Abstract
Research Highlights: It was found that both tree species and ground vegetation affected soil carbon stock in boreal forests. Carbon stocks in the mineral layers were related negatively to the C/N ratio in the organic horizon and pine proportion in the growing stock volume, and positively to the share of herbaceous plants and the proportion of spruce. Background and Objectives: Existing research showed the effects of tree species on soil carbon stocks in organic horizons, but these effects were less clear in mineral horizons. Little is known about the effects of ground vegetation on soil carbon stock. This study aims to identify associations between the forest vegetation composition and soil carbon stocks in northwestern Russia. Materials and Methods: Research data from 109 pine, spruce and birch forests of different Cajander’s and Sukachev’s types with different functional compositions of ground vegetation at autonomous positions are discussed in this paper. The V-test was used to assess the impact of vegetation on soil carbon stocks. Results: Variations in Carbon stocks in the mineral layers were associated with the soil types and vegetation composition. Carbic Albic Podzols accumulated the least amount of carbon in the mineral profile. Carbon stock in the mineral layers in pine forests was considerably lower than in spruce and birch forests. Spruce forests with the highest share of herbaceous plants were characterised by the highest carbon stocks in the mineral layers, while pine forests with dwarf shrubs and green mosses accumulated more carbon in the organic layers, but carbon stocks in the mineral layers here were the lowest. Conclusions: Differences in soil carbon stocks between and within northern and middle taiga in northwestern Russia were associated not only with soil types but also with the proportions of forest types dominated by different tree species and ground vegetation functional groups.
1. Introduction
Boreal forest soils are a reservoir for long-term storage of carbon in the boreal biome and a significant contributor to global carbon storage [1]. The soil carbon stock is regulated by well-known soil-forming factors: climate, soil-forming rocks, topography, biota, as well as anthropogenic impact, primarily, forest management and climate change. As expected, climate change and associated disturbances can affect the plant species composition of boreal forests [2], and therefore it is very important to understand how changing vegetation will affect the ability of forest soils to accumulate organic carbon. The synthesis of existing research showed consistent tree species effects on soil carbon stocks in the forest floor but these effects were less clear in mineral horizons [3].
The results of the estimates of soil carbon in boreal forests of Scandinavian countries indicate that the greatest variety of soil carbon stocks, associated with the impact of environmental factors, is revealed in organic horizons. The variability of forest biomass and carbon stocks depends on tree species, stand age, site productivity and the history of natural (fires, insect attacks, fungal diseases, windfalls) and anthropogenic (forest management practices, air pollution, etc.) disturbances [4]. It has been shown that the contribution of the organic horizon to total soil carbon stock of boreal forests depends on the age of stands, the amount of litterfall and its decomposition rate. In Scandinavia, an increase in carbon stock in mineral soils towards the south has been established [5] which was attributed to nitrogen deposition [6]. Soil carbon stocks in Swedish forests showed a positive correlation with temperature and site productivity, and soil carbon stocks in spruce forests have been found to be significantly higher than those in pine forests are. According to field data, the national mean soil organic carbon (SOC) stock was 9.2 kg m−2 in spruce dominated stands and 5.7 kg m−2 in pine-dominated stands [7]. The simulations demonstrated that the build-up of SOC over several rotations was 22% higher in spruce forests than in pine forests under similar environmental conditions. It has been suggested that removal of harvest residues may have a greater impact on the SOC stock under spruce than in pine stands. In Norway, soil carbon stocks were higher in more productive forests than in less productive forests, which was explained by differences in soil layer thickness [8]. Although significant variability in soil carbon stock has been found, no significant differences in the influence of individual tree species have been established. In Finland, the amounts of carbon stocks in soil varied with changes in forest litter production, weather conditions and felling [9]. It was shown that more productive forest site types accumulated more carbon in the soil of Finnish forests [10].
In Canada, mineral soil carbon stocks in boreal mixed-wood forests on sandy loam (northeastern Ontario) averaged 51 t ha−1 and held up to 30% of total carbon stocks, while the forest floor organic layer contained from 22 to 36 tons of carbon per hectare [11]. It was shown that compared to aspen, black spruce soils, characterised by a slow carbon turnover, stored more soil organic carbon in the Canadian boreal biome [12].
Russia accounts for more than 20% of the world’s forest resources and more than half of the world’s boreal forests. According to existing estimates, the average carbon stock ranged from 10.6 to 17.2 t ha−1 in organic horizons while it varied from 46.6 to 122.2 t ha−1 in the 30-cm layer of soil mineral profile in boreal forests of the European part of Russia [13]. In the Republic of Komi, which lies to the northeast of the European part of Russia, the carbon stock in the 1-meter soil layer varied from 29 t ha−1 to 121 t ha−1, depending on the soil type [14]. In the Republic of Karelia, northwest Russia, soil carbon stocks in the 1-m layer also varied significantly, and amounted to 24–434 t ha−1 in pine forests and 39–402.4 t ha−1 in spruce forests, depending on the soil type and moisture [15].
Thus, a significant variation in soil carbon stocks was found in the boreal forests of the European part of Russia; however, the relationships between the composition of vegetation and soil carbon stock were not studied. Soil organic matter is originated from the aboveground and belowground litterfall of both woody plants and ground vegetation. The abundance and composition of ground vegetation differ among different types of boreal forests, but little is known about the effect of these plants on soil carbon stock.
This study aimed to estimate soil carbon stocks and to identify associations between forest vegetation composition and soil carbon stocks in northwestern Russia.
The following scientific questions are raised in this study.
(1) Are there links between the species composition of woody plants and carbon stocks not only in the organic but also in the mineral horizons of the soils of boreal forests?
(2) Are there links between the functional composition of ground vegetation and carbon stocks in both the organic and mineral horizons of the soils of boreal forests?
2. Materials and Methods
2.1. Study Areas
The results of an analysis of data from 109 boreal forest plots, including 34 Norway spruce, 59 Scots pine, and 16 silver birch or downy birch plots, established in the northern and middle taiga of the Republic of Karelia (NK and MK, respectively) and the Karelian Isthmus, Leningrad region (MKI) are discussed in this paper. This monitoring network was organised during the implementation of the International Cooperative Programme on Assessment and Monitoring of Air Pollution Effects on Forests (ICP Forests) in 2008 (Figure 1; [16]). The permanent plots were established in the junction points of the 16 × 16 km regular network in the Karelian Isthmus and 32 × 32 km network in the Republic of Karelia. The plots represent taiga forests at autonomous positions in northwest Russia. Plots are clusters with four circular sample subplots, on which at least six trees of the dominant species grow. The circular subplots are located at a distance of 25 m and are oriented to the cardinal points from the plot centre (central tree) [17,18].
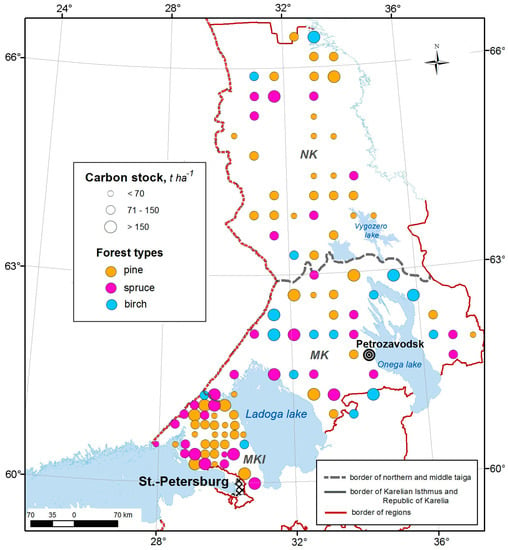
Figure 1.
Soil carbon stocks in pine, spruce and birch forests at 109 monitoring plots. NK—northern taiga, Karelia, MK—middle taiga, Karelia, MKI—middle taiga, the Karelian Isthmus, Leningrad region.
2.1.1. Climatic Conditions
The average annual temperature in NK is close to 0 °C, while in MK and MKI, it can reach 4 °C [16]. The total amount of annual precipitation reaches up to 450–500 mm per year in NK, 600–700 mm in MK, and is the highest (700–800 mm) in MKI due to the influence of the sea. The border between the northern and middle taiga is close to the isotherm of 1400 degree days with an average daily temperature above 5 °C.
2.1.2. Vegetation
Forests cover 54% of the Republic of Karelia [19]. They are dominated by Scots pine (Pinus sylvestris L.) (64%), while the proportions of Norway spruce (Picea abies (L.) H.Karst.) and birch (Betula pendula Roth, Betula pubescens Ehrh.) forests are considerably lower (24% and 11%, respectively). The NK forests are dominated by Scots pine forests with dwarf shrubs, green mosses, and lichens, while Norway spruce forests with dwarf shrubs and green mosses are widespread in MK [20]. The forested area of the Karelian Isthmus (MKI) reaches almost 70% [21]; forests are dominated by Scots pine (51%), while the proportions of spruce and birch forests are relatively low (29% and 16%, respectively). Forest stand productivity increases from north to south [16]. Like in the northern taiga, dwarf shrubs and green mosses dominate in the ground vegetation of the middle taiga, but herb nemoral species are more common here [16]. In MKI, some species of broadleaved trees and shrubs (Acer platanoides L., Quercus robur L., Corylus avellana L., Lonicera xylosteum L.) occur.
The main drivers of forest dynamics in northwestern Russia are felling and fires [19,22]. Slash-and-burn agriculture was practiced widely in the Karelian Isthmus until the beginning of the 20th century [22]. The result was a destruction of spruce forests on the most fertile soils, large-scale and severe fires favored the regeneration of pine. The slash-and-burn practice had a very limited spatial distribution in the Republic of Karelia [19].
2.1.3. Soil
There is about 4.8 million ha of podzols in boreal forests, which are over 30% of the boreal biome [23]. The predominant type of soil in Karelian forests (NK and MK) is Carbic Albic Podzols (Arenic) (CAP) on till (60 plots), and Carbic Entic Podzols (Arenic) (CEP) (9 plots) which in the middle taiga develop on carbon-containing rock—shungite and on till. In the Karelian Isthmus (MKI forests), Albic Retisols (Arenic)—AR (39 plots) and Carbic Entic Podzols (Arenic) (1 plot) were identified. The predominant soil-forming rock in MKI is polymictic sandstone [24]. The fraction, less than 0.002 mm in soil-forming rock averaged 1.5–2.1% in NK, and 0.8–1.4% and 0.1–1.4% in MK and MKI, respectively [16].
2.2. Methods
2.2.1. Vegetation
Vegetation was described in four square subplots (100 m2) within each plot (Figure 2).
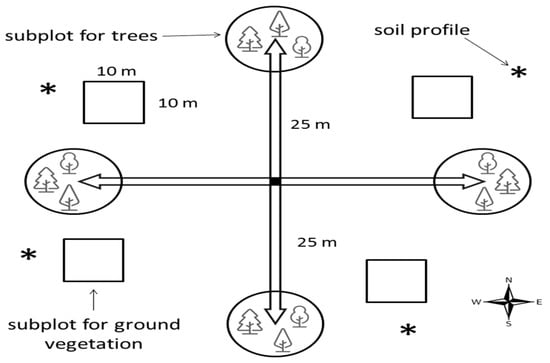
Figure 2.
Monitoring plot design.
Four vegetation layers were identified: A—a tree layer (woody plants >5 m in height), B—a shrub layer (woody plants, from 0.5 to 5 m in height), C—an herb layer (all non-woody and woody plants <0.5 m in height, including tree seedlings), and D—a moss and lichen layer (terricolous bryophytes and lichens). For each of the vegetation layers, the total projective cover (in percent) was assessed visually. The classification of plant functional groups based on the division of plant species according to their life forms and taxonomic affiliation [25] was adopted: 1—gramineous plants and sedges, 2—herbs (all herbaceous plants excluding those from 1), 3—dwarf shrubs (low-growing perennial shrubs), 4—green mosses and 5—lichens. A complete list of species was made for each sample plot; then, the lists for four sample plots were combined into a general list for a plot with the average values of species abundance [16].
For each sample plot, the shares of all functional groups in the total coverage of ground vegetation were estimated. According to the quality of plant residues (contents of nutrients and secondary metabolites), these functional groups were assigned to the three main contrasting combined functional types: (1) with a high proportion of herbaceous plants (herbs + grasses)—H, (2) dwarf shrubs + green mosses—SM, (3) green mosses + lichens/green mosses—ML. In cases where the proportion of herbs with grasses exceeded the proportion of any other functional group, the first group with a high proportion of herbaceous plants was identified. The second type was identified by the dominance of dwarf shrubs and green mosses, which most often grow together in different layers (C and D layers) of vegetation and form a separate element of the vegetation cover in boreal forests. The third type combines green mosses and lichens that often grow together and form a separate cover element. These three functional types of ground vegetation were used to highlight the effect of plants with different litter quality on soil carbon stocks within the areas.
Cajander’s forest site types were identified according to the composition of understory vegetation and the occurrence of certain indicator species of site productivity [26]. The following acronyms for the identified site types were used: 2—OMT—Oxalis-Myrtillus, 3—MT—Myrtillus, 4—VT—Vaccinium vitis-idaea. Site type 2 is co-dominated by Oxalis acetosella and Vaccinium myrtillus L. with a significant addition of mesophilous herb species (including Fragaria vesca L., Maianthemum bifolium (L.) F.W.Schmidt and Rubus saxatilis L.) and boreal green mosses (Hylocomium splendens (Hedw.) Schimp. and Pleurozium schreberi (Willd. ex Brid.) Mitt.). Site type 3 is dominated by V. myrtillus and several boreal moss species. Site type 4 is characterised by abundant Vaccinium vitis-idaea L. and V. myrtillus. The layer D is dominated by P. schreberi, some lichen species also occur. Cajander’s forest site types have been used to highlight the effect of site productivity on soil carbon stocks. The C/N ratio in the organic horizon increased from 30 in the more productive Cajander’s site type 2 to 38 and 46 in the less productive site types 3 and 4, respectively [16].
Sukachev’s forest types are distinguished taking into account the dominance of tree and ground vegetation species. In this study, Sukachev’s forest types were identified within Cajander’s forest site types by the tree species composition, which was done following the approach suggested by the author himself [27]. Sukachev’s forest types dominated by spruce, pine and birch were identified within Cajander’s forest site types 3 and 4. Sukachev’s forest types were used to capture the effect of both ground vegetation and tree species on soil carbon stocks within the areas.
2.2.2. Soil
Data on the soil carbon stock in taiga forests were obtained during the implementation of ICP Forests in 2009–2010 [17,28]. According to the national methodical guidelines [17], samples of the organic horizon were taken using a frame of 0.25 × 0.25 m, and at the same site, samples from mineral soil horizons (E/A, B, and BC) were excavated from 0.5 × 0.5 m holes in each test plot. The holes were dug near the four vegetation plots. Individual samples were mixed to obtain an averaged sample taking into account genetic soil horizons.
In the laboratory, soil samples were dried and sieved through a 2-mm sieve. The fraction <2 mm was subjected to analytical processing. The content of carbon and nitrogen in soil samples was determined using a PE-2040 CHNS-analyser (Perkin Elmer, Waltham, MA, USA).
To determine the mass of the organic horizon and carbon stock in it, the samples were taken with a 0.25 × 0.25 m frame. The bulk density and thickness of mineral horizons were measured using four soil profiles in each plot. In the laboratory, all samples were dried at 105 °C and weighed. The carbon stock in the organic horizon was calculated by multiplying the sample weight by the carbon content. Carbon stock in soil mineral profiles was calculated by multiplying bulk density, carbon content and mineral layer thickness [13]:
where BD—bulk density, g cm−3, C—carbon content, g kg−1 and H—layer thickness, cm.
Since the studied soils were characterised by a sharp vertical differentiation of soil profile into genetic horizons, the calculations of carbon stock for fixed mineral layers (30-cm and 50-cm layers) were based on its estimation in the horizons, accordingly officially accepted national guidelines [13].
2.2.3. Statistical Processing
Statistical processing was performed using the R statistical software [29].
The V-test [30] was used to assess the impact of the forest soil types, forest tree species, forest site type/forest type, and plant functional groups of ground vegetation on soil carbon stocks. The test and the associated descriptive statistics were calculated using the function catdes of the “FactoMine” R package [31]. One-way analysis of variance (ANOVA) was conducted to estimate the contribution of different factors such as areas, soil types, Cajander site types, trees, and functional types of ground vegetation to variation of carbon stocks in the mineral layers of soil (Table 1).

Table 1.
One-way ANOVA of the main factors affecting soil carbon stock.
The identification of variables that are most associated with the carbon stock in the mineral layers, taking into account their possible relationships, was carried out based on multiple linear regression. The goal was to find optimal models, both by statistical criteria and by an expert assessment based on existing knowledge.
Before the selection of models, the variables were transformed following the statistical requirements of the analysis. The selection of potentially the most important predictor variables for the response was as follows. Using the functions of the “MuMIn” package [32], all possible combinations of predictors were completely enumerated, a regression model was built for each possible combination, and the corrected Akaike information criterion (AICc) was calculated. The most interpretable model with the AICc value not more than the 2ΔAICc was taken as the final one. The contributions to the explained variance of predictors in the final models were calculated by the hierarchical partitioning method implemented in the “hier.part” package [33].The results of the analysis are presented in Table 2, which contains regression coefficients, their significance (based on t-statistics), and the proportions of explained variance. In addition, the table presents the general characteristics of the models: the adjusted coefficient of determination, F-statistics, degrees of freedom and the p-value.

Table 2.
Results of regression analysis for carbon stock in the mineral layer 0–50 cm.
3. Results
3.1. Vegetation
The NK forests were dominated by pine (71%), while shares of spruce and birch forests were considerably lower there: 20% and 9%, respectively (Figure 3). In the MK forests, the proportions of pine, spruce and birch forests were comparable (31–37%). The MKI forests were dominated by pine forests (66%); spruce (35%) and birch (5%) forests were much less common. The NK and MK forest stands were characterised by a wide age range: from 25 to 230 years old (from young to old-growth stands). In the MKI forests, the age range of tree stands was narrower: 60–120 years, i.e., young and old-growth stands were absent; the groups of premature and mature forests predominated [16].
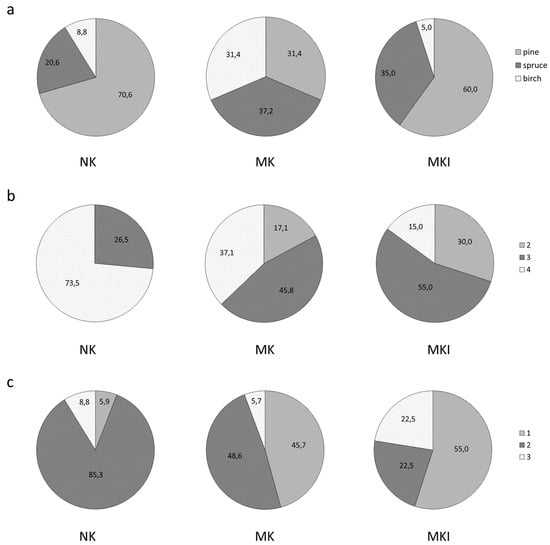
Figure 3.
The proportion of (a) forest types, (b) Cajander’s site types: 2—Oxalis-Myrtillus, 3—Myrtillus, 4—Vaccinium vitis-idaea, and (c) plant functional types: 1—with a high proportion of herbaceous plants, 2—with dwarf shrubs and green mosses, and 3—with green mosses and lichens/green mosses in forests of three areas: NK—northern taiga, Karelia, MK—middle taiga, Karelia, MKI—middle taiga, the Karelian Isthmus, Leningrad region.
The herb and grass cover increased pronouncedly from the NK forests (2% and 1%) to the middle taiga plots (13% and 20%, 6% and 8%, in the MK and MKI, respectively), while in NK, the lichen and dwarf shrub cover were higher (7% and 44%, respectively) than in the MK and MKI (2% and 26%, 0 and 24%, respectively) (Table S1).
Pine forests had a greater coverage of dwarf shrubs (35%) and lichens (5%), and birch forests had a larger coverage of herbs (21%) and grasses/sedges (13%) and a lower coverage of mosses (33%). Spruce forests were characterised by a great coverage of mosses (56%) and relatively low coverage of dwarf shrubs (24%) (Table S2).
There were different proportions of Cajander’s forest site types within taiga subzones. In NK, Cajander’s site type 4 was predominant (73.5%) while site type 3 was also common (26.5%) but site type 2 was absent (Figure 3) [16]. The MKI forests were characterised by a prevalence of site type 3 (55%), a high proportion of site type 2 (30%), and a relatively low share of site type 4 (15%). The MK forests occupied an intermediate position: site type 3 was predominant (46%) and site type 4 was common (37%), while the proportion of site type 2 was lower (17%) than in MKI. The less productive Cajander’s site type 4 was characterised by the dominance of pine in the forest stand (76%), while spruce more often dominated in site type 3, and birch in site type 2 (Table S3). The share of herbs was most high in site type 2 (41%) and very low in type 4 (2%). In site type 4, the proportion of lichens was noticeable (7%), and in site types 2 and 3, their abundance was insignificant. The high coverage of mosses and dwarf shrubs was typical for all three types, but it was lower in site type 2 compared to site types 3 and 4. The proportion of plant functional type 1 was most high in the MKI forests, while functional type 2 dominated in the NK (Figure 3).
When identifying Sukachev’s forest types within Cajander’s forest site types, it was revealed that Cajander’s site type 4 dominated by pine (86%) was characterised by a slight admixture of spruce and birch (6 and 8%, respectively), while in this site type dominated by spruce, the proportion of pine and birch was noticeably higher (31 and 16%, respectively). In the cases of the dominance of birch in site type 4, the proportion of pine was high (32%), unlike the share of spruce (9%) (Table S4). In Cajander’s site type 3, the dominance of the main tree species was brightly pronounced, while the shares of other species varied from 6–16% (Table S5).
3.2. Soil Carbon Stock
Results of one-way ANOVA demonstrated a significant contribution of different factors such as areas, soil types, Cajander site types, trees, and functional types of ground vegetation to variation of carbon stocks in the mineral layers of soil (Table 1). The influence of the last two factors is most pronounced in the forests of Karelia, and the impact of Cajander’s site types is manifested only when all three areas are included in the analysis.
A comparison between the three areas showed that the lowest carbon stock in the organic horizon was in the MKI, while in the NK and MK, it was comparable and more than two times higher than in the MKI: 19 t ha−1 and about 43 t ha−1, respectively. However, MKI was characterised by the highest carbon stock in the 30-cm and 50-cm mineral layers (80 and 107 t ha−1, respectively). The total soil carbon stock, calculated taking into account the organic horizon and the 50-cm mineral layer, was comparable in the MK and MKI (Figure 4). The share of the organic horizon in the total carbon stock decreased from 38% in the NK to 33% in the MK and 15% in the MKI.
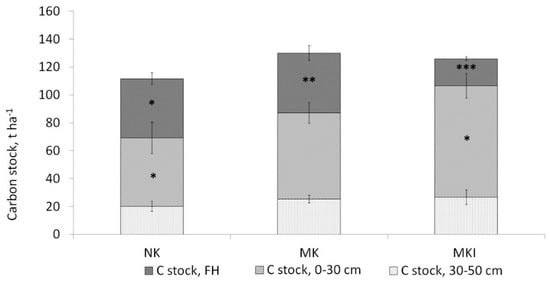
Figure 4.
Soil carbon stocks in forests of three areas. NK—northern taiga, Republic of Karelia (n = 34), MK—middle taiga, Republic of Karelia (n = 35), MKI—middle taiga, the Karelian Isthmus, Leningrad region (n = 40). Here and in Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9, asterisks indicate the significance of the differences between the mean in category and overall mean: *—p ≤ 0.1, **—p ≤ 0.01, ***—p ≤ 0.001.
As one would expect, the soil carbon stocks were associated with the soil types: the carbon stocks in Carbic Entic Podzols were the highest (Figure 5).
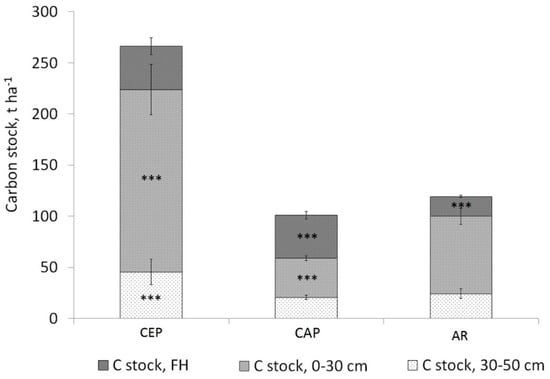
Figure 5.
Soil carbon stocks in Carbic Entic Podzols (CEP, n = 10), Carbic Albic Podzols (CAP, n = 60) and (3) Albic Retisols (AR, n = 39).
When considering soil carbon stock in forests dominated by different tree species, it was found that pine forests accumulated the least amount of carbon in the 0–30 and 0–50 cm layers (54 and 73 t ha−1, respectively, while birch forests were characterised by the highest carbon stock in these layers (96 and 125 t ha−1, respectively) due to the high proportion of Carbic Entic Podzols (31%). Spruce forests occupied an intermediate position between the pine and birch forests (69 and 99 t ha−1, respectively) (Table S2). The total soil carbon stocks, calculated taking into account the organic horizon and the 50-cm mineral layer, were lowest in pine forests (Figure 1). It should be noted that the differences in soil carbon stock in mineral layers associated with coniferous tree species in the MKI forests were less pronounced than in Karelian (NK + MK) forests (Figure 6).
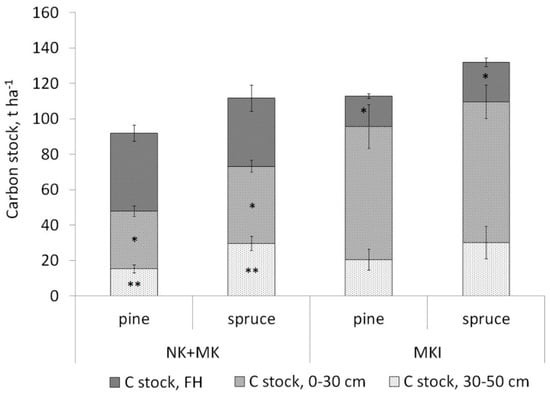
Figure 6.
Soil carbon stocks in pine (n = 34) and spruce (n = 17) Karelian forests (NK + MK) on Carbic Albic Podzols and in pine (n = 23) and spruce (n = 14) forests on Albic Retisols in the Karelian Isthmus (MKI). The significance of the differences between the mean in category and overall mean was estimated taking into account the areas (NK + MK and MKI).
Differences in the soil carbon stock between the predominant Cajander’s site types were also evident: Cajander’s site type 4 was characterised by the lowest carbon stock in the mineral layers 0–30 and 0–50 cm (51 and 71 t ha−1, respectively) among all sites (Table S3, Figure 7).
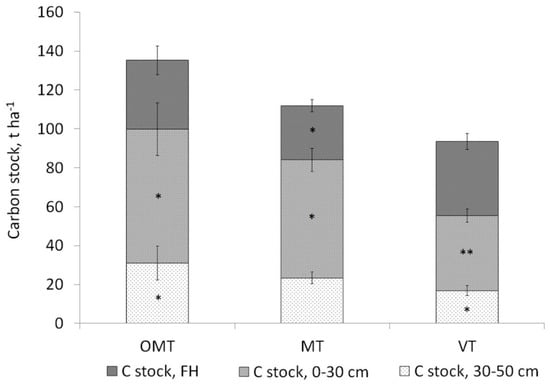
Figure 7.
Soil carbon stocks in Cajander’s forest site types on Carbic Albic Podzols and Albic Retisols: OMT—Cajander’s site type 2 (n = 16), MT—Cajander’s site type 3 (n = 43), VT—Cajander’s site type 4 (n = 40).
When considering Sukachev’s forest types identified within Cajander’s site types in the Karelian forests (NK and MK), it turned out that site type 4 dominated by pine was characterised by the lowest carbon stocks in the mineral layers 0–30 and 0–50 cm (33.6 and 49.7 t ha−1, respectively) among all forests dominated by different tree species (Table S4). Pine-dominated sites of type 3 were also characterised by the lowest carbon stock in the mineral layers, while spruce-dominated sites accumulated the greatest carbon amount in the mineral layer 30–50 cm (29 t ha−1) (Table S5).
When analysing the links of the three dominating functional types, identified based on the functional groups of plants of similar residues quality, with soil carbon stock for all three areas together, it was revealed that the type with a high proportion of herbaceous plants (herbs + grasses) was associated to the highest carbon stock in the 30-cm and 50-cm mineral layers of soil (Table S6). However, a more detailed analysis showed that these differences are provided by the forests of Karelia, but do not appear on the Karelian Isthmus (Figure 8).
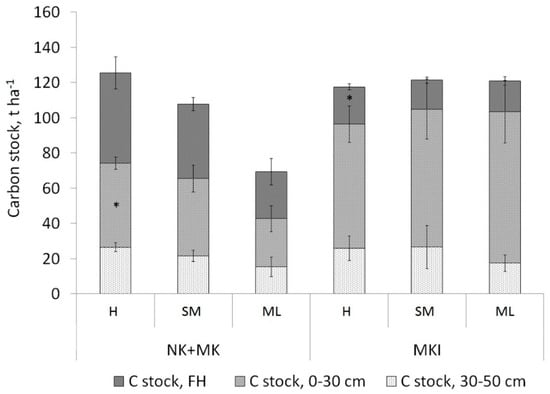
Figure 8.
Soil carbon stocks in Karelian forests (NK + MK) on Carbic Albic Podzols with a high proportion of herbaceous plants (H, n = 18), with dwarf shrubs + green mosses (SM, n = 46), and with green mosses+ lichens/green mosses (ML, n = 5), and in the forests of the Karelian Isthmus on Albic Retisols with a high proportion of herbaceous plants (H, n = 22), with dwarf shrubs + green mosses (SM, n = 9), and with green mosses + lichens/green mosses (ML, n = 9). The significance of the differences between the mean in category and overall mean was estimated taking into account the areas (NK + MK and MKI).
The regression analysis showed that carbon stock in the mineral layer 0–50 cm was related negatively to the C/N ratio in the organic horizon (37% out of total variance) and related positively to the share of herbaceous plants in vegetation cover (47% out of total variance) and the share of spruce in the total growing stock volume (18.7% out of total variance) (Table 2). When the proportion of pine was considered instead of the proportion of spruce as a predictor, the relationship with carbon stock in the mineral layers became negative.
Thus, spruce forests with the highest share of herbaceous plants were characterised by the highest carbon stocks in the mineral layers, while pine forests with dwarf shrubs and green mosses accumulated more carbon in the organic layers, but carbon stocks in the mineral layers here were the lowest (Figure 9).
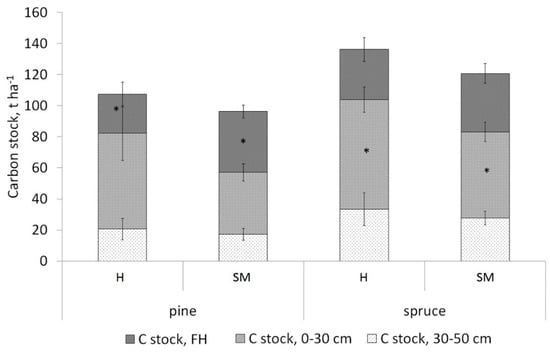
Figure 9.
Soil carbon stocks in forests on Carbic Albic Podzols and Albic Retisols with a high proportion of herbaceous plants—H (n = 12 in pine forests, n = 12 in spruce forests) and with dwarf shrubs and green mosses—SM (n = 35 in pine forests, n= 15 in spruce forests). The significance of the differences between the mean in category and overall mean was estimated taking into account trees.
4. Discussion
4.1. Soil Type and Soil Carbon Stocks
Variations in soil carbon stocks were associated with the soil type. Due to the presence of the humus horizon A, the Albic Retisols dominating in the Karelian Isthmus accumulated significantly more carbon in the upper part of the mineral profile than the Carbic Albic Podzols dominating in Karelian forests did. As shown by the example of Karelia, Carbic Entic Podzols, which, unlike other soils, do not have carbon-poor eluvial (E) horizons, accumulated more carbon not only in the lower part of the profile, where this can be determined by the carbon-containing rock—schungite in some cases in the middle taiga, but also in the upper part, formed by illuvial horizons, where this is also the result of a soil-forming process (illuviation). However, it should be noted that the proportion of these soils in the area was low (about 13%).
4.2. Soil Carbon Stock in the Sites with Different Functional Groups and in Cajander’s Site Types
According to the results of regression analysis, the most informative predictors of carbon stock changes in the 0–50 cm mineral layer were the C/N ratio in the organic horizons and the proportion of herbaceous plants in vegetation cover. The results of this study show that carbon stocks in the mineral layers of sites with a high contribution of herbaceous plants (herbs + grasses) were the highest, while the sites with functional type of dwarf shrubs + green mosses accumulated more carbon in the organic horizons. Therefore, the share of certain plant functional groups is important for the processes of soil carbon accumulation.
Soil carbon stock is regulated by both litter production and decomposition processes. The rate of decomposition of plant residues is related to litter quality, the indices of which are concentrations of nutrients and secondary metabolites, such as lignin and polyphenols [34,35,36]. It is also well known that the rate of plant residues decomposition depends on climatic factors but the influence of climatic factors on the soil properties could be mediated by vegetation. In the forests of northwestern Russia, the herb and grass species richness and cover correlated positively with temperature and total precipitation, while negative correlations with these climate variables were found for lichens and dwarf shrubs [36]. According to meta-analysis, local interspecific variation in litter quality may have stronger effects on litter decomposition than climate parameters [37]. Certainly, plant species within the functional groups [25], as well as functional groups within the functional types, differ in the quality of plant residues, as shown below. However, differences in the quality of litter between groups and types are much more significant than between individual species within the groups and individual groups within the types. In addition, the functional groups combined in this study into functional types grow together in different layers of vegetation and form separate units of vegetation cover in boreal forests.
The dependence of the mass-loss rate on the initial concentrations of nutrients, the C/N and lignin/N ratios in plant residues have been demonstrated earlier for forests in northwestern Russia [38]. The mass-loss rate of bilberry leaves was 1.3–1.5 and 2–4 times higher than that of lingonberry and green mosses, respectively, which was associated with differences in the content of nutrients. These findings confirmed other scientific results on the quality of plant residues. A high amount of phenolics and a low concentration of nitrogen in plant tissues of the Ericaceae family impede litter decomposition [39]. Bilberry produces leaf litter with high concentrations of N and low concentrations of polyphenols, which is known to decompose more rapidly than other Erica shrubs [40,41,42]. Mosses are characterised by a very low content of nutrients, as well as water-soluble extractives [42]. It was shown that mosses, representing a large soil carbon input in the black spruce forests of Canada, are slow to decompose [12]. Both bryophytes and lichens tend to invest strongly in secondary metabolites [43] that can inhibit the activity of soil microorganisms.
The tissues of herbaceous plants (herbs and grasses) contain much more nitrogen and other nutrients than dwarf shrubs, mosses, and lichens, which can promote faster litter decomposition in the sites with a high contribution of herbaceous plants. A higher share of herbaceous plants results in the higher decomposition rate in the organic horizons, which leads to active migration of dissolved organic carbon down the soil profile. It is worth emphasising that dissolved organic carbon can account for 11% of an ecosystem’s net productivity [44], and is one of the important components of carbon balance [3]. In the illuvial soil horizons, the organic carbon can be fixed by sesquioxides [45,46], or humus accumulation in illuvial horizons can be a result of combined effects of translocation, root deposition and vertical mixing [47].
Thus, the higher the share of herbaceous plants in the forest vegetation cover, the higher the soil carbon stock. The highest carbon stock in the organic horizon associated with the functional types of dwarf shrubs + green mosses could be due to the low rate of litter decomposition, which is explained by the low quality of plant residues.
The results of this study confirm the findings for Finnish forests that more productive forest site types are characterised by a higher carbon stock in the mineral layers of soils [10]. Cajander’s site types 2 and 3 demonstrated higher carbon stocks in the mineral layers of soil than the less productive site type 4, which was characterised by the highest C/N ratio in the organic horizon. Unlike in Finnish forests, significant differences in carbon stock were not found in the organic horizons of soil between Cajander’s site types in this study.
4.3. Soil Carbon Stock in Forests Dominated by Different Tree Species
As the results of the regression analysis showed, along with plant functional groups, tree species are an important predictor of soil carbon accumulation. A positive relationship between carbon stock in the mineral layer 0–50 cm and the share of spruce in the total growing stock volume was found. However, when the proportion of pine was considered as a predictor, the relationship with carbon stock in the mineral layers became negative.
The higher carbon stock in the mineral layers of spruce forests compared to that of pine forests could be explained by the higher spruce needle litter quality, including a lower C/N ratio than in pine needles [38,48]. The amount of precipitation below the crowns of Scots pine trees, especially the amount of water flowing down along the trunks, was considerable, by several times greater compared to that in the Norway spruce forests in the northern taiga of Russia [49]. This effect was explained by differences in the crown structure of these coniferous tree species: the crowns of pine trees are open and short, whereas the crowns of old spruce trees are long and dense. Low amounts of precipitation penetrating through a dense and low canopy can prevent carbon loss from the soil horizons in spruce forests. This could explain differences in the soil washing profile rates between the pine and spruce forests, and the lower carbon stock in the mineral layers of soil in pine forests. The content of organic carbon in the illuvial horizons of soil in spruce forests is one and a half times higher than in pine forests on the soil-forming rock of similar mechanical and chemical composition [16].
In Sweden, the decomposition rate was much higher under birch than under spruce and pine resulting in lower carbon stocks in the organic horizon [50]. Similar differences in the mass-loss rates between the residues of birch leaves and conifer needles have been shown for forests in the northern taiga of Russia [38,48], but no statistical difference in carbon stock in the organic horizons was found between the birch and coniferous forests in this study. Although the carbon content and the C/N ratio were significantly lower in the organic horizons of birch forests, the horizon mass in birch and coniferous forests was comparable or the horizon mass was even greater in birch forests. Moreover, birch forests demonstrated a high level of carbon stock in the mineral layers. It must be emphasised that very high carbon stock in the mineral layers of soil in the birch forests of Cajander’s site type 4 (Table S4) is related to soil type Carbic Entic Podzols and specific carbon-containing soil-forming rock: eluvium-diluvium of shungite schists [51]. However, birch forests identified in Cajander’s site 3, developing on Carbic Albic Podzols on till, also demonstrated a high carbon stock in the organic horizons and in the layer 0–30 cm (Table S5), which is comparable to those in spruce forests. In these areas, 50-year-old birch stands developed after felling in coniferous—mainly spruce—forests [52]. High organic horizon mass in birch forests could be related to the accumulation of coniferous tree residues after felling. High carbon stock in the upper 30-cm mineral layer of soil in birch forests could be explained by a high content of nitrogen and other nutrients and a lower C/N ratio in birch leaves, which resulted in accelerated decomposition of the birch residues, subsequent migration of the organic carbon from the organic horizon and accumulation in the upper part of the mineral profile.
4.4. Soil Carbon Stock Comparison Taking into Account Both Tree Species and Ground Vegetation
As the results of this study demonstrated, both tree species and ground vegetation affect soil carbon accumulation. Sukachev’s forest types take into account both of these components of vegetation. Cajander’s site types dominated by pine, i.e., Sukachev’s pine forests, were characterised by the lowest carbon stock in the mineral layers. Karelian forests (NK and MK) dominated by different tree species with similar plant functional groups also demonstrated the lowest carbon stock in the mineral layers of soil in forests dominated by pine. Moreover, among all pine forests, forests with a large proportion of mosses and dwarf shrubs were characterised by the lowest carbon stock in the mineral layers. However, spruce forests with the highest share of herbaceous plants were characterised by the highest carbon stock in the mineral soil horizons. The explanations for that could be differences in (1) the quality of plant residues and (2) the amount of precipitation penetrating through the canopy of these coniferous forests. High carbon accumulation in the mineral layers of birch forests could be explained by a higher quality of plant residues, except for forests, which were on four plots with Carbic Entic Podzols on carbon-containing soil-forming rock.
4.5. Soil Carbon Stock Comparison between the Areas
Differences in soil carbon stock between the three areas—NK, MK and MKI—were associated with the proportions of forest types dominated by different tree species and ground vegetation species belonging to different functional groups. Lower carbon stocks in the organic horizon of the MKI forests were due to the low mass of this horizon, which was half of that in forests of the NK and MK forests (Table 1). The organic horizon mass depends on different factors, including the decomposition rate, as well as both natural and human-induced disturbances. The rate of decomposition depends on the quality of plant litter.
The NK forests were dominated by pine forests (71%) and by Cajander’s site type 4 (74%), and among the functional groups of ground vegetation, mosses and dwarf shrubs dominated there (85%). Therefore, the quality of plant litter in these forests was low, and one would expect a large mass of the organic horizon, but that was not the case; the organic horizon masses in the NK and MK were comparable. The reason for this could be periodical forest fires; pine forests with lingonberry dominating in NK often indicate post-fire succession in Karelian forests [53]. The C/N ratio in the organic horizon, reflecting the quality of litter, was higher in the NK than in the MKI. Thus, the lowest carbon stock in the mineral layers in the NK forests could be explained by the predominance of pine forests with lingonberry and mosses characterised by plant residues of low quality and by carbon emissions due to periodical fires. One more reason could be the intense washing of the soil profile in pine forests because of a large amount of precipitation penetrating through the open and short pine crowns.
Higher carbon stocks in the mineral layers of soil in the MK forests compared to forests in the NK could be explained by higher forest productivity and litter quality. In the MK forests, the proportions of spruce, pine and birch forests were comparable; the shares of site types 2 and 3 were 17% and 46%, respectively.
In this study, like in Scandinavian countries [5], carbon stock in soil was the highest in more productive forests growing in more southern conditions, i.e., in the MK and MKI forests. Although carbon stocks in the mineral layer 30–50 cm of soil in the MK and MKI forests were comparable, the upper soil mineral layers (0–30 cm) of the MKI forests were characterised by the higher carbon contents and stocks. The main explanation for this is carbon accumulation in the humus (A) horizon of Albic Retisols. Additionally, it should be noted that although the MKI forests were dominated by pine with a litter of low quality, Cajander’s site type 2 reached a level of 30%, and the proportion of plant functional type with a high share of herbaceous plants was more than 50%. It was shown that the result of slash-and-burn practice, widely distributed in the Karelian Isthmus until the beginning of the 20th century, was the destruction of spruce forests on the most fertile soils, and large-scale and severe fires favored the regeneration of pine [22]. Climatic conditions in the MKI forests are favourable for the development of herb plant cover, which could lead to accelerated litter decomposition and subsequent migration of dissolved organic carbon down the soil profile. Moreover, long-term agricultural practices [22,54] could lead to humus accumulation in the upper mineral horizons of soil in some areas. According to an analysis of land cover change on the Karelian Isthmus in the period 1939–2005, the share of forests increased by 5% at the expense of agricultural areas and open peatland [54]. Thus, the less pronounced differences in soil carbon stocks associated with the impact of different coniferous tree species in MKI forests may be related to widely distributed slash-and-burn agricultural practice in the past resulted in the prevalence of pine forests, including those with a high proportion of herbaceous plants, that could smooth out these natural differences found in Karelian forests.
5. Conclusions
In northwestern Russia, carbon stocks in the soil organic horizon and mineral layer 0–50 cm increased from 106 t ha−1 in the northern taiga of Karelia to 163 t ha−1 in the middle taiga of the Karelian Isthmus. Variations in soil carbon stocks were associated with the soil types. The higher carbon stocks in the upper part of the soil mineral profile in the Karelian Isthmus were explained by the presence of the carbon-rich humus horizon A in Albic Retisols. Locally found in Karelian forests, Carbic Entic Podzols accumulated more carbon in the overall mineral profile than typical Carbic Albic Podzols.
Like in Scandinavian countries, in more productive forests growing in more southern conditions, the soil carbon stock was the highest. Differences in soil carbon stocks between and within the three areas—the northern and middle taiga of Karelia and the middle taiga of the Karelian Isthmus—were associated with the proportions of forest types dominated by different tree species and by ground vegetation species belonging to different functional groups.
Pine forests were characterised by lower carbon stocks in the mineral layers of soil than spruce and birch forests. The associations between the soil carbon stocks and Cajander’s forest site types were confirmed; the less productive site type accumulated less carbon in the mineral layers. However, significant differences in soil carbon stock were found within the same Cajander’s site types dominated by different tree species, i.e., between Sukachev’s forest types; the sites dominated by spruce accumulated significantly more carbon in the mineral layers than the sites of the same Cajander’s type dominated by pine.
Carbon stocks in the mineral layers were related negatively to the C/N ratio in the organic horizon and proportion of pine in growing stock volume and positively to the share of herbaceous plants and the proportion of spruce. Spruce forests with the highest share of herbaceous plants were characterised by the highest carbon stocks in the mineral soil horizons, while pine forests with dwarf shrubs and green mosses accumulated more carbon in the organic layers, and the carbon stock in the mineral layers there was the lowest. The explanations for these differences in soil carbon stocks could be differences in (1) the quality of plant residues and (2) the amount of precipitation penetrating through the canopy, which depends on the crown structure of the tree species.
Thus, both tree species and ground vegetation affect the soil carbon accumulation, which should be taken into account when assessing and forecasting the dynamics of soil carbon stocks.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/11/9/979/s1. Table S1: Soil carbon stocks and shares of dominating tree species and plant functional groups in forests of three areas, Table S2: Soil carbon stocks and shares of dominating tree species and plant functional groups in pine, spruce and birch forests, Table S3: Soil carbon stocks and shares of dominating tree species and plant functional groups in Cajander’s site types, Table S4: Soil carbon stocks and shares of dominating tree species and plant functional groups in Cajander’s site type 4 (VT) dominated by different tree species in Karelia, Table S5: Soil carbon stocks and shares of dominating tree species and plant functional groups in Cajander’s site type 3 (MT) dominated by different tree species in Karelia, Table S6: Soil carbon stocks and shares of dominating tree species and plant functional groups in sites with main functional vegetation types.
Author Contributions
Conceptualization, N.L., A.K. (Anastasia Kuznetsova) and E.T.; Data curation, M.D., D.T. and M.S.; Formal analysis, V.S.; Investigation, A.G., O.B., A.K. (Aleksandr Kryshen) and M.S.; Visualization, A.K. (Anastasia Kuznetsova) and S.K.; Writing—original draft, N.L., A.K. (Anastasia Kuznetsova) and E.T.; Writing—review & editing, N.L., A.K. (Anastasia Kuznetsova) and E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the RUSSIAN SCIENCE FOUNDATION, project number 16-17-10284 and by MINISTRY OF SCIENCE AND HIGH EDUCATION OF RUSSIAN FEDERATION, project number AAAA-A18-118052590019-7, and was carried out under state order to the Karelian Research Centre of the Russian Academy of Sciences (Forest Research Institute).
Acknowledgments
We thank Juha-Pekka Hotanen from Natural Resources Institute Finland (Luke) for helping us in identification of Cajander’s site types in forests, located within the regular grid of plots in Karelia and the Karelian Isthmus.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jones, C.; Falloon, P. Sources of uncertainty in global modelling of future soil organic carbon storage. In Uncertainties in Environmental Modelling and Consequences for Policy Making; Springer: Dordrecht, The Netherlands, 2009; pp. 283–315. [Google Scholar]
- Lindner, S.; Peterson, S.; Windhorst, W. An economic and environmental assessment of carbon capture and storage (CCS) power plants: A case study for the City of Kiel. J. Environ. Plan. Manag. 2010, 53, 1069–1088. [Google Scholar] [CrossRef]
- Vesterdal, L.; Clarke, N.; Sigurdsson, B.D.; Gundersen, P. Do tree species influence soil carbon stocks in temperate and boreal forests? For. Ecol. Manag. 2013, 309, 4–18. [Google Scholar] [CrossRef]
- Framstad, E.; Wit, H.; Mäkipää, R.; Larjavaara, M.; Vesterdal, L.; Karltun, E. Biodiversity, Carbon Storage and Dynamics of Old Northern Forests; Nordic Council of Ministers: Copenhagen, Denmark, 2013; p. 130. [Google Scholar]
- Callesen, I.; Liski, J.; Raulund-Rasmussen, K.; Olsson, M.T.; Tau-Strand, L.; Vesterdal, L.; Westman, C.J. Soil carbon stores in Nordic well-drained forest soils—Relationships with climate and texture class. Glob. Chang. Biol. 2003, 9, 358–370. [Google Scholar] [CrossRef]
- Svensson, M.; Jansson, P.-E.; Berggren Kleja, D. Modelling soil C sequestration in spruce forest ecosystems along a Swedish transect based on current conditions. Biogeochemistry 2008, 89, 95–119. [Google Scholar] [CrossRef]
- Stendahl, J.; Johansson, M.B.; Eriksson, E.; Nilsson, A.; Langvall, O. Soil organic carbon in Swedish spruce and pine forests–differences in stock levels and regional patterns. Silv. Fenn. 2010, 44, 5–21. [Google Scholar] [CrossRef]
- De Wit, H.A.; Kvindesland, S. Carbon stocks in Norwegian forest soils and effects of forest management on carbon storage. Rapp. Fraskogforskningen Suppl. 1999, 14, 52. [Google Scholar]
- State of Finland’s Forests 2012 Criterion 1 Forest Resources. Carbon Stock on Forest Land (1.4). Available online: http://www.metla.fi/metinfo/sustainability/c1-carbon-stock.htm (accessed on 7 July 2020).
- Liski, J.; Westman, J. Carbon storage in forest soil of Finland. 1. Effect of thermoclimate. Biogeochemistry 1997, 36, 239–260. [Google Scholar] [CrossRef]
- Payne, N.J.; Cameron, D.A.; Leblanc, J.D.; Morrison, I.K. Carbon storage and net primary productivity in Canadian boreal mixedwood stands. J. For. Res. 2019, 30, 1667–1678. [Google Scholar] [CrossRef]
- Laganiere, J.; Paré, D.; Bergeron, Y.; Chen, H.Y.; Brassard, B.W.; Cavard, X. Stability of soil carbon stocks varies with forest composition in the Canadian boreal biome. Ecosystems 2013, 16, 852–865. [Google Scholar] [CrossRef]
- Order of the Ministry of Natural Resources and Ecology of Russian Federation no. 20-r of June 30, 2017 on Recommendations for Qualitative Measurements of Absorption Volume of Greenhouse Gases. Available online: https://www.garant.ru/products/ipo/prime/doc/71612096/ (accessed on 7 July 2020).
- Dymov, A.A. Soils of Post-Cutting, Post-Pyrogenic and Post-Agrogenic Forest Ecosystems of the Northeast of the European Part of Russia; Moscow State University: Moscow, Russia, 2018; p. 46. (In Russian) [Google Scholar]
- Bakhmet, O.N. Carbon storages in soils of pine and spruce forests in Karelia. Russ. J. For. Sci. 2018, 1, 48–55. [Google Scholar] [CrossRef]
- Lukina, N.V.; Tikhonova, E.V.; Orlova, M.A.; Bakhmet, O.N.; Kryshen, A.M.; Tebenkova, D.N.; Kuznetsova, A.I.; Smirnov, V.E.; Braslavskaya, T.Y.; Gornov, A.V.; et al. Associations between forest vegetation and the fertility of soil organic horizons in northwestern Russia. Ecosystem 2019, 6, 34. [Google Scholar] [CrossRef]
- UNECE ICP Forests Programme Co-Ordinating Centre (Ed.) Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of air Pollution on Forests; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2016; Available online: http://www.icp-forests.net/page/icp-forests-manual (accessed on 7 July 2020).
- Tikhonova, E.; Tikhonov, G.; Shevchenko, N.; Knyazeva, S.; Plotnikova, A.; Lukina, N.; Shashkov, M. Tree diversity patterns along the latitudinal gradient in the northwestern Russia. For. Ecosyst. 2017, 4, 27. [Google Scholar] [CrossRef]
- Volkov, A.D. Karelian Forest Types; Karelian Research Centre of RAS: Petrozavodsk, Russia, 2008; 180p. (In Russian) [Google Scholar]
- Kryshen, A.M. Types of forest vegetation conditions on automorphic soils in Karelia. Botani. J. 2010, 95, 281–297. (In Russian) [Google Scholar]
- Doronina, A.Y. Vascular Plants of the Karelian Isthmus; Association of Scientific Publications; KMK: Moscow, Russia, 2007; p. 574. (In Russian) [Google Scholar]
- Fedorchuk, V.N.; Neshatayev, V.Y.; Kuznetsova, M.L. Forest Ecosystems of the Northwest Regions of Russia: Typology, Dynamics, Economic Features; Hromis: St.-Petersburg, Russia, 2005; 382p. (In Russian) [Google Scholar]
- Deluca, T.H.; Boisvenue, C. Boreal forest soil carbon: Distribution, function and modelling. Forestry 2012, 85, 161–184. [Google Scholar] [CrossRef]
- Chertov, O.G. Ecology of Forest Land (Soil and Ecological Research of Forest Habitats); Nauka: Leningrad, Soviet Union, 1981. (In Russian) [Google Scholar]
- Salemaa, M.; Derome, J.; Nojd, P. Response of boreal forest vegetation to the fertility status of the organic layer along a climatic gradient. Boreal Environ. Res. 2008, 13, 48–66. [Google Scholar]
- Hotanen, J.P.; Maltamo, M.; Reinikainen, A. Canopy stratification in Peatland Forests in Finland. Silva Fennica 2008, 40, 53–76. [Google Scholar] [CrossRef][Green Version]
- Sukachev, V.N. Concepts Forest Biogeocoenology. Selected Works; Science: Leningrad, Soviet Union, 1972; pp. 311–356. (In Russian) [Google Scholar]
- Lukina, N.V.; Orlova, M.A.; Gornov, A.V.; Kryshen’, A.M.; Kuznetsov, P.V.; Knyazeva, S.V.; Smirnov, V.E.; Bakhmet, O.N.; Eydlina, S.P.; Ershov, V.V.; et al. Assessment of Sustainable Forest Management Criteria with the ICP Forests Indicators. Russ. J. For. Sci. 2013, 5, 62–75. [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 7 July 2020).
- Husson, F.; Le, S.; Pagès, J. Exploratory Multivariate Analysis by Example Using R, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2017. [Google Scholar]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, Issue1. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.42.1. 2018. Available online: https://CRAN.Rproject.org/package=MuMIn (accessed on 7 July 2020).
- Walsh, C.; Mac Nally, R. Hier.Part: Hierarchical Partitioning. R Package Version 1.0-4. 2013. Available online: https://CRAN.R-project.org/package=hier.part (accessed on 7 July 2020).
- Vitousek, P.M. Beyond global warming: Ecology and global change. Ecology 1994, 75, 1861–1876. [Google Scholar] [CrossRef]
- Berg, B. Litter decomposition and organic matter turnover in northern forest soil. For. Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]
- Coq, S.; Souquet, J.M.; Meudec, E.; Cheynier, V.; Hättenschwiler, S. Interspecific variation in leaf litter tannins drives decomposition in a tropical rain forest of French Guiana. Ecology 2010, 91, 2080–2091. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Lukina, N.V.; Orlova, M.A.; Steinnes, E.; Artemkina, N.A.; Gorbacheva, T.T.; Smirnov, V.E.; Belova, E.A. Mass-loss rates from decomposition of plant residues in spruce forests near the northern tree line subject to strong air pollution. Environ. Sci. Pollut. Res. 2017, 24, 19874–19887. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, S.; Zhang, Y. Indirect effects of precipitation variation on the decomposition process of Mongolian oak (Quercus mongolica) leaf litter. Front. China 2007, 2, 417–423. [Google Scholar] [CrossRef]
- Nilsson, M.C.; Wardle, D.A. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 2005, 3, 421–428. [Google Scholar] [CrossRef]
- Hilli, S.; Stark, S.; Derome, J. Litter decomposition rates in relation to litter stocks in boreal coniferous forests along climatic and soil fertility gradients. Appl. Soil Ecol. 2010, 46, 200–208. [Google Scholar] [CrossRef]
- Hilli, S. Significance of Litter Production of Forest Stands and Ground Vegetation in the Formation of Organic Matter and Storage of Carbon in Boreal Coniferous Forests; Forest Condition Monitoring in Finland-National Report; Merilä, P., Jortikka, S., Eds.; The Finnish Forest Research Institute: Vantaa, Finland, 2013; Available online: http://www.metla.fi/metinfo/forest-condition/intensive-monitoring/foliar-chemistry.htm (accessed on 7 July 2020).
- Cornelissen, J.H.; Lang, S.I.; Soudzilovskaia, N.A.; During, H.J. Comparative cryptogam ecology: A review of bryophyte and lichen traits that drive biogeochemistry. Ann. Bot. 2007, 99, 987–1001. [Google Scholar] [CrossRef]
- Gielen, B.; Neirynck, J.; Luyssaert, S.; Janssens, I.A. The importance of dissolved organic carbon fluxes for the carbon balance of a temperate Scots pine forest. Agric. For. Meteorol. 2011, 151, 270–278. [Google Scholar] [CrossRef]
- Ponomareva, V.V. Theory of the Podzol-Forming Process (Biochemical Aspects); Nauka: Moscow, Soviet Union, 1964. (In Russian) [Google Scholar]
- Dalsgaard, L.; Lange, H.; Strand, L.T.; Callesen, I.; Borgen, S.K.; Liski, J.; Astrup, R. Underestimation of boreal forest soil carbon stocks related to soil classification and drainage. Can. J. For. Res. 2016, 46, 1413–1425. [Google Scholar] [CrossRef]
- Buurman, P.; Jongmans, A.G. Podzolisation and soil organic matter dynamics. Geoderma 2005, 125, 71–83. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Lukina, N.V.; Danilova, M.A.; Artemkina, N.A.; Smirnov, V.E.; Ershov, V.V.; Isaeva, L.G. The effect of air pollution on the rate of decomposition of plant litter at the northern limit of pine forests. Russ. J. For. Sci. 2019, 6, 533–546. [Google Scholar] [CrossRef]
- Lukina, N.V.; Ershov, V.V.; Gorbacheva, T.T.; Orlova, M.A.; Isaeva, L.G.; Tebenkova, D.N. Assessment of soil water composition in the Northern taiga coniferous forests of background territories in the industrially developed region. Eurasian Soil Sci. 2018, 51, 277–289. [Google Scholar] [CrossRef]
- Hansson, K.; Fröberg, M.; Helmisaari, H.S.; Kleja, D.B.; Olsson, B.A.; Olsson, M.; Persson, T. Carbon and nitrogen pools and fluxes above and below ground in spruce, pine and birch stands in southern Sweden. For. Ecol. Manag. 2013, 309, 28–35. [Google Scholar] [CrossRef]
- Fedorets, N.G.; Morozova, R.M.; Bakhmet, O.N. Soils and soil cover of Zaonezhie Karelia. Tr. Karel. Nauchnogo Cent. Ross. Akad. Nauk. 2004, 6, 69–89. (In Russian) [Google Scholar]
- Gromtsev, A.N. Secondary forests in the west of the taiga zone of Russia: Origin, current status and dynamics. Boreal For. Status Dyn. Ecosyst. Serv. 2019, 5, 5–16. (In Russian) [Google Scholar]
- Valyaev, V.N. Age structure of pine forests of Karelia. Russ. J. For. Sci. 1968, 6, 36–41. (In Russian) [Google Scholar]
- Rautiainen, A.; Virtanen, T.; Kauppi, P.E. Land cover change on the isthmus of Karelia 1939–2005: Agricultural abandonment and natural succession. Environ. Sci. Pol. 2016, 55, 127–134. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).