spp., Dokdonella ginsengisoli Ten et al., Mesorhizobium loti (Jarvis et al.) Jarvis et al., Methylibium spp., Mucilaginibacter spp., and Rhizobacter spp.) were found exclusively in control soils.
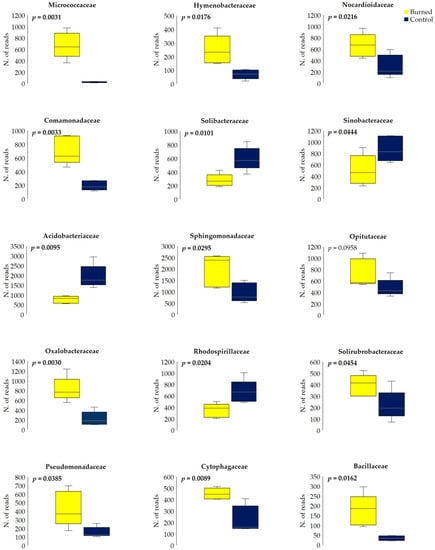
Figure 9.
Variations in bacterial richness within main families, between burned and control (n. of row reads; rectangles define 25th and 75th percentiles, horizontal lines show median values, and t-test p < 0.05). Raw data and differential analysis results are available in Table S4.
4. Discussion
4.1. Fire Effect on Vascular Plants and Bryophyte Diversity
At the plant community level, the fire disturbance on beech forest of the Lattari Mountains which occurred in late August 2017, has removed previously established forest vegetation, releasing space for new pioneer species. The fire triggered a secondary succession process, known as “pyrogenic succession”, that started in the days following the fire and which will last for a time that is difficult to quantify. Indeed, immediately after the wildfire, the disturbed areas showed a canopy of trees and the layers of the undergrowth almost completely burned, including the litter, with part of dead beech stems standing (A. Stinca, personal observations).
Two years after the fire event, the vascular plant species richness and Shannon–Wiener diversity index values were much higher in these gaps than in unburned communities. These results, the first for Mediterranean beech forests at the southern limit of their range, are fully in agreement with the general model of early post-fire succession in Mediterranean ecosystems [104,105,106,107,108,109]. Furthermore, our results are also in accordance to similar studies that were carried out in a burned Spanish mountain beech forest [110] and other Mediterranean forest ecosystems that were affected by fires and dominated by coniferous species, such as Abies cephalonica Loudon [111], Pinus halepensis Mill. [112,113,114], Pinus sylvestris L. [110], and Pinus nigra J.F.Arnold subsp. pallasiana (D.Don.) Holmboe [115]. Although the stimulating effect of fire on plant diversity seems to be consistent to wind disturbance in the canopy gaps [116,117], the plant succession is strongly influenced by the presence of ash and biomass (i.e., live plants, uprooted trees, and litter), respectively. In fact, fire-derived ashes may have increased the content of mineral nitrogen and other nutrients in the surface layer of the soil, compared to the undisturbed understory [27,118,119].
Plant species spreading can be explained by changes in the micro-environmental conditions within the gaps. Our data confirmed the hypothesis that in the gaps the absence of forest vegetation produced higher light flux-density at ground level, higher temperature with larger diurnal, and seasonal fluctuations and lower moisture. Effectively, PPFD within the disturbed area was significantly higher than the control area along all vertical profile of vegetation. These new environmental conditions allowed the colonization of the area by short-lived species which will later disappear [105,110]. Indeed, the higher floristic richness was mainly due to the increased presence of vascular species into the herbaceous layer (especially therophytes), which are also increasing their coverage and, secondarily, to the increased number of species in the shrub and bryophytes layers. The greater number of species is also reflected in the higher taxonomic levels with a consistent increase in the number of families of vascular plants and bryophytes and their diversity. More specifically, in the burned areas, the highest numbers of Asteraceae and Fabaceae species were observed with a major increase in richness, as also observed in other meso-Mediterranean forests [111,115]. The colonization of Asteraceae species in the burned plots, for the most part, is due to their anemochorous dispersal capabilities, whose regeneration depends on long-distance seed dispersal [32]. The increase in Fabaceae species is mainly due to the soil seed bank whose dormancy is broken by fire [120,121,122,123]. In addition to soil protection against water erosion, during the first years after a fire, many Fabaceae species play an important ecological role related to their nitrogen-fixing capability. The input of organic N from legumes can stimulate the recovery of microbial activity and N cycling which further can serve to facilitate community succession and promote ecosystem resilience [124,125]. However, two years after the fire event, no Asteraceae and Fabaceae plant species showed a significant change in terms of frequency and cover in the burned areas compared to control. At species level, we observed a great frequency increase for Scrophularia vernalis, Rubus hirtus group, and Clematis vitalba, which were found in all the burned plots, but not in the unburned ones. Rubus hirtus group, which includes R. hirtus s.str. and R. glandulosus Bellardi, according to Bartolucci et al. [66], is a Eurasiatic shrub able to reproduce both vegetatively by tip-rooting, and sexually by seeds. Its seeds are easily dispersed by animals and the rate of its expansion and relation to the amount of light are well reported [126,127].
Within the gaps, changes in the micro-environmental conditions also induced the disappearance of Sesleria autumnalis, Neottia nidus-avis, Epipactis helleborine (L.) Crantz, and Cephalanthera rubra (L.) Rich. All these species require shady and fresh environments and they are normally present in the undisturbed beech wood of the study area. Regarding the orchid species, in addition to changing local microclimatic conditions, fire may have damaged their mycorrhizal fungi (mostly basidiomycetes), which are necessary for seed germination and often useful for nutrition throughout autotrophic plants’ lives [128]. Additionally, smoke from fires has antifungal properties [129,130], and hence may have reduced the activities of mycorrhizal fungi, as soil micro-organism abundance is greatest in the topsoil [17]. Two years after the fire, the lack of presence of all orchid species in the burned areas highlights the short-term negative effect on these plants of high conservation interest (Annex II of the Convention on International Trade in Endangered Species of wild fauna and flora-CITES and Regional Law of Campania 40/94 [131]). In the next years, orchids colonization and establishment in the burned areas, as well as the other undergrowth sciaphilous species, will be mostly dependent on the restoration of the trees layer.
Fire disturbance, in addition to causing the death of Fagus sylvatica trees and a clear reduction in the overall coverage of this species with a presumable reduction in slopes stability [132]), allowed significant colonization of Verbascum thapsus subsp. thapsus and Digitalis micrantha, as well as an increase of the bryophytes frequency (e.g., Funaria hygrometrica and Lunularia cruciata subsp. cruciata), species generally not present in the studied beech forest. In the Lattari Mountains, Verbascum thapsus subsp. thapsus generally occurs in open environments and was absent in the unburned beech forest due to its light requirements. Therefore, in the native range of Lattari Mountains, Verbascum thapsus subsp. thapsus colonized early post-fire communities, as it was observed in the territories where it was introduced as an exotic plant [133,134]. Similar to the observations of other authors [135,136], we also observed a germination of on-site seed in spring 2018 (A. Stinca, personal observations) probably due to the richness of its soil seed bank. In fact, Verbascum thapsus subsp. thapsus is a Eurasiatic biennial herb with rosette leaves in the first year which overwinters and is followed in the succeeding growing season by a stout flowering stem, and its seeds possess no specialized morphological adaptations for dispersal by wind or animals [137]. The Italian endemic Digitalis micrantha, which has biological traits similar to Verbascum thapsus subsp. thapsus (hemicryptophyte scapose vs. hemicryptophyte biennial), is common in deciduous forests located below 1000 m a.s.l. and more rarely grows in beech forests of the Lattari Mountains. Additionally, for this species, in the gaps of the forest, we have observed many seedlings in spring 2018 (A. Stinca, personal observations) due to its soil seed bank. Moreover, its biological form, as a hemicryptophyte, with a perennial biological cycle with overwintering buds at ground level and protected by bedding or snow, can give it fire resistance and vegetative regrowth capacity, as reported for related Digitalis thapsi L. in the burned beech forest areas from Spain [110].
Change in bryophyte species composition after the fire also follows the general model due to the environmental post-fire conditions with an increase in the frequency of acrocarpous species in burned soil and pleurocarpous in unburned soil [138,139,140]. The high frequency of cosmopolitan moss Funaria hygrometrica in the burned ground, mainly those affected by high intensity fire, is in accordance with several previous studies on post-fire bryophyte dynamics in the Mediterranean vegetation [140,141,142,143].
As for the regeneration of trees, the higher number of total seedling species found in the canopy gaps is in accordance to van Gils et al. [34]. Our result on Fagus sylvatica subsp. sylvatica is in accordance with a study regarding the regeneration after wind disturbance in an old-growth Fagus-Abies forest in Slovenia [144]. Effectively, in European beech forests, Fagus sylvatica subsp. sylvatica regeneration by seeds occurs mainly in the canopy gaps [42,116,145]. Here, the absence of litter burned by the fire may limit root necrosis of seedlings, due to autotoxic effects by extracellular self-DNA [146]. However, Fagus sylvatica subsp. sylvatica is also relatively shade-tolerant, so regeneration can survive for long periods under a closed canopy [34,36,147]. In the burned plots, we also observed many dead Fagus sylvatica seedlings, probably as a result of water and thermal stress that occurred in the summer period. On the other hand, the abundance of early post-fire colonizers also observed in our study sites, can limit beech regeneration [40]. Both these factors can explain why the cover of seedlings in the gaps was not significantly different from the unburned areas.
The change in the micro-environmental conditions produced by the fire in the investigated beech forest, in addition to a significant increase of therophytes and chamaephytes, also caused a strong reduction in geophytes, such as orchids. In alpine forests, Alberti et al. [148] found that the abundance of geophytes groups is strictly related to the degree of shading of the forest stand (e.g., tree density and canopy cover).
One of the more surprising findings in this study was the lack of alien species in the burned plots, two years post-fire. On the other hand, non-native vascular plants generally spread in the disturbed forests [117,149,150]. Our study area was located in the altitudinal range of 1176–1384 m a.s.l. within a territory little altered by human activities and this could have prevented the spread of exotic plants. Indeed, it is known that alien species richness typically declines along the elevation [151,152,153] and increases with the anthropogenic pressure [154]. Fire disturbance anyway produced a significant change in chorological features in the plant communities. The increase in the burned areas in cosmopolite (e.g., Arabidopsis thaliana (L.) Heynh., Hypericum perforatum L. subsp. perforatum, and Pteridium aquilinum (L.) Kuhn subsp. aquilinum), steno-Mediterranean (e.g., Petrosedum sediforme (Jacq.) Grulich subsp. sediforme, Silene vulgaris (Moench) Garcke subsp. tenoreana (Colla) Soldano and F.Conti, and Vicia pseudocracca Bertol.) and Atlantic species (e.g., Helleborus foetidus L. subsp. foetidus), as well as the decrease in Eurasiatic plants percentage (e.g., Cephalanthera rubra, Epipactis helleborine, Lathyrus venetus (Mill.) Wohlf., Neottia nidus-avis, and Sesleria autumnalis) was related to the colonization of many opportunistic species, typical of the initial stages of a secondary succession.
Changes in the micro-environmental conditions within the canopy gaps fit perfectly with the ecological characteristics of post-fire plant community found. Here, we recorded significant changes in EIVs related to the climatic variables: an increase in species with greater needs for light and temperatures, and reduction of plants typical of the continental climates. In contrast, changes in the specific composition did not induce any significant shifts in the Ellenberg Indicator Values (EIVs) related to the edaphic conditions: moisture, reaction (i.e., pH), and nutrient availability. These results seem to agree with the soil organic matter content and functional features of soil community (total microbial biomass, fungal mycelium, respiration, and indexes of microbial metabolism) which have been mildly or not affected by the fire.
4.2. Fire Effect on Soil Chemical and Microbial Properties
The studied soil had typical properties of Allophanic Andisol, i.e., volcanic soil with high organic C content (8%–12%) and CEC (20–50 cmol kg−1), and acidic pH (4.0–6.0) [155]. Moreover, it was non-saline (EC < 2 dS m−1 [156]).
Two years after the wildfire occurred in the beech forest of the Lattari Mountains, a significant increase in pH was observed, as also reported previously in other forest soils affected by wildfire [157,158,159,160,161]. The pH may increase up to three units as a consequence of the production of K and Na oxides, hydroxides, and carbonates, but this increase is only temporary because these compounds are very soluble and do not persist through the wet season. On the contrary, the neo-formed calcite is much less soluble and may be retained in the soil three years after burning, maintaining moderately alkaline pH in soils that were neutral to strongly acidic before burning [162]. The effect of fire on pH may depend on soil temperature reached during the burning event. Badía and Martí [163] observed a decrease in pH at 250 °C, but an increase at 500 °C.
The significant increase in EC, which is a measure of soil salinity, in burned plots may depend on the use of seawater to put out the fire [164,165], as was the case in the 2017 fire here (A. Stinca, personal observation).
A decrease in CEC was observed in the burned soil (although not statistically significant, p = 0.054), notwithstanding the organic carbon content did not change. CEC reduction, reported also by other authors [163], may be due to a decrease in the clay content. Badía and Martí [163] observed a continuous increase of the sand fraction with the increase in temperature from 25 °C to 500 °C, corresponding to a simultaneous decrease of the clay fraction; this could be due to transformations of mineral compounds, such as the thermal modifications of the Fe- and Al-silicates, which cause the fusion of clay particles into sand-sized particles.
Total organic C and labile organic (Cext and mineralizable C) contents were similar in the burned and control soils, probably because a recovery during the two years following the wildfire occurred. However, it cannot be excluded that no change in the organic matter occurred due to fire. Indeed, available studies on this topic are equivocal, as no effect, increases, or decreases, have been reported [118,119,157,158,160] during the first year after a wildfire. The effect of fire on soil organic matter depends on the fire intensity [119] and it may vary from volatilization of minor constituents, charring, or complete oxidation. During each wildfire, a fraction of burning biomass, instead of being emitted to the atmosphere as CO2 and other gases and aerosols, may be converted to “pyrogenic organic matter”, which is resistant to microbial decomposition and includes partly charred organic materials, charcoal, and soot [166]. Substantial loss of organic matter begins at 200–250 °C to complete at about 460 °C. Badía and Martí [163] reported no loss in organic matter at soil heating to 150 °C, a significant decrease at 250 °C; and a loss of 9/10 of the initial soil organic matter after a soil heating at 500 °C. It is unlikely that two years after the wildfire that we studied there was no trace of a significant loss of organic matter due to fire event; therefore, soil temperature probably was not very high during the wildfire.
Based on the observations of no change in organic C pool and the probable low temperature reached by soil during the fire, significant differences were not observed in this study between burned and unburned soil in total microbial biomass nor in the fungal mycelium. The latter microbial group is more sensitive to fire disturbance compared to bacteria, according to lower temperature thresholds in dry and moist soil (80 °C and 60 °C, respectively) compared to bacteria (120 °C and 100 °C, respectively [167]). Indeed, in contrast to the total microbial biomass, fungal mycelium was reduced during the first two years after experimental fires carried out in Mediterranean maquis with consequent reduction of the fungal component on the microbial community, evaluated as Cfung%Cmic after [27]; notwithstanding, an increase in xerotolerant and heat-stimulated fungi abundance occurred [168]. In this experiment, the temperature reached the maximum values of 219 °C at the soil surface, and of 31 °C at 5-cm depth [169]. The persistent decrease in fungal mycelium observed by these authors may be explained not only by soil heating during the fire but also by indirect effects, such as higher fluctuations in water content and temperature in the top centimeters of soils (due to plant cover reduction), the reduced availability of litter, and qualitative changes in organic substrates induced by fire and/or increased availability of toxic substance to fungi [27]. Probably, in our soil, none of these effects occurred. Similarly, no fire effect was observed, two years after the event, on microbial activity (i.e., soil respiration) and metabolic quotient (qCO2) indicating the efficiency in the use of C resource by microorganisms [170]. Fierro et al. [171] observed an increase in soil respiration and qCO2 only in the first three months after an experimental fire in Mediterranean maquis, but no differences successively. In our study we did not observe alterations in quotient of mineralization (qM) which is the percentage of organic C that can be mineralized by microorganisms in a short time in optimal conditions; thus, indicating the labile organic fraction rather than the total one.
4.3. Fire Effect on Bacterial Diversity
Proteobacteria, Acidobacteria, and Actinobacteria were the most abundant phyla in the studied soils. Our findings were supported by observations from previous studies of forests affected by wildfire. Li et al. [172] observed the same trend in the top soil of Pinus tabuliformis Carrière forest of northern China, six months after a high-severity wildfire. The same trend was observed in an Australian wet sclerophyll forest [159], where Acidobacteria (36%–42%), Verrucomicrobia (24%–39%), and Proteobacteria (12%–23%) were the most abundant phyla found in the soil under long-term frequent prescribed fire. Unlike biomass (Cmic) and total microbial activity (respiration and carbon mineralization), the composition of the bacterial community was strongly affected by the fire as evidenced by net separation of bacterial communities from burned and control soils. Five phyla were over-represented and five others were under-represented in burned soils compared to control. It is interesting to note that the most abundant phyla (Proteobacteria, Acidobacteria, Bacteroidetes, Planctomycetes, Firmicutes, Gemmatimonadetes, and Chloroflexi) were not significantly affected by fire, except for Actinobacteria, which were over-represented in burned soil. Similarly, Sáenz de Miera et al. [173] observed a clear increase in the relative frequency of Actinobacteria two months after wildfire occurred in Mediterranean ecosystems (shrublands and forest) of North-West Spain, as well as in Proteobacteria and Firmicutes, but a decrease in the Acidobacteria, Bacteroidetes, Verrucomicrobia (which disappeared in forest soil), and Chloroflexi. Xiang et al. [18] reported a significant decrease in Acidobacteria, Deltaproteobacteria, and Planctomycetes, until one year after wildfire in coniferous forests of North-East China, and in Alphaproteobacteria, until 11 years after fire. On the contrary, Betaproteobacteria significantly increased one year after fire and Actinobacteria was not affected. In Chinese Pinus tabuliformis forest, Li et al. [172] observed an increase in Proteobacteria and Actinobacteria abundances, but significantly lower relative abundances of Acidobacteria, Verrucomicrobia, and Chloroflexi after a high- or moderate-severity wildfire.
While the bacterial family richness did not change, the composition in the two different conditions was significantly different. As other authors also reported, soil pH was one of the major factors contributing to the observed bacterial community variations [18,159]. Some families were under-represented (Acidobacteriaceae, Sinobacteraceae, Rhodospirillaceae, and Solibacteraceae), whereas others were over-represented (Sphingomonadaceae, Iamiaceae, Nocardioidaceae, Opitutaceae, Comamonadaceae, Oxalobacteraceae, Micrococcaceae, Pseudomonadaceae, Cytophagaceae, Solirubrobacteraceae, Bacillaceae, and Hymenobacteraceae) in burned soil compared to control. The increase in pH could explain the reduction in Acidobacteriaceae, as suggested also by other authors [18,159].
Within Micrococcaceae, Arthrobacter spp. were found only in burned soil and lacking in the control; the same occurred also for Bacillus species. Similarly, Sáenz de Miera et al. [173] observed an increase in the relative abundance of Arthrobacter. Some species of the genera Arthrobacter and Bacillus are capable of degrading polycyclic aromatic hydrocarbons, some of which (deriving from the organic matter) accumulate in the soil during the fires [173]. In a burned woodland soil, Andreolli et al. [174] found phenanthrene, benzo(a)anthracene, chrysene, benzo(k)fluoranthene, and benzo(a)pyrene. Whitman et al. [175] identified Arthrobacter sp. and Massilia sp. as positive fire responders. Arthrobacter, which can survive fires because of its ability to resist starvation, desiccation, and oxidative stress and to thrive on the fire-linked aromatic C sources, may play a role in post-fire nitrogen cycling and phosphorus solubilization; therefore, it has important effects on plant growth [175].
5. Conclusions
Although high-intensity wildfires frequently threaten the Mediterranean forest ecosystems, few studies regarding their impacts on beech forests have been conducted. As far as we know, our research is the first on the change in relationships between multi-level beech forest biodiversity and key soil features linked to soil functioning in secondary succession process after a wildfire. Moreover, this study is the first in which total vascular flora is analyzed to understand the impact of fire and the recovery of vegetation in burned beech forests. In this research we demonstrated the dramatic effect on plant composition and soil bacterial community composition, even two years after the wildfire occurred in a coastal beech forest located in southern Italy. In the forest gaps, changes in micro-environmental conditions due to wildfire have triggered a plant secondary succession process with the colonization by many pioneer species which generally grow in the surrounding study area in open environments and/or at lower altitudes. Noteworthy was the increase in the coverage or frequency of the vascular species Verbascum thapsus subsp. thapsus and Digitalis micrantha, as well as the bryophyte Funaria hygrometrica. At the same time, we observed the disappearance of all orchid species and significant changes in the structural (increase of therophytes and chamaephytes, and reduction of geophytes), chorological (increase of cosmopolites, steno-Mediterraneans and Atlantics, and decrement of Eurasiatics) and ecological features (increase of species with greater needs for light and temperatures, reduction of those typical of continental climates) of the studied plant community.
Furthermore, we also identified an effect of fire on the microbial community composition. As far as we know, this is the first studies conducted, in which the quantitative and qualitative composition of bacteria is significantly altered following a fire in a beech forest. Our results identify specific fire-responsive microbial taxa and provide support for possible successful post-fire ecological strategies with potentially important implications for ecosystem functions. Microorganisms can be good indicators of the health status of a forest ecosystem, and thus used to monitor soil recovery. Indeed, some bacterial groups that are likely to play key functional roles in these soils, can be lost following a fire. Recovering and inoculating them from unaltered soils could help accelerate forest recovery.
Soil pH change was the main chemical driver of bacterial community composition and microbial community structure in terms of relative abundance of fungal vs. total microbial biomass. While the effect of the fire on soil microbial community composition was still detectable two years after the fire, functional features of this community (total microbial biomass, fungal mycelium, respiration, and indexes of microbial metabolism) appeared unchanged between burned and control plots, reflecting the unchanged organic matter resource both as total pool (Corg) and its more labile fractions (Cext, mineralizable C). This result suggests that soil functions, such as organic matter decomposition and consequent nutrient mineralization, were guaranteed in burned soil, so that nutrient availability for the plant community should not be modified compared to unburned plots. Therefore, it can be expected that the re-establishment of this beech forest will not be limited by the alteration in soil properties, and it may occur even if it takes a long time, which is typical of ecological succession.
In conclusion, in the study area, where current climatic changes could include an increase in drought periods and, consequently, cause an increase in fire frequency and intensity, the disturbance effects of wildfires on less fire-prone vegetation, such as Mediterranean beech forests, represent an escalating problem. However, to date, very little is known about the temporal trends and factors that trigger post-fire regeneration processes and biodiversity conservation. We stress that further comprehensive mid-term monitoring studies are needed to properly assess the fire effects and plan subsequent management actions for the coastal beech forests restoration after wildfires.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/11/9/983/s1, Table S1: Geographic coordinates, site conditions, vegetation, and soil features of the sampling plots, Table S2: Vegetation relevés performed in the study area, Table S3: Taxonomic, biological, and ecological features of the vascular plants surveyed in the study area, Table S4: Bacterial sequencing reads (phyla, families, and species) identified in the soils of the study area, Figure S1: Rarefaction curves for 16S rRNA gene sequences from soils of the study area, and Figure S2: Total, differential, over- and under-represented OTUs from soils of the study area.
Author Contributions
Conceptualization, A.S.; methodology, A.S.; investigation, A.S., M.R. and R.M.; formal analysis, A.S., M.R., R.M., G.M., A.C., F.A.R. and A.E.; data curation, A.S., M.R., R.M., G.M., A.C., F.A.R. and A.E.; writing—original draft preparation, A.S., M.R., R.M. and F.A.R.; and writing—review and editing, A.S., M.R., R.M., G.M., A.C., F.A.R. and A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful to Claudia Campobasso (Civil Emergency and Post-Emergency Protection of the Campania Region, Italy) for providing some 2017 fire data relating to the study area, Giacomo Mei (Marche Polytechnic University, Ancona, Italy) for suggestions on vascular plants diversity data elaboration, and Anindya Mukhopadhya for English language checking.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bowman, D.M.J.S.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.S.; Doyle, J.C.; Harrison, S.P.; et al. Fire in the Earth System. Science 2009, 324, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Van Mantgem, P.J.; Nesmith, J.C.B.; Keifer, M.; Knapp, E.E.; Flint, A.; Flint, L. Climatic stress increases forest fire severity across the western United States. Ecol. Lett. 2013, 16, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Diffenbaugh, N.S.; Swain, D.L.; Touma, D. Anthropogenic warming has increased drought risk in California. Proc. Natl. Acad. Sci. USA 2015, 112, 3931–3936. [Google Scholar] [CrossRef] [PubMed]
- Abatzoglou, J.T.; Williams, A.P. Impact of anthropogenic climate change on wildfire across western US forests. Proc. Natl. Acad. Sci. USA 2016, 113, 11770–11775. [Google Scholar] [CrossRef]
- Schumacher, S.; Reineking, B.; Sibold, J.; Bugmann, H. Modeling the impact of climate and vegetation on fire regimes in mountain landscapes. Landsc. Ecol. 2006, 21, 539–554. [Google Scholar] [CrossRef]
- Flannigan, M.D.; Stocks, B.J.; Wotton, B.M. Climate change and forest fires. Sci. Total Environ. 2000, 262, 221–229. [Google Scholar] [CrossRef]
- San-Miguel-Ayanz, J.; Durrant, T.; Boca, R.; Libertà, G.; Branco, A.; de Rigo, D.; Ferrari, D.; Maianti, P.; Artés Vivancos, T.; Costa, H.; et al. Forest Fires in Europe, Middle East and North Africa 2017; European Commission, JRC Technical Reports; Join Research Center: Ispra (Varese), Italy, 2018; p. 139. [Google Scholar]
- Ganteaume, A.; Camia, A.; Jappiot, M.; San-Miguel-Ayanz, J.; Long-Fournel, M.; Lampin, C. A Review of the Main Driving Factors of Forest Fire Ignition over Europe. Environ. Manag. 2013, 51, 651–662. [Google Scholar] [CrossRef]
- Broncano, M.J.; Retana, J. Topography and forest composition affecting the variability in fire severity and post-fire regeneration occurring after a large fire in the Mediterranean basin. Int. J. Wildland Fire 2004, 13, 209–216. [Google Scholar] [CrossRef]
- Birch, D.S.; Morgan, P.; Kolden, C.A.; Abatzoglou, J.T.; Dillon, G.K.; Hudak, A.T.; Smith, A.M.S. Vegetation, topography and daily weather influenced burn severity in central Idaho and western Montana forests. Ecosphere 2015, 6, 1–23. [Google Scholar] [CrossRef]
- Rogers, B.M.; Soja, A.J.; Goulden, M.L.; Randerson, J.T. Influence of tree species on continental differences in boreal fires and climate feedbacks. Nat. Geosci. 2015, 8, 228–234. [Google Scholar] [CrossRef]
- Fares, S.; Bajocco, S.; Salvati, L.; Camarretta, N.; Dupuv, J.L.; Xanthopoulos, G.; Guijarro, M.; Madrigal, J.; Hernando, C.; Corona, P. Characterizing potential wildland fire fuel in live vegetation in the Mediterranean region. Ann. For. Sci. 2017, 74, 1. [Google Scholar] [CrossRef]
- Fang, L.; Yang, J.; White, M.; Liu, Z. Predicting Potential Fire Severity Using Vegetation, Topography and Surface Moisture Availability in a Eurasian Boreal Forest Landscape. Forests 2018, 9, 130. [Google Scholar] [CrossRef]
- Pausas, J.G. Simulating Mediterranean landscape pattern and vegetation dynamics under different fire regimes. Plant Ecol. 2006, 187, 249–259. [Google Scholar] [CrossRef]
- He, T.; Lamont, B.B.; Pausas, J.G. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef]
- Ducrey, M.; Duhoux, F.; Huc, R.; Rigolot, E. The ecophysiological and growth responses of Aleppo pine (Pinus halepensis) to controlled heating applied to the base of the trunk. Can. J. For. Res. 1996, 26, 1366–1374. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Xiang, X.; Shi, Y.; Yang, J.; Kong, J.; Lin, X.; Zhang, H.; Zeng, J.; Chu, H. Rapid recovery of soil bacterial communities after wildfire in a Chinese boreal forest. Sci. Rep. 2014, 4, 3829. [Google Scholar] [CrossRef]
- Sharma, S.K.; Ramesh, A.; Sharma, M.P.; Joshi, O.P.; Govaerts, B.; Steenwerth, K.L.; Karlen, D.L. Microbial community structure and diversity as indicators for evaluating soil quality. In Biodiversity, Biofuels, Agroforestry and Conservation Agriculture; Lichtfouse, E., Ed.; Sustainable Agriculture Reviews; Springer: Amsterdam, The Netherlands, 2010; Volume 5, pp. 317–358. [Google Scholar]
- Dominati, E.; Patterson, M.; Mackay, A. A framework for classifying and quantifying the natural capital and ecosystem services of soils. Ecol. Econ. 2010, 69, 1858–1868. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Matei, G.M.; Matei, S.; Mocanu, V.; Dumitru, S. Microbiological characterization of suppressive forest soil from Enisala. Ann. Univ. Craiova-Agric. Montanol. Cadastre Ser. 2016, 46, 341–347. [Google Scholar]
- Weller, D.M.; Raaijmakers, J.M.; McSpadden Gardener, B.B.; Thomashow, L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002, 40, 309–348. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Gu, J.; Li, Y.; Qian, X.; Sun, W.; Wang, X.; Gao, H.; Zhen, L.; Lei, Y. Analyses of microbial biomass and community diversity in kiwifruit orchard soils of different planting ages. Acta Ecol. Sin. 2015, 35, 22–28. [Google Scholar] [CrossRef]
- Giuditta, E.; Marzaioli, R.; Esposito, A.; Ascoli, D.; Stinca, A.; Mazzoleni, S.; Rutigliano, F.A. Soil microbial diversity, biomass, and activity in two pine plantations of Southern Italy treated with prescribed burning. Forests 2020, 11, 19. [Google Scholar] [CrossRef]
- Hamman, S.T.; Burke, I.C.; Stromberger, M.E. Relationships between microbial community structure and soil environmental conditions in a recently burned system. Soil Biol. Biochem. 2007, 39, 1703–1711. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; De Marco, A.; D’Ascoli, R.; Castaldi, S.; Gentile, A.; Virzo De Santo, A. Impact of fire on fungal abundance and microbial efficiency in C assimilation and mineralisation in a Mediterranean maquis soil. Biol. Fertil. Soils 2007, 44, 377–381. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, M.; Wang, S. A meta-analysis on the response of microbial biomass, dissolved organic matter, respiration, and N mineralization in mineral soil to fire in forest ecosystems. For. Ecol. Manag. 2012, 271, 91–97. [Google Scholar] [CrossRef]
- Pietikainen, J.; Fritze, H. Clear-cutting and prescribed burning in coniferous forest—Comparison of effects on soil fungal and total microbial biomass, respiration activity and nitrification. Soil Biol. Biochem. 1995, 27, 101–109. [Google Scholar] [CrossRef]
- Bárcenas-Moreno, G.; Bååth, E. Bacterial and fungal growth in soil heated at different temperatures to simulate a range of fire intensities. Soil Biol. Biochem. 2009, 41, 2517–2526. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire in Mediterranean climate ecosystems-A comparative overview. Isr. J. Ecol. Evol. 2012, 58, 123–135. [Google Scholar]
- Paula, S.; Arianoutsou, M.; Kazanis, D.; Tavsanoglu, Ç.; Lloret, F.; Buhk, C.; Ojeda, F.; Luna, B.; Moreno, J.M.; Rodrigo, A.; et al. Fire-related traits for plant species of the Mediterranean Basin. Ecology 2009, 90, 1420. [Google Scholar] [CrossRef]
- Madrigal, J.; Souto-García, J.; Calama, R.; Guijarro, M.; Picos, J.; Hernando, C. Resistance of Pinus pinea L. bark to fire. Int. J. Wildland Fire 2019, 28, 342–353. [Google Scholar] [CrossRef]
- Van Gils, H.; Odoi, J.; Andrisano, T. From monospecific to mixed forest after fire? For. Ecol. Manag. 2010, 259, 433–439. [Google Scholar] [CrossRef]
- Walentowski, H.; Müller-Kroehling, S.; Bergmeier, E.; Bernhardt-Römermann, M.; Gossner, M.; Reif, A.; Schulze, E.; Bußler, H.; Strätz, C.; Adelmann, W. Faunal diversity of Fagus sylvatica forests: A regional and European perspective based on three indicator groups. Ann. For. Res. 2014, 57, 215–231. [Google Scholar]
- Wagner, S.; Collet, C.; Madsen, P.; Nakashizuka, T.; Nyland, R.D.; Sagheb-Talebi, K. Beech regeneration research: From ecological to silvicultural aspects. For. Ecol. Manag. 2010, 259, 2172–2182. [Google Scholar] [CrossRef]
- Bär, A.; Nardini, A.; Mayr, S. Post-fire effects in xylem hydraulics of Picea abies, Pinus sylvestris and Fagus sylvatica. New Phytol. 2018, 217, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Packham, J.R.; Thomas, P.; Atkinson, M.; Degen, T. Biological flora of the British Isles: Fagus sylvatica. J. Ecol. 2012, 100, 1557–1608. [Google Scholar] [CrossRef]
- Maringer, J.; Wohlgemuth, T.; Neff, C.; Pezzatti, G.; Conedera, M. Postfire spread of alien plant species in a mixed broad-leaved forest of the Insubric region. Flora 2012, 1, 19–29. [Google Scholar] [CrossRef]
- Maringer, J.; Conedera, M.; Ascoli, D.; Schmatz, D.R.; Wohlgemuth, T. Resilience of European beech forests (Fagus sylvatica L.) after fire in a global change context. Int. J. Wildland Fire 2016, 25, 699–710. [Google Scholar] [CrossRef]
- Ascoli, D.; Castagneri, D.; Valsecchi, C.; Conedera, M.; Bovio, G. Postfire restoration of beech stands in the Southern Alps by natural regeneration. Ecol. Eng. 2013, 54, 210–217. [Google Scholar] [CrossRef]
- Ascoli, D.; Vacchiano, G.; Maringer, J.; Bovio, G.; Conedera, M. The synchronicity of masting and intermediate severity fire effects favor beech recruitment. For. Ecol. Manag. 2015, 353, 126–135. [Google Scholar] [CrossRef]
- Gimeno-Garcia, E.; Andreu, V.; Rubio, J.L. Influence of vegetation recovery on water erosion at short and medium-term after experimental fires in a Mediterranean shrubland. Catena 2007, 69, 150–160. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Penas, Á.; Díaz, T.E. Bioclimatic & Biogeographic Maps of Europe. 2004. Available online: http://www.globalbioclimatics.org/form/maps.htm (accessed on 10 June 2020).
- Blasi, C.; Marignani, M.; Copiz, R.; Fipaldini, M.; Del Vico, E. (Eds.) Le Aree Importanti per le Piante Nelle Regioni d’Italia: Il Presente e il Futuro Della Conservazione del Nostro Patrimonio Botanico; Progetto Artiser: Roma, Italy, 2010; p. 224. [Google Scholar]
- CARG. Carta Geologica d’Italia. Scala 1:50.000. Foglio 466–485: Sorrento-Termini. Note Illustrative alla Carta Geologica d’Italia alla Scala 1:50.000. Fogli 466–485 Sorrento-Termini.ISPRA, CNR. 2014. Available online: https://www.isprambiente.gov.it/Media/carg/campania.html (accessed on 10 June 2020).
- Di Gennaro, A. I Sistemi di Terre della Campania; Risorsa: Firenze, Italy, 2002; p. 64. [Google Scholar]
- Zitti, S.; Frattaroli, A.R.; Carli, E.; Cutini, M. 9210 *Faggeti degli Appennini con Taxus e Ilex. In Manuali per il Monitoraggio di Specie e Habitat di Interesse Comunitario (Direttiva 92/43/CEE) in Italia: Habitat; Angelini, P., Casella, L., Grignetti, A., Genovesi, P., Eds.; Serie Manuali e Linee Guida 142/2016; ISPRA: Roma, Italy, 2016; pp. 234–235. [Google Scholar]
- Cancellieri, L.; Caneva, G.; Cutini, M. Phytosociology and ecology of the Mediterranean forests ecosystems in the Amalfi Coast (Monti Lattari, Italy). Rend. Lincei 2017, 28, 651–671. [Google Scholar] [CrossRef]
- Di Pietro, R.; Izco, J.; Blasi, C. Contribute to the nomenclatural knowledge of the beech-woodland syntaxa of southern Italy. Plant Biosyst. 2004, 138, 27–36. [Google Scholar] [CrossRef]
- Peruzzi, L.; Domina, G.; Bartolucci, F.; Galasso, G.; Peccenini, S.; Raimondo, F.M.; Albano, A.; Alessandrini, A.; Banfi, E.; Barberis, G.; et al. An inventory of the names of vascular plants endemic to Italy, their loci classici and types. Phytotaxa 2015, 196, 1–217. [Google Scholar] [CrossRef]
- Stinca, A. Lonicera stabiana Guss. & Pasq. In La Flora Endemica Minacciata delle Montagne Italiane; Conti, F., Bartolucci, F., Di Martino, L., Manzi, A., Eds.; Club Alpino Italiano: Milano, Italy, 2019; pp. 340–342. [Google Scholar]
- Orsenigo, S.; Montagnani, C.; Fenu, G.; Gargano, D.; Peruzzi, L.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Bovio, M.; et al. Red Listing plants under full national responsibility: Extinction risk and threats in the vascular flora endemic to Italy. Biol. Conserv. 2018, 224, 213–222. [Google Scholar] [CrossRef]
- Orsenigo, S.; Fenu, G.; Gargano, D.; Montagnani, C.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Carta, A.; Castello, M.; et al. Red list of threatened vascular plant species in Italy. Plant Biosyst. 2020. [Google Scholar] [CrossRef]
- Battipaglia, G.; Tognetti, R.; Valese, E.; Ascoli, D.; De Luca, P.F.; Basile, S.; Ottaviano, M.; Mazzoleni, S.; Marchetti, M.; Esposito, A. Incendi 2017: Un’importante lezione. For. J. Silvic. For. Ecol. 2017, 14, 231–236. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde, 3rd ed.; Springer: Wien, Austria; New York, NY, USA, 1964; p. 865. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea, Volume 2; Cambridge University Press: Cambridge, UK, 1968; p. 465. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea, Volume 3; Cambridge University Press: Cambridge, UK, 1972; p. 381. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea, Volume 4; Cambridge University Press: Cambridge, UK, 1976; p. 515. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea, Volume 5; Cambridge University Press: Cambridge, UK, 1980; p. 463. [Google Scholar]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993; Volume 1, p. 581. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, Volume 1; Edagricole: Bologna, Italy, 2017; p. 1064. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, Volume 2; Edagricole: Bologna, Italy, 2017; p. 1178. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, Volume 3; Edagricole: Bologna, Italy, 2018; p. 1288. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, Volume 4; Edagricole: Bologna, Italy, 2019; p. 1054. [Google Scholar]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Smith, A.J.E. The Liverworts of Britain and Ireland; Cambridge University Press: Cambridge, UK, 1991; p. 378. [Google Scholar]
- Smith, A.J.E. The Moss Flora of Britain and Ireland; Cambridge University Press: Cambridge, UK, 2010; p. 1026. [Google Scholar]
- Cortini Pedrotti, C. Flora dei Muschi d’Italia. Sphagnopsida. Andreaeopsida. Bryopsida (I Parte); Antonio Delfino Editore: Roma, Italy, 2001; p. 817. [Google Scholar]
- Cortini Pedrotti, C. Flora dei muschi d’Italia. Bryopsida (II Parte); Antonio Delfino Editore: Roma, Italy, 2006; pp. 818–1235. [Google Scholar]
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An annotated checklist of bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Pignatti, S.; Menegoni, P.; Pietrosanti, S. Valori di indicazione delle piante vascolari della Flora d’Italia. Braun-Blanquetia 2005, 9, 1–97. [Google Scholar]
- Vacchiano, G.; Lonati, M.; Berretti, R.; Motta, R. Drivers of Pinus sylvestris L. regeneration following small, high-severity fire in a dry, inner-alpine valley. Plant Biosyst. 2015, 149, 354–363. [Google Scholar] [CrossRef]
- Khapugin, A.A.; Vargot, E.V.; Chugunov, G.G. Vegetation recovery in fire damaged forests: A case study at the southern boundary of the taiga zone. For. Stud. 2016, 64, 39–50. [Google Scholar] [CrossRef]
- Calabrese, V.; Carranza, M.L.; Evangelista, A.; Marchetti, M.; Stinca, A.; Stanisci, A. Long term changes in the composition, ecology and structure of Pinus mugo scrubs in the Apennines (Italy). Diversity 2018, 10, 70. [Google Scholar] [CrossRef]
- Mei, G.; Pesaresi, S.; Corti, G.; Cocco, S.; Colpi, C.; Taffetani, F. Changes in vascular plant species composition, top-soil and seed-bank along coppice rotation in an Ostrya carpinifolia forest. Plant Biosyst. 2020, 154, 259–268. [Google Scholar] [CrossRef]
- USDA-NRCS. Soil Survey Laboratory Methods Manual; Soil Survey Investigations Report No. 42, Version 4.0; Burc, R., Ed.; National Soil Survey Center: Lincoln, NE, USA, 2004; p. 487.
- Allen, S.E. Chemical Analysis of Ecological Materials, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1989; p. 368. [Google Scholar]
- Springer, U.; Klee, J. Prüfung der Leistungsfähigkeit von einigen wichtigeren Verfahren zur Bestimmung des Kohlemstoffs mittels Chromschwefelsäure sowie Vorschlag einer neuen Schnellmethode. Z. Pflanz. Düngung Bodenkd. 1954, 64, 1–26. [Google Scholar] [CrossRef]
- Sleutel, S.; De Neve, S.; Singier, B.; Hofman, G. Quantification of Organic Carbon in Soils: A Comparison of Methodologies and Assessment of the Carbon Content of Organic Matter. Commun. Soil Sci. Plant Anal. 2007, 38, 2647–2657. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Sundman, V.; Sivelä, S. A comment on the membrane filter technique for estimation of length of fungal hyphae in soil. Soil Biol. Biochem. 1978, 10, 399–401. [Google Scholar] [CrossRef]
- Olson, F.C.W. Quantitative estimates of filamentous algae. Trans. Am. Microsc. Soc. 1959, 69, 272–279. [Google Scholar] [CrossRef]
- Berg, B.; Söderström, B. Fungal biomass and nitrogen in decomposing Scots pine needle litter. Soil Biol. Biochem. 1979, 11, 339–341. [Google Scholar] [CrossRef]
- ISO 16072. Soil Quality—Laboratory Methods for Determination of Microbial Soil Respiration; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Pell, M.; Stenström, J.; Granhall, U. Monitoring and evaluating soil quality. In Microbiological Methods for Assessing Soil Quality; Bloem, J., Benedetti, A., Hopkins, D.W., Eds.; CAB International: Wallingford, UK, 2006; pp. 117–127. [Google Scholar]
- Riffaldi, R.; Saviozzi, A.; Levi-Minzi, R. Carbon mineralization kinetics as influenced by soil properties. Biol. Fertil. Soils 1996, 22, 293–298. [Google Scholar] [CrossRef]
- D’Ascoli, R.; Rutigliano, F.A.; De Pascale, R.A.; Gentile, A.; Virzo De Santo, A. Functional diversity of the microbial community in Mediterranean maquis soils as affected by fires. Int. J. Wildland Fire 2005, 14, 355–363. [Google Scholar] [CrossRef]
- Killham, K. Soil Ecology; Cambridge University Press: Cambridge, UK, 1994; p. 242. [Google Scholar]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; Blackwell Scientific Publications: Oxford, UK, 1979; p. 372. [Google Scholar]
- Anderson, T.H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Canullo, R.; Starlinger, F.; Granke, O.; Fischer, R.; Aamlid, D. Part VI.1: Assessment of Ground Vegetation. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Co-Ordinating Centre, Ed.; Thunen Institute of Forest Ecosystems: Eberswalde, Germany, 2016; p. 15. Available online: http://icp-forests.net/page/icp-forests-manual (accessed on 15 June 2020).
- Knapp, E.E.; Ritchie, M.W. Response of understory vegetation to salvage logging following a high-severity wildfire. Ecosphere 2016, 7, e01550. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. ACM SIGMOBILE Mob. Comput. Commun. Rev. 1948, 27, 379–423. [Google Scholar]
- Sokal, R.R.; Rholf, F.J. Biometry: The Principles and Practices of Statistic in Biological Research, 3rd ed.; W.H. Freeman & Company: New York, NY, USA, 1995; p. 887. [Google Scholar]
- Podani, J. SYN-TAX 2000. Computer Programs for Data Analysis in Ecology and Systematics; User’s Manual; Scientia Publishing: Budapest, Hungary, 2001; p. 452. [Google Scholar]
- Van der Maarel, E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar]
- Paytuví, A.; Battista, E.; Scippacercola, F.; Aiese Cigliano, R.; Sanseverino, W. GAIA: An integrated metagenomics suite. bioRxiv 2019. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016, 44, D7–D19. [Google Scholar] [CrossRef]
- Mcmurdie, P.J.; Holmes, S.; Watson, M. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Purdie, R.W.; Slatyer, R.O. Vegetation succession after fire in sclerophyll woodland communities in south-eastern Australia. Aust. J. Ecol. 1976, 1, 223–236. [Google Scholar] [CrossRef]
- Trabaud, L. Dynamics after fire of sclerophyllous plant communities in the mediterranean basin. Ecol. Mediterr. 1987, 13, 25–37. [Google Scholar] [CrossRef]
- Guo, Q. Early post-fire succession in California chaparral: Changes in diversity, density, cover and biomass. Ecol. Res. 2001, 16, 471–485. [Google Scholar] [CrossRef]
- Harvey, B.J.; Holzman, B.A. Divergent successional pathways of stand development following fire in a Californian closed-cone pine forest. J. Veg. Sci. 2014, 25, 88–99. [Google Scholar] [CrossRef]
- Naveh, Z. The Role of Fire and Its Management in the Conservation of Mediterranean Ecosystems and Landscapes. In The Role of Fire in Mediterranean-Type Ecosystems; Moreno, J.M., Oechel, W.C., Eds.; Springer: New York, NY, USA, 1995; pp. 163–185. [Google Scholar]
- Capitanio, R.; Carcaillet, C. Post-fire Mediterranean vegetation dynamics and diversity: A discussion of succession models. For. Ecol. Manag. 2008, 255, 431–439. [Google Scholar] [CrossRef]
- Herranz, J.M.; Martinez-Sanchez, J.J.; De Las Heras, J.; Ferrandis, P. Stages of plant succession in Fagus sylvatica L. and Pinus sylvestris L. forest of Tejera Negra National Park (Central Spain), three years after fire. Isr. J. Plant Sci. 1996, 44, 347–358. [Google Scholar] [CrossRef]
- Christopoulou, A.; Kazanis, D.; Fyllas, N.M.; Arianoutsou, M. Post-fire recovery of Abies cephalonica forest communities: The case of Mt Parnitha National Park, Attica, Greece. iFor.-Biogeosci. For. 2018, 11, 757–764. [Google Scholar] [CrossRef]
- Kazanis, D.; Arianoutsou, M. Vegetation composition in a post-fire successional gradient of Pinus halepensis forests in Attica, Greece. Int. J. Wildland Fire 1996, 6, 83–91. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Neeman, G. Post-fire regeneration of natural Pinus halepensis forests in the East Mediterranean Basin. In Ecology, Biogeography and Management of Pinus halepensis and P. brutia Forest Ecosystems in the Mediterranean Basin; Ne’Eman, G., Trabaud, L., Eds.; Backhuys: Leiden, The Netherlands, 2000; pp. 269–290. [Google Scholar]
- Ganatsas, P.; Zagas, T.; Tsakaldimi, M.; Tsitsoni, T. Post-fire regeneration dynamics in a Mediterranean type ecosystem in Sithonia, northern Greece: Ten years after the fire. In Proceedings of the 10th MEDECOS Conference, Rhodes, Greece, 25 April–1 May 2004; Arianoutsou, M., Papanastasis, V., Eds.; Millpress: Rotterdam, The Netherlands, 2004; pp. 1–9. [Google Scholar]
- Ocak, A.; Kurt, L.; Oz, M.; Tug, N. Floristic al and ecological studies on burned black pine (Pinus nigra Arn. subsp. pallasiana (Lamb) Holboe) forest area at Central Anatolia. Asian J. Plant Sci. 2007, 6, 892–905. [Google Scholar]
- Nagel, T.A.; Svoboda, M.; Diaci, J. Regeneration patterns after intermediate wind disturbance in an old-growth Fagus–Abies forest in southeastern Slovenia. For. Ecol. Manag. 2006, 226, 268–278. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Abd El-Gawad, A.M.; Sarker, T.C.; Stinca, A.; Motti, R.; Cesarano, G.; Teobaldelli, M.; Saulino, L.; Cona, F.; et al. Windstorm disturbance triggers multiple species invasion in an urban Mediterranean forest. iFor.-Biogeosci. For. 2018, 11, 64–71. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Fierro, A.; De Pascale, R.A.; De Marco, A.; Virzo De Santo, A. Role of fire on soil organic matter turnover and microbial activity in a mediterranean burned area. Dev. Soil Sci. 2002, 28B, 205–215. [Google Scholar]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Auld, T.D.; O’Connell, M.A. Predicting patterns of post-fire germination in 35 eastern Australian Fabaceae. Aust. J. Ecol. 1991, 16, 53–70. [Google Scholar] [CrossRef]
- Herranz, J.M.; Ferrandis, P.; Martinez-Sanchez, J.J. Influence of heat on seed germination of seven Mediterranean Leguminosae species. Plant Ecol. 1998, 136, 95–103. [Google Scholar] [CrossRef]
- Baeza, M.J.; Vallejo, V.R. Ecological mechanisms involved in dormancy breakage in Ulex parviflorus seeds. Plant Ecol. 2006, 183, 191–205. [Google Scholar] [CrossRef]
- Bekdouche, F.; Sahnoune, M.; Krouchi, F.; Achour, S.; Guemati, N.; Derridj, A. The contribution of legumes to post-fire regeneration of Quercus suber and Pinus halepensis forests in northeastern Algeria. Rev. D’écol. 2011, 66, 29–42. [Google Scholar]
- Johnson, D.W.; Susfalk, R.B.; Caldwell, T.G.; Murphy, J.F.; Miller, W.W.; Walker, R.F. Fire effects on carbon and nitrogen budgets in forests. In Biogeochemical Investigations of Terrestrial, Freshwater, and Wetland Ecosystems across the Globe; Kelman Wieder, R., Novák, M., Vile, M.A., Eds.; Reprinted from Water, Air, & Soil Pollution: Focus 4; Springer: Dordrecht, The Netherlands, 2004; pp. 263–275. [Google Scholar]
- Goergen, E.M.; Chambers, J.C. Influence of a native legume on soil N and plant response following prescribed fire in sagebrush steppe. Int. J. Wildland Fire 2009, 18, 665–675. [Google Scholar] [CrossRef]
- Pancer-Koteja, E.; Szwagrzyk, J.; Bodziarczyk, J. Small scale spatial pattern and size structure of Rubus hirtus in a canopy gap. J. Veg. Sci. 1998, 9, 755–762. [Google Scholar] [CrossRef]
- Gazda, A.; Szwagrzyk, J.; Nybom, H.; Werlemark, G. Morphological and genetical variability of Rubus hirtus (Waldst. & Kitt.) plants under partly open forest canopy. Pol. J. Ecol. 2007, 55, 49–55. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Elsevier, Academic Press: London, UK, 2008; p. 800. [Google Scholar]
- Zagory, D.; Parmeter, J.R. Fungitoxicity of smoke. Phytopathology 1984, 74, 1027–1031. [Google Scholar] [CrossRef]
- Lin, H.-L.; Chumpookam, J.; Shiesh, C.-C.; Chung, W.-H. Smoke-water controls Pythium damping-off in papaya seedling. HortScience 2012, 47, 1453–1456. [Google Scholar] [CrossRef]
- Stinca, A. Le Orchidee della Penisola Sorrentina e dei Monti Lattari; Regione Campania, Assessorato all’Ecologia e alla Tutela dell’Ambiente; Nicola Longobardi Editore: Castellammare di Stabia (Napoli), Italy, 2014; p. 127. [Google Scholar]
- Gehring, E.; Conedera, M.; Maringer, J.; Giadrossich, F.; Guastini, E.; Schwarz, M. Shallow landslide disposition in burnt European beech (Fagus sylvatica L.) forests. Sci. Rep. 2019, 9, 8638. [Google Scholar] [CrossRef] [PubMed]
- Catling, P.M.; Sinclair, A.; Cuddy, D. Plant community composition and relationship of disturbed and undisturbed alvar woodland. Can. Field Nat. 2002, 116, 571–579. [Google Scholar]
- Bataineh, A.L.; Oswald, B.P.; Bataineh, M.M.; Williams, H.M.; Coble, D.W. Changes in understory vegetation of a ponderosa pine forest in northern Arizona 30 years after a wildfire. For. Ecol. Manag. 2006, 235, 283–294. [Google Scholar] [CrossRef]
- Hunter, M.E.; Omi, P.N. Seed supply of native and cultivated grasses in pine forests of the southwestern United States and the potential for vegetation recovery following wildfire. Plant Ecol. 2006, 183, 1–8. [Google Scholar] [CrossRef]
- Stark, K.E.; Arsenault, A.; Bradfield, G.E. Soil seed banks and plant community assembly following disturbance by fire and logging in interior Douglas-fir forests of south-central British Columbia. Can. J. Bot. 2006, 84, 1548–1560. [Google Scholar] [CrossRef]
- Gross, K.L.; Werner, P.A. Biology of Canadian Weeds. 28. Verbascum Thapsus L. and Verbascum Blattaria L. Can. J. Plant Sci. 1978, 58, 401–413. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Esposito, A. Vegetation regrowth after fire and cutting of Mediterranean macchia species. In Fire in Mediterranean Ecosystems; Trabaud, L., Prodod, R., Eds.; ECSC-EEC-EAEC: Brussels, Belgium; Luxembourg, 1993; pp. 87–99. [Google Scholar]
- Esposito, A.; Strumia, S.; Buonanno, M.; Castaldo-Cobianchi, R.; Mazzoleni, S. Analysis of bryophyte dynamics after fires of Pine woodlands and Mediterranean macchia, southern Italy. In Fire Management and Landscape Ecology; Trabaud, L., Ed.; International Association of Wildland Fire: Washington, DC, USA, 1998; pp. 77–87. [Google Scholar]
- Esposito, A.; Mazzoleni, S.; Strumia, S. Post-fire bryophyte dynamics in Mediterranean vegetation. J. Veg. Sci. 1999, 10, 261–268. [Google Scholar] [CrossRef]
- Hoffman, G.R. Ecological Studies of Funaria hygrometrica Hedw. In Eastern Washington and Northern Idaho. Ecol. Monogr. 1966, 36, 157–180. [Google Scholar] [CrossRef]
- Brasell, H.M.; Mattay, J.P. Colonization by Bryophytes of Burned Eucalyptus Forest in Tasmania, Australia: Changes in Biomass and Element Content. Bryologist 1984, 87, 302–307. [Google Scholar] [CrossRef]
- Smith, R.J.; Abella, S.R.; Stark, L.R. Post-fire recovery of desert bryophyte communities: Effects of fires and propagule soil banks. J. Veg. Sci. 2014, 25, 447–456. [Google Scholar] [CrossRef]
- Zeibig, A.; Diaci, J.; Wagner, S. Gap disturbance patterns of a Fagus sylvatica virgin forest remnant in the mountain vegetation belt of Slovenia. For. Snow Landsc. Res. 2005, 79, 69–80. [Google Scholar]
- Collet, C.; Piboule, A.; Leroy, O.; Frochot, H. Advance Fagus sylvatica and Acer pseudoplatanus seedlings dominate tree regeneration in a mixed broadleaved former coppice-with-standards forest. Forestry 2008, 81, 135–150. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Bonanomi, G.; Incerti, G.; Chiusano, M.L.; Termolino, P.; Mingo, A.; Senatore, M.; Giannino, F.; Cartenì, F.; Rietkerk, M.; et al. Inhibitory and toxic effects of extracellular self-DNA in litter: A mechanism for negative plant–soil feedbacks? New Phytol. 2015, 205, 1195–1210. [Google Scholar] [CrossRef]
- Szwagrzyk, J.; Szewczyk, J.; Bodziarczyk, J. Dynamics of seedling banks in beech forest: Results of a 10-year study on germination, growth and survival. For. Ecol. Manag. 2001, 141, 237–250. [Google Scholar] [CrossRef]
- Alberti, G.; Boscutti, F.; Pirotti, F.; Bertacco, C.; De Simon, G.; Sigura, M.; Cazorzi, F.; Bonfanti, P. A LiDAR-based approach for a multi-purpose characterization of Alpine forests: An Italian case study. iFor.-Biogeosci. For. 2013, 6, 156–170. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire Management Impacts on Invasive Plants in the Western United States. Conserv. Biol. 2006, 20, 375–384. [Google Scholar] [CrossRef]
- Stinca, A.; Motti, R. Alien plant invasions in Astroni crater, a decades-long unmanaged forest in southern Italy. Atti della Soc. Toscana Sci. Nat. Mem. Ser. B 2017, 124, 101–108. [Google Scholar]
- Becker, T.; Dietz, H.; Billeter, R.; Buschmann, H.; Edwards, P.J. Altitudinal distribution of alien plant species in the Swiss Alps. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 173–183. [Google Scholar] [CrossRef]
- Alexander, J.M.; Kueffer, C.; Daehler, C.C.; Edwards, P.J.; Pauchard, A.; Seipel, T.; MIREN Consortium. Assembly of nonnative floras along elevational gradients explained by directional ecological filtering. Proc. Natl. Acad. Sci. USA 2011, 108, 656–661. [Google Scholar] [CrossRef]
- Guo, Q.; Fei, S.; Shen, Z.; Iannone, B.V.; Knott, J.; Chown, S.L. A global analysis of elevational distribution of non-native versus native plants. J. Biogeogr. 2018, 45, 793–803. [Google Scholar] [CrossRef]
- Aronson, M.F.; Handel, S.N.; La Puma, I.P.; Clemants, S.E. Urbanization promotes non-native woody species and diverse plant assemblages in the New York metropolitan region. Urban Ecosyst. 2015, 18, 31–45. [Google Scholar] [CrossRef]
- McDaniel, P.A.; Lowe, D.J.; Arnalds, O.; Ping, C.-L. Andisols. In Handbook of Soil Sciences, Vol. 1: Properties and Processes, 2nd ed.; Huang, P.M., Li, Y., Sumner, M.E., Eds.; CRC Press (Taylor & Francis): Boca Raton, FL, USA, 2012; pp. 33.29–33.48. [Google Scholar]
- Soil Science Division Staff. Soil Survey Manual; USDA, Agriculture Handbook No. 18; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; Government Printing Office: Washington, DC, USA, 2017; p. 603.
- Certini, G.; Nocentini, C.; Knicker, H.; Arfaioli, P.; Rumpe, C. Wildfire effects on soil organic matter quantity and quality in two fire-prone Mediterranean pine forests. Geoderma 2011, 167–170, 148–155. [Google Scholar] [CrossRef]
- Chen, H.Y.H.; Shrestha, B.M. Stand age, fire and clearcutting affect soil organic carbon and aggregation of mineral soils in boreal forests. Soil Biol. Biochem. 2012, 50, 149–157. [Google Scholar] [CrossRef]
- Shen, J.P.; Chen, C.R.; Lewis, T. Long term repeated fire disturbance alters soil bacterial diversity but not the abundance in an Australian wet sclerophyll forest. Sci. Rep. 2016, 6, 19639. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; de Menezes, A.B.; Macdonald, L.M.; Toscas, P.; Bissett, A.; Baker, G.; Farrell, M.; Richardson, A.E.; Wark, T.; Thrall, P.H. Wildfire impact: Natural experiment reveals differential short-term changes in soil microbial communities. Soil Biol. Biochem. 2017, 109, 1–13. [Google Scholar] [CrossRef]
- Knelman, J.E.; Schmidt, S.K.; Garayburu-Caruso, V.; Kumar, S.; Graham, E.B. Multiple, compounding disturbances in a forest ecosystem: Fire increases susceptibility of soil edaphic properties, bacterial community structure, and function to change with extreme precipitation event. Soil Syst. 2019, 3, 40. [Google Scholar] [CrossRef]
- Ulery, A.L.; Graham, R.C.; Amrhein, C. Wood-ash composition and soil pH following intense burning. Soil Sci. 1993, 156, 358–364. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C. Effect of Simulated Fire on Organic Matter and Selected Microbiological Properties of Two Contrasting Soils. Arid Land Res. Manag. 2003, 17, 55–69. [Google Scholar] [CrossRef]
- Bogunović, I.; Kisić, I.; Jurišić, A. Influence of wildfire and fire suppression by seawater on soil properties. Appl. Ecol. Environ. Res. 2015, 13, 1157–1169. [Google Scholar] [CrossRef]
- Park, J.S.; Koo, K.-S.; Lee, E.J. The changes of soil salinity in the Pinus densiflora forest after seawater spread using a fire-fight helicopter. J. Ecol. Environ. 2015, 38, 443–450. [Google Scholar] [CrossRef]
- Santín, C.; Stefan, H.; Doerr, S.H.; Preston, C.M.; González-Rodríguez, G. Pyrogenic organic matter production from wildfires: A missing sink in the global carbon cycle. Glob. Chang. Biol. 2014, 21, 1621–1633. [Google Scholar] [CrossRef]
- Hart, S.C.; DeLuca, T.H.; Newman, G.S.; MacKenzie, M.D.; Boyle, S.I. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For. Ecol. Manag. 2005, 220, 166–184. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Migliorini, M.; Maggi, O.; D’Ascoli, R.; Fanciulli, P.P.; Persiani, A.M. Dynamics of fungi and fungivorous microarthropods in a Mediterranean maquis soil affected by experimental fire. Eur. J. Soil Biol. 2013, 56, 33–34. [Google Scholar] [CrossRef]
- De Marco, A.; Gentile, A.E.; Arena, C.; Virzo De Santo, A. Organic matter, nutrient content and biological activity in burned and unburned soils of a Mediterranean maquis area of southern Italy. Int. J. Wildland Fire 2005, 14, 367–377. [Google Scholar] [CrossRef]
- Wardle, D.A.; Ghani, A. A critique of the microbial metabolic quotient (qCO2) as a bioindicator of disturbance and ecosystem development. Soil Biol. Biochem. 1995, 27, 1601–1610. [Google Scholar] [CrossRef]
- Fierro, A.; Rutigliano, F.A.; De Marco, A.; Castaldi, S.; Virzo De Santo, A. Post-fire stimulation of soil biogenic emission of CO2 in a sandy soil of a Mediterranean shrubland. Int. J. Wildland Fire 2007, 16, 573–583. [Google Scholar] [CrossRef]
- Li, W.; Niu, S.; Liu, X.; Wang, J. Short-term response of the soil bacterial community to differing wildfire severity in Pinus tabulaeformis stands. Sci. Rep. 2019, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Sáenz de Miera, L.E.; Pinto, R.; Gutierrez-Gonzalez, J.J.; Calvo, L.; Ansola, G. Wildfire effects on diversity and composition in soil bacterial communities. Sci. Total Environ. 2020, 726, 138636. [Google Scholar] [CrossRef] [PubMed]
- Andreolli, M.; Lampis, S.; Brignoli, P.; Vallini, G. Bioaugmentation and biostimulation as strategies for the bioremediation of a burned woodland soil contaminated by toxic hydrocarbons: A comparative study. J. Environ. Manag. 2015, 153, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Whitman, T.; Whitman, E.; Woolet, J.; Flannigan, M.D.; Thompson, D.K.; Parisien, M.-A. Soil bacterial and fungal response to wildfires in the Canadian boreal forest across a burn severity gradient. Soil Biol. Biochem. 2019, 138, 107571. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).