Herbivore Gender Effects on Volatile Induction in Aspen and on Olfactory Responses in Leaf Beetles
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Insects
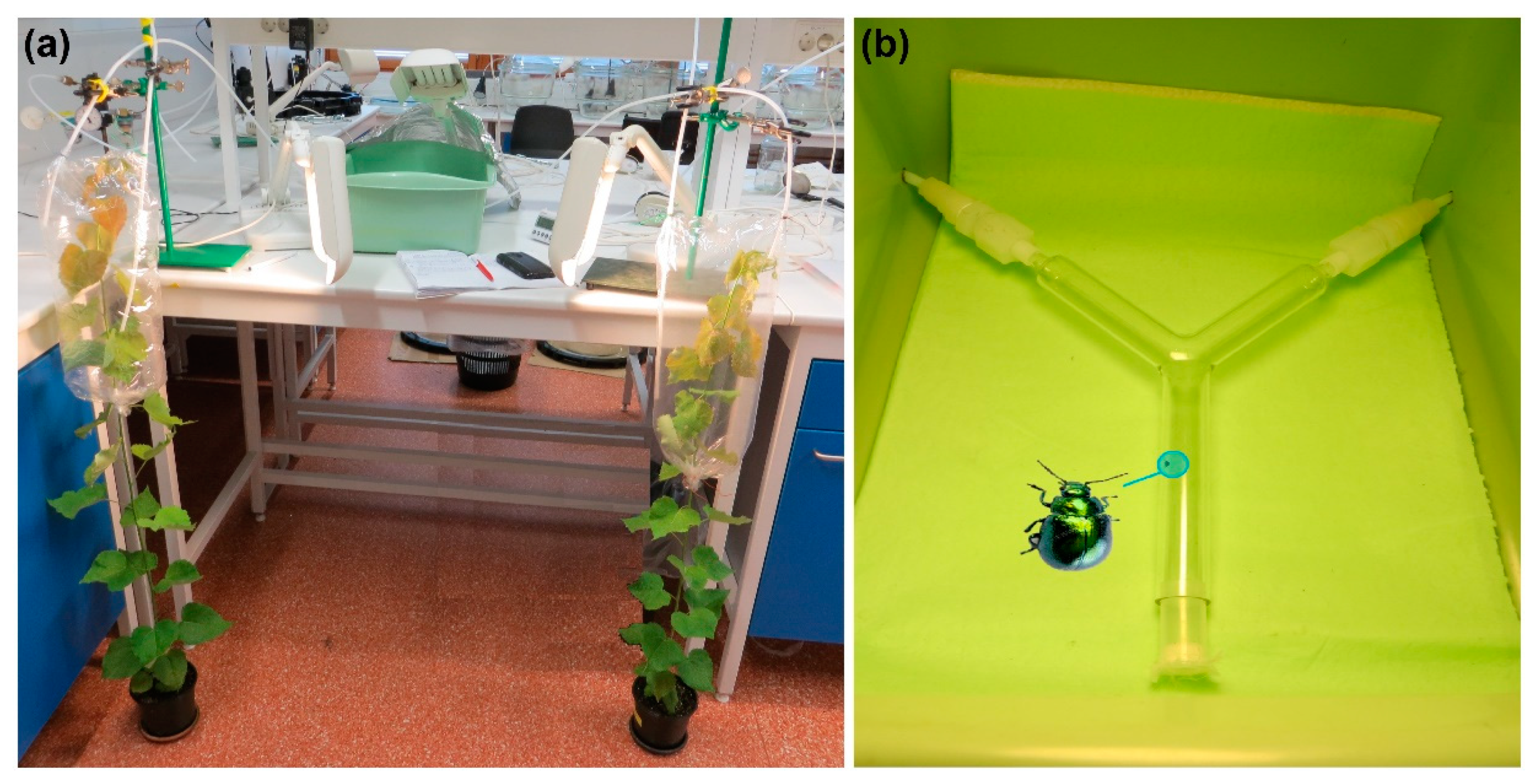
2.2. Odor Sources for Dual-Choice Olfactory Bioassays
2.3. Dual-Choice Olfactory Bioassays
2.4. VOC Collection and Analysis
2.5. Statistical Analysis
3. Results
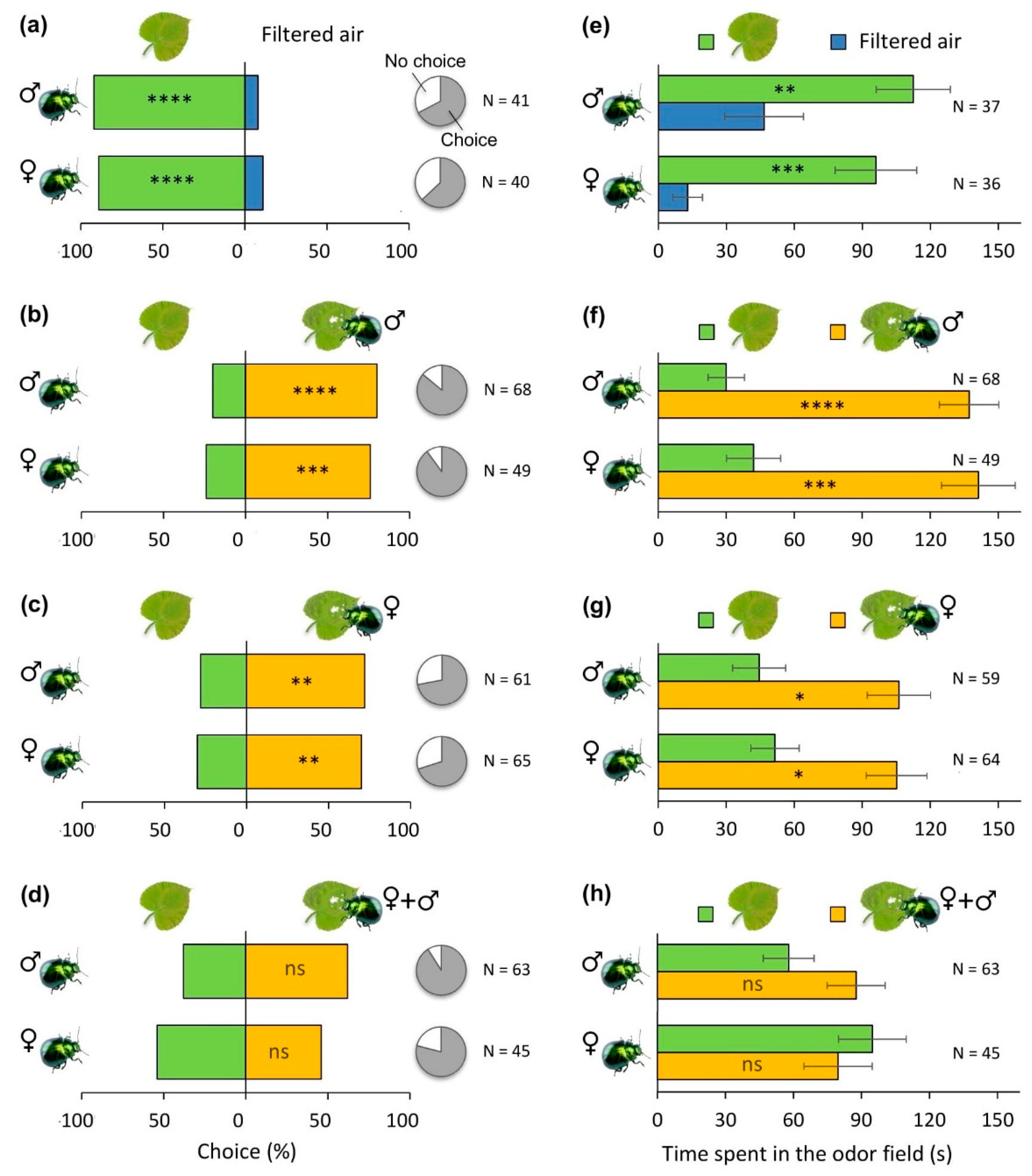
3.1. Role of Plant Volatiles in Host Orientation Behaviour of P. laticollis Adults
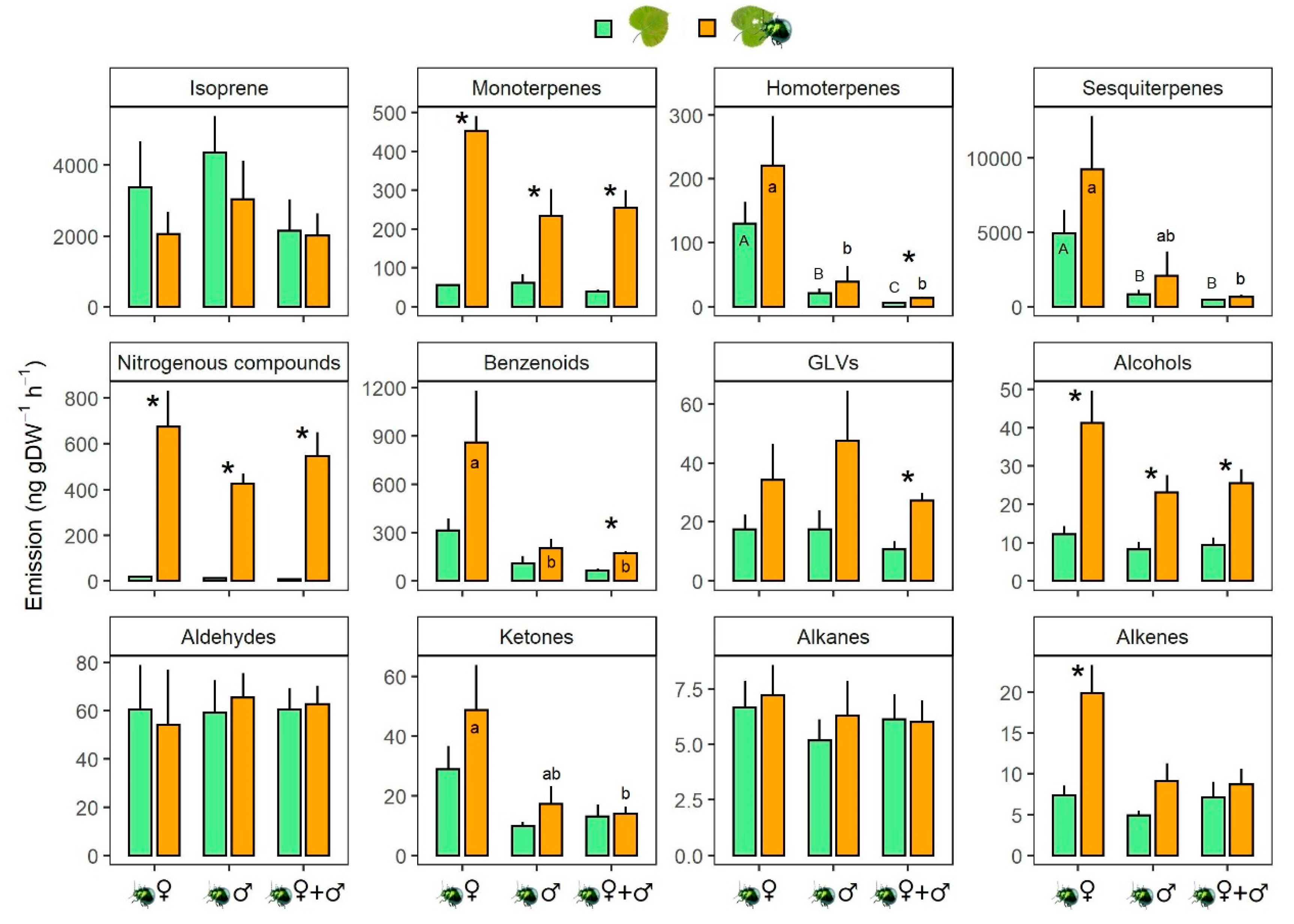
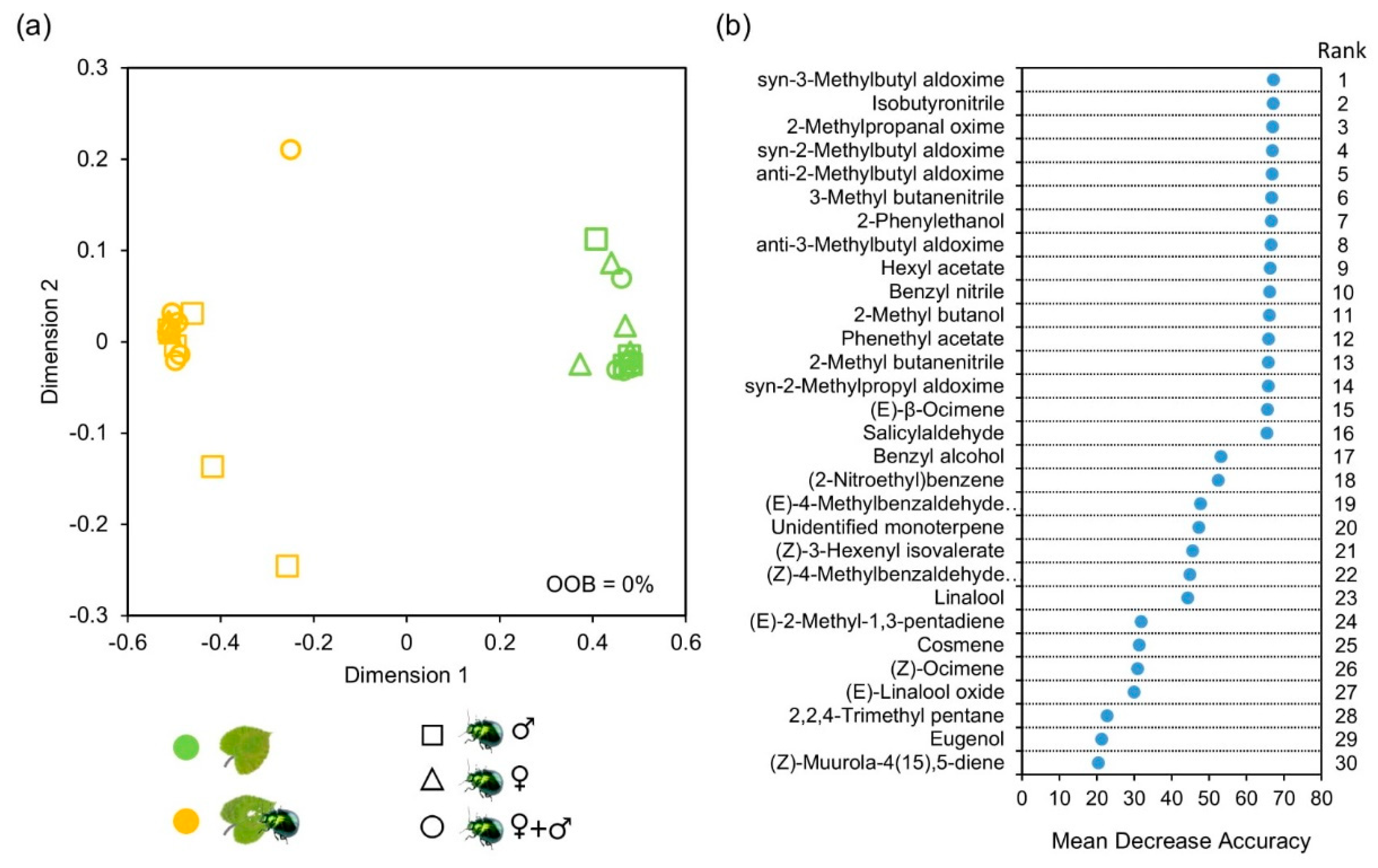
3.2. Effects of P. laticollis Herbivory on Host Volatile Emission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aljbory, Z.; Chen, M. Indirect plant defense against insect herbivores: A review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Xiao, L.; Carrillo, J.; Siemann, E.; Ding, J. Herbivore-specific induction of indirect and direct defensive responses in leaves and roots. Aob. Plants 2019, 11, plz003. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Huber, D.; Bohlmann, J. Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa × deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (-)-germacrene D synthase, PtdTPS1. Plant J. 2004, 37, 603–616. [Google Scholar] [CrossRef]
- Staudt, M.; Lhoutellier, L. Volatile organic compound emission from holm oak infested by gypsy moth larvae: Evidence for distinct responses in damaged and undamaged leaves. Tree Physiol. 2007, 27, 1433–1440. [Google Scholar] [CrossRef]
- Wu, J.; Baldwin, I.T. Herbivory-induced signalling in plants: Perception and action. Plant Cell Environ. 2009, 32, 1161–1174. [Google Scholar] [CrossRef]
- Li, T.; Holopainen, J.K.; Kokko, H.; Tervahauta, A.I.; Blande, J.D. Herbivore-induced aspen volatiles temporally regulate two different indirect defences in neighbouring plants. Funct. Ecol. 2012, 26, 1176–1185. [Google Scholar] [CrossRef]
- Frost, C.J.; Appel, M.; Carlson, J.E.; De Moraes, C.M.; Mescher, M.C.; Schultz, J.C. Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol. Lett. 2007, 10, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Kost, C. Priming of indirect defences. Ecol. Lett. 2006, 9, 813–817. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Schultz, J.C. Rapid changes in tree leaf chemistry induced by damage: Evidence for communication between plants. Science 1983, 221, 277–279. [Google Scholar] [CrossRef]
- Lackner, S.; Lackus, N.D.; Paetz, C.; Koellner, T.G.; Unsicker, S.B. Aboveground phytochemical responses to belowground herbivory in poplar trees and the consequence for leaf herbivore preference. Plant Cell Environ. 2019, 42, 3293–3307. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.C.; Boeckler, G.A.; Koellner, T.G.; Gershenzon, J.; Unsicker, S.B. The timing of herbivore-induced volatile emission in black poplar (Populus nigra) and the influence of herbivore age and identity affect the value of individual volatiles as cues for herbivore enemies. BMC Plant Biol. 2014, 14, 304. [Google Scholar] [CrossRef]
- McCormick, A.C.; Irmisch, S.; Boeckler, G.A.; Gershenzon, J.; Koellner, T.G.; Unsicker, S.B. Herbivore-induced volatile emission from old-growth black poplar trees under field conditions. Sci. Rep. 2019, 9, 7714. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.C.; Irmisch, S.; Reinecke, A.; Boeckler, G.A.; Veit, D.; Reichelt, M.; Hansson, B.S.; Gershenzon, J.; Koellner, T.G.; Unsicker, S.B. Herbivore-induced volatile emission in black poplar: Regulation and role in attracting herbivore enemies. Plant Cell Environ. 2014, 37, 1909–1923. [Google Scholar] [CrossRef]
- Cope, O.L.; Lindroth, R.L. Clonal Saplings of Trembling Aspen Do Not Coordinate Defense Induction. J. Chem. Ecol. 2018, 44, 1045–1050. [Google Scholar] [CrossRef]
- Fabisch, T.; Gershenzon, J.; Unsicker, S.B. Specificity of Herbivore Defense Responses in a Woody Plant, Black Poplar (Populus nigra). J. Chem. Ecol. 2019, 45, 162–177. [Google Scholar] [CrossRef]
- Li, T.; Blande, J.D. Volatile-Mediated within-Plant Signaling in Hybrid Aspen: Required for Systemic Responses. J. Chem. Ecol. 2017, 43, 327–338. [Google Scholar] [CrossRef]
- Frost, C.J.; Mescher, M.C.; Dervinis, C.; Davis, J.M.; Carlson, J.E.; De Moraes, C.M. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 2008, 180, 722–733. [Google Scholar] [CrossRef]
- Williams, L.; Rodriguez-Saona, C.; Pare, P.W.; Crafts-Brandner, S.J. The piercing-sucking herbivores Lygus hesperus and Nezara viridula induce volatile emissions in plants. Arch. Insect Biochem. Physiol. 2005, 58, 84–96. [Google Scholar] [CrossRef]
- Rim, H.; Uefune, M.; Ozawa, R.; Takabayashi, J. An omnivorous arthropod, Nesidiocoris tenuis, induces gender-specific plant volatiles to which conspecific males and females respond differently. Arthropod Plant Interact 2018, 12, 495–503. [Google Scholar] [CrossRef]
- Gols, R. Direct and indirect chemical defences against insects in a multitrophic framework. Plant Cell Environ. 2014, 37, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Aartsma, Y.; Bianchi, F.J.; van der Werf, W.; Poelman, E.H.; Dicke, M. Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol. 2017, 216, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Blande, J.D. Plant communication with herbivores. Adv. Bot. Res. 2017, 82, 281–304. [Google Scholar] [CrossRef]
- Dicke, M. Behavioural and community ecology of plants that cry for help. Plant Cell Environ. 2009, 32, 654–665. [Google Scholar] [CrossRef]
- Vet, L.; Wackers, F.L.; Dicke, M. How to hunt for hiding hosts-the reliability-detectability problem in foraging parasitoids. Neth. J. Zool. 1991, 41, 202–213. [Google Scholar]
- Xu, H.; Desurmont, G.; Degen, T.; Zhou, G.; Laplanche, D.; Henryk, L.; Turlings, T.C.J. Combined use of herbivore-induced plant volatiles and sex pheromones for mate location in braconid parasitoids. Plant Cell Environ. 2017, 40, 330–339. [Google Scholar] [CrossRef]
- Fahlvik, N.; Rytter, L.; Stener, L. Production of hybrid aspen on agricultural land during one rotation in southern Sweden. J. For. Res. 2019, 1–9. [Google Scholar] [CrossRef]
- Tullus, A.; Rytter, L.; Tullus, T.; Weih, M.; Tullus, H. Short-rotation forestry with hybrid aspen (Populus tremula L. × P. tremuloides Michx.) in Northern Europe. Scand. J. For. Res. 2012, 27, 10–29. [Google Scholar] [CrossRef]
- Hytonen, J.; Beuker, E.; Vihera-Aarnio, A. Clonal variation in basic density, moisture content and heating value of wood, bark and branches in hybrid aspen. Silva Fenn. 2018, 52, 9938. [Google Scholar] [CrossRef]
- Freiwald, V.; Haikio, E.; Julkunen-Tiitto, R.; Holopainen, J.K.; Oksanen, E. Elevated ozone modifies the feeding behaviour of the common leaf weevil on hybrid aspen through shifts in developmental, chemical, and structural properties of leaves. Entomol. Exp. Appl. 2008, 128, 66–72. [Google Scholar] [CrossRef]
- Kopf, A.; Rank, N.E.; Roininen, H.; Julkunen-Tiitto, R.; Pasteels, J.M.; Tahvanainen, J. The evolution of host-plant use and sequestration in the leaf beetle genus Phratora (Coleoptera: Chrysomelidae). Evolution 1998, 52, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Saalas, U. Suomen Metsähyönteiset [Finnish Forest Insects]; WSOY: Helsinki, Finland, 1949; p. 719. [Google Scholar]
- Johnsen, L.G.; Skou, P.B.; Khakimov, B.; Bro, R. Gas chromatography—Mass spectrometry data processing made easy. J. Chromatogr. A 2017, 1503, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, Y.; Borges, R.M. Reducing the babel in plant volatile communication: Using the forest to see the trees. Plant Biol. 2010, 12, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Zalucki, M.P.; Madden, J.L.; Patel, V.S.; Paterson, S.C. Local dispersion of the Eucalyptus leaf beetle Chrysophtharta bimaculata (Coleoptera: Chrysomelidae), and implications for forest protection. J. Appl. Ecol. 1997, 34, 807–816. [Google Scholar] [CrossRef]
- Moayeri, H.R.S.; Ashouri, A.; Brodsgaard, H.F.; Enkegaard, A. Males of the predatory mirid bug Macrolophus caliginosus exploit plant volatiles induced by conspecifics as a sexual synomone. Entomol. Exp. Appl. 2007, 123, 49–55. [Google Scholar] [CrossRef]
- Brilli, F.; Ciccioli, P.; Frattoni, M.; Prestininzi, M.; Spanedda, A.F.; Loreto, F. Constitutive and herbivore-induced monoterpenes emitted by Populus × euroamericana leaves are key volatiles that orient Chrysomela populi beetles. Plant Cell Environ. 2009, 32, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Austel, N.; Bjorkman, C.; Hilker, M.; Meiners, T. Phenotypic plasticity in host plant preference of the willow leaf beetle Phratora vulgatissima: The impact of experience made by adults. Agric. For. Entomol. 2014, 16, 417–425. [Google Scholar] [CrossRef]
- Copolovici, L.; Kaennaste, A.; Remmel, T.; Vislap, V.; Niinemets, U. Volatile emissions from Alnus glutionosa induced by herbivory are quantitatively related to the extent of damage. J. Chem. Ecol. 2011, 37, 18–28. [Google Scholar] [CrossRef]
- Miresmailli, S.; Gries, R.; Gries, G.; Zamar, R.H.; Isman, M.B. Population density and feeding duration of cabbage looper larvae on tomato plants alter the levels of plant volatile emissions. Pest Manag. Sci. 2012, 68, 101–107. [Google Scholar] [CrossRef]
- Yli-Pirilä, P.; Copolovici, L.; Kannaste, A.; Noe, S.; Blande, J.D.; Mikkonen, S.; Klemola, T.; Pulkkinen, J.; Virtanen, A.; Laaksonen, A.; et al. Herbivory by an Outbreaking Moth Increases Emissions of Biogenic Volatiles and Leads to Enhanced Secondary Organic Aerosol Formation Capacity. Environ. Sci. Technol. 2016, 50, 11501–11510. [Google Scholar] [CrossRef]
- Kos, M.; Houshyani, B.; Overeem, A.; Bouwmeester, H.J.; Weldegergis, B.T.; van Loon, J.J.; Dicke, M.; Vet, L.E.M. Genetic engineering of plant volatile terpenoids: Effects on a herbivore, a predator and a parasitoid. Pest Manag. Sci. 2013, 69, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ikeda, A.; Shiojiri, K.; Ozawa, R.; Shiki, K.; Nagai-Kunihiro, N.; Fujita, K.; Sugimoto, K.; Yamato, K.T.; Dohra, H.; et al. Identification of a hexenal reductase that modulates the composition of green leaf volatiles. Plant Physiol. 2018, 178, 552–564. [Google Scholar] [CrossRef]
- Mäntylä, E.; Alessio, G.A.; Blande, J.D.; Heijari, J.; Holopainen, J.K.; Laaksonen, T.; Piirtola, P.; Klemola, T. From plants to birds: Higher avian predation rates in trees responding to insect herbivory. PLoS ONE 2008, 3, e2832. [Google Scholar]
- Allmann, S.; Baldwin, I.T. Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science 2010, 329, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Loughrin, J.H.; Potter, D.A.; Hamilton-Kemp, T.R. Volative Compounds Induced by Herbivory Act as Aggregation Kairomones for the Japanese-Beetle (Popillia japonica Newman). J. Chem. Ecol. 1995, 21, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, G.; Cai, X.; Jin, S.; Gao, Y.; Chen, Z. The Tea Weevil, Myllocerinus aurolineatus, is Attracted to Volatiles Induced by Conspecifics. J. Chem. Ecol. 2010, 36, 388–395. [Google Scholar] [CrossRef]
- Copolovici, L.; Kaennaste, A.; Remmel, T.; Niinemets, U. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ. Exp. Bot. 2014, 100, 55–63. [Google Scholar] [CrossRef]
- Maja, M.M.; Kasurinen, A.; Yli-Pirila, P.; Joutsensaari, J.; Klemola, T.; Holopainen, T.; Holopainen, J.K. Contrasting responses of silver birch VOC emissions to short- and long-term herbivory. Tree Physiol. 2014, 34, 241–252. [Google Scholar] [CrossRef]
- Kugimiya, S.; Shimoda, T.; Tabata, J.; Takabayashi, J. Present or Past Herbivory: A screening of volatiles released from Brassica rapa under caterpillar attacks as attractants for the solitary parasitoid, Cotesia vestalis. J. Chem. Ecol. 2010, 36, 620–628. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Grauer-Gray, K.; Holopainen, J.K.; Blande, J.D. Herbivore Gender Effects on Volatile Induction in Aspen and on Olfactory Responses in Leaf Beetles. Forests 2020, 11, 638. https://doi.org/10.3390/f11060638
Li T, Grauer-Gray K, Holopainen JK, Blande JD. Herbivore Gender Effects on Volatile Induction in Aspen and on Olfactory Responses in Leaf Beetles. Forests. 2020; 11(6):638. https://doi.org/10.3390/f11060638
Chicago/Turabian StyleLi, Tao, Kristen Grauer-Gray, Jarmo K. Holopainen, and James D. Blande. 2020. "Herbivore Gender Effects on Volatile Induction in Aspen and on Olfactory Responses in Leaf Beetles" Forests 11, no. 6: 638. https://doi.org/10.3390/f11060638
APA StyleLi, T., Grauer-Gray, K., Holopainen, J. K., & Blande, J. D. (2020). Herbivore Gender Effects on Volatile Induction in Aspen and on Olfactory Responses in Leaf Beetles. Forests, 11(6), 638. https://doi.org/10.3390/f11060638






