Drought Resistance of Norway Spruce (Picea abies [L.] Karst) and European Beech (Fagus sylvatica [L.]) in Mixed vs. Monospecific Stands and on Dry vs. Wet Sites. From Evidence at the Tree Level to Relevance at the Stand Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Data Sampling and Tree Level Evaluation
2.3. Stand Level Evaluation
2.4. Detrending Procedures
2.5. Quantification of Resistance, Resilience and the Stand Level Growth Losses
2.6. Statistical Analyses
- Tree level resilience per se: Beech in 2003
- Tree level resistance: Beech in 2015
2.6.1. Tree Level Models
2.6.2. Stand Level Models
Non-consideration of the Stand Density
3. Results
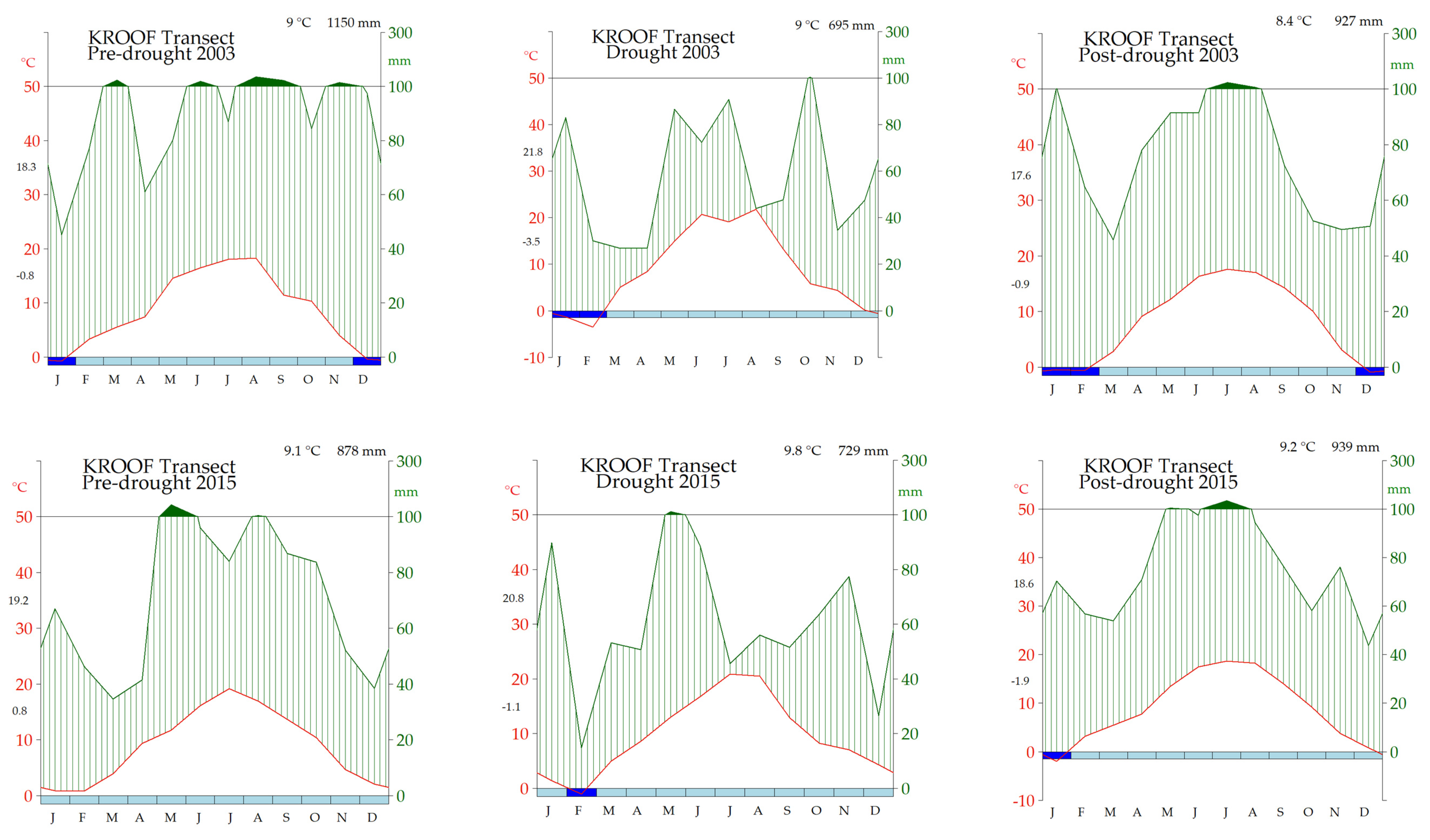
3.1. Weather Conditions along the Gradient
3.2. Effect of Individual Tree Size on the Drought Response
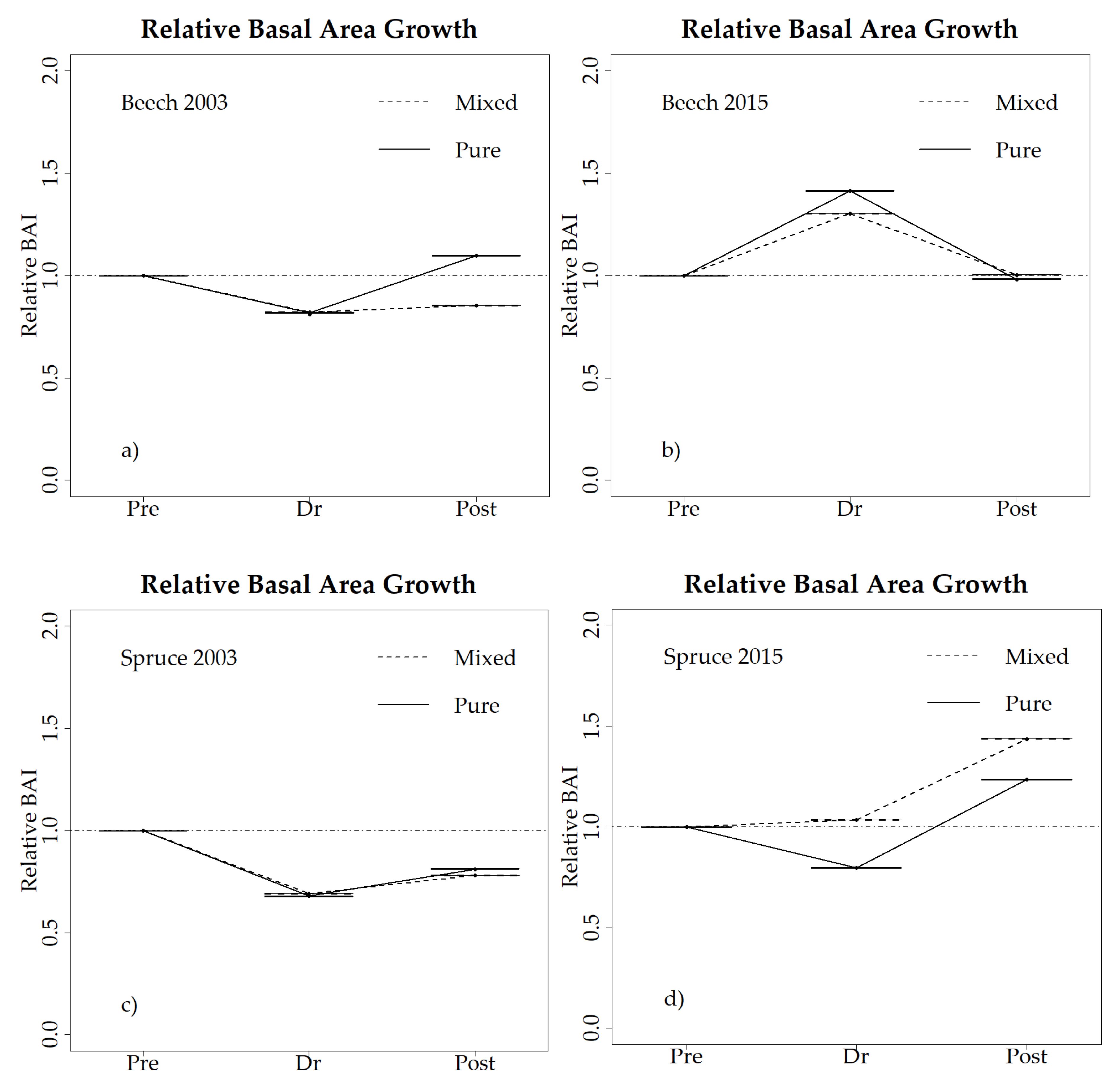
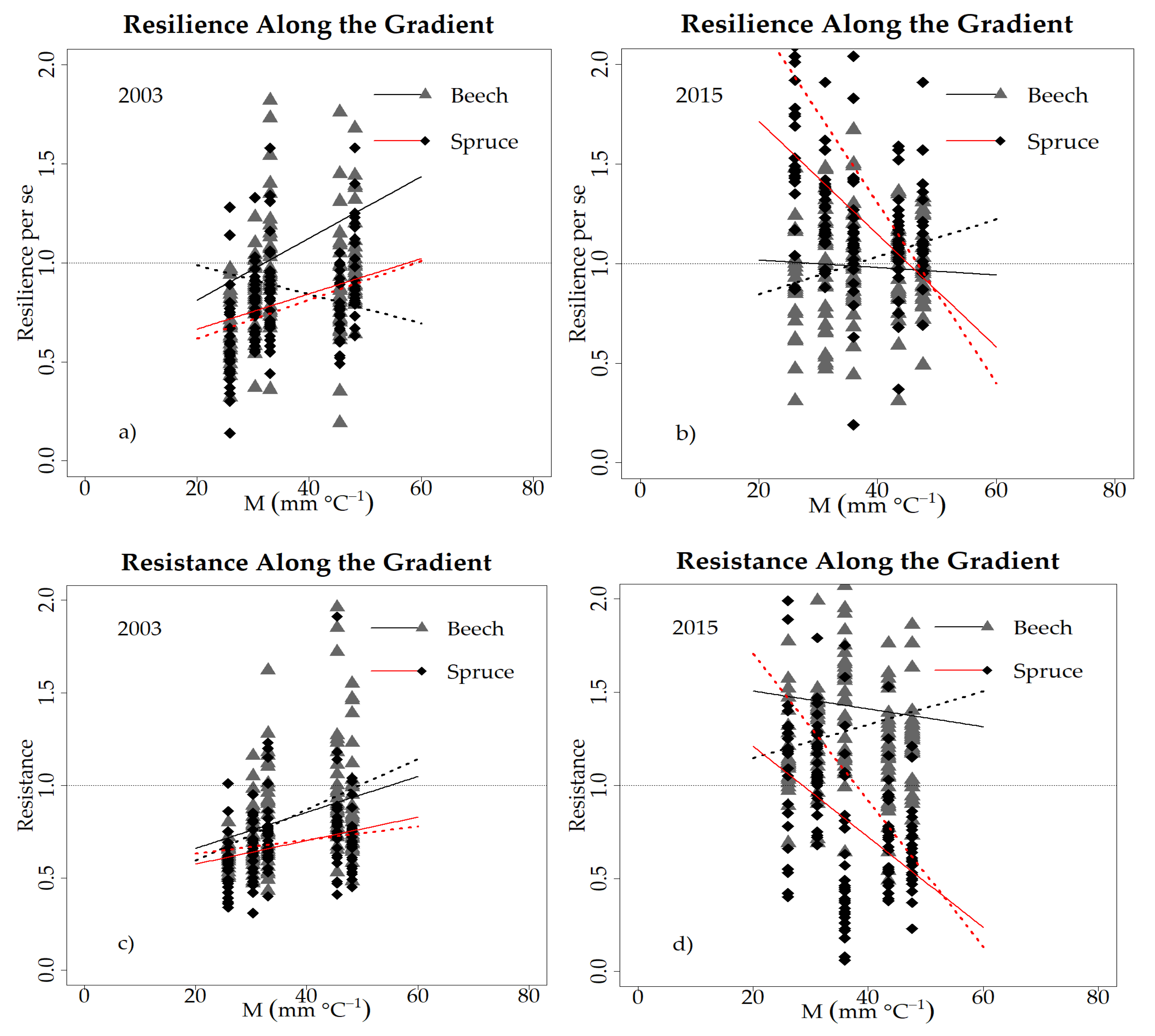
3.3. Drought Responses at the Tree Level
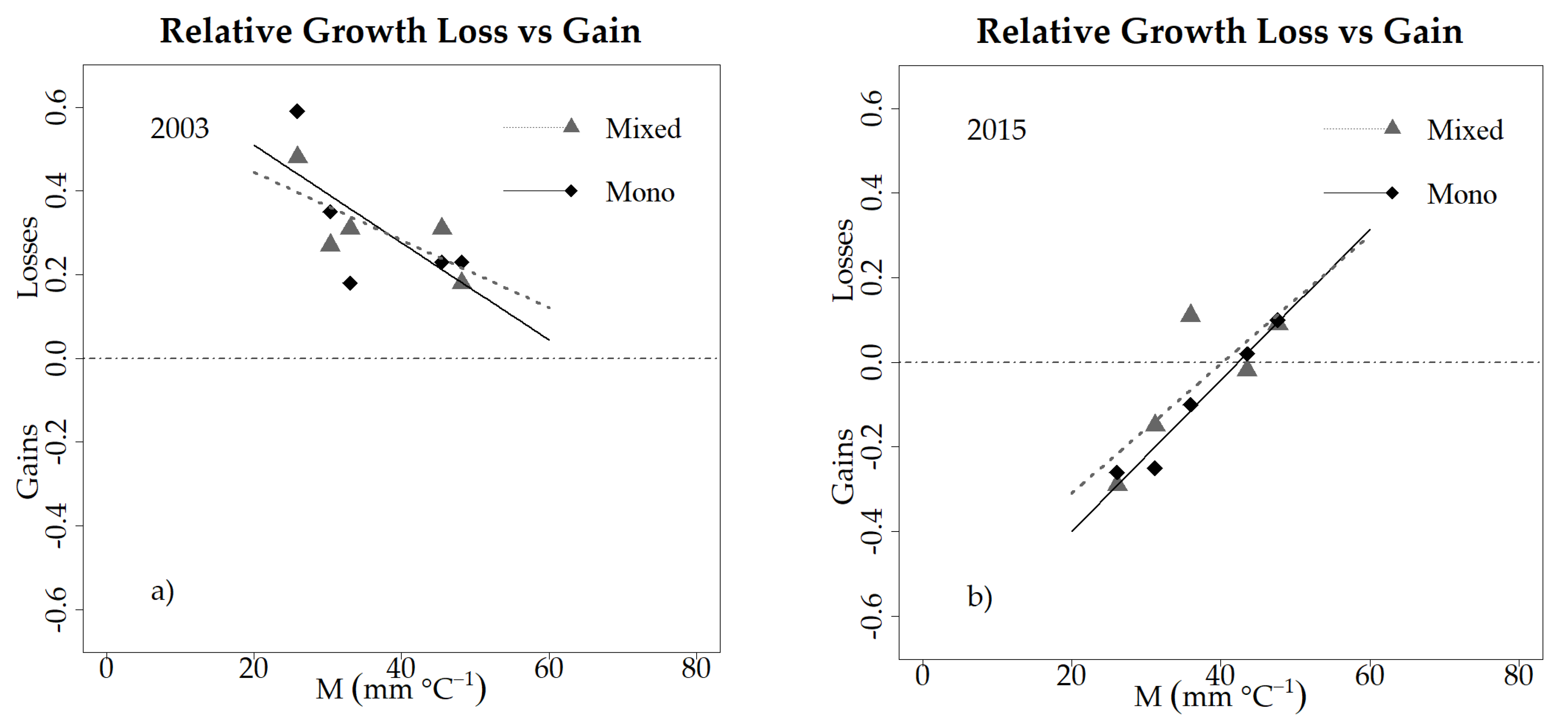
3.4. Drought Responses at the Stand Level
4. Discussion
4.1. Species-Specific Drought Response in Mixtures (H1)
4.2. Drought Response along an Ecological Gradient (H2)
4.3. Relevance for Forest Management (H3 & H4)
4.4. Comparison between the Findings at the Tree and the Stand Level
4.5. Methodological Considerations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ibáñez, I.; Acharya, K.; Juno, E.; Karounos, C.; Lee, B.R.; McCollum, C.; Schaffer-Morrison, S.; Tourville, J. Forest Resilience under Global Environmental Change: Do We Have the Information We Need? A Systematic Review. PLoS ONE 2019, 14, e0222207. [Google Scholar] [CrossRef]
- Bauhus, J.; Forrester, D.I.; Gardiner, B.; Jactel, H.; Vallejo, R.; Pretzsch, H. Ecological Stability of Mixed-Species Forests. In Mixed Species Forests; Springer: Berlin, Germany, 2017; pp. 337–382. [Google Scholar]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Kerhoulas, L.P.; Kolb, T.E.; Hurteau, M.D.; Koch, G.W. Managing Climate Change Adaptation in Forests: A Case Study from the U.S. Southwest. J. Appl. Ecol. 2013, 50, 1311–1320. [Google Scholar] [CrossRef]
- Grossiord, C.; Granier, A.; Ratcliffe, S.; Bouriaud, O.; Bruelheide, H.; Chećko, E.; Forrester, D.I.; Dawud, S.M.; Finér, L.; Pollastrini, M.; et al. Tree Diversity Does Not Always Improve Resistance of Forest Ecosystems to Drought. Proc. Natl. Acad. Sci. USA 2014, 111, 14812–14815. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, C.; Rötzer, T.; Thurm, E.A.; Biber, P.; Kallenbach, C.; Pretzsch, H. Growth and Tree Water Deficit of Mixed Norway Spruce and European Beech at Different Heights in a Tree and under Heavy Drought. Forests 2019, 10, 577. [Google Scholar] [CrossRef]
- Griess, V.C.; Knoke, T. Growth Performance, Wind Throw, and Insects: Meta-Analyses of Parameters Influencing Performance of Mixed-Species Stands in Boreal and Northern Temperate Biomes. Can. J. For. Res. 2011, 41, 1141–1159. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate Change and Forest Disturbances. Bioscience 2001, 51, 723. [Google Scholar] [CrossRef]
- Grossiord, C. Having the Right Neighbors: How Tree Species Diversity Modulates Drought Impacts on Forests. New Phytol. 2019. [Google Scholar] [CrossRef]
- Del Río, M.; Pretzsch, H.; Ruíz-Peinado, R.; Ampoorter, E.; Annighöfer, P.; Barbeito, I.; Bielak, K.; Brazaitis, G.; Coll, L.; Drössler, L.; et al. Species Interactions Increase the Temporal Stability of Community Productivity in Pinus sylvestris–Fagus sylvatica Mixtures across Europe. J. Ecol. 2017, 105, 1032–1043. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Snall, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Froberg, M.; Stendahl, J.; Philipson, C.D.; et al. Higher Levels of Multiple Ecosystem Services Are Found in Forests with More Tree Species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef]
- Steckel, M.; Heym, M.; Wolff, B.; Reventlow, D.O.J.; Pretzsch, H. Forest Ecology and Management Transgressive Overyielding in Mixed Compared with Monospecific Scots Pine (Pinus sylvestris L.) and Oak (Quercus robur L., Quercus petraea (Matt.) Liebl.) Stands—Productivity Gains Increase with Annual Water Supply. For. Ecol. Manag. 2019, 439, 81–96. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G. Transgressive Overyielding in Mixed Compared with Pure Stands of Norway Spruce and European Beech in Central Europe: Evidence on Stand Level and Explanation on Individual Tree Level. Eur. J. For. Res. 2009, 128, 183–204. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G. Effect of Tree Species Mixing on the Size Structure, Density, and Yield of Forest Stands. Eur. J. For. Res. 2016, 135, 1–22. [Google Scholar] [CrossRef]
- Ammer, C. Diversity and Forest Productivity in a Changing Climate. New Phytol. 2019, 221, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European Tree Species to Drought Stress in Mixed versus Pure Forests: Evidence of Stress Release by Inter-Specific Facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.A.; Bauhus, J. Benefits of Mixtures on Growth Performance of Silver Fir (Abies alba) and European Beech (Fagus sylvatica) Increase With Tree Size Without Reducing Drought Tolerance. Front. For. Glob. Chang. 2019, 2. [Google Scholar] [CrossRef]
- Serra-Maluquer, X.; Mencuccini, M.; Martínez-Vilalta, J. Changes in Tree Resistance, Recovery and Resilience across Three Successive Extreme Droughts in the Northeast Iberian Peninsula. Oecologia 2018, 187, 343–354. [Google Scholar] [CrossRef]
- Steckel, M.; del Río, M.; Heym, M.; Aldea, J.; Bielak, K.; Brazaitis, G.; Černý, J.; Coll, L.; Collet, C.; Ehbrecht, M.; et al. Species Mixing Reduces Drought Susceptibility of Scots Pine (Pinus sylvestris L.) and Oak (Quercus robur L., Quercus petraea (Matt.) Liebl.)—Site Water Supply and Fertility Modify the Mixing Effect. For. Ecol. Manag. 2020, 461, 117908. [Google Scholar] [CrossRef]
- Thurm, E.A.; Uhl, E.; Pretzsch, H. Mixture Reduces Climate Sensitivity of Douglas-Fir Stem Growth. For. Ecol. Manag. 2016, 376, 205–220. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of Tree Resilience: Effects of Successive Low-Growth Episodes in Old Ponderosa Pine Forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Ding, H.; Pretzsch, H.; Schütze, G.; Rötzer, T. Size-Dependence of Tree Growth Response to Drought for Norway Spruce and European Beech Individuals in Monospecific and Mixed-Species Stands. Plant Biol. 2017, 19, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Schütze, G.; Biber, P. Drought Can Favour the Growth of Small in Relation to Tall Trees in Mature Stands of Norway Spruce and European Beech. For. Ecosyst. 2018, 5. [Google Scholar] [CrossRef]
- Schäfer, C.; Grams, T.E.E.; Rötzer, T.; Feldermann, A.; Pretzsch, H. Drought Stress Reaction of Growth and Δ13C in Tree Rings of European Beech and Norway Spruce in Monospecific versus Mixed Stands along a Precipitation Gradient. Forests 2017, 8, 177. [Google Scholar] [CrossRef]
- Pretzsch, H.; Grams, T.; Häberle, K.H.; Pritsch, K.; Bauerle, T.; Rötzer, T. Growth and Mortality of Norway Spruce and European Beech in Monospecific and Mixed-Species Stands under Natural Episodic and Experimentally Extended Drought. Results of the KROOF Throughfall Exclusion Experiment. Trees 2020. [Google Scholar] [CrossRef]
- Neuner, S.; Albrecht, A.; Cullmann, D.; Engels, F.; Griess, V.C.; Hahn, W.A.; Hanewinkel, M.; Härtl, F.; Kölling, C.; Staupendahl, K.; et al. Survival of Norway Spruce Remains Higher in Mixed Stands under a Dryer and Warmer Climate. Glob. Chang. Biol. 2015, 21, 935–946. [Google Scholar] [CrossRef]
- Čermák, P.; Rybníček, M.; Žid, T.; Andreassen, K. Impact of Climate Change on Growth Dynamics. Silva Fenn. 2017, 51, 1–16. [Google Scholar] [CrossRef]
- Krupková, L.; Havránková, K.; Krejza, J.; Sedlák, P.; Marek, M.V. Impact of Water Scarcity on Spruce and Beech Forests. J. For. Res. 2019, 30, 899–909. [Google Scholar] [CrossRef]
- Bolte, A.; Hilbrig, L.; Grundmann, B.; Kampf, F.; Brunet, J.; Roloff, A. Climate Change Impacts on Stand Structure and Competitive Interactions in a Southern Swedish Spruce-Beech Forest. Eur. J. For. Res. 2010, 129, 261–276. [Google Scholar] [CrossRef]
- Pretzsch, H.; Rötzer, T.; Matyssek, R.; Grams, T.E.E.; Häberle, K.-H.; Pritsch, K.; Kerner, R.; Munch, J.C. Mixed Norway Spruce (Picea abies [L.] Karst) and European Beech (Fagus sylvatica [L.]) Stands under Drought: From Reaction Pattern to Mechanism. Trees 2014. [Google Scholar] [CrossRef]
- Forrester, D.I. The Spatial and Temporal Dynamics of Species Interactions in Mixed-Species Forests: From Pattern to Process. For. Ecol. Manag. 2014, 312, 282–292. [Google Scholar] [CrossRef]
- Forrester, D.I. Transpiration and Water-Use Efficiency in Mixed-Species Forests versus Monocultures: Effects of Tree Size, Stand Density and Season. Tree Physiol. 2015, 35, 289–304. [Google Scholar] [CrossRef]
- Bosela, M.; Tobin, B.; Šeben, V.; Petráš, R.; Larocque, G.R. Different Mixtures of Norway Spruce, Silver Fir, and European Beech Modify Competitive Interactions in Central European Mature Mixed Forests. Can. J. For. Res. 2015, 45, 1577–1586. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Rathgeber, C.B.K.; Ulrich, E. Sensitivity of French Temperate Coniferous Forests to Climate Variability and Extreme Events (Abies alba, Picea abies and Pinus sylvestris). J. Veg. Sci. 2010, 21, 364–376. [Google Scholar] [CrossRef]
- Malkinson, D.; Tielbörger, K. What Does the Stress-Gradient Hypothesis Predict? Resolving the Discrepancies. Oikos 2010, 119, 1546–1552. [Google Scholar] [CrossRef]
- Bertness, M.D.; Callaway, R. Positive Interactions in Communities. Trends Ecol. Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef]
- Pretzsch, H.; del Río, M.; Ammer, C.; Avdagic, A.; Barbeito, I.; Bielak, K.; Brazaitis, G.; Coll, L.; Dirnberger, G.; Drössler, L.; et al. Growth and Yield of Mixed versus Pure Stands of Scots Pine (Pinus sylvestris L.) and European Beech (Fagus sylvatica L.) Analysed along a Productivity Gradient through Europe. Eur. J. For. Res. 2015, 134, 927–947. [Google Scholar] [CrossRef]
- Wetterdienst, D. Climate Data. Available online: http://ftp-cdc.dwd.de/climate_environment/CDC/ (accessed on 27 February 2019).
- Häberle, K.-H.; (Technical University of Munich, Freising, Bavaria, Germany). Soil Description. Personal Communication, 2020. [Google Scholar]
- Martonne, D. Une Novelle Fonction Climatologique: L’indice d’aridité. La Métérologie 1926, 21, 449–458. [Google Scholar]
- Assmann, E.; Franz, F. Vorläufige Fichten-Ertragstafel Für Bayern (Mittleres Ertragsniveau). In Hilfstafeln für die Forsteinrichtung 1966; Bayerisches Staatsministerium für Ernährung Landwirtschaft und Forsten, Ed.; Hilfstafeln für die Forsteinrichtung: Munich, Germany, 1963; pp. 52–63. [Google Scholar]
- Schober, R. Buchen-Ertragstfel Für Mäßige Und Starke Durchforstung. In Schober R (1972) Die Rotbuche 1971; Schr Forstl Fak Univ Göttingen u Niedersächs Forstl Versuchsanst, Ed.; Sauerländer’s Verlag: Frankfurt am Main, Germany, 1967; p. 333. [Google Scholar]
- Sweden, H. Increment Borer. Available online: http://www.haglofsweden.com/index.php/en/products/instruments/survey (accessed on 21 April 2020).
- GmbH, B. Digital Positiometer Type II. Available online: https://www.biritz.at/messgeräte/digitalpositiometer/digitalpositiometer-typ-2/ (accessed on 21 April 2020).
- Sweden, H. Vertex IV Hypsometer. Available online: http://www.haglofsweden.com/index.php/en/products/instruments/height/341-vertex-iv (accessed on 21 April 2020).
- Johann, K. Normen Der Sektion Ertragskunde Im Deutschen Verband Forstlicher Forschungsanstalten Zur Aufbereitung von Waldwach- Stumskundlichen Dauerversuchen; Proc Dt Verb Forstl Forschungsanst, Sektion Ertragskunde: Unterreichenbach- Kapfenhardt, Germany, 1993. [Google Scholar]
- Franz, F. Funktionen Und Tabellen Der Derbholzformhöhen Für Die Wichtigsten Baumarten in Bayern—Manuskript Druck, Munich. Unpublished.
- Freese, F. US Forest Service Research Paper FPL-17. In Linear Regression Methods for Forest Research; Forest Products Laboratory: Madison, WI, USA, 1964. [Google Scholar]
- Korsuň, F. Život Normálního Porostu Ve Vzorcích. Lesn. Práce 1935, 14, 289–300. [Google Scholar]
- Michailoff, I. Zahlenmäßiges Verfahren Für Die Ausführung Der Bestandeshöhenkurven. Cbl. Thar. Forstl. Jahrb. 1943, 65, 273–279. [Google Scholar]
- Petterson, H. Die Massenproduktion Des Nadelwaldes. Mittlg. Der Forstlichen Versuchsanstalten Schwedens; Statens Skogsforskningsinstitut: Stockholm, Sweden, 1955; Volume 45. [Google Scholar]
- Prodan, M. Messung Der Waldbestände; JD Sauerländer‘s Verlag: Frankfurt am Main, Germany, 1951. [Google Scholar]
- Heym, M.; Bielak, K.; Wellhausen, K.; Uhl, E.; Biber, P.; Perkins, D.; Steckel, M.; Andreas Thurm, E.; Rais, A.; Pretzsch, H. A New Method to Reconstruct Recent Tree and Stand Attributes of Temporary Research Plots: New Opportunity to Analyse Mixed Forest Stands. In Conifers; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Kennel, R. Die Buchendurchforstungsversuche in Bayern von 1870 Bis 1970. Forstl. Vers. München 1972, 7, 77–80. [Google Scholar]
- Franz, F.; Bachler, J.; Deckelmann, E.; Kennel, E.; Kennel, R.; Schmidt, A.; Wotschikowsky, U. Bayerische Waldinventur 1970/71. Inventurabschnitt I: Großrauminventur Aufnahme- Und Auswer- Tungsverfahren. Forstl. Vers. München 1973, 11, 143. [Google Scholar]
- Zang, C.; Pretzsch, H.; Rothe, A. Size-Dependent Responses to Summer Drought in Scots Pine, Norway Spruce and Common Oak. Trees 2012, 26, 557–569. [Google Scholar] [CrossRef]
- Bošela, M.; Kulla, L.; Marušák, R. Detrending Ability of Several Regression Equations in Tree-Ring Research: A Case Study Based on Tree-Ring Data of Norway Spruce (Picea Abies [L.]). J. For. Sci. 2011, 57, 491–499. [Google Scholar] [CrossRef]
- Cook, E.; Peters, K. The Smoothing Spline, a New Approach to Standardising Forest Interior Tree-Ring. Tree-Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Reinsch, C.H. Smoothing by Spline Functions. II. Numer. Math. 1971, 16, 451–454. [Google Scholar] [CrossRef]
- Pretzsch, H. Diagnose von Wachstumsstörungen. In Grundlagen der Waldwachstumsforschung; Springer Spektrum: Heidelberg, Germany, 2019; pp. 571–627. [Google Scholar]
- Bunn, A.G. A Dendrochronology Program Library in R. Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- R Core Team. R Version 3.5.3.; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Reineke, L.H. Perfecting a Stand-Density Index for Even-Aged Forests. J. Agric. Res. 1933, 46, 627–638. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-Plus; Springer: Berlin, Germany, 2000. [Google Scholar]
- Pinheiro, J.C.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, C.R. nlme: Linear and Nonlinear Mixed Effects Models, Version 3.1-145. Available online: https://CRAN.R-project.org/package=nlme (accessed on 2 April 2020).
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Soc. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Ryan, M.G. Tree Responses to Drought. Tree Physiol. 2011, 31, 237–239. [Google Scholar] [CrossRef]
- Stovall, A.E.L.; Shugart, H.; Yang, X. Tree Height Explains Mortality Risk during an Intense Drought. Nat. Commun. 2019, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-Taking Plants: Anisohydric Behavior as a Stress-Resistance Trait. Plant Signal. Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Guan, H.; Zhang, X.; Zhang, C.; Liu, N.; Li, G. Responses of Plant Water Use to a Severe Summer Drought for Two Subtropical Tree Species in the Central Southern China. J. Hydrol. Reg. Stud. 2016, 8, 1–9. [Google Scholar] [CrossRef]
- Hafner, B.D.; Tomasella, M.; Häberle, K.H.; Goebel, M.; Matyssek, R.; Grams, T.E.E. Hydraulic Redistribution under Moderate Drought among English Oak, European Beech and Norway Spruce Determined by Deuterium Isotope Labeling in a Split-Root Experiment. Tree Physiol. 2017, 37, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Ryel, R.J. Hydraulic Redistribution. In Progress in Botany: Genetics Physiology Systematics Ecology; Esser, K., Lüttge, U., Beyschlag, W., Murata, J., Eds.; Springer: Berlin, Germany, 2004; pp. 413–435. [Google Scholar]
- Zang, C.; Hartl-Meier, C.; Dittmar, C.; Rothe, A.; Menzel, A. Patterns of Drought Tolerance in Major European Temperate Forest Trees: Climatic Drivers and Levels of Variability. Glob. Chang. Biol. 2014, 20, 3767–3779. [Google Scholar] [CrossRef] [PubMed]
- Szejner, P.; Belmecheri, S.; Ehleringer, J.R.; Monson, R.K. Recent Increases in Drought Frequency Cause Observed Multi-Year Drought Legacies in the Tree Rings of Semi-Arid Forests. Oecologia 2020, 192, 241–259. [Google Scholar] [CrossRef] [PubMed]
- D’Orangeville, L.; Maxwell, J.; Kneeshaw, D.; Pederson, N.; Duchesne, L.; Logan, T.; Houle, D.; Arseneault, D.; Beier, C.M.; Bishop, D.A.; et al. Drought Timing and Local Climate Determine the Sensitivity of Eastern Temperate Forests to Drought. Glob. Chang. Biol. 2018, 24, 2339–2351. [Google Scholar] [CrossRef]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate Forest Trees and Stands under Severe Drought: A Review of Ecophysiological Responses, Adaptation Processes and Long-Term Consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Jactel, H.; Koricheva, J.; Castagneyrol, B. Responses of Forest Insect Pests to Climate Change: Not so Simple. Curr. Opin. Insect Sci. 2019, 35, 103–108. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Bentz, J.; Carroll, A.L.; Hicke, J.A.; Kolb, T.E. Beetles to a Changing Climate. In Climate Change and Insect Pests; Björkman, C., Niemelä, P., Eds.; CABI International: Wallingford, UK, 2015; pp. 173–201. [Google Scholar] [CrossRef]
- Myburg, A.A.; Sederoff, R.R. Xylem Structure and Function. eLS 2001, 1–9. [Google Scholar] [CrossRef]
- Goisser, M.; Geppert, U.; Rötzer, T.; Paya, A.; Huber, A.; Kerner, R.; Bauerle, T.; Pretzsch, H.; Pritsch, K.; Häberle, K.H.; et al. Does Belowground Interaction with Fagus sylvatica Increase Drought Susceptibility of Photosynthesis and Stem Growth in Picea abies? For. Ecol. Manag. 2016, 375, 268–278. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Zhao, J. Seasonal Drought Effects on Intra-Annual Stem Growth of Taiwan Pine along an Elevational Gradient in Subtropical China. Forests 2019, 10, 1128. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Oliveras, I.; Rifai, S.; Fauset, S.; Adu-Bredu, S.; Affum-Baffoe, K.; Baker, T.R.; Feldpausch, T.R.; Gvozdevaite, A.; Hubau, W.; et al. Drier Tropical Forests Are Susceptible to Functional Changes in Response to a Long-Term Drought. Ecol. Lett. 2019, 22, 855–865. [Google Scholar] [CrossRef]
- Ashton, I.W.; Miller, A.E.; Bowman, W.D.; Suding, K.N. Niche Complementarity Due to Plasticity in Resource Use: Plant Partitioning of Chemical N Forms. Ecology 2010, 91, 3252–3260. [Google Scholar] [CrossRef]
- Fargione, J.; Tilman, D.; Dybzinski, R.; Lambers, J.H.R.; Clark, C.; Harpole, W.S.; Knops, J.M.H.; Reich, P.B.; Loreau, M. From Selection to Complementarity: Shifts in the Causes of Biodiversity-Productivity Relationships in a Long-Term Biodiversity Experiment. Proc. R. Soc. B Biol. Sci. 2007, 274, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.A.; Kruuk, L.E.B.; Nicotra, A.B. How to Analyse Plant Phenotypic Plasticity in Response to a Changing Climate. New Phytol. 2019, 222, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Gratani, L. Plant Phenotypic Plasticity in Response to Environmental Factors. Adv. Bot. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Zhang, Z.; Papaik, M.J.; Wang, X.; Hao, Z.; Ye, J.; Lin, F.; Yuan, Z. The Effect of Tree Size, Neighborhood Competition and Environment on Tree Growth in an Old-Growth Temperate Forest. J. Plant Ecol. 2016, 10, rtw126. [Google Scholar] [CrossRef]
- Matsushita, M.; Takata, K.; Hitsuma, G.; Yagihashi, T.; Noguchi, M.; Shibata, M.; Masaki, T. A Novel Growth Model Evaluating Age-Size Effect on Long-Term Trends in Tree Growth. Funct. Ecol. 2015, 29, 1250–1259. [Google Scholar] [CrossRef]
- Ryan, M.G. Tree Mortality: Large Trees Losing out to Drought. Nat. Plants 2015, 1, 9–11. [Google Scholar] [CrossRef]
- Bennett, A.C.; Mcdowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.J. Larger Trees Suffer Most during Drought in Forests Worldwide. Nat. Plants 2015, 1. [Google Scholar] [CrossRef]
- Shenkin, A.; Bolker, B.; Peña-Claros, M.; Licona, J.C.; Ascarrunz, N.; Putz, F.E. Interactive Effects of Tree Size, Crown Exposure and Logging on Drought-Induced Mortality. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef]
- Kölling, C.; Knoke, T.; Schall, P.; Ammer, C. Überlegungen Zum Risiko Des Fichtenanbaus in Deutschland Vor Dem Hintergrund Des Klimawandels. Forstarchiv 2009, 80, 42–54. [Google Scholar]
- Jactel, H.; Gritti, E.S.; Drössler, L.; Forrester, D.I.; Mason, W.L.; Morin, X.; Pretzsch, H.; Castagneyrol, B. Positive Biodiversity–Productivity Relationships in Forests: Climate Matters. Biol. Lett. 2018, 14, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive Biodiversity-Productivity Relationship Predominant in Global Forests. Science 2016, 354, 196. [Google Scholar] [CrossRef] [PubMed]
- Forrester, D.I.; Bonal, D.; Dawud, S.; Gessler, A.; Granier, A.; Pollastrini, M.; Grossiord, C. Drought Responses by Individual Tree Species Are Not Often Correlated with Tree Species Diversity in European Forests. J. Appl. Ecol. 2016, 53, 1725–1734. [Google Scholar] [CrossRef]
- Pretzsch, H.; Forrester, D.I. Stand Dynamics of Mixed-Species Stands Compared with Monocultures. In Mixed Species Forests; Springer: Berlin, Germany, 2017; pp. 117–209. [Google Scholar]
- Pretzsch, H. Size-Structure Dynamics in Mixed Versus Monospecific Stands. In Mixed Species Forests; Springer: Berlin, Germany, 2017; pp. 211–269. [Google Scholar]
- Pretzsch, H.; del Río, M.; Schütze, G.; Ammer, C.; Annighöfer, P.; Avdagic, A.; Barbeito, I.; Bielak, K.; Brazaitis, G.; Coll, L.; et al. Mixing of Scots Pine (Pinus sylvestris L.) and European Beech (Fagus sylvatica L.) Enhances Structural Heterogeneity, And the Effect Increases with Water Availability. For. Ecol. Manag. 2016, 373, 149–166. [Google Scholar] [CrossRef]
- Assmann, E. The Principles of Forest Yield Study; Pergamon Press: Oxford, UK, 1970. [Google Scholar]
- Walter, H.; Lieth, H. Klimadiagramm. In Weltatlas; VEB Gustaf Fischer Verlag: Jena, Germany, 1967. [Google Scholar]
- Guijarro, J.A. “Climatol”: Climate Tools (Series Homogenization and Derived Products); R Package Version 3.1. 1; R Core Team: Vienna, Austria, 2019. [Google Scholar]
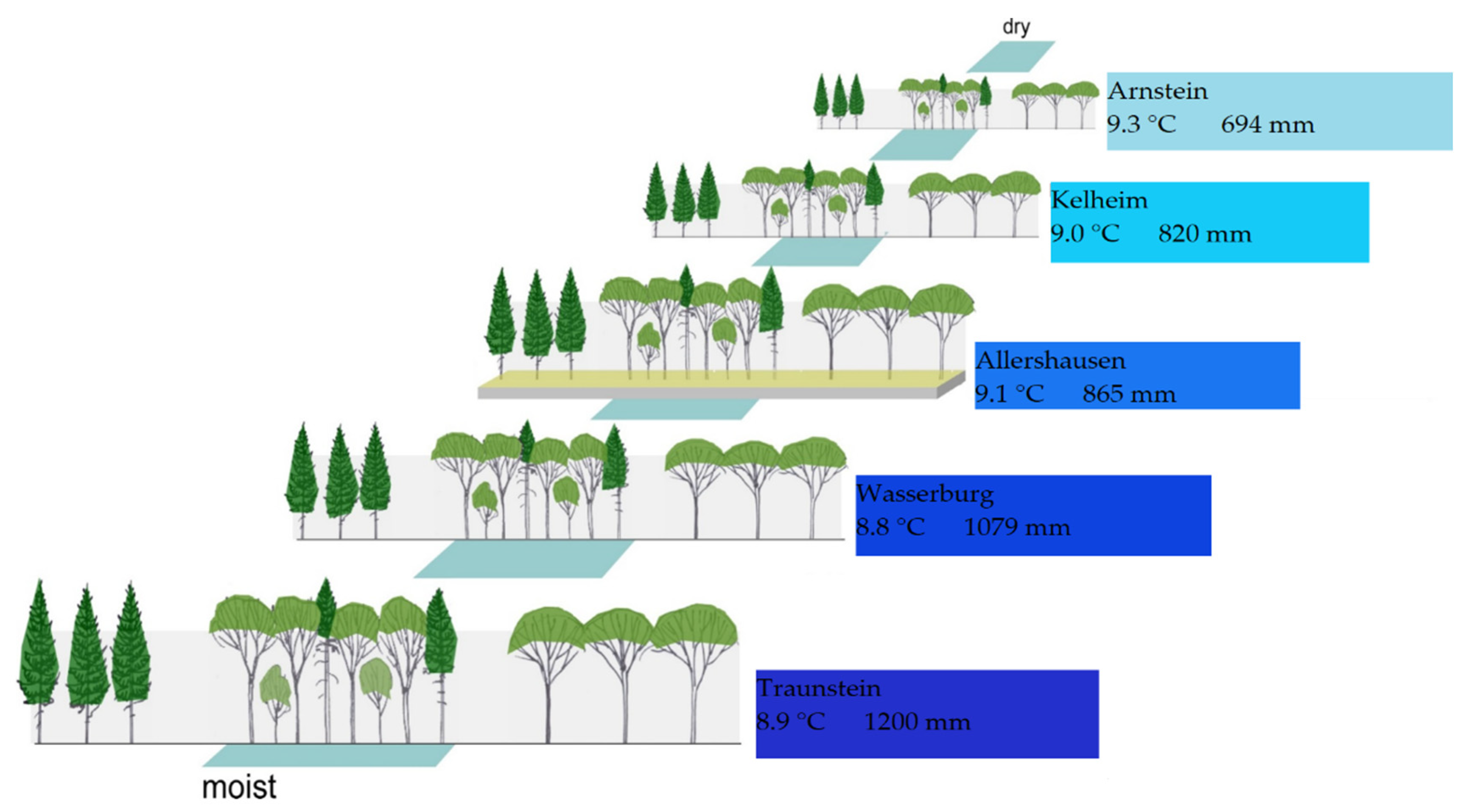
| Site | Code | Age | Geographical Location | E | A | T | P | M | Sub | ST | SI | SI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (years) | Lon | Lat | (°) | (m) | °C | (mm year−1) | (mm °C−1) | Be (mono) | Sp (mono) | ||||
| Arnstein | 1021 | 82 | 09°58′37.20″ | 49°54′10.80″ | - | 330 | 9.3 | 694 | 36 | LS | LUV | 25.4 | 36.4 |
| Kelheim | 1022 | 89 | 11°49′19.20″ | 48°56′08.16″ | 315 | 550 | 9 | 820 | 43 | LS | CAM | 33.4 | 38.4 |
| Allershausen | 1023 | 72 | 11°37′18.17″ | 48°25′53.23″ | - | 490 | 9.1 | 865 | 45 | TS | CAM | 32.8 | 31.5 |
| Wasserburg | 1024 | 61 | 12°04′22.80″ | 48°08′31.20″ | 180 | 620 | 8.8 | 1079 | 57 | MO | CAM | 35.3 | 35.8 |
| Traunstein | 1025 | 67 | 12°40′19.20″ | 47°56′20.40″ | 270 | 600 | 8.9 | 1200 | 63 | MO | CAM | 35.0 | 40.1 |
| Beech Mixed | Beech Pure | Spruce Mixed | Spruce Pure | Total | |
|---|---|---|---|---|---|
| All Cored | |||||
| N | 96 | 96 | 94 | 93 | 379 |
| Mean dbh | 35.4 ± 1.0 | 31.7 ± 1.0 | 37.0 ± 1.0 | 32.7 ± 0.9 | 34.2 ± 0.6 |
| Min–Max | 12.7–60.7 | 11–54.8 | 14.6–58.2 | 13–51.6 | 11–60.7 |
| Subsampled | |||||
| N | 77 | 81 | 71 | 75 | 304 |
| Mean dbh | 35.6 ± 1.2 | 31.7 ± 1.1 | 36.5 ± 1.1 | 33.0 ± 1.0 | 34.1 ± 0.6 |
| Min–Max | 12.7–60.7 | 11–54.8 | 15–58.2 | 13–51.6 | 11–60.7 |
| Species | Age | N | dq | hq | G | V | SDI | Mixing Proportion | IG | IV |
|---|---|---|---|---|---|---|---|---|---|---|
| years | (ha−1) | (cm) | (m) | (m2 ha−1) | (m3 ha−1) | (ha−1) | (%) | (m2 ha−1 year−1) | (m3 ha−1 year−1) | |
| Be (mixed) | 80 | 528 | 31.4 | 27.8 | 37 | 538 | 686.6 | 0.59 | 0.9 | 17.1 |
| 65–95 | 308–931 | 23.7–37.7 | 23.9–33.7 | 28.2–46.2 | 349.9–642.6 | 574–855 | 0.41–0.73 | 0.3–2.2 | 7.9–32.6 | |
| Be (pure) | 76 | 684 | 27.8 | 25.9 | 38 | 512 | 745.1 | 0.8 | 15.1 | |
| 59–100 | 365–1018 | 22.4–33.8 | 21.6–33.4 | 32.8–45.1 | 410.9–660.1 | 593–929 | 0.3–1.5 | 9.2–28.4 | ||
| Sp (mixed) | 80 | 545 | 36.4 | 30.6 | 56 | 813 | 980.7 | 0.41 | 1.2 | 21 |
| 65–95 | 427–699 | 31.7–44.1 | 27–34.8 | 45.8–74.9 | 622.5–1190.9 | 839–1217 | 0.27–0.59 | 0.5–2.3 | 12.2–33.3 | |
| Sp (pure) | 62 | 787 | 31.2 | 27.1 | 57 | 758 | 1073.8 | 1.5 | 25.2 | |
| 55–75 | 523–1000 | 24–35.9 | 22.2–31.3 | 45.4–75.6 | 499.7–1103.1 | 904–1415 | 0.5–2.9 | 12.7–41.4 | ||
| Be + Sp | 43 | 635 | 791.9 | 1 | 18.4 | |||||
| (mixed) | 35.6–46.5 | 450.1–795.2 | 701–891 | 0.5–2 | 11.1–30 |
| Tree Level | Beech | Spruce | |||||||
| Resilience | 2003 | 2015 | 2003 | 2015 | |||||
| H1 | |||||||||
| Value | SE | Value | SE | Value | SE | Value | SE | ||
| a0 | 1.406 ** | 0.207 | 1.034 ** | 0.055 | 0.845 ** | 0.071 | 1.440 ** | 0.208 | |
| C | a1 | −0.244 . | 0.131 | 0.021 | 0.053 | −0.031 | 0.037 | 0.202 * | 0.095 |
| G | a2 | −4.934 * | 2.270 | −0.568 | 0.521 | −0.508 | 0.562 | −2.179 * | 1.058 |
| H2 | |||||||||
| a0 | 0.869 | 0.845 | 1.094 ** | 0.188 | 0.515 * | 0.245 | 2.502 * | 0.682 | |
| C | a1 | 0.635 | 0.606 | −0.397 | 0.263 | −0.066 | 0.156 | 0.833 . | 0.453 |
| M | a2 | 0.016 | 0.022 | −0.002 | 0.005 | 0.009 | 0.006 | −0.028 | 0.018 |
| C*M | a3 | −0.023 | 0.015 | 0.011 | 0.007 | 0.001 | 0.004 | −0.017 | 0.012 |
| G | a4 | −5.924 * | 2.280 | −0.438 | 0.523 | −0.449 | 0.562 | −2.336 * | 1.054 |
| Resistance | |||||||||
| H1 | |||||||||
| a0 | 0.976 ** | 0.082 | 1.493 ** | 0.113 | 0.796 ** | 0.047 | 1.177 ** | 0.185 | |
| C | a1 | 0.003 | 0.053 | −0.111 | 0.066 | 0.015 | 0.032 | 0.240 ** | 0.106 |
| G | a2 | −2.527 ** | 0.749 | −0.887 | 0.832 | −1.826 ** | 0.470 | −4.04 . | 1.181 |
| H2 | |||||||||
| a0 | 0.611 ** | 0.245 | 1.66 ** | 0.497 | 0.562 ** | 0.162 | 2.078 ** | 0.545 | |
| C | a1 | −0.147 | 0.237 | −0.635 | 0.385 | 0.110 | 0.133 | 0.798 | 0.509 |
| M | a2 | 0.010 | 0.006 | −0.005 | 0.013 | 0.006 | 0.004 | −0.024 | 0.014 |
| C*M | a3 | 0.004 | 0.006 | 0.014 | 0.010 | −0.003 | 0.004 | −0.015 | 0.013 |
| G | a4 | −2.339 ** | 0.755 | −0.636 | 0.899 | −1.775 ** | 0.470 | −4.05 ** | 1.169 |
| Stand Level | Resilience | Resistance | Growth Loss | ||||
|---|---|---|---|---|---|---|---|
| H3 | |||||||
| Value | SE | Value | SE | Value | SE | ||
| 2003 | a0 | 0.884 ** | 0.042 | 0.800 ** | 0.030 | 0.316 ** | 0.063 |
| C | a1 | 0.004 | 0.034 | 0.002 | 0.026 | −0.006 | 0.047 |
| 2015 | a0 | 1.058 ** | 0.039 | 1.040 ** | 0.049 | −0.098 | 0.074 |
| C | a1 | 0.010 | 0.022 | −0.056 | 0.028 | 0.046 | 0.048 |
| H4 | |||||||
| 2003 | a0 | 0.64 * | 0.171 | 0.617 * | 0.113 | 0.74 * | 0.224 |
| C | a1 | 0.107 | 0.156 | 0.029 | 0.126 | −0.136 | 0.221 |
| M | a2 | 0.007 | 0.005 | 0.004 | 0.003 | −0.011 | 0.006 |
| C*M | a3 | −0.003 | 0.004 | −0.001 | 0.003 | 0.004 | 0.006 |
| 2015 | a0 | 1.315 ** | 0.150 | 1.442 ** | 0.170 | −0.76 * | 0.187 |
| C | a1 | 0.012 | 0.124 | −0.155 | 0.146 | 0.143 | 0.260 |
| M | a2 | −0.007 | 0.004 | −0.011 . | 0.005 | 0.018 * | 0.005 |
| C*M | a3 | −0.000 | 0.003 | 0.003 | 0.004 | −0.003 | 0.007 |
| Tree Level | Stand Level | ||||||
|---|---|---|---|---|---|---|---|
| Beech | Spruce | Mixed/Mono | |||||
| Resilience | 2003 | 2015 | 2003 | 2015 | Resilience | 2003 | 2015 |
| Equation (8) | Equation (10) | ||||||
| C | -. | + | C | ||||
| G | - | - | G | ||||
| Equation (9) | Equation (11) | ||||||
| C | +. | C | |||||
| M | M | ||||||
| C*M | C*M | ||||||
| G | - | - | |||||
| Resistance | Resistance | ||||||
| Equation (10) | Equation (10) | ||||||
| C | ++ | C | |||||
| G | -- | -- | -. | ||||
| Equation (11) | Equation (11) | ||||||
| C | C | ||||||
| M | M | -. | |||||
| C*M | C*M | ||||||
| G | -- | -- | -- | ||||
| Growth Losses | |||||||
| Equation (10) | |||||||
| C | |||||||
| Equation (11) | |||||||
| C | |||||||
| M | + | ||||||
| C*M | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rukh, S.; Poschenrieder, W.; Heym, M.; Pretzsch, H. Drought Resistance of Norway Spruce (Picea abies [L.] Karst) and European Beech (Fagus sylvatica [L.]) in Mixed vs. Monospecific Stands and on Dry vs. Wet Sites. From Evidence at the Tree Level to Relevance at the Stand Level. Forests 2020, 11, 639. https://doi.org/10.3390/f11060639
Rukh S, Poschenrieder W, Heym M, Pretzsch H. Drought Resistance of Norway Spruce (Picea abies [L.] Karst) and European Beech (Fagus sylvatica [L.]) in Mixed vs. Monospecific Stands and on Dry vs. Wet Sites. From Evidence at the Tree Level to Relevance at the Stand Level. Forests. 2020; 11(6):639. https://doi.org/10.3390/f11060639
Chicago/Turabian StyleRukh, Shah, Werner Poschenrieder, Michael Heym, and Hans Pretzsch. 2020. "Drought Resistance of Norway Spruce (Picea abies [L.] Karst) and European Beech (Fagus sylvatica [L.]) in Mixed vs. Monospecific Stands and on Dry vs. Wet Sites. From Evidence at the Tree Level to Relevance at the Stand Level" Forests 11, no. 6: 639. https://doi.org/10.3390/f11060639
APA StyleRukh, S., Poschenrieder, W., Heym, M., & Pretzsch, H. (2020). Drought Resistance of Norway Spruce (Picea abies [L.] Karst) and European Beech (Fagus sylvatica [L.]) in Mixed vs. Monospecific Stands and on Dry vs. Wet Sites. From Evidence at the Tree Level to Relevance at the Stand Level. Forests, 11(6), 639. https://doi.org/10.3390/f11060639





