Modeling Climatic Influences on Three Parasitoids of Low-Density Spruce Budworm Populations. Part 1: Tranosema rostrale (Hymenoptera: Ichneumonidae)
Abstract
:1. Introduction
2. Materials and Methods
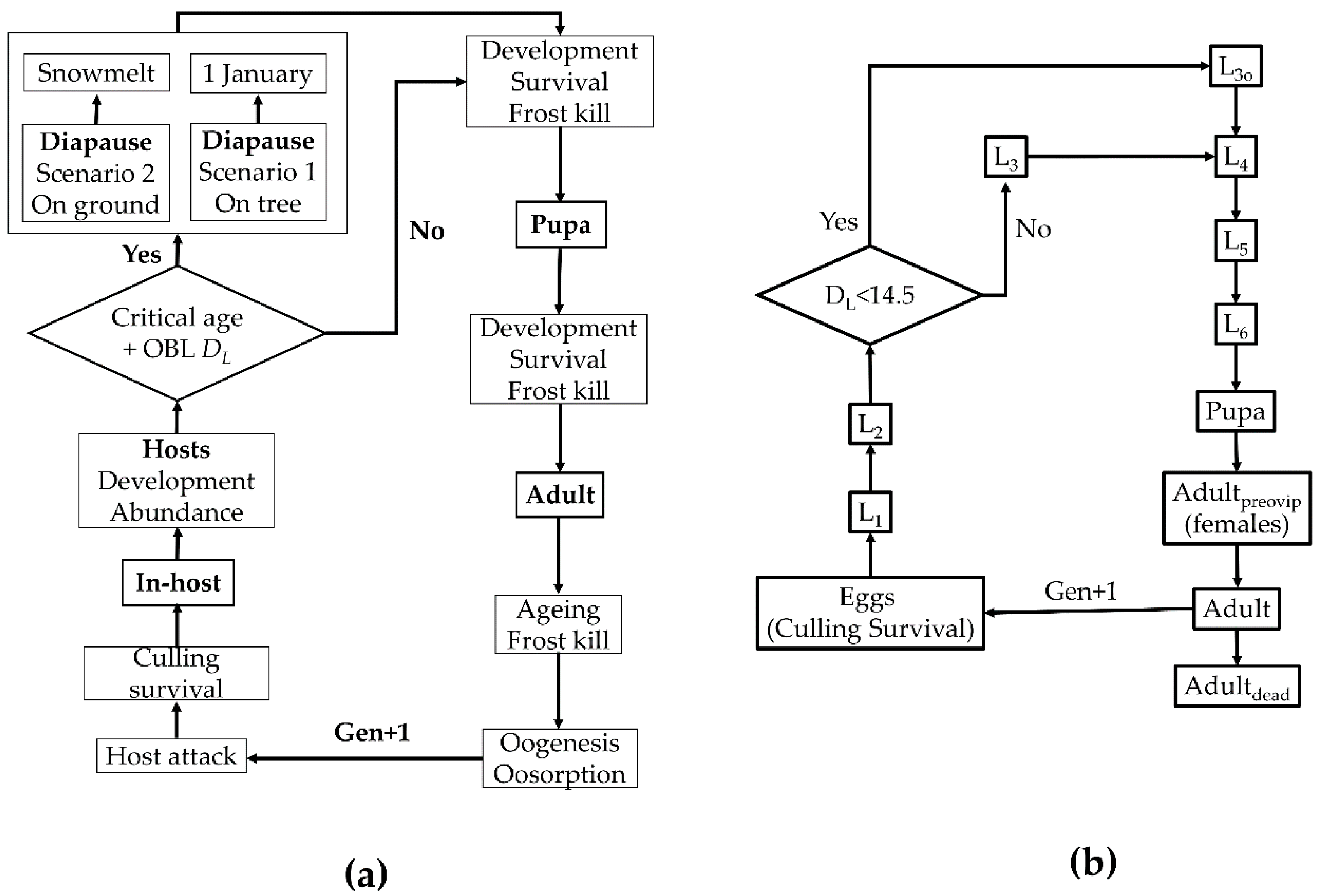
2.1. Modeling the Seasonal Biology of T. rostrale
2.2. Modeling the Seasonal Biology of the Hosts
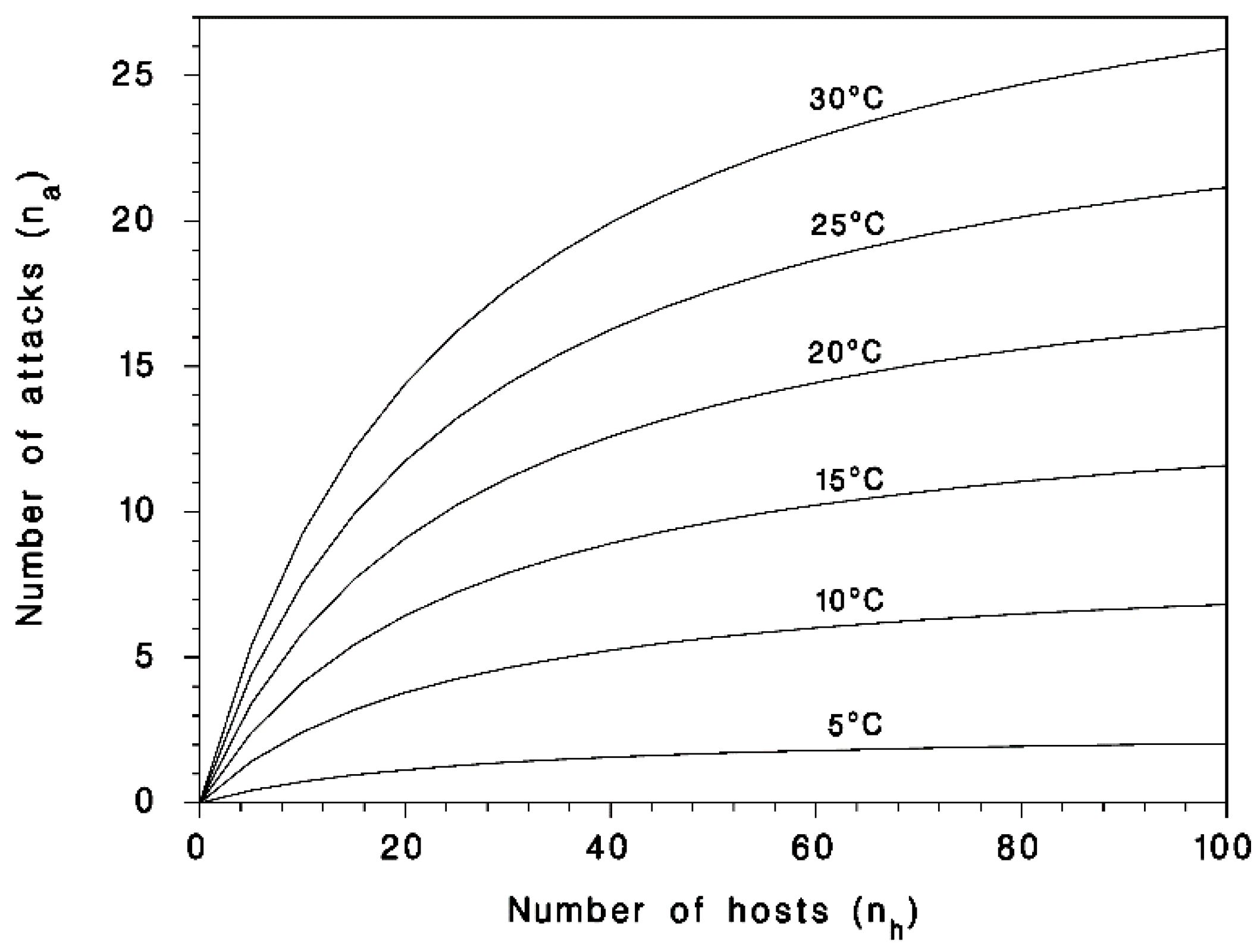
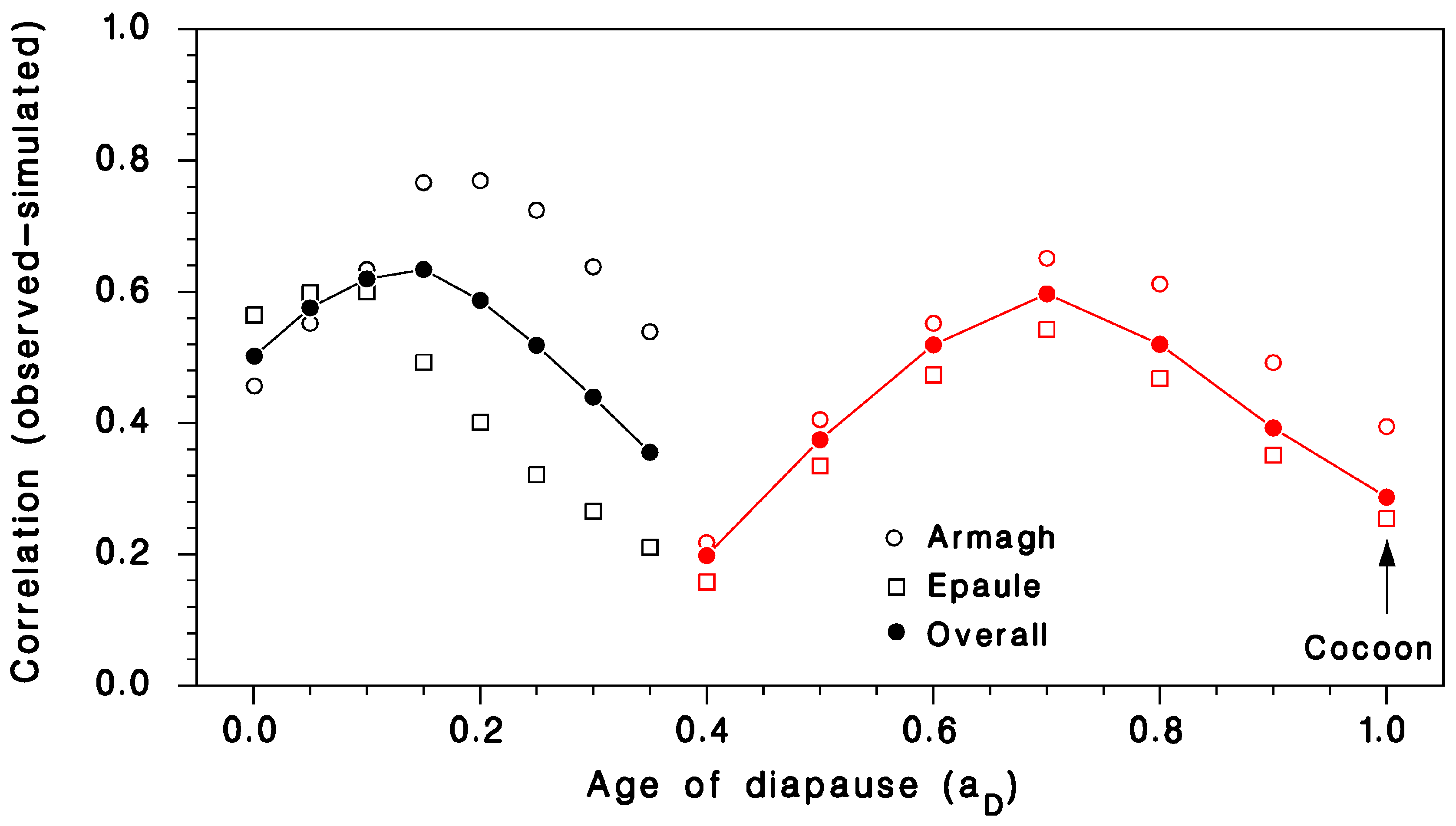
2.3. Model Calibration
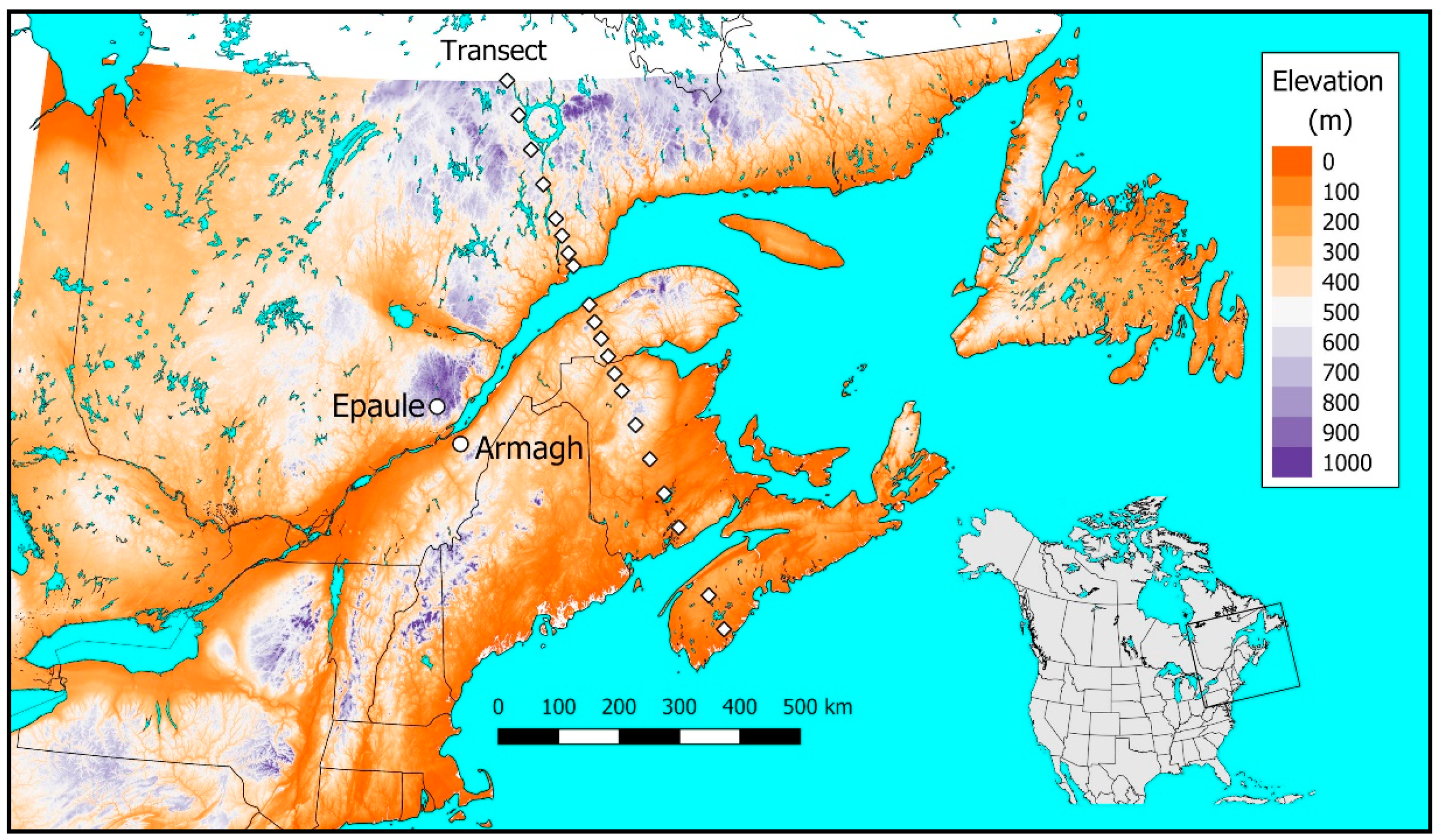
2.4. Tranosema Rostrale Performance over Northeastern North America, Present and Future
3. Results
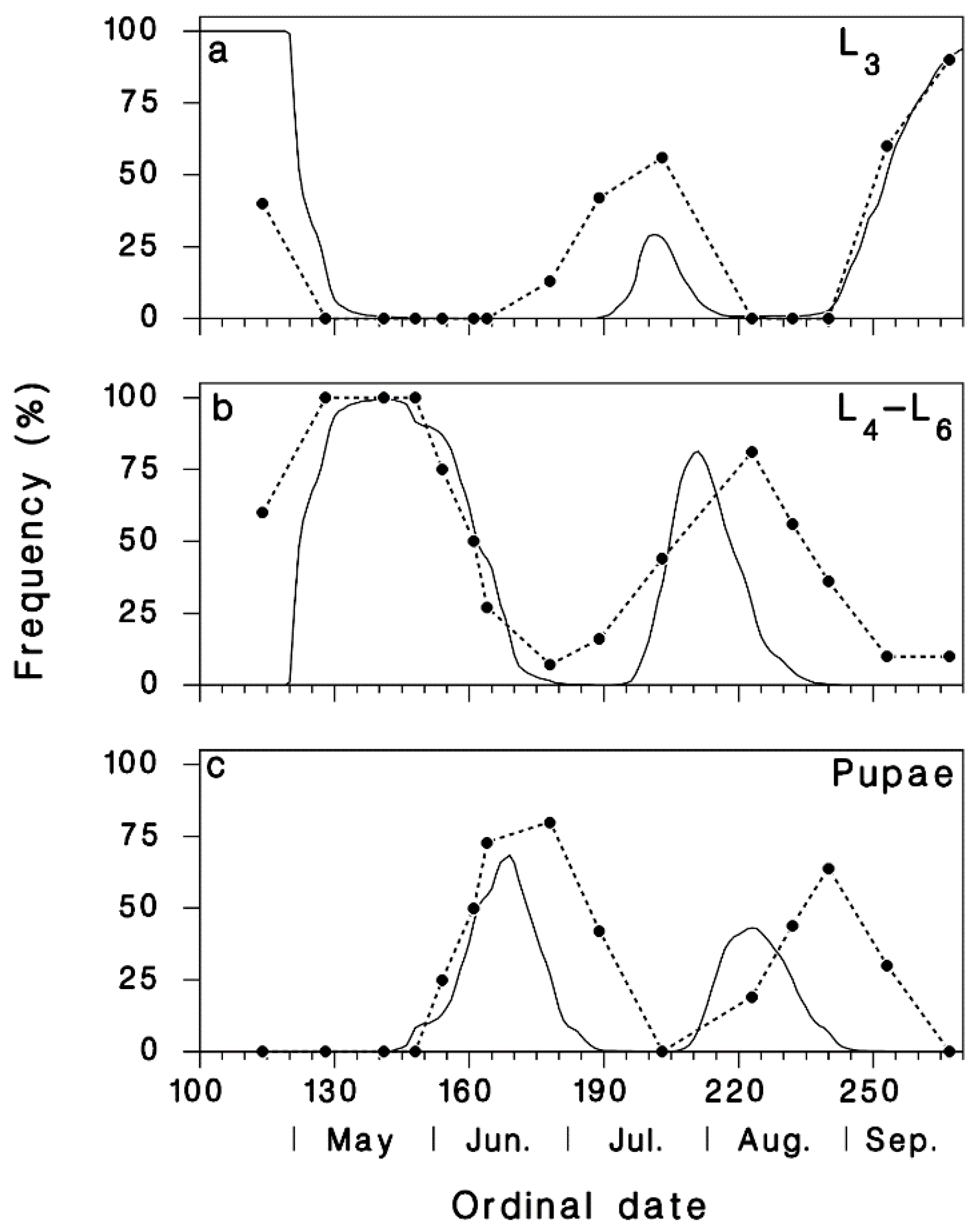
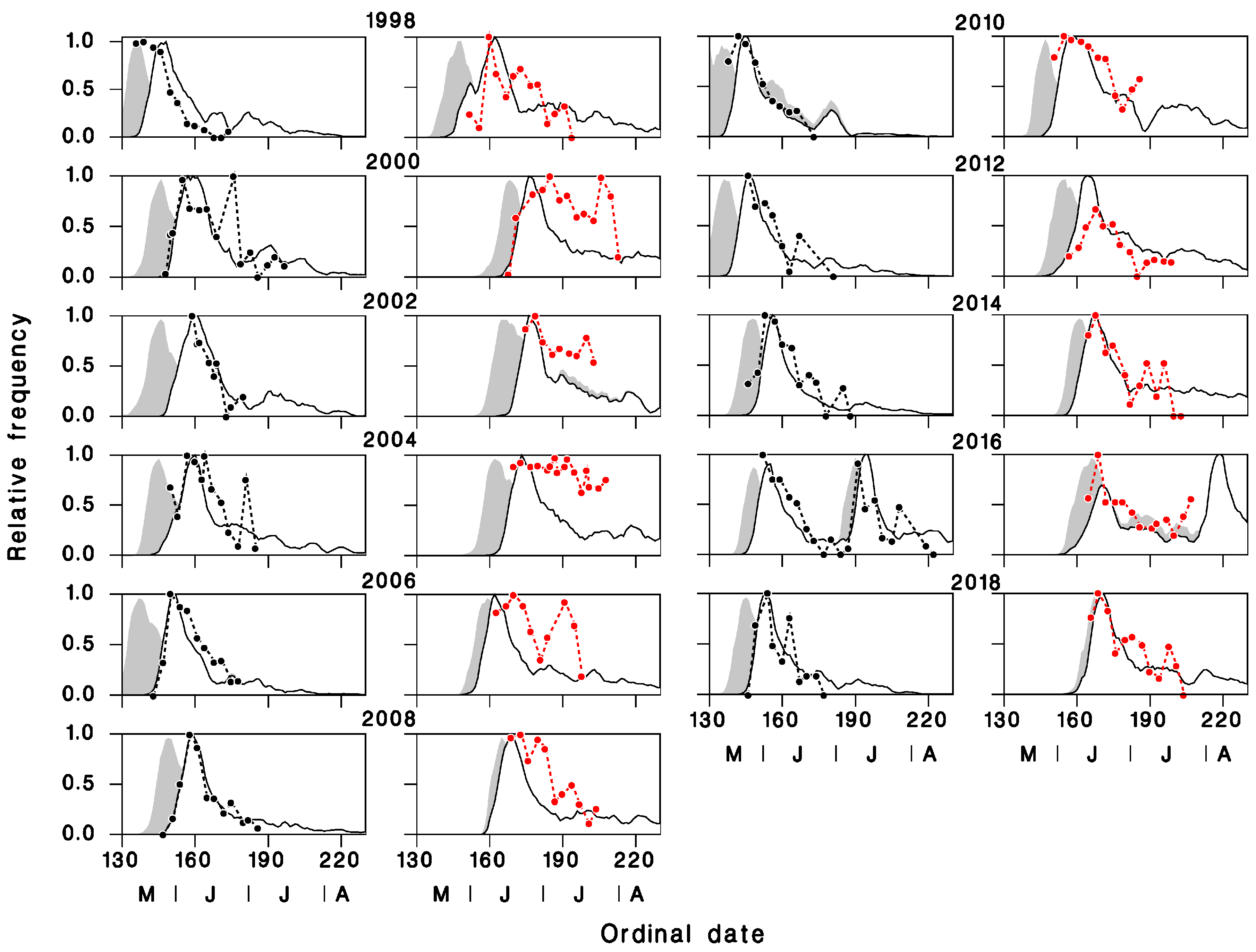
3.1. Validation of Predicted Seasonality of T. rostrale Attack Rates
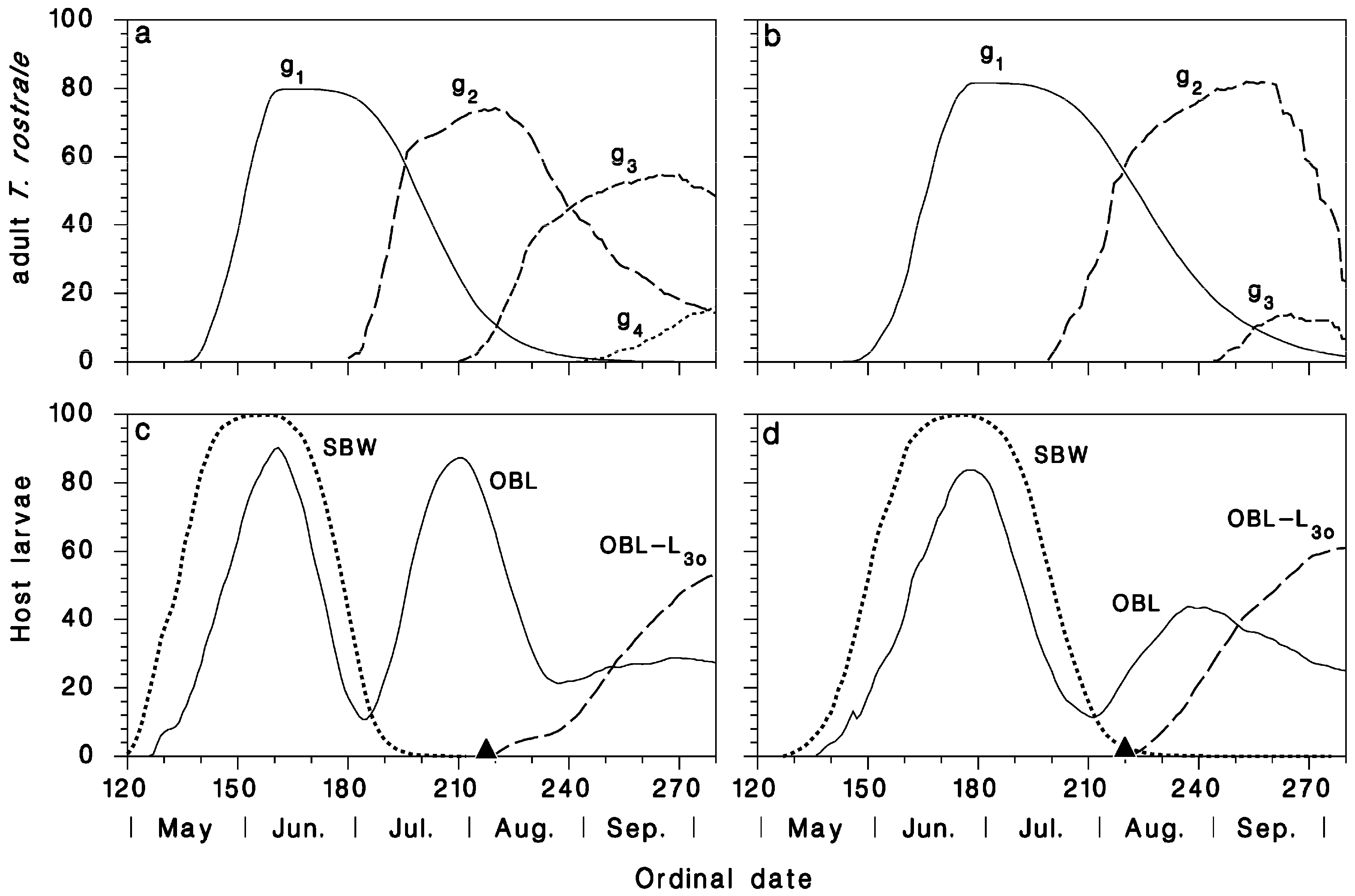
3.2. Seasonal Biology of T. rostrale, SBW, and OBL in Armagh and Epaule
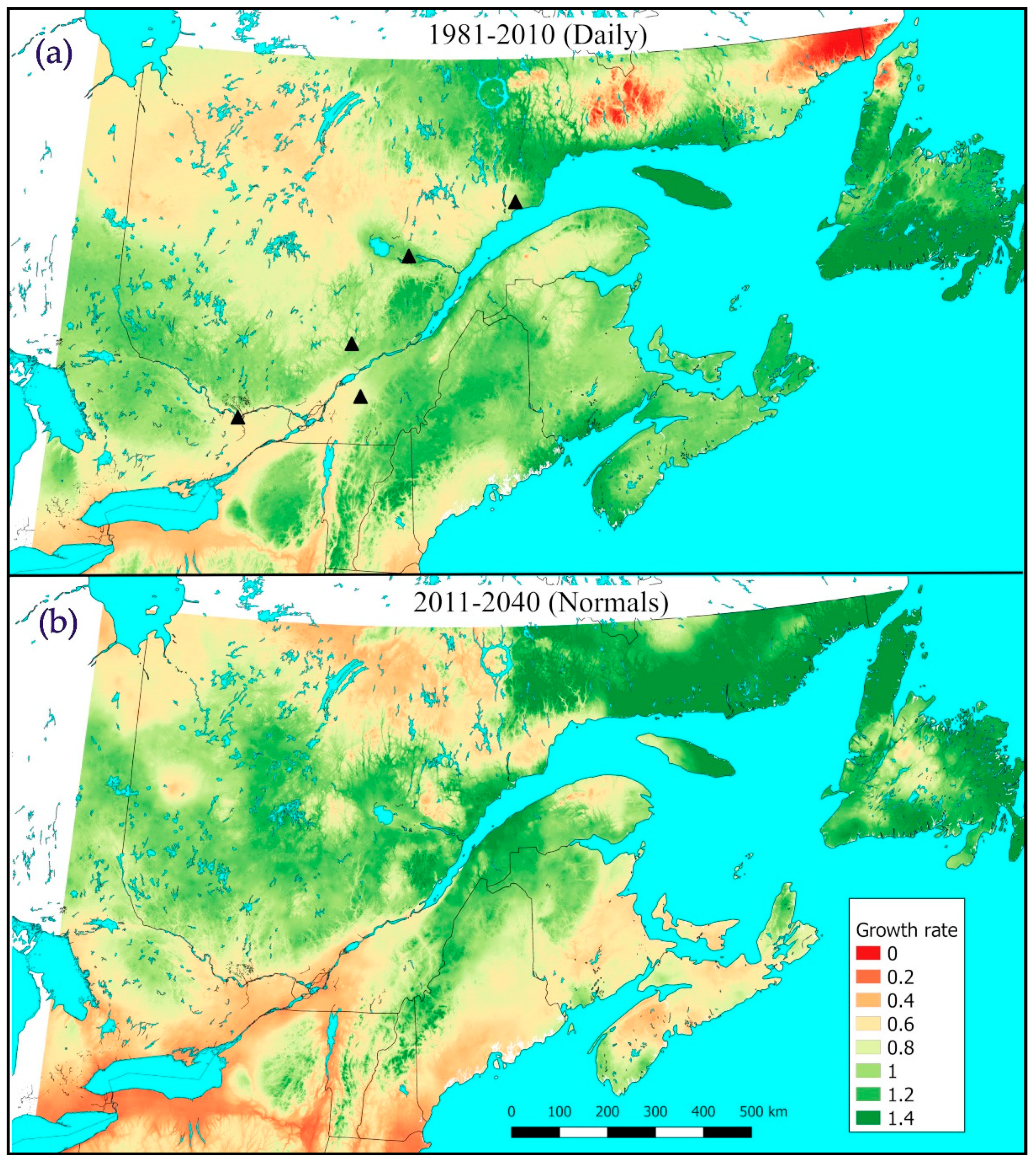
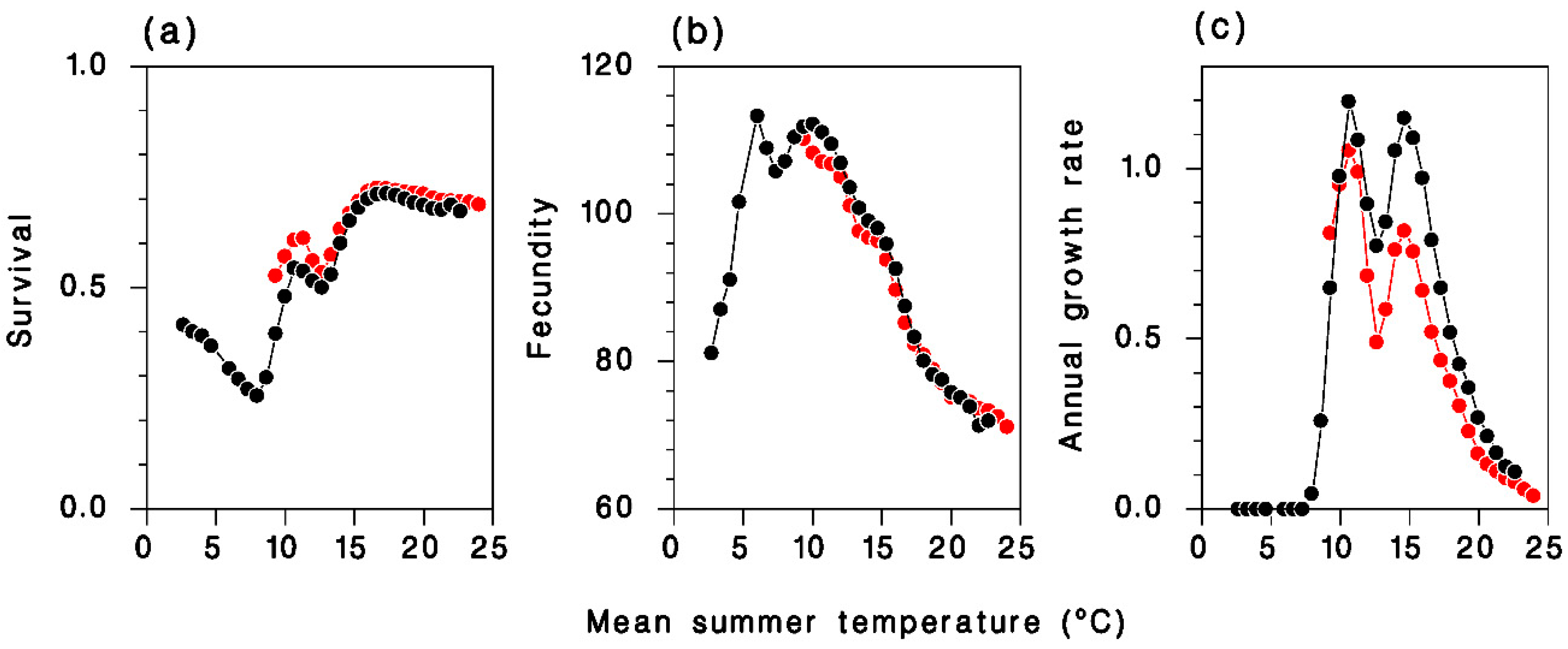
3.3. Tranosema Rostrale Performance over Northeastern North America, Present and Future
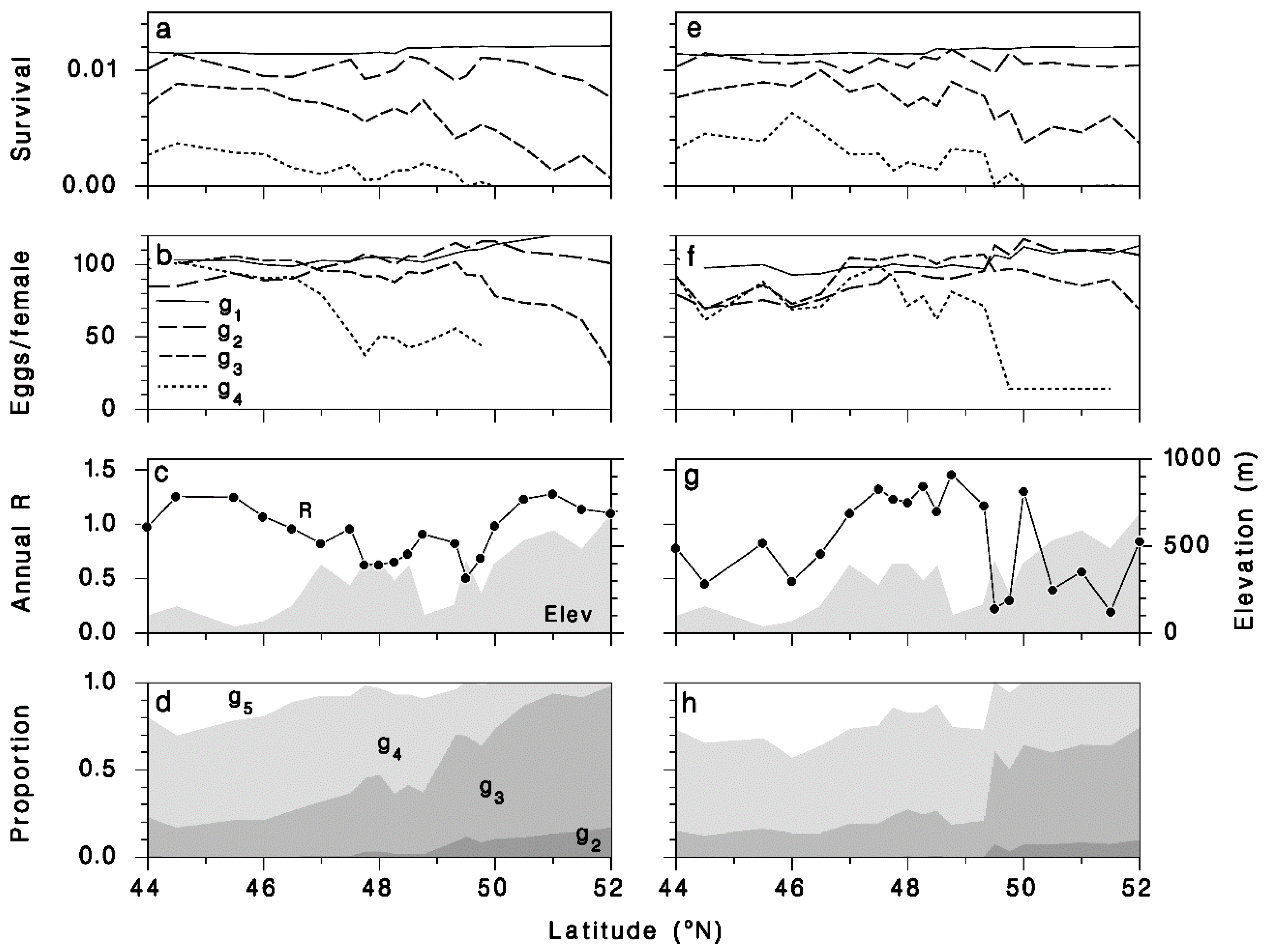
3.4. Performance of T. rostrale Along a North-South Transect
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hawkins, B.A.; Cornell, H.V.; Hochberg, M.E. Predators, parasitoids, and pathogens as mortality agents in phytophagous insect populations. Ecology 1997, 78, 2145–2152. [Google Scholar] [CrossRef]
- LaSalle, J.; Gauld, I.D. Parasitic Hymenoptera and the biodiversity crisis. Redia 1991, 74, 315–334. [Google Scholar]
- Quicke, D.L.J. We know too little about parasitoid wasp distributions to draw any conclusions about latitudinal trends in species richness, body size and biology. PLoS ONE 2012, 7, e32101. [Google Scholar] [CrossRef] [PubMed]
- Eveleigh, E.S.; McCann, K.S.; McCarthy, P.C.; Pollock, S.J.; Lucarotti, C.J.; Morin, B.; McDougall, G.A.; Strongman, D.B.; Huber, J.T.; Umbanhowar, J.; et al. Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proc. Natl. Acad. Sci. USA 2007, 104, 16976–16981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Régnière, J.; Griffiths, K.J. La modélisation en lutte biologique: Un exemple d’utilisation dans l’étude du synchronisme des cycles vitaux d’un parasitoïde et de son hôte. In La Lutte Biologique; Vincent, C., Coderre, D., Eds.; Gaëtan Morin: Boucherville, QC, Canada, 1992; pp. 265–284. [Google Scholar]
- Chuine, I.; Régnière, J. Process-based models of phenology for plants and animals. Ann. Rev. Ecol. Evol. Syst. 2017, 48, 159–182. [Google Scholar] [CrossRef]
- Régnière, J.; St-Amant, R.; Duval, P. Predicting insect distributions under climate change from physiological responses: Spruce budworm as an example. Biol. Invasions 2012, 14, 1571–1586. [Google Scholar] [CrossRef]
- Flinn, P.W.; Hagstrum, D.W.; Muir, W.E.; Sudayappa, K. Spatial model for simulating changes in temperature and insect population dynamics in stored grain. Environ. Entomol. 1992, 21, 1351–1356. [Google Scholar] [CrossRef]
- Drummond, F.A.; Van Driesche, R.G.; Logan, P.A. Model for the temperature-dependent emergence of overwintering Phyllorycter crataegella (Clements) (Lepidoptera: Gracillariidae), and its parasitoid, Sympiesis marylandensis Girault (Hymenoptera: Eulophidae). Environ. Entomol. 1985, 14, 305–311. [Google Scholar] [CrossRef]
- de Souza, A.A.; Martins, S.G.F.; Zacarias, M.S. Computer simulation applied to the biological control of the insect Aphis gossypii for the parasitoid Lysiphlebus testaceipes. Ecol. Model. 2009, 220, 756–763. [Google Scholar] [CrossRef] [Green Version]
- Hance, T.; van Baaren, J.; Vernon, P.; Boivin, G. Impact of extreme temperatures on parasitoids in a climate change perspective. Ann. Rev. Entomol. 2007, 52, 107–126. [Google Scholar] [CrossRef]
- Jeffs, C.T.; Lewis, O.T. Effects of climate warming on host-parasitoid interactions. Ecol. Entomol. 2013, 38, 209–218. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Voigt, W.; Perner, J.; Davis, A.J.; Eggers, T.; Schumacher, J.; Bährmann, R.; Fabian, B.; Heinrich, W.; Köhler, G.; Lichter, D.; et al. Trophic levels are differentially sensitive to climate. Ecology 2003, 84, 2444–2453. [Google Scholar] [CrossRef] [Green Version]
- Harvey, J.A. Conserving host–parasitoid interactions in a warming world. Curr. Opin. Insect Sci. 2015, 12, 79–85. [Google Scholar] [CrossRef]
- Stireman, J.O.; Dyer, L.A.; Janzen, D.H.; Singer, M.S.; Lill, J.T.; Marquis, R.J.; Ricklefs, R.E.; Gentry, G.L.; Hallwachs, W.; Coley, P.D.; et al. Climatic unpredictability and parasitism of caterpillars: Implications of global warming. Proc. Natl. Acad. Sci. USA 2005, 102, 17384–17387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tougeron, K.; Le Lann, C.; Brodeur, J.; Van Baaren, J. Are aphid parasitoids from mild winter climates losing their winter diapause? Oecologia 2017, 183, 619–629. [Google Scholar] [CrossRef] [Green Version]
- Senior, V.L.; Evans, L.C.; Leather, S.R.; Oliver, T.H.; Evans, K.L. Phenological responses in a sycamore–aphid–parasitoid system and consequences for aphid population dynamics: A 20 year case study. Glob. Chang. Biol. 2020, 26, 2814–2828. [Google Scholar] [CrossRef]
- Van Nouhuys, S.; Lei, G. Parasitoid–host metapopulation dynamics: The causes and consequences of phenological asynchrony. J. Anim. Ecol. 2004, 73, 526–535. [Google Scholar] [CrossRef]
- Nealis, V.G. Comparative ecology of conifer-feeding spruce budworms (Lepidoptera: Tortricidae). Can. Entomol. 2016, 148 (Suppl. 1), S33–S57. [Google Scholar] [CrossRef] [Green Version]
- Royama, T.; Eveleigh, E.S.; Morin, J.R.B.; Pollock, S.J.; McCarthy, P.C.; McDougall, G.A.; Lucarotti, C.J. Mechanisms underlying spruce budworm outbreak processes as elucidated by a 14-year study in New Brunswick, Canada. Ecol. Monogr. 2017, 87, 600–631. [Google Scholar] [CrossRef]
- Bouchard, M.; Martel, V.; Régnière, J.; Therrien, P.; Correia, D.L.P. Do natural enemies explain fluctuations in low-density spruce budworm populations? Ecology 2018, 99, 2047–2057. [Google Scholar] [CrossRef]
- Régnière, J.; Cooke, B.; Béchard, A.; Dupont, A.; Therrien, P. Dynamics and management of rising outbreak spruce budworm populations. Forests 2019, 10, 748. [Google Scholar] [CrossRef] [Green Version]
- Cusson, M.; Barron, J.R.; Goulet, H.; Régnière, J.; Doucet, D. Biology and status of Tranosema rostrale rostrale (Hymenoptera: Ichneumonidae), a parasitoid of the eastern spruce budworm (Lepidoptera: Tortricidae). Ann. Entomol. Soc. Am. 1998, 91, 87–93. [Google Scholar] [CrossRef]
- Fidgen, J.G.; Eveleigh, E.S. Life history characteristics of Elachertus cacoeciae (Hymenoptera: Eulophidae), an ectoparasitoid of spruce budworm larvae, Choristoneura fumiferana (Lepidoptera: Tortricidae). Can. Entomol. 1998, 130, 215–229. [Google Scholar] [CrossRef]
- Shaw, M.R. Anatomy, reach and classification of the parasitoid complex of a common British moth, Anthophila fabriciana (L.) (Choreutidae). J. Nat. Hist. 2017, 51, 1119–1149. [Google Scholar] [CrossRef]
- Seehausen, M.L. Life-History traits and temperature-dependent performance of Tranosema rostrale (Hym.: Ichneumonidae), a parasitoid of low-density spruce budworm (Lepidoptera: Tortricidae) populations. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2017. [Google Scholar]
- Eveleigh, E.S.; Royama, T.; Lucarotti, C.J.; McCarthy, P.C.; Morin, B.; Pollock, S. Endemic spruce budworm populations in New Brunswick. In Proceedings of the 17th Eastern Spruce Budworm Research Work Conference, Quebec, QC, Canada, 2–3 April 1997; Delisle, J., Régnière, J., Eds.; Info. Rep. LAU-X-113B. Canadian Forest Service, Laurentian Forestry Centre: Quebec, QC, Canada, 1997. [Google Scholar]
- Miller, C.A. Parasites and the spruce budworm. Mem. Entomol. Soc. Can. 1963, 95, 228–244. [Google Scholar] [CrossRef]
- Miller, C.A.; Renault, T.R. Incidence of parasitoids attacking endemic spruce budworm (Lepidoptera: Tortricidae) populations in New Brunswick. Can. Entomol. 1976, 108, 1045–1052. [Google Scholar] [CrossRef]
- Seehausen, M.L.; Labrecque, M.; Martel, V.; Régnière, J.; Mansour, A.; Smith, S.M. Reproductive biology and behavior of Tranosema rostrale (Hymenoptera: Ichneumonidae), a parasitoid of low-density spruce budworm (Lepidoptera: Tortricidae) populations. J. Insect Behav. 2016, 29, 500–514. [Google Scholar] [CrossRef]
- Seehausen, M.L.; Régnière, J.; Martel, V.; Smith, S.M. Seasonal parasitism and host instar preference by the spruce budworm (Lepidoptera: Tortricidae) larval parasitoid Tranosema rostrale (Hymenoptera: Ichneumonidae). Environ. Entomol. 2016, 45, 1123–1130. [Google Scholar] [CrossRef]
- Shaw, M.R.; Horstmann, K.; Whiffin, A.L. Two hundred and twenty-five species of reared western Palaearctic Campopleginae (Hymenoptera: Ichneumonidae) in the National Museums of Scotland, with descriptions of new species of Campoplex and Diadegma, and records of fifty-five species new to Britain. Entomol. Gaz. 2016, 66, 245–247. [Google Scholar]
- Gangavalli, R.R.; Aliniazee, T. Diapause induction in the oblique-banded leafroller Choristoneura rosaceana (Lepidoptera: Tortricidae): Role of photoperiod and temperature. J. Insect Phys. 1985, 31, 831–835. [Google Scholar] [CrossRef]
- Seehausen, M.L.; Régnière, J.; Martel, V.; Smith, S.M. Developmental and reproductive responses of the spruce budworm (Lepidoptera: Tortricidae) parasitoid Tranosema rostrale (Hymenoptera: Ichneumonidae) to temperature. J. Insect Physiol. 2017, 98, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Gangavalli, R.R.; Aliniazee, T. Temperature requirements for development of the obliquebanded leafroller, Choristoneura rosaceana (Lepidoptera: Tortricidae). Environ. Entomol. 1985, 14, 17–19. [Google Scholar] [CrossRef]
- Jones, V.P.; Doerr, M.D.; Brunner, J.F.; Baker, C.C.; Wilburn, T.D.; Wiman, N.G. A synthesis of the temperature-dependent development rate of the obliquebanded leafroller, Choristoneura rosaceana. J. Insect Sci. 2005, 5, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliniazee, M.T. Seasonal history, adult flight activity, and damage of the obliquebanded leafroller, Choristoneura rosaceana (Lepidoptera: Torticidae), in filbert orchards. Can. Entomol. 1986, 118, 353–361. [Google Scholar] [CrossRef]
- Lethiecq, J.L.; Régnière, J. CFS Spruce Budworm Population Studies: Sites Descriptions; Info. Rep. LAU-X-83; Canadian Forest Service, Laurentian Forestry Centre: Quebec, QC, Canada, 1988.
- Seehausen, M.L.; Régnière, J.; Bauce, E. Does spruce budworm (Lepidoptera: Tortricidae) rearing diet influence larval parasitism? Can. Entomol. 2013, 345, 539–542. [Google Scholar] [CrossRef]
- Roe, A.R.; Demidovich, M.; Dedes, J. Origins and history of laboratory insect stocks in a multispecies insect production facility, with the proposal of standardized nomenclature and designation of formal standard names. J. Insect Sci. 2018, 18, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Holling, C.S. The functional response of invertebrate predators to prey density. Mem. Entomol. Soc. Can. 1966, 48, 1–88. [Google Scholar] [CrossRef]
- Miller, C.A. The measurement of spruce budworm populations and mortality during the first and second larval instars. Can. J. Zool. 1958, 36, 409–422. [Google Scholar] [CrossRef]
- Tougeron, K.; Brodeur, J.; Le Lann, C.; van Baaren, J. How climate change affects the seasonal ecology of insect parasitoids. Ecol. Entomol. 2020, 45, 167–181. [Google Scholar] [CrossRef] [Green Version]
- Régnière, J.; Powell, J.A. Animal life cycle models (poikilotherms). In Phenology: An Integrative Environmental Science, 2nd ed.; Schwartz, M., Ed.; Springer: New York, NY, USA, 2013; pp. 295–316. [Google Scholar] [CrossRef]
- Régnière, J.; St-Amant, R.; Béchard, A. BioSIM 10—User’s Manual; Info. Rep. LAU-X-155; Canadian Forest Service, Laurentian Forestry Centre: Quebec, QC, Canada, 2014.
- Régnière, J.; Bolstad, P. Statistical simulation of daily air temperature patterns in eastern North America to forecast seasonal events in insect pest management. Environ. Entomol. 1994, 23, 1368–1380. [Google Scholar] [CrossRef]
- Régnière, J. Generalized approach to landscape-wide seasonal forecasting with temperature-driven simulation models. Environ. Entomol. 1996, 25, 869–881. [Google Scholar] [CrossRef]
- Brown, R.D.; Brasnett, B.; Robinson, D. Gridded North American snow depth and snow water equivalent for GCM evaluation. Atmos. Ocean 2003, 41, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Régnière, J.; St-Amant, R. Stochastic simulation of daily air temperature and precipitation from monthly normals in North America north of Mexico. Int. J. Biometeorol. 2007, 51, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Scinocca, J.F.; Kharin, V.V.; Jiao, Y.; Qian, M.W.; Lazare, M.; Solheim, L.; Flato, G.M. Coordinated global and regional climate modeling. J. Clim. 2016, 29, 17–35. [Google Scholar] [CrossRef]
- Arora, V.K.; Scinocca, J.F.; Boer, G.J.; Christian, J.R.; Denman, K.L.; Flato, G.M.; Kharin, V.V.; Lee, W.G.; Merryfield, W.J. Carbon emission limits required to satisfy future representative concentration pathways of greenhouse gases. Geophys. Res. Lett. 2011, 38, L05805. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; International Panel on Climate Change: Geneva, Switzerland, 2014; p. 151. Available online: https://www.ipcc.ch/report/ar5/syr (accessed on 3 August 2020).
- Canadian Centre for Climate Modelling and Analysis (CCCma) Climate Model Data, CanESM2/CGCM4 Model Output, 2018. Available online: http://climate-modelling.canada.ca/climatemodeldata/cgcm4/CanESM2/rcp45/ (accessed on 3 August 2020).
- Liebhold, A.M.; Rossi, R.E.; Kemp, W.P. Geostatistics and geographic information systems in applied insect ecology. Ann. Rev. Entomol. 1993, 38, 303–327. [Google Scholar] [CrossRef]
- Cusson, M.; Laforge, L.; Régnière, J.; Béliveau, C.; Trudel, D.; Thireau, J.-C.; Bellemare, G.; Keirstead, N.; Stoltz, D. Multiparasitism of Choristoneura fumiferana by the ichneumonid Tranosema rostrale and the tachinid Actia interrupta: Occurrence in the field and outcome of competition under laboratory conditions. Entomol. Exp. Appl. 2002, 102, 125–133. [Google Scholar] [CrossRef]
- Weisser, W.W.; Volkl, W.; Hassell, M.P. The importance of adverse weather conditions for behaviour and population ecology of an aphid parasitoid. J. Anim. Ecol. 1997, 66, 386–400. [Google Scholar] [CrossRef]
- Seehausen, M.L.; Cusson, M.; Régnière, J.; Bory, M.; Stewart, D.; Djoumad, A.; Smith, S.M.; Martel, V. High temperature induces downregulation of polydnavirus gene transcription in lepidopteran host and enhances accumulation of host immunity transcripts. J. Insect Physiol. 2017, 98, 126–133. [Google Scholar] [CrossRef]
- Seehausen, M.L.; Naumann, P.H.; Béliveau, C.; Martel, V.; Cusson, M. Impact of rearing temperature on encapsulation and the accumulation of transcripts putatively involved in capsule formation in a parasitized lepidopteran host. J. Insect Physiol. 2018, 107, 244–249. [Google Scholar] [CrossRef]
- Bouchard, M.; Auger, I. Influence of environmental factors and spatio-temporal covariates during the initial development of a spruce budworm outbreak. Landsc. Ecol. 2014, 29, 111–126. [Google Scholar] [CrossRef]
- Bouchard, M.; Régnière, J.; Therrien, P. Bottom-up factors contribute to large-scale synchrony in spruce budworm populations. Can. J. For. Res. 2017, 48, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Régnière, J.; Nealis, V.G. Density dependence of egg recruitment and moth dispersal in spruce budworms. Forests 2019, 10, 706. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.W.; Liebhold, A.M. Latitudinal shifts in spruce budworm (Lepidoptera: Tortricidae) outbreaks and spruce-fir forest distributions with climate change. Acta Phytopathol. Entomol. Hung. 1997, 32, 205–215. [Google Scholar]
- Candau, J.-N.; Fleming, R.A. Forecasting the response of spruce budworm defoliation to climate change in Ontario. Can. J. For. Res. 2011, 41, 1948–1960. [Google Scholar] [CrossRef]
- Gray, D.R. The influence of forest composition and climate on outbreak characteristics of the spruce budworm in eastern Canada. Can. J. For. Res. 2013, 43, 1181–1195. [Google Scholar] [CrossRef]
- Fleming, R.A.; Volney, W.J.A. Effects of climate change on insect defoliator population processes in Canada’s boreal forest: Some plausible scenarios. Water Air Soil Pollut. 1995, 82, 445–454. [Google Scholar] [CrossRef]
- Régnière, J.; Powell, J.; Bentz, B.; Nealis, V.G. Effects of temperature on development, survival and reproduction of insects: Experimental design, data analysis and modeling. J. Insect Physiol. 2012, 58, 634–647. [Google Scholar] [CrossRef]
- Fleming, R.A. A mechanistic perspective of possible influences of climate change on defoliating insects in North America’s boreal forests. Silva Fenn. 1996, 30, 281–294. [Google Scholar] [CrossRef] [Green Version]
- Klapwijk, M.J.; Gröbler, B.C.; Ward, K.; Wheeler, D.; Lewis, O.T. Influence of experimental warming and shading on host–parasitoid synchrony. Glob. Chang. Biol. 2010, 16, 102–112. [Google Scholar] [CrossRef]
- Johnston, A.S.A.; Boyd, R.J.; Watson, J.W.; Paul, A.; Evans, L.C.; Gardner, E.L.; Boult, V.L. Predicting population responses to environmental change from indivual-level mechanisms: Towards a standardized mechanistic approach. R. Soc. Proc. B 2019, 286, 20191916. [Google Scholar] [CrossRef] [Green Version]
- Maltais, J.; Régnière, J.; Cloutier, C.; Hébert, C.; Perry, D.F. Seasonal biology of Meteorus trachynotus Vier. (Hymenoptera: Braconidae) and of its overwintering host Choristoneura rosaceana (Harr.)(Lepidoptera: Tortricidae). Can. Entomol. 1989, 121, 745–756. [Google Scholar] [CrossRef]
- Johns, C.R.; Bowden, J.B.; Carleton, R.D.; Cooke, B.J.; Edwards, S.; Emilson, E.J.S.; James, P.M.A.; Kneeshaw, D.; MacLean, D.A.; Martel, V.; et al. A conceptual framework for the spruce budworm early intervention strategy: Can outbreaks be stopped? Forests 2019, 10, 910. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, O.J.; Barton, B.T. Climate change effects on behavioral and physiological ecology of predator-prey interactions: Implications for conservation biological control. Biol. Control 2014, 75, 87–96. [Google Scholar] [CrossRef]
- Gherman, A.-L.M.; Hall, R.J.; Byers, J.E. Host and parasite thermal ecology jointly determine the effect of climate warming on epidemic dynamics. Proc. Natl. Acad. Sci. USA 2018, 115, 744–749. [Google Scholar] [CrossRef] [Green Version]
- Durant, J.M.; Molinera, J.-C.; Ottersen, G.; Reygondeau, G.; Stige, L.C.; Langangen, O. Contrasting effects of rising temperatures on trophic interactions in marine ecosystems. Sci. Rep. 2019, 9, 15213. [Google Scholar] [CrossRef] [Green Version]
- Daugaard, U.; Petchey, O.L.; Pennekamp, F. Warming can destabilize predator-prey interactions by shifting the functional response from Type III to Type II. J. Anim. Ecol. 2019, 88, 1575–1586. [Google Scholar] [CrossRef]
| Life Stage | a | b | TL = −a/b |
|---|---|---|---|
| EGG † | −0.0833 | 0.008744 | 9.5 |
| L1 ‡ | −0.14279 | 0.013027 | 11 |
| L2 | −0.18377 | 0.018627 | 9.9 |
| L3 | −0.17239 | 0.0173625 | 9.9 |
| L3D | −0.11872 | 0.01069 | 11.1 |
| L4 | −0.08368 | 0.011875 | 7.1 |
| L5 | −0.12157 | 0.0138175 | 8.8 |
| L6 (males) | −0.12648 × 1.23 | 0.011125 × 1.23 | 11.4 |
| L6 (females) | −0.12648 × 0.84 | 0.011125 × 0.84 | 11.4 |
| PUPA † | −0.07901 | 0.008305 | 9.6 |
| ADULT_PREOVIP | −0.33918 | 0.028427 | 11.9 |
| ADULT * | −0.02667 | 0.0064 | 4.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Régnière, J.; Seehausen, M.L.; Martel, V. Modeling Climatic Influences on Three Parasitoids of Low-Density Spruce Budworm Populations. Part 1: Tranosema rostrale (Hymenoptera: Ichneumonidae). Forests 2020, 11, 846. https://doi.org/10.3390/f11080846
Régnière J, Seehausen ML, Martel V. Modeling Climatic Influences on Three Parasitoids of Low-Density Spruce Budworm Populations. Part 1: Tranosema rostrale (Hymenoptera: Ichneumonidae). Forests. 2020; 11(8):846. https://doi.org/10.3390/f11080846
Chicago/Turabian StyleRégnière, Jacques, M. Lukas Seehausen, and Véronique Martel. 2020. "Modeling Climatic Influences on Three Parasitoids of Low-Density Spruce Budworm Populations. Part 1: Tranosema rostrale (Hymenoptera: Ichneumonidae)" Forests 11, no. 8: 846. https://doi.org/10.3390/f11080846
APA StyleRégnière, J., Seehausen, M. L., & Martel, V. (2020). Modeling Climatic Influences on Three Parasitoids of Low-Density Spruce Budworm Populations. Part 1: Tranosema rostrale (Hymenoptera: Ichneumonidae). Forests, 11(8), 846. https://doi.org/10.3390/f11080846





