The Use of Wood Chips for Revitalization of Degraded Forest Soil on Young Scots Pine Plantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Experiment
2.2. Scots Pine Growth Measurement
2.3. Acarological Research
2.4. Statistical Analysis
3. Results
3.1. Scots Pine Growth Characteristics
3.2. Acarological Research
4. Discussion
4.1. Scots Pine Growth Characteristics
4.2. Acarological Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Note
References
- CSO. Statistical Information and Elaborations: Environment; Central Statistical Office Regional and Environmental Surveys Department: Warszawa, Poland, 2017; p. 122. [Google Scholar]
- Koreleski, K. Ecological, legal and planning problems of afforestation in the rural areas. Inż. Roln. 2003, 3, 251–260. [Google Scholar]
- Milewski, W. Lasy w Polsce [Forests in Poland]; CILP: Warszawa, Poland, 2017. [Google Scholar]
- Nakicenovic, N. Emissions Scenarios. In The Intergovernmental Panel on Climate Change (IPCC) Special Report on Emissions Scenarios; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Lindberg, N.; Bengtsson, J. Population responses of oribatid mites and collembolans after drought. Appl. Soil. Ecol. 2005, 28, 163–174. [Google Scholar] [CrossRef]
- Gulvik, M.E. Mites (Acari) as indicators of soil biodiversity and land use monitoring: A review. Pol. J. Ecol. 2007, 55, 415–440. [Google Scholar]
- Brzezińska, M. Aktywność biologiczna oraz procesy jej towarzyszące w glebach organicznych nawadnianych oczyszczonymi ściekami miejskimi (badania polowe i modelowe) [Biological activity and associated processes in organic soils irrigated with treated municipal wastewater (field and model studies)]. Acta Agrophys. 2006, 131, 164. [Google Scholar]
- Olszowska, G.; Zwoliński, J.; Matuszczyk, I.; Syrek, D.; Zwolińska, B.; Pawlak, U.; Kwapis, Z.; Dudzińska, M. Wykorzystanie badań aktywności biologicznej do wyznaczenia wskaźnika żyzności gleb w drzewostanach sosnowych na siedliskach boru świeżego i boru mieszanego świeżego [The use of biological activity for testing the soil fertility index in pine stands on fresh and fresh mixed forest habitats]. Leśne Prace Badawcze 2005, 3, 17–37. [Google Scholar]
- Axelsson, B.; Lohm, U.; Lundkvist, H.; Persson, T.; Sköglund, J.; Wiren, A. Effects of nitrogen fertilization on the abundance of soil fauna populations in a Scots pine stand. Res. Notes R. Coll. For. 1973, 14, 5–10. [Google Scholar]
- Seniczak, S.; Bukowski, G.; Seniczak, A. Mechowce (Acari, Oribatida) glebowe strefy ekotonowej pomiedzy borem sosnowym a brzegiem Jeziora Lobeliowego Wielkie Gacno w Borach Tucholskich [Oribatid mites (Acari, Oribatida) of ecotone between scots pine forest and Lobelias Lake Wielkie Gacno in Tuchola Forest]. Zesz. Nauk. Zootechnika 2006, 248, 39–44. [Google Scholar]
- Ruf, A.; Beck, L. The use of predatory soil mites in ecological soil classification and assessment concepts, with perspectives for oribatid mites. Ecotox. Environ. Safe. 2005, 62, 290–299. [Google Scholar] [CrossRef]
- Behan-Pelletier, V.M. Oribatid mite biodiversity in agroecosystems: Role of bioindication. Agric. Ecosyst. Environ. 1999, 74, 411–423. [Google Scholar] [CrossRef]
- Behan-Pelletier, V.M. Acari and Collembola biodiversity in Canadian agricultural soils. Can. J. Soil Sci. 2003, 83, 279–288. [Google Scholar] [CrossRef]
- Luxton, M. Studies on the oribatid mites of a Danish beech wood soil. I. Nutritional biology. Pedobiologia 1972, 12, 434–463. [Google Scholar]
- Ponge, I.F. Succession of fungi and fauna during decomposition of needles in a small area of Scots pine litter. Plant. Soil 1991, 138, 99–113. [Google Scholar] [CrossRef]
- Renker, C.; Otto, P.; Schneider, K.; Zimdars, B.; Maraun, M.; Buscot, F. Oribatid Mites as Potential Vectors for Soil Microfungi: Study of Mite-Associated Fungal Species. Microb. Ecol. 2005, 50, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Renker, C.; Maraun, M. Oribatid mite (Acari, Oribatida) feeding on ectomycorrhizal fungi. Mycorrhiza 2005, 16, 67–72. [Google Scholar] [CrossRef]
- Remén, C.; Fransson, P.; Persson, T. Population responses of oribatids and enchytraeids to ectomycorrhizal and saprotrophic fungi in plantesoil microcosms. Soil Biol. Biochem. 2010, 42, 978–985. [Google Scholar] [CrossRef]
- Schneider, K.; Renker, C.; Scheu, S.; Maraun, M. Feeding biology of oribatid mites: A minireview. Phytophaga 2004, XIV, 247–256. [Google Scholar]
- Werner, M.R.; Dindal, D.L. Effects of conversion to organic practices agricultural on soil biota. Am. J. Alternative Agr. 1990, 5, 24–32. [Google Scholar] [CrossRef]
- Hilszczańska, D. Mycorrhizal development and growth of inoculated Scot pine seedlings in different soil moisture conditions in the glasshouse. Sylwan 2001, 7, 89. [Google Scholar]
- Kropp, B.R.; Langloi, S.G.C. Ectomycorrhiza in reforestation. Can. J. For. Res. 1990, 20, 438. [Google Scholar] [CrossRef]
- Marx, D.H. Ectomycorrhizal fungus inoculation: A tool for improving forestation practices. In Tropical Mycorrhiza Research; Mikola, P., Ed.; Oxford University Press: New York, NY, USA, 1980; pp. 13–71. [Google Scholar]
- Molina, R.; Trappe, J.P. Mycorrhiza management in bare-root nurseries. In Forest Nursery Manual Production of Bareroot Seedlings; Duryea, M., Landis, T.D., Eds.; Nijhoff/Junk: The Hague, The Netherlands, 1984; pp. 211–223. [Google Scholar]
- Klimek, A.; Chachaj, B. Comparison of seasonal dynamics of mite (Acari) aggregation in pine forest litter and pine chips. Infrastruct. Ecol. Rural Areas 2015, 2, 405–417. [Google Scholar]
- Klimek, A.; Chachaj, B. Colonization of hardwood and pine wood chips by mites (Acari), with particular reference to oribatid mites (Oribatida). Folia For. Pol. Ser. A For. 2018, 60, 22–33. [Google Scholar] [CrossRef]
- Klimek, A.; Chachaj, B.; Gackowski, G. The presence of mites (Acari) in pine wood chips enriched with peat and lignite inoculated with forest litter and irrigated. Infrastruct. Ecol. Rural Areas 2017, II/1, 483–493. [Google Scholar]
- Klimek, A.; Chachaj, B.; Gackowski, G.; Kosakowski, L. Mite (Acari) colonization of pine chips alone and pine chips supplemented with forest litter, peat and lignite in revitalization of degraded forest soils. Infrastruct. Ecol. Rural Areas 2017, IV/2, 1577–1590. [Google Scholar]
- Berthet, P.; Gerard, G. A statistical study of microdistribution of Oribatei (Acari) I. The distribution pattern. Oikos 1965, 16, 214–227. [Google Scholar] [CrossRef]
- Orzeł, S. Biomasa sadzonek sosny zwyczajnej w uprawie doświadczalnej na rekultywowanym wyrobisku piasku i w terenie silnie skażonym imisjami przemysłowymi [Biomass of Scots pine seedlings in experimental cultivation on reclaimed sand pits and in areas heavily contaminated with industrial immissions]. In Ektomikoryzy. Nowe biotechnologie w polskim szkółkarstwie leśnym [Ectomycorrhiza. New biotechnologies in Polish forestry nursery]; Kowalski, S., Ed.; CILP: Warszawa, Poland, 2007; pp. 336–358. [Google Scholar]
- Klimek, A.; Rolbiecki, S.; Rolbiecki, R.; Malczyk, P. Impact of chosen bare root nursery practices on white birch seedling quality and soil mites (Acari). Pol. J. Environ. Stud. 2009, 18, 1013–1020. [Google Scholar]
- Klimek, A.; Rolbiecki, S. Growth of Scots pine (Pinus sylvestris L.) and occurrence of soil mites (Acari) on the reclaimed post-military area at forest district Żołędowo. Infrastruct. Ecol. Rural Areas 2011, 1, 249–262. [Google Scholar]
- Klimek, A.; Rolbiecki, S.; Rolbiecki, R.; Hilszczańska, D.; Malczyk, P. Impact of chosen bare root nursery practices in Scots pine seedling quality and soil mites (Acari). Pol. J. Environ. Stud. 2008, 17, 247–255. [Google Scholar]
- Prescott, C.E.; Maynard, D.G.; Laiho, R. Humus in northern forests: Friend or foe? For. Ecol. Manag. 2000, 133, 23–36. [Google Scholar] [CrossRef]
- Klimek, A.; Rolbiecki, S.; Rolbiecki, R.; Hilszczańska, D.; Malczyk, P. Effects of organic fertilization and mulching under micro-sprinkler irrigation on growth and mycorrhizal colonization of European larch seedlings, and occurrence of soil mites. Pol. J. Environ. Stud. 2011, 5, 1211–1219. [Google Scholar]
- Klimek, A.; Rolbiecki, S.; Rolbiecki, R.; Długosz, J.; Musiał, M. The use of compost from sewage sludge and forest ectohumus for enrichment of soils in the nursery cultivation of littleleaf linden (Tilia cordata Mill.). Annu. Set Environ. Prot. 2013, 15, 2811–2828. [Google Scholar]
- Szabla, K. Cechy morfologiczno-rozwojowe oraz przeżywalność sadzonek różnych gatunków drzew leśnych w uprawach doświadczalnych na gruntach nieleśnych i leśnych o różnym stopniu degradacji [Morphological and developmental features and survival of seedlings of various forest tree species in experimental crops on non-forest and forest land with varying degrees of degradation]. In Ektomikoryzy. Nowe biotechnologie w polskim szkółkarstwie leśnym [Ectomycorrhiza. New biotechnologies in Polish forestry nursery]; Kowalski, S., Ed.; CILP: Warszawa, Poland, 2007; pp. 289–336. [Google Scholar]
- Haimi, J. Decomposer animals and bioremediation of soils. Environ. Pollut. 2000, 107, 233–238. [Google Scholar] [CrossRef]
- Klimek, A. Possibility of use of ectohumus for revitalization of soils in forest nurseries. Manag. Environ. Prot. Forests 2010, 4, 80–93. [Google Scholar]
- Klimek, A.; Rolbiecki, S. Moss mites (Acari: Oribatida) in soil revitalizing: A chance for practical application in silviculture. Biol. Lett. 2014, 51, 71–82. [Google Scholar] [CrossRef][Green Version]
- Klimek, A.; Rolbiecki, S.; Rolbiecki, R.; Hilszczańska, D.; Malczyk, P. The effect of nursery measures on mycorrhizal colonisation of Scots pine and occurrence of soil mites. Sci. Res. Essays. 2012, 7, 2380–2389. [Google Scholar]
- Beckmann, M. Die Entwicklung der Bodenmesofauna eines Ruderal Ökosystems und ihre Beeinflussung durch Rekultivierung: 1. Oribatiden. Pedobiologia 1988, 31, 391–408. [Google Scholar]
- Wanner, M.; Dunger, W. Primary immigration and succession of soil organisms on reclaimed opencast coal mining areas in eastern Germany. Eur. J. Soil Biol. 2002, 38, 137–143. [Google Scholar] [CrossRef]
- Lehmitz, R.; Russell, D.; Hohberg, K.; Christian, A.; Xylander, W.E.R. Wind dispersal of oribatid mites as a mode of migration. Pedobiologia 2011, 54, 201–207. [Google Scholar] [CrossRef]
- Gwiazdowicz, D.; Zawieja, B.; Olejniczak, I.; Coulson, S.J. Changing Microarthropod Communities in Front of a Receding Glacier in the High Arctic. Insects 2020, 11, 226. [Google Scholar] [CrossRef]
- Dunger, W. The return of soil fauna to coal mined areas in the German Democratic Republic. In Animals Primary Succession: The Role of Fauna in Reclaimed Lands; Mayer, D.J., Ed.; Cambridge University Press: Cambridge, UK, 1989; pp. 307–337. [Google Scholar]
- Klironomos, J.N.; Kendrick, W.B. Palatability of microfungi to soil arthropods in relation to the functioning of arbuscular mycorrhizae. Biol. Fertil. Soils 1996, 21, 43–52. [Google Scholar] [CrossRef]
- Weigmann, G.; Kratz, W. Die deutschen Hornmilbenarten und ihre ökologische Charakteristik. Zool. Beitr. 1981, 27, 459–489. [Google Scholar]
- Siepel, H. Life–history tactics of soil microarthropods. Biol. Fertil. Soils 1994, 18, 263–278. [Google Scholar] [CrossRef]
- Skubała, P.; Gulvik, M. Pioneer oribatid mite communities (Acari: Oribatida) in natural (glacier foreland) and anthropogenic (post-industrial dumps) habitats. Pol J. Ecol 2005, 53, 105–111. [Google Scholar]
- Skubała, P. Colonization of a dolomitic dump by oribatid mites (Acari, Oribatida). Pedobiologia 1999, 43, 145–159. [Google Scholar]
- Rolbiecki, S.; Stypczyńska, Z.; Klimek, A.; Długosz, J.; Rolbiecki, R. Flora and some properties of fallow soil which was previously under arable cultivation in conditions of sprinkler irrigation. Infrastruct. Ecol. Rural Areas 2006, 2/1, 183–194. [Google Scholar]
- Weigmann, G. Oribatid communities in transects from bogs to forests in Berlin indicating the bio-tope qualities. In Proceedings of 8th International Congress on Acarology. Modern Acarology; Dusbanek, F., Bukva, V., Eds.; Academia, Prague and SPB Academic Publishing: The Hague, The Netherlands, 1991; pp. 359–364. [Google Scholar]
- Rajski, A. Autecological-zoogeographical analy-sis of moss mites (Acari, Oribatei) on the basis of fauna in the Poznań environs. Part II. Fragm. Faun. 1968, 12, 277–405. [Google Scholar]
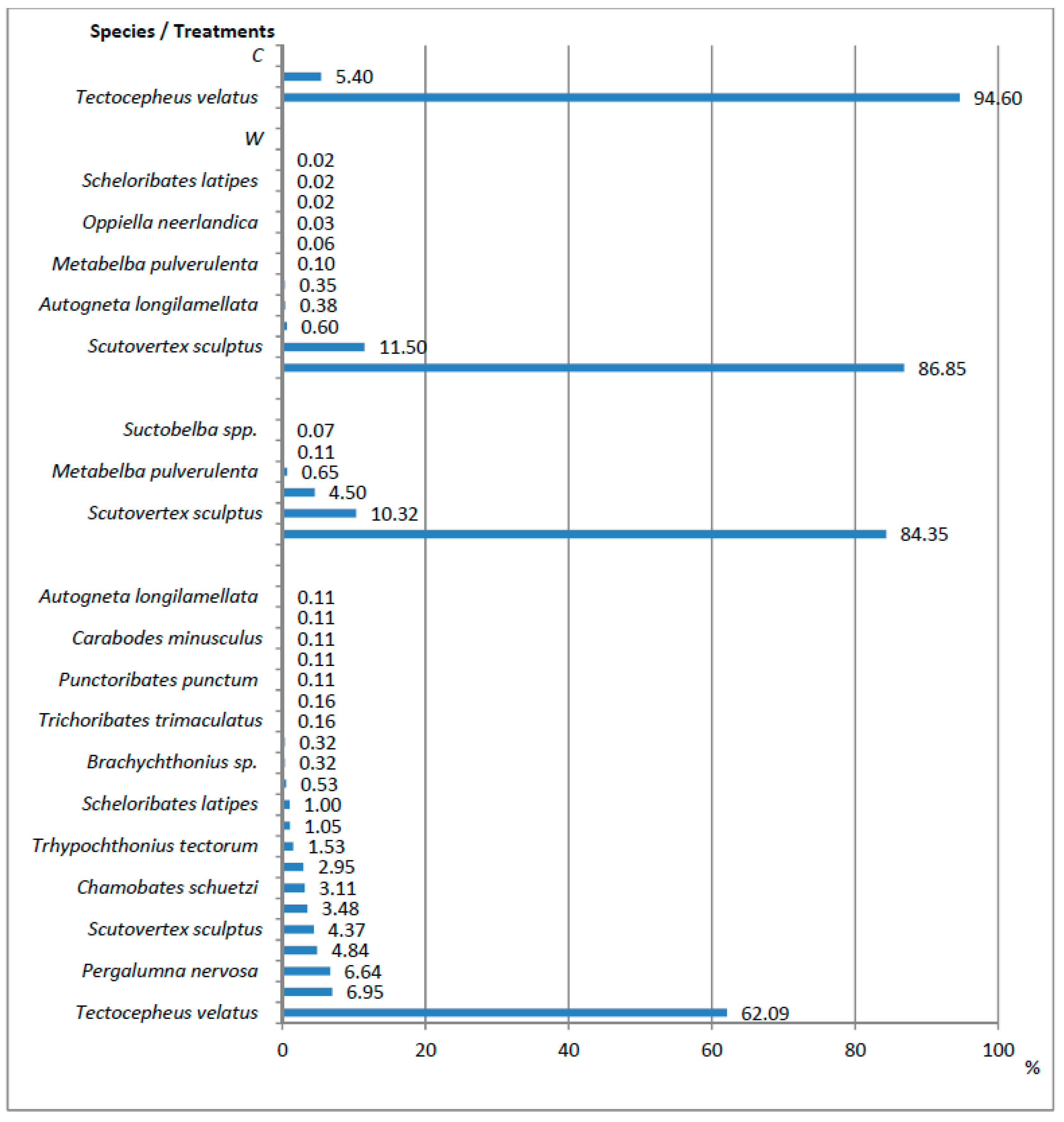
| Treatment | Length of Annual Increment of the Main Stem (cm) | Stem Base Diameter (mm) | Number of Lateral Shoots in the Annual Whorl (pcs) | Sum of Lengths of Lateral Shoots in the Annual Whorl (cm) |
|---|---|---|---|---|
| C | 17.8 | 17.4 | 6.2 | 124.1 |
| W | 18.7 | 13.6 | 5.4 | 95.4 |
| WM | 17.8 | 15.5 | 5.9 | 115.9 |
| WL | 14.0 | 14.8 | 4.8 | 95.4 |
| Mean | 17.1 | 15.3 | 5.6 | 107.7 |
| LSD0.05 | ns | 2.835 | ns | ns |
| Index—Taxon | Treatments | Kruskal-Wallis Test | ||||
|---|---|---|---|---|---|---|
| C | W | WM | WL | H | p | |
| N—Acaridida | 0.00 | 0.00 | 0.03 a | 1.53 b | 31.09 | 0.000 |
| N—Actinedida | 3.56 a | 8.71 b | 6.78 b | 7.13 b | 29.53 | 0.000 |
| N—Mesostigmata | 0.09 a | 0.09 a | 1.39 b | 1.45 b | 26.73 | 0.000 |
| N—Oribatida | 0.37 a | 60.44 b | 27.80 c | 18.99 c | 71.56 | 0.000 |
| N—Tarsonemida | 0.00 | 0.00 | 0.57 a | 0.53 a | 8.69 | 0.034 |
| N—Acari (Total) | 4.02 a | 70.76 b | 36.57 c | 29.63 c | 72.28 | 0.000 |
| N—Oppiella nova (Oudemans) | 0.00 | 0.36 a | 1.25 a | 1.32 a | 12.88 | 0.005 |
| N—Oribatula tibialis (Nicolet) | 0.02 | - | - | 0.06 | - | - |
| N—Pergalumna nervosa (Berlese) | - | - | - | 1.26 | - | - |
| N—Scutovertex sculptus (Michael) | 0.00 | 6.95 a | 2.87 ab | 0.83 b | 34.90 | 0.000 |
| N—Tectocepheus velatus (Michael) | 0.35 a | 52.48 b | 23.45 c | 11.79 c | 66.43 | 0.000 |
| Others Oribatida (N < 1.0) | 0.02 | 0.65 | 0.23 | 3.79 | - | - |
| N—Or/Ac abundance ratio | 0.10 | 6.94 | 4.10 | 2.66 | - | - |
| S—Oribatida | 2 | 11 | 6 | 21 | - | - |
| s—Oribatida | 0.23 a | 1.98 b | 1.58 b | 3.70 c | 89.47 | 0.000 |
| H’—Oribatida | 0.22 | 0.47 | 0.56 | 1.52 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimek, A.; Rolbiecki, S.; Rolbiecki, R.; Gackowski, G.; Stachowski, P.; Jagosz, B. The Use of Wood Chips for Revitalization of Degraded Forest Soil on Young Scots Pine Plantation. Forests 2020, 11, 683. https://doi.org/10.3390/f11060683
Klimek A, Rolbiecki S, Rolbiecki R, Gackowski G, Stachowski P, Jagosz B. The Use of Wood Chips for Revitalization of Degraded Forest Soil on Young Scots Pine Plantation. Forests. 2020; 11(6):683. https://doi.org/10.3390/f11060683
Chicago/Turabian StyleKlimek, Andrzej, Stanisław Rolbiecki, Roman Rolbiecki, Grzegorz Gackowski, Piotr Stachowski, and Barbara Jagosz. 2020. "The Use of Wood Chips for Revitalization of Degraded Forest Soil on Young Scots Pine Plantation" Forests 11, no. 6: 683. https://doi.org/10.3390/f11060683
APA StyleKlimek, A., Rolbiecki, S., Rolbiecki, R., Gackowski, G., Stachowski, P., & Jagosz, B. (2020). The Use of Wood Chips for Revitalization of Degraded Forest Soil on Young Scots Pine Plantation. Forests, 11(6), 683. https://doi.org/10.3390/f11060683








