1. Introduction
The activity of reforestation and wood use in Brazil has experienced a continuous growth in the last years registering a 13.1% increase in 2018, in relation to the previous year, reaching a sectorial revenue of R
$ 86.6 billion. This, represents 1.3% of the national GDP and 6.9% of industrial GDP. The total planted area covers 7.83 million hectares of the Brazilian territory of which
Eucalyptus plantations accounts for almost 73%, pine 20% and other species 7% of the total cultivated area in the country [
1].
Eucalyptus plantations in Brazil are mainly concentrated in the states of Minas Gerais (24%), São Paulo (17%) and Mato Grosso do Sul (16%) [
1], in soils of low natural fertility [
2,
3] fertilization for obtaining high yields.
The main products obtained from forest products are—cellulose, paper, charcoal, industrialized wood panels, plywood, sawn wood and others of a smaller scale such as fence posts, posts and firewood. Brazil is the world’s largest producer of charcoal and the second largest producer of pulp [
1].
According to Reference [
4], the most cultivated genetic material, in general clones and hybrids, is originated from:
Eucalyptus grandis,
Eucalyptus saligna,
Eucalyptus urophylla,
Eucalyptus camaldulensis, Eucalyptus viminalis and
Eucalyptus dunnii (southern region of the Brazil).
One of the advantages of
Eucalyptus compared to other forest species is its ability to sprout after the tree is felled, which enables the stands to be managed in a regime called coppicing. After the tree is cut, the stump can produce several shoots, due to the stimulus of adventitious buds [
5,
6], originating several trunks.
In Brazil, most Eucalyptus stands are managed by coppicing, although there are specific situations, such as the replacement of one genetic material by another, in which a new planting is carried out. In the management by coppicing, depending on the use of the wood, one or more shoots can be left per stump, while the others are eliminated. When there are stumps that do not sprout, more than one shoot can be left to recompose the original number of trunks.
The production obtained in
Eucalyptus stands managed by coppicing is, in principle, similar to the original production, provided that the availability of growth factors (water, light, nutrients, oxygen, temperature) is not reduced [
7,
8]. What has been observed in most conditions of
Eucalyptus cultivation in Brazil is the reduction of production in successive harvests in stands managed by coppicing. In general, such reduction can be attributed to the lower availability of nutrients [
6,
8], due to their export; soil compaction [
9,
10]; damage to the buds during harvest [
11]; gradual increase in the number of stumps that do not sprout [
5]; water regime [
5], among others.
Reference [
12] reported yield reduction that reached approximately 50% of the initial one when
Eucalyptus plots were not re-fertilized with potassium after the first harvest. When nutrient availability is low, the efficiency in their use by trees increases until it reaches the critical efficiency [
13], above which nutritional deficiency and reduction of growth occur. Thus, the efficiency of nutrient use by
Eucalyptus stands managed by coppicing is expected to increase with the number of rotations, unless the exported nutrients are replaced by fertilization.
The number of shoots per stump influences the industrial wood production. According to Reference [
5], each shoot left on the stump behaves as a single plant and contributes to increasing the pressure on the environmental resources as the number increases. Therefore, the permanence of more than one shoot per stump stimulates competition between them for growth resources and, probably, differences in size between shoots, if the amount of resources at the site is limited [
14]. If so, a smaller number of shoots per stump allows for greater growth of the remaining shoots. Reference [
15] observed that the average diameter at breast height (DBH) and the average height of shoots were inversely proportional to the number of shoots per stump. Similarly, delaying the elimination of excess shoots contributes to intensifying the competition between shoots from the same stump. Conversely, early thinning causes growth resources to be allocated to the remaining shoot(s), which will probably have a higher individual growth rate than if multiple shoots remain on the same stump. Although such procedure may not affect the final production, it effects the diameter and height of the remaining stems, as pointed out by the results of Reference [
15].
Coppicing has lower cost than replanting the stand thus becoming an alternative to reduce the costs of reform, which justifies the increase in the number of stands managed by coppicing [
16].
This study aimed to: (1) to assess nutrient demand of the Eucalyptus stand under a coppice regime; (2) to test the effect of fertilization on the production of eucalyptus wood, distribution and compartmentalization of nutrients in stands managed by coppice; (3) to test the effect of the height and number of shoots, on the occasion of the sprouting, in the production of eucalyptus managed by coppice and (4) to compare the productivity of high eucalyptus wood with a coppice management regime.
2. Materials and Methods
The study was carried out in Lassance county, Minas Gerais State, Brazil, in the Cerrado Biome (a Savannah type of vegetation), with geographical coordinates of 18°01’ South latitude and 44°47′ longitude West and an altitude of 850 m. The climate of the region is Aw, according to the Köppen-Geiger classification, that is, tropical with a dry winter season (megathermal climate) in which the average monthly temperature is greater than 18 °C in all months of the year and, in at least one month, there is average rainfall below 60 mm [
17]. According to local data, the average annual rainfall is 1250 mm, with dry period from May to October. The average temperature is 21.3 °C, reaching maximum of 38 °C and minimum of 10 °C. In the dry period, relative humidity fluctuates from 10% to 40%.
The soil was classified as Latossolo Vermelho-Amarelo (Oxisol) with sandy loam texture, drained, deep and with low natural fertility. The values of chemical and particle-size characteristics were determined, from the sampling of 25 simple samples in the layer 0–40 cm deep randomly traversed in the field, in the area of the first cut of the clone GG 157, then obtained the composite soil sample (
Table 1). The following soil properties were determined for the samples collected in pre-mining and for the post-mined site—soil pH was determined in water (1:2.5
v/v); soil organic matter by the Walkley-Black method; exchangeable Al and H+Al extracted by KCl (1 mol L
−1) and calcium acetate (0.5 mol L
−1) at pH 7.0, respectively and quantified by titration. P and K were extracted by Mehlich-1 solution and quantified by colorimetry and flame photometry, respectively. Ca
2+ and Mg
2+ were extracted by KCl (1 mol L
−1) and quantified by atomic absorption spectrophotometry (AAS).
The experiment was conducted using stumps remaining from the first cut (high forest) of the clone GG157, Eucalyptus urophylla hybrid. The experimental plots were randomly allocated, within commercial stands of pure eucalyptus, with an area around 25 ha.
In the high-forest management (first rotation), 1,000 kg ha−1 of dolomitic limestone were applied broadcast in total area and 350 kg ha−1 of single superphosphate were applied in a continuous strip. Immediately after planting the seedlings, 120 kg ha−1 of formulation 10-30-10 + 0.5% B + 0.5% Zn were applied inside pits, 15 cm from the seedlings. A top-dressing fertilization was divided into two applications, at first they applied 150 kg ha−1 of 14-00-28 + 0.5% B + 0.3% Zn, eight months after planting and the second one with 200 kg ha−1 of 14-00-28 + 0.5% B + 0.3% Cu, twenty months after planting. Both applications were performed manually, distributing the fertilizers under the crown projection. Planting spacing was 3.8 × 2.4 m. After the first harvest at the age of 7, the slash (leaf and branch) was left in the area. The cultivation of eucalyptus in this area is intended to produce bioreductors (charcoal).
In the coppicing management, limestone and reactive natural phosphate from Gafsa were applied 60 days (in March) prior to stand harvesting; a top-dressing fertilization was applied after harvesting, according to
Table 2, referred to as treatments—liming/fertilization. 1000 kg ha
−1 of dolomitic limestone and 250 kg ha
−1 of Gafsa reactive natural phosphate were applied in a continuous strip, without incorporation, only in the fertilized treatments (
Table 2). Top-dressing fertilization consisted of 450 kg ha
−1 of a misture NPK (10-00-30) split into two portions; the first half was applied after six months from the cutting of the high forest (in November) and the other half after 13 months (in June) from the cutting of the high forest, only in treatments called—liming/fertilization, according to
Table 2.
The experiment was conducted in split plots, with three replicates, testing eight treatments (
Table 2). The plot is represented by application or not of limestone, Gafsa natural phosphate and top- dressing fertilization, whereas the subplot is represented by the thinning technique (pole selection).
The selection was performed when the poles were 1, 2 or 4 m high, leaving one or two per stump, according to the treatment (
Table 2). Care was taken in this procedure to leave the most vigorous and best positioned poles, considering also their uniform distribution on the stump.
Each plots consisted of 720 stumps (90 × 8), occupying a total area of 0.66 ha. Each subplot contained 80 (10 × 8) stumps, of which only the 48 (8 × 6) in the center of the plot (0.04 ha) were measured, to eliminate the border effect and constituted a subplot.
When the shoots reached the age of five years, the diameter at breast height (DBH) 1.30 m from the soil, the commercial height (Hc), defined by the height up to the point of the trunk with diameter of 4 cm and the total height (Ht) were measured in the trees of the usable plot, the age at which the harvest was carried out. The trunk section with diameter of less than 4 cm was considered as slash. Subsequently, the volumes of trunk, wood and bark were calculated. Three medium-size trees were felled in each subplot and their leaves, branches, wood and bark were separated to determine the fresh and dry matter weights. The aerial part or above ground portion corresponds to the sum of the trunk, leaves and branches compartments. The trunk was divided into four sections—0–25%, 25–50%, 50–75% and 75–100% of commercial height, from which 5-cm-thick discs were collected to determine dry weight and conduct chemical analysis.
In the laboratory, the diameter of the disc, with and without bark, was measured separating the bark from the wood.
Samples of bark, wood, leaves and branches, were taken to laboratory for N, P, K, Ca and Mg determination. The samples of the plant material, after oven- dried to constant weight, were ground in a Wiley-type mill, passed through a 0.5-mm-mesh sieve and subjected to digestion by a nitric- perchloric acids solution. Phosphorus was determined by colorimetry [
18], K by flame emission photometry, Ca and Mg by atomic absorption spectrometry and N by titration after digestion using the Kjeldahl method [
19].
Then the following parameters were calculated: content of nutrients in the plant (multiplying the dry matter by the content of each nutrient) and nutrient use efficiency in the trunk (obtained by dividing the trunk weight (t ha
−1) by the content of each nutrient in this compartment (kg ha
−1), according to Reference [
20].
The volume of each pole (shoot), with and without bark, was determined using the Smalian’s formula [
21].
The data were subjected to analysis of variance and the means of the effects of the studied factors were compared by Tukey test at 5% probability level.
4. Discussion
The positive effect of fertilization on the DBH of the shoots indicates that the soil and high forest harvesting residues (slash), in the unfertilized plots, did not supply all the amount of nutrients required by eucalyptus sprouts for high production. This fact frequently occurs in eucalyptus stands growing in the Cerrado soils regardless the reproductive method, coppicing [
15,
23] or in seed stands [
24,
25]. The diameters of fertilized trees were on average 22.5% larger than those of unfertilized trees (
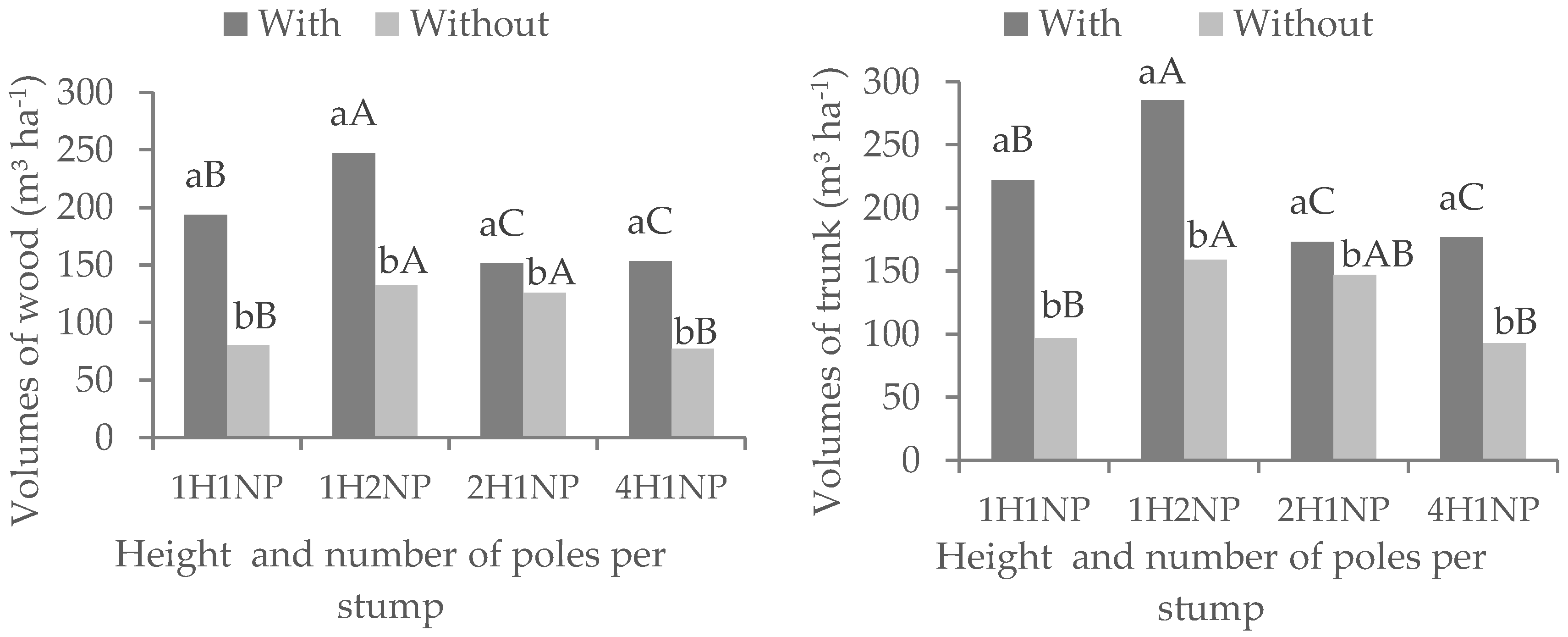
Figure 1).
The effect of pole height at the time of pole selection on DBH depended on fertilization. In fertilized trees, selecting poles with smaller height (1H1NP) enabled greater growth in diameter (15 cm) compared to the selection of taller poles (2H1NP and 4H1NP) (
Figure 1). However, in the unfertilized plots the largest diameter was obtained when 2.0-m-high poles were selected. This indicates that fertilization intensified the growth of young shoots as it concentrated resources, while in the older ones there may have been competition between shoots, with division of growth resources, before the elimination of excess shoots. The treatment 2H1NP probably had better balance between production and requirement of reserves to form leaves and roots, resulting in larger diameter. The higher the number of poles remaining on the stump, the lower the DBH (
Figure 1), due to the greater competition between poles for growth resources (water, light and nutrients) and smaller individual leaf area. Our results are supported by the findings of other researchers [
15,
23,
26,
27].
Reference [
23] found that fertilizer application, in the furrow between tree rows before harvesting the stand in the first rotation, was the treatment with best results. They observed a direct and highly significant relationship between top-dressing fertilization, performed in second rotation, and shoot development. In our study, the application of Gafsa phosphate before planting and the use of soluble fertilizers also resulted in growth gains, besides meeting the demand for N, P, K, Ca and Mg of the trees.
Fertilization increased the yield (trunk volume) of the stands managed by coppicing compared to the control without fertilization (
Figure 2). The highest trunk production was found in the treatment 1H2NP, followed by 1H1NP, 2H1NP and 4H1NP in the fertilized plots; while in the unfertilized plots the sequence was 1H2NP, 2H1NP, 4H1NP and 1H1NP. This result confirms those obtained by References [
23] and [
15]. These authors observed that wood volume increased in those plots that received fertilization and that there is a tendency of increase in the volume per area with the number of shoots per stump. Depending on the use of the wood, these authors recommended two shoots/stump, which is supported by results of the best treatment of our study. The number of shoots to be left depends on the use intended for the wood, since there is an inverse relationship between the number of shoots and their individual volume [
23].
The gains in both wood and trunk yields ranged from 20.2% to 140.2% and from 18% to 128.6%, respectively, according to the treatments. The lowest gain with fertilization occurred in the 2H1NP treatment, while the highest gain was obtained in the 1H1NP treatment, for both, wood and trunk volumes (
Figure 2). Reference [
15] found differences of volume between fertilized and unfertilized treatments ranging from 27% to 76%, depending on the number of shoots per stump.
In the Cerrado region fertilization is necessary to restore or improve soil nutrient to the level of the previous rotation [
5]. Reference [
12] found that the non-replacement of K in stand of
E. grandis led to a 54% reduction in volumetric production and mean annual increment (MAI) of the second rotation. The high response to K application is often found in high forest systems, as it is the second most limiting nutrient in the Brazilian soils used for eucalyptus plantation [
6,
28]. For the second rotation coppicing management eucalyptus fast growth and high production and nutrient exportation, contribute to high responses to fertilization. Another point to consider, according to Reference [
25], is the earlier nutritional demand of the shoots since their initial growth rate is higher when compared to that of first-rotation stands, due to the existence of an already established root system. Earlier demand also occurs when a higher number of shoots is left per stump. Supplying nutrients through fertilizer to maintain high growth rate will favor the achievement of maximum production at younger ages, leading to a higher rate of return of the managed forest [
5].
Another fact that may have further aggravated the lack of nutrients is their immobilization in the shoots eliminated by thinning, because the rate of decomposition of this material can be slow [
29]. In general, later thinning, that is, when the shoots are taller, caused reduction in yield, probably due to the greater immobilization of nutrients in the removed material. The return of nutrients to the plant growth process may take years, depending on the degree of lignification of the material.
The higher yield obtained in the 1H2NP treatment is related to the sum of the trunk volumes of the two poles left at the time of pole selection (
Figure 2). However, it should be noted that the DBH of each pole is lower when compared to those of the other treatments (
Figure 1). The practice of leaving more than one pole per stump is usually used to recompose the original plant population, thus compensating for stumps that do not sprout.
The amount of nutrients at the time of forest harvesting represents the nutrient requirement that the plant needs to complete the rotation. Nutrient allocation in the various tree components informs about their possible export. Trees that received fertilization had higher accumulation of nutrients in the shoots (
Table 3,
Table 4 and
Table 5) and, consequently, higher yield (
Figure 3). Shoot management was also influenced for the accumulation of nutrients and the trees with two poles per stump (1H2NP treatment) accumulated the largest amount of nutrients.
For stumps that remained with one pole but were thinned at different heights, greater accumulation of nutrients was found in those that were thinned earlier. Late thinning leads to the elimination of shoots that had already accumulated nutrients, which are temporarily immobilized. The effect of height at the time of pole selection on nutrient accumulation in the biomass indicates that the nutrients in the eliminated poles had not been fully mineralized at the end of the experiment. The relative differences between extreme treatments (1H1NP x 4H1NP) are 24% for the amount of N, 35.5% for P, 47% for K, 29% for Ca and 24% for Mg. Therefore, these percentage differences indicate an order of nutritional limitation, which would be higher for K, followed by P. As K is quickly released by the harvesting residues [
30,
31], one can rule out the possibility of its loss by leaching, in view of the sandy texture of the soil [
32,
33]. The studies of References [
30] and [
31] determined the renewal times for N, P, K, Ca and Mg and showed that K has shorter renewal time.
Considering the 1H1NP treatment as a reference, because it is the one similar to what of the first production cycle, when the bark is removed from the trunk and left in the area, a relevant amount of nutrients is no longer exported, which implies savings of fertilizers. In plots that received fertilization, exporting only the wood would maintain in the area 76, 62, 56, 33 and 29% of Ca, Mg, P, N and K, respectively, of the amount that would be exported with the removal of the trunk (
Table 3 and
Table 4). In plots that did not receive fertilization, the contribution would be greater, because 79, 64, 61, 42 and 39% of these nutrients would be maintained (
Table 3 and
Table 4). These percentages indicate a higher proportion of these nutrients in the bark in unfertilized stands, especially N and Mg, as compared with the fertilized trees. As most Brazilian soils are poor in Ca, P and K, removing the bark from the trunk would reduce the export of these nutrients, contributing to a reduction in fertilizer use in the next plantings or rotation.
The amount of nutrients in the crown informs about what would be left in the area after harvest and could become available for the next production cycle. Nitrogen and Ca are the most accumulated nutrients in the trunk by eucalyptus trees [
34,
35], with values ranging from 64.8% to 73.6% in relation to the total accumulated in the above ground. Therefore, the amount of these two nutrients in the rown is relatively low. Higher content of Ca in the trunk is due to its high concentration in the bark [
34,
36]. In our study, the most accumulated nutrients in the trunk were N, K and Ca; in fertilized plots, they corresponded to 58.8%, 75.5% and 74.1%, respectively, of the total accumulated in the above ground part, while in unfertilized plot, the values were 9%, 73% and 71% of N, K and Ca, respectively (
Table 4 and
Table 5). These results confirm the importance of fertilization in new plantings or in the growing of sprouts due to the great export of these nutrients.
In general, in plots that received fertilization, the trunk had higher values of EU for the nutrients, except for K, indicating higher efficiency in their use for dry matter production (
Table 7). Higher trunk production in fertilized plots results from the higher supply of nutrients in the soil and nutrient contents in the trunk of the shoots (
Figure 3 and
Table 4) grown in these plots. In the works of References [
37] and [
38] the use of fertilizers in eucalyptus plantations provide gains that can vary from 5% to more than 90% in wood production, depending on the nutrient, the age of the stand and the edaphoclimatic conditions.
Higher values of EU in fertilized plots indicate higher efficiency in the conversion of nutrients into dry matter, that is, with relatively lower nutrient amount in the trunk of the shoots from the fertilized plot, the biomass production was higher. In unfertilized plot, nutrient amount was relatively higher but biomass production was lower.
Higher values of EU in the trunk for K, in the unfertilized plot, as well as in some cases for P, Ca and Mg (
Table 7), may indicate nutritional restriction. Water restriction can also prevent or hamper their transport to the roots and lead to higher values of EU. Alternatively, the root system may not have grown in such a way to explore a larger soil volume, making the absorption of nutrients difficult. Thus, all these factors can compromise the efficiency of nutrient utilization for production. In general, EU values were also higher where thinning was performed later, which corroborates the hypothesis of immobilization of nutrients in the poles for a long time.
According to the studies conducted by Reference [
22], the reference values for the critical leaf contents for macronutrients in eucalyptus are: N 17.0; P 0.80; K 7.0; Ca 4.8 and Mg 1.6 g kg
−1. Leaf N contents are below the critical level (
Table 6), in both fertilized and unfertilized plots; for P, the contents are adequate; for K, the contents are below the critical level in both plots; for Ca, the contents are above the recommended but higher in the unfertilized plot than in the fertilized plot; and finally Mg, whose contents are adequate for both conditions.
The chemical analysis (
Table 1) shows that the nutrient contents in the soil are all below what is recommended for the crop [
20]. However, in fertilized plots, P, K and Ca were supplied in order to meet the adequate demand of eucalyptus. Nevertheless, it was not enough for N and Mg. In the unfertilized plot, the nutrient contents in the soil and high-forest residues left were not sufficient to meet the demand of eucalyptus sprouts, leading to low contents in the leaves, as well as for the other nutrients that are below the critical content. Another hypothesis is that, as N and K are mobile in the plant, the internal cycling has been intensified, therefore, these nutrients may not reach a critical level in the leaf because they were more intensively transferred to the other plant organs.
The adequate leaf content of P as compared to its critical level in the both, fertilized and unfertilized plots (
Table 6), is due to the supply of P via fertilization, in the first and second rotation (coppicing). Phosphorus fertilizers present longer residual effects than N and K fertilizers [
39].
The critical leaf content of Mg was below the adequate level in the fertilized plot (
Table 6), while K and Ca contents meet sufficient levels in the fertilized plots (
Table 6). The low leaf content of Mg is not expected considering the application of dolomitic limestone (
Table 6). The competition between Ca and Mg for the absorption site, was suggested by Reference [
40] as a possible reason for, high Ca content and low Mg content in eucalyptus seedlings which received different proportions of these nutrients. The study conducted by Reference [
41] shows that a higher Ca:Mg ratio had a negative effect on leaf Mg content, as also observed by References [
42] and [
43]. The high contents of Ca and Mg in the unfertilized plot maybe related to the effect of concentration because the plants grew less in these plots.
The difference between the amount of in the trunk of fertilized and unfertilized trees (
Table 4) and EU at the end of the cycle (
Table 7), as compared with these characteristic for the other nutrients, indicate K as the main nutrient limiting the growth and development of the shoots.
The trunk production of clone GG157 in the first rotation, at 60 months of age, was on average 172.5 m
3 ha
−1 (
Figure 4), while under coppicing, at the same age, it was 222.1 m
3 ha
−1 in the 1H1NP treatment and 285.4 m
3 ha
−1 in the 1H2NP treatment in the plot that received fertilization and 97.2 and 159.0 m
3 ha
−1 in the plot without fertilization in the same treatments, respectively. Tree survival rate was not much different, i.e., 96% and 90% in the 1H1NP treatment and to 93% and 85% in the 1H2NP treatment in plots with and without fertilization, respectively. The corrected production, considering the survival rate, was equal to 213.2 m
3 ha
−1 with fertilization and to 87.5 m
3 ha
−1 without fertilization in the 1H1NP treatment and to 265.5 m
3 ha
−1 with fertilization and to 135.1 m
3 ha
−1 without fertilization in the 1H2NP treatment (
Figure 4). Thus, in coppicing there were production gains of 24 and 54% in the treatments 1H1NP and 1H2NP, in the fertilized plot, respectively and reductions of 49% and 22% in the treatments 1H1NP and 1H2NP, in the unfertilized plot, respectively, when compared to the trunk production of the high forest (
Figure 4). These results confirm those obtained by References [
44] and [
12], for cases in which fertilization is not carried out in the coppice system. Reference [
44] found reduction in trunk yield of 46%, compared to the first rotation, when the shoots were not re-fertilized with K, in a study conducted in Red Yellow Podzolic (Spodosol) with medium/sandy texture. Similar results were obtained by Reference [
12], who reported yield reductions that reached approximately 50% of the initial yield when eucalyptus plots were not re-fertilized with K after the first harvest, in a clay-textured Red Yellow Latosol (Oxisol). In both studies it is concluded that the reduction of could be attributed to low soil K supply and high export of K during high forest harvesting.
According to Reference [
25], in many Brazilian regions there has been a reduction in the yield of forests managed by coppicing, not necessarily due to the decrease in plant population but due to the lower growth, as a result of nutrients limitation in the soil compared to the first rotation. The results obtained in this study confirm and indicate that the yield reduction in the second rotation observed by References [
44] and [
12] are related to the inadequate supply of nutrients, considering the significant gain of yield shown in
Figure 4.
Another important indication obtained in this study is the possibility of earlier harvest in the coppicing system. The trunk production obtained in the second rotation, in fertilized plots, in the treatments 1H1NP and 1H2NP would only be achieved in the first rotation at 68 and 81 months of age, respectively. Thus, depending on the treatment applied in the coppicing, whether 1H1NP or 1H2NP, the forest could be harvested 8 to 21 months earlier. The management of eucalyptus stands by coppicing is justified by the rapid initial growth of shoots, in general, as compared with that of stands established from seedlings. This is mainly due to the presence of an already established root system, which facilitates the absorption of water and nutrients and the use of organic and inorganic reserves, present in the stump or roots [
45,
46].
In the coppicing system, the trees used the nutrients for trunk production more efficiently than in the high-forest system, except for Mg (
Table 7). Higher values of EU for nutrients in the trunk in the coppicing system indicate that higher trunk production can be achieved with relatively smaller amount of nutrients. Higher efficiency in nutrient use may result from genetic control or lower nutrient availability in the soil. The present study does not rule out this second possibility given the export of nutrients in the first harvest. This would reinforce the concern about yield reduction in the next rotation if nutrients are not rationally replaced via fertilizer.