Implications of Reduced Stand Density on Tree Growth and Drought Susceptibility: A Study of Three Species under Varying Climate
Abstract
1. Introduction
2. Material and Methods
2.1. Study Sites and Sampling Design
2.2. Data Collection and Preparation
2.3. Dendrochronological Evaluation
2.4. Meteorological Data and Selection of Drought Events
2.5. Quantification of Tree Population Density
2.6. Assessment of Mean Tree Growth, Mean Tree Growth Sensitivity and Short-Term Growth Responses to Episodic Drought Stress
2.7. Statistical Testing
3. Results
3.1. Tree Ring Series and Their Basic Statistics
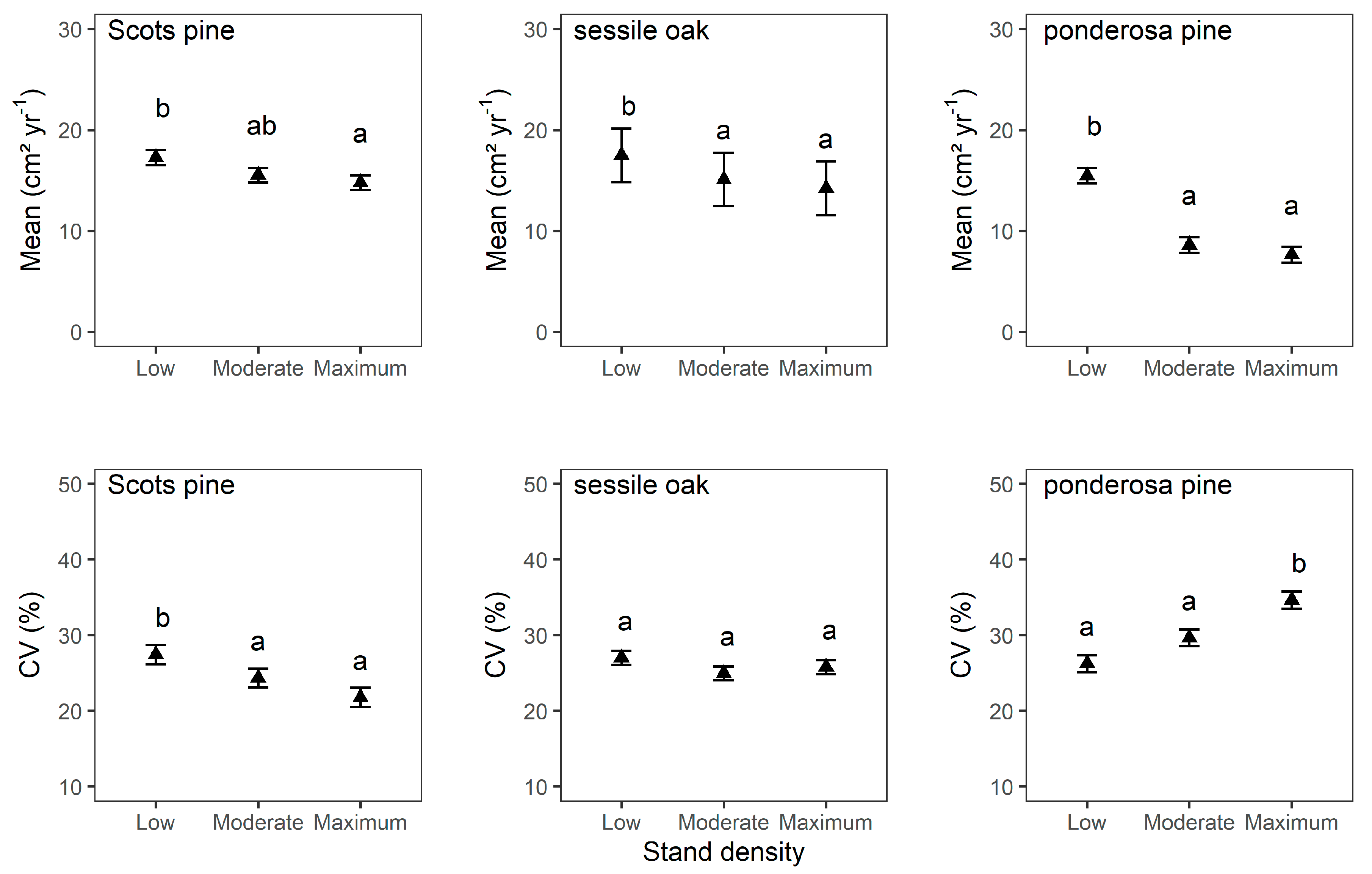
3.2. Mean Tree Growth and Mean Tree Growth Sensitivity
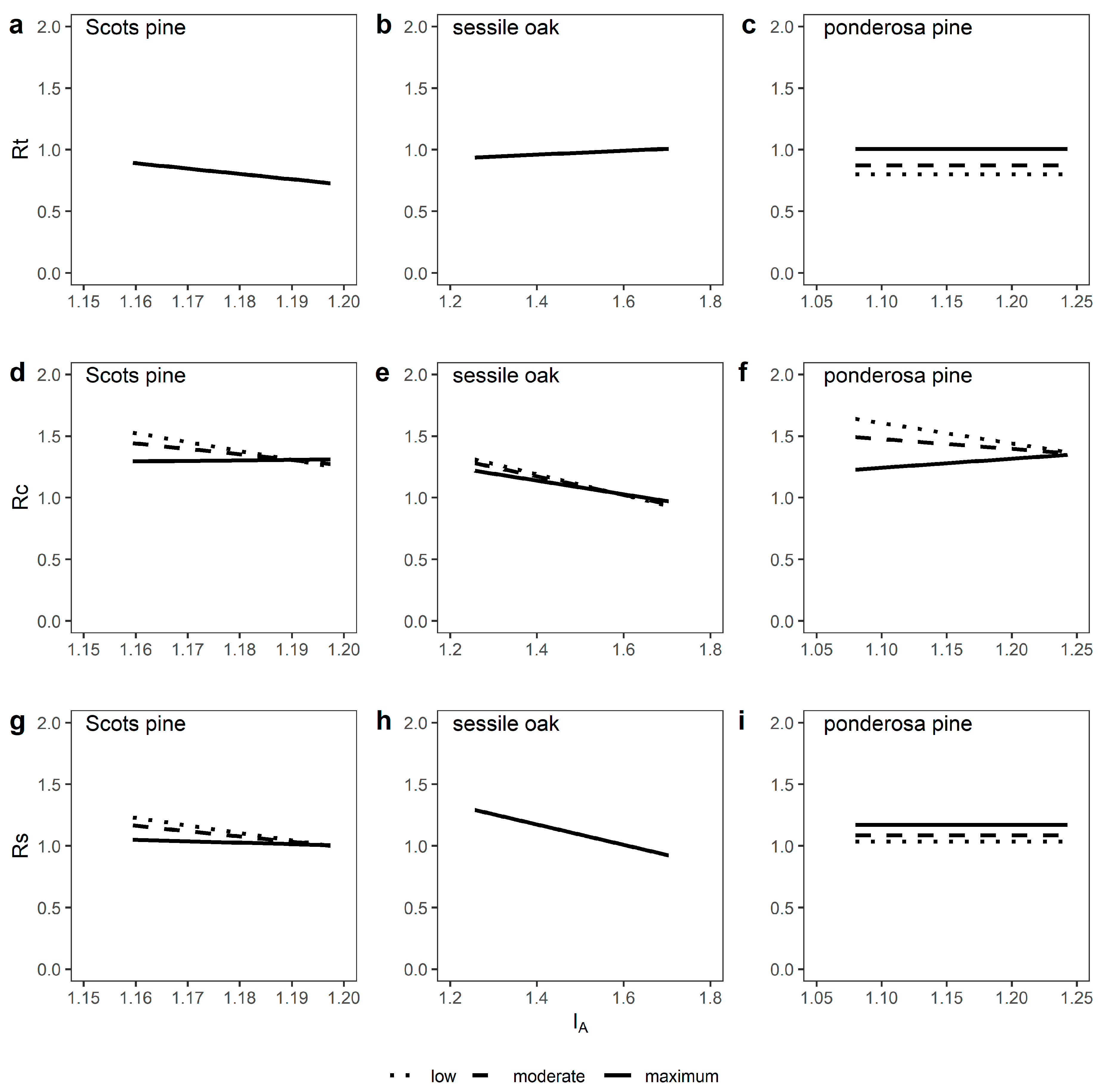
3.3. Growth Responses to Episodic Drought Stress
4. Discussion
4.1. Stand Density Influences Mean Tree Growth and Mean Tree Growth Sensitivity
4.2. Tree Growth Reactions to Episodic Drought Stress are Shaped by Relative Stand Density, Site Water Availability and Stand Age
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Site | Species | Thinning History (Year of Thinning and Sampling) | Drought Years (SPEI) |
|---|---|---|---|
| DE 1 | sessile oak | 2004, 2009, 2014 | 2015 (−1.5) |
| DE 2 | sessile oak | 1981, 1986, 1991, 1996, 2002, 2010 | 2003 (−1.6), 2015 (−1.8) |
| DE 3 | sessile oak | 1934, 1942, 1952, 1958, 1966, 1976, 1986, 1996, 2006, 2013 | 2003 (−1.6), 2015 (−1.8) |
| DE 4 | Scots pine | 1966, 1977, 1986, 1993, 1998, 2003, 2008, 2015 | 1976 (−1.2) |
| DE 5 | Scots pine | 2002 | 2003 (−2.1), 2015 (−1.4) |
| DE 6 | Scots pine | 1987, 1992, 1997, 2002, 2007, 2014 | 2003 (−1.7), 2015 (−1.6) |
| US 1 | ponderosa pine | 1925, 1934 | 1989 (−1.4), 2002 (−2.1), 2009 (−1.3) |
| US 2 | ponderosa pine | 1924, 1935, 1946, 1967, 1968, 1988, 1997 | 2002 (−2.2), 2009 (−1.3) |
| US 3 | ponderosa pine | 1925, 1936 | 1989 (−1.0), 2002 (−2.2), 2009 (−1.3) |
| Species | Site | SD | n | dbh | h | MRBA | Rbar | EPS |
|---|---|---|---|---|---|---|---|---|
| cm | m | |||||||
| sessile oak | DE 1 | Low | 15 | 21.8 | 18.5 | 0.44 | 0.55 | 0.95 |
| Mod | 15 | 18.5 | 18.3 | 0.65 | 0.45 | 0.92 | ||
| Max | 15 | 18.3 | 19.0 | 1.00 | 0.56 | 0.95 | ||
| DE 2 | Low | 15 | 38.4 | 26.2 | 0.60 | 0.54 | 0.94 | |
| Mod | 15 | 34.8 | 27.2 | 0.73 | 0.56 | 0.94 | ||
| Max | 15 | 28.2 | 28.3 | 1.00 | 0.52 | 0.94 | ||
| DE 3 | Low | 15 | 48.7 | 31.1 | 0.80 | 0.50 | 0.92 | |
| Mod | 15 | 44.0 | 30.5 | 0.86 | 0.59 | 0.95 | ||
| Max | 15 | 35.5 | 29.0 | 1.00 | 0.54 | 0.94 | ||
| Scots pine | DE 4 | Low | 15 | 30.4 | 20.8 | 0.47 | 0.41 | 0.90 |
| Mod | 16 | 25.5 | 20.7 | 0.58 | 0.44 | 0.92 | ||
| Max | 15 | 28.1 | 24.7 | 1.00 | 0.43 | 0.91 | ||
| DE 5 | Low | 17 | 38.3 | 23.5 | 0.30 | 0.40 | 0.91 | |
| Mod | 15 | 37.0 | 24.7 | 0.56 | 0.44 | 0.91 | ||
| Max | 15 | 30.6 | 24.9 | 1.00 | 0.52 | 0.94 | ||
| DE 6 | Low | 15 | 24.1 | 18.2 | 0.50 | 0.57 | 0.95 | |
| Mod | 15 | 15.6 | 13.7 | 0.70 | 0.48 | 0.93 | ||
| Max | 17 | 14.9 | 14.5 | 1.00 | 0.49 | 0.94 | ||
| ponderosa pine | US 1 | Low | 15 | 40.8 | 17.1 | 0.30 | 0.47 | 0.93 |
| Mod | 15 | 35.1 | 19.2 | 0.52 | 0.29 | 0.84 | ||
| Max | 15 | 27.6 | 19.4 | 1.00 | 0.33 | 0.88 | ||
| US 2 | Low | 15 | 31.3 | 14.0 | 0.32 | 0.55 | 0.90 | |
| Mod | 15 | 27.4 | 13.4 | 0.61 | 0.58 | 0.95 | ||
| Max | 15 | 22.3 | 12.7 | 1.00 | 0.44 | 0.92 | ||
| US 3 | Low | 15 | 36.8 | 13.8 | 0.31 | 0.32 | 0.84 | |
| Mod | 15 | 33.6 | 17.9 | 0.56 | 0.35 | 0.88 | ||
| Max | 15 | 23.7 | 15.6 | 1.00 | 0.18 | 0.74 |
| Species | Response Variable | Statistic | Intercept | ba | SDmod | SDmax |
|---|---|---|---|---|---|---|
| a0 | a1 | a2 | a3 | |||
| Scots pine | Mean | Estimate | 1.45 | 0.02 *** | −1.73 . | −2.48 * |
| SE | 1.70 | 0.00 | 0.98 | 1.01 | ||
| CV | Estimate | 31.59 *** | −0.01 *** | −3.09 * | −5.65 *** | |
| SE | 2.64 | 0.00 | 1.19 | 1.23 | ||
| sessile oak | Mean | Estimate | 3.93 | 0.01 *** | −2.41 ** | −3.27 *** |
| SE | 4.85 | 0.00 | 0.80 | 0.92 | ||
| CV | Estimate | 27.68 *** | −0.00 | −2.03 | −1.21 | |
| SE | 2.30 | 0.00 | 1.30 | 1.46 | ||
| ponderosa pine | Mean | Estimate | 9.24 *** | 0.01 *** | −6.87 *** | −7.84 *** |
| SE | 1.69 | 0.00 | 0.83 | 0.96 | ||
| CV | Estimate | 27.91 *** | −0.00 | 3.40 * | 8.38 *** | |
| SE | 2.74 | 0.00 | 1.62 | 1.87 |
References
- Spinoni, J.; Vogt, J.V.; Naumann, G.; Barbosa, P.; Dosio, A. Will drought events become more frequent and severe in Europe? Int. J. Climatol. 2018, 38, 1718–1736. [Google Scholar] [CrossRef]
- Wilhite, D.A. (Ed.) Drought Assessment, Management, and Planning, Theory and Case Studies; Kluwer Acad Publ Boston: Boston, MA, USA, 1993; ISBN 0792393376. [Google Scholar]
- Gleason, K.E.; Bradford, J.B.; Bottero, A.; D’Amato, A.W.; Fraver, S.; Palik, B.J.; Battaglia, M.A.; Iverson, L.; Kenefic, L.; Kern, C.C. Competition amplifies drought stress in forests across broad climatic and compositional gradients. Ecosphere 2017, 8, e01849. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D.; et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang. 2013, 3, 292–297. [Google Scholar] [CrossRef]
- Knoke, T.; Ammer, C.; Stimm, B.; Mosandl, R. Admixing broadleaved to coniferous tree species A review on yield, ecological stability and economics. Eur. J. Forest. Res. 2008, 127, 89–101. [Google Scholar] [CrossRef]
- Bolte, A.; Degen, B. Forest adaptation to climate change-Options and limitations. Landbauforschung Volkenrode 2010, 60, 111–118. [Google Scholar]
- Brang, P.; Spathelf, P.; Larsen, J.B.; Bauhus, J.; Boncčìna, A.; Chauvin, C.; Drössler, L.; García-Güemes, C.; Heiri, C.; Kerr, G.; et al. Suitability of close-to-nature silviculture for adapting temperate European forests to climate change. Forestry 2014, 87, 492–503. [Google Scholar] [CrossRef]
- Bottero, A.; D’Amato, A.W.; Palik, B.J.; Bradford, J.B.; Fraver, S.; Battaglia, M.A.; Asherin, L.A. Density-dependent vulnerability of forest ecosystems to drought. J. Appl. Ecol. 2017, 54, 1605–1614. [Google Scholar] [CrossRef]
- Pretzsch, H. Grundlagen der Waldwachstumsforschung, 2nd ed.; Springer: Berlin, Germany, 2019; ISBN 978-3-662-58155-1. [Google Scholar]
- Guillemot, J.; Klein, E.K.; Davi, H.; Courbet, F. The effects of thinning intensity and tree size on the growth response to annual climate in Cedrus atlantica a linear mixed modeling approach. Ann. For. Sci. 2015, 72, 651–663. [Google Scholar] [CrossRef]
- Smith, D.M. The practice of silviculture. In Applied Forest Ecology, 9th ed.; Wiley: New York, NY, USA, 1997; ISBN 978-0-471-10941-9. [Google Scholar]
- Linder, M. Developing adaptive forest management strategies to cope with climate change. Tree Phys. 2000, 20, 299–307. [Google Scholar] [CrossRef]
- Papadopol, C.S. Impacts of climate warming on forests in Ontario: Options for adaptation and mitigation. For. Chron 2000, 76, 139–149. [Google Scholar] [CrossRef][Green Version]
- Spittlehouse, D.L.; Stewart, R.B. Adapting to Climate Change in Forest Management. 2003. Available online: http://41.73.194.134/xmlui/handle/123456789/493 (accessed on 30 May 2020).
- Thomas, Z.; Waring, K.M. Enhancing Resiliency and Restoring Ecological Attributes in Second-Growth Ponderosa Pine Stands in Northern New Mexico, USA. For. Sci. 2014, 61, 93–104. [Google Scholar] [CrossRef]
- Ammer, C. Unraveling the Importance of Inter- and Intraspecific Competition for the Adaptation of Forests to Climate Change. In Progress in Botany; Cánovas, F.M., Lüttge, U., Matyssek, R., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; Volume 78, pp. 345–367. ISBN 978-3-319-49490-6. [Google Scholar]
- Bréda, N.; Granier, A.; Aussenac, G. Effects of thinning on soil and tree water relations, transpiration and growth in an oak forest (Quercus petraea (Matt.) Liebl.). Tree Phys. 1995, 15, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Aussenac, G.; Granier, A. Effects of thinning on water stress and growth in Douglas-fir. Can. J. For. Res. 1988, 18, 100–105. [Google Scholar] [CrossRef]
- Lagergren, F.; Lankreijer, H.; Kučera, J.; Cienciala, E.; Mölder, M.; Lindroth, A. Thinning effects on pine-spruce forest transpiration in central Sweden. For. Ecol. Manag. 2008, 255, 2312–2323. [Google Scholar] [CrossRef]
- Brooks, J.R.; Mitchell, A.K. Interpreting tree responses to thinning and fertilization using tree-ring stable isotopes. New Phytol. 2011, 190, 770–782. [Google Scholar] [CrossRef]
- Aussenac, G. Interactions between forest stands and microclimate Ecophysiological aspects and consequences for silviculture. Ann. For. Sci. 2000, 57, 287–301. [Google Scholar] [CrossRef]
- Nilsen, E.T.; Clinton, B.D.; Lei, T.T.; Miller, T.T.; Semones, S.W.; Walker, J.F. Does Rhododendron maximum L. (Ericaceae) Reduce the Availability of Resources Above and Belowground for Canopy Tree Seedlings. Am. Midl. Nat. 2001, 145, 325–343. [Google Scholar] [CrossRef]
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
- D’Amato, A.W.; Bradford, J.B.; Fraver, S.; Palik, B.J. Effects of thinning on drought vulnerability and climate response in north temperate forest ecosystems. Ecol. Appl. 2013, 23, 1735–1742. [Google Scholar] [CrossRef]
- Pretzsch, H. Density and growth of forest stands revisited. Effect of the temporal scale of observation, site quality, and thinning. For. Ecol. Manag. 2020, 460, 117879. [Google Scholar] [CrossRef]
- Eaton, E.; Caudullo, G.; Oliveira, S.; Rigo, D. Quercus robur and Quercus petraea in Europe distribution, habitat, usage and threats. In European Atlas of Forest Tree Species, 1st ed.; San-Miguel-Ayanz, J., Rigo, D.D., Caudullo, G., Durrant, T.H., Mauri, A., Eds.; Publication Office of the European Union Luxembourg: Luxembourg, 2016; ISBN 978-92-79-36740-3. [Google Scholar]
- Houston Durrant, T.; Rigo, D.; Caudullo, G. Pinus sylvestris in Europe 965 distribution, habitat, usage and threats. In European Atlas of Forest Tree Species, 1st ed.; San-Miguel-Ayanz, J., Rigo, D.d., Caudullo, G., Durrant, T.H., Mauri, A., Eds.; Publication Office of the European Union Luxembourg: Luxembourg, 2016; ISBN 978-92-79-36740-3. [Google Scholar]
- Spellmann, H.; Albert, M.; Schmidt, M.; Sutmöller, J.; Overbeck, M. Waldbauliche Anpassungsstrategien für veränderte Klimaverhältnisse. AFZ-Der Wald. 2011, 11, 19–23. [Google Scholar]
- Zang, C.; Rothe, A.; Weis, W.; Pretzsch, H. Zur Baumarteneignung bei Klimawandel Ableitung der Trockenstress-Anfälligkeit wichtiger Waldbaumarten aus Jahrringbreiten. Environ. Sci. Policy 2011, 14, 100–110. [Google Scholar]
- Albert, M.; Hansen, J.; Nagel, J.; Schmidt, M.; Spellmann, H. Assessing risks and uncertainties in forest dynamics under different management scenarios and climate change. For. Ecosyst. 2015, 2, 14. [Google Scholar] [CrossRef]
- Krakau, U.K.; Liesebach, M.; Aronen, T.; Lelu-Walter, M.-A.; Schneck, V. Scots Pine (Pinus sylvestris L.). In Forest Tree Breeding in Europe Current State-of-the-Art and Perspectives; Pâques, L.E., Ed.; Springer Dordrecht: New York, NY, USA, 2013; ISBN 9400761457. [Google Scholar]
- Irvine, J.; Perks, M.P.; Magnani, F.; Grace, J. The response of Pinus sylvestris to drought Stomatal control of transpiration and hydraulic conductance. Tree Physiol. 1998, 18, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Piñol, J. Drought-induced mortality and hydraulic architecture in pine populations of the NE Iberian Peninsula. For. Ecol. Manag. 2002, 161, 247–256. [Google Scholar] [CrossRef]
- Bigler, C.; Bräker, O.U.; Bugmann, H.; Dobbertin, M.; Rigling, A. Drought as an Inciting Mortality Factor in Scots Pine Stands of the Valais, Switzerland. Ecosystems 2006, 9, 330–343. [Google Scholar] [CrossRef]
- Kölling, C.; Zimmermann, L. Die Anfälligkeit der Wälder Deutschlands gegenüber Klimawandel. Gefahrstoffe-Reinhaltung der Luft 2007, 67, 259–268. [Google Scholar]
- Galiano, L.; Martínez-Vilalta, J.; Lloret, F. Drought-Induced Multifactor Decline of Scots Pine in the Pyrenees and Potential Vegetation Change by the Expansion of Co-occurring Oak Species. Ecosystems 2010, 13, 978–991. [Google Scholar] [CrossRef]
- Matías, L.; Jump, A.S. Interactions between growth, demography and biotic interactions in determining species range limits in a warming world The case of Pinus sylvestris. For. Ecol. Manag. 2012, 282, 10–22. [Google Scholar] [CrossRef]
- Sohn, J.A.; Hartig, F.; Kohler, M.; Huss, J.; Bauhus, J. Heavy and frequent thinning promotes drought adaptation in Pinus sylvestris forests. Ecol. Appl. 2016, 26, 2190–2205. [Google Scholar] [CrossRef] [PubMed]
- Del Río, M.; Bravo-Oviedo, A.; Pretzsch, H.; Löf, M.; Ruiz-Peinado, R. A review of thinning effects on Scots pine stands From growth and yield to new challenges under global change. For. Syst. 2017, 26, eR03S. [Google Scholar] [CrossRef]
- Praciak, A.; Pasiecznik, N.; Sheil, D.; van Heist, M.; Sassen, M.; Correia, C.S.; Dixon, C.; Fyson, G.; Rushford, K.; Teeling, C. The CABI Encyclopedia of Forest Trees; CABI Oxfordshire: Wallingford, UK, 2013; ISBN 978-1-78064-236-9. [Google Scholar]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Steckel, M.; del Río, M.; Heym, M.; Aldea, J.; Bielak, K.; Brazaitis, G.; Černý, J.; Coll, L.; Collet, C.; Ehbrecht, M.; et al. Species mixing reduces drought susceptibility of Scots pine (Pinus sylvestris L.) and oak (Quercus robur L., Quercus petraea (Matt.) Liebl.)—Site water supply and fertility modify the mixing effect. For. Ecol. Manag. 2020, 461, 117908. [Google Scholar] [CrossRef]
- Merlin, M.; Perot, T.; Perret, S.; Korboulewsky, N.; Vallet, P. Effects of stand composition and tree size on resistance and resilience to drought in sessile oak and Scots pine. For. Ecol. Manag. 2015, 339, 22–33. [Google Scholar] [CrossRef]
- Pretzsch, H.; Steckel, M.; Heym, M.; Biber, P.; Ammer, C.; Ehbrecht, M.; Bielak, K.; Bravo, F.; Ordóñez, C.; Collet, C.; et al. Stand growth and structure of mixed-species and monospecific stands of Scots pine (Pinus sylvestris L.) and oak (Q. robur L., Quercus petraea (Matt.) Liebl.) analysed along a productivity gradient through Europe. Eur. J. Forest Res. 2019, 162, 141. [Google Scholar] [CrossRef]
- Steckel, M.; Heym, M.; Wolff, B.; Reventlow, D.O.J.; Pretzsch, H. Transgressive overyielding in mixed compared with monospecific Scots pine (Pinus sylvestris L.) and oak (Quercus robur L., Quercus petraea (Matt.) Liebl.) stands—Productivity gains increase with annual water supply. For. Ecol. Manag. 2019, 439, 81–96. [Google Scholar] [CrossRef]
- Jacob, D.; Petersen, J.; Eggert, B.; Alias, A.; Christensen, O.B.; Bouwer, L.M.; Braun, A.; Colette, A.; Déqué, M.; Georgievski, G.; et al. EURO-CORDEX new high-resolution climate change projections for European impact research. Reg. Environ. Chang. 2014, 14, 563–578. [Google Scholar] [CrossRef]
- Perkins, D.; Uhl, E.; Biber, P.; Du Toit, B.; Carraro, V.; Rötzer, T.; Pretzsch, H. Impact of Climate Trends and Drought Events on the Growth of Oaks (Quercus robur L. and Quercus petraea (Matt.) Liebl.) within and beyond Their Natural Range. Forests 2018, 9, 108. [Google Scholar] [CrossRef]
- Wennerberg, S. Plant Guide. Ponderosa Pine. 2004. Available online: https://plants.usda.gov/plantguide/pdf/pg_pipo.pdf (accessed on 6 March 2020).
- Schütt, P.; Schuck, H.J.; Stimm, B. Lexikon der Baum- und Straucharten, Das Standardwerk der Forstbotanik, Morphologie, Pathologie, Ökologie und Systematik Wichtiger Baum- und Straucharten; Lizenzausgabe 2007; Nikol Verlagsgesellschaft: Hamburg, Germany, 2007; ISBN 3933203538. [Google Scholar]
- Giuggiola, A.; Bugmann, H.; Zingg, A.; Dobbertin, M.; Rigling, A. Reduction of stand density increases drought resistance in xeric Scots pine forests. For. Ecol. Manag. 2013, 310, 827–835. [Google Scholar] [CrossRef]
- Fernández-de-Uña, L.; Cañellas, I.; Gea-Izquierdo, G. Stand competition determines how different tree species will cope with a warming climate. PLoS ONE 2015, 10, e0122255. [Google Scholar] [CrossRef] [PubMed]
- Trouvé, R.; Bontemps, J.D.; Collet, C.; Seynave, I.; Lebourgeois, F. Radial growth resilience of sessile oak after drought is affected by site water status, stand density, and social status. Trees 2017, 31, 517–529. [Google Scholar] [CrossRef]
- Fernández, M.E.; Gyenge, J.E.; Urquiza, M.M.; Varela, S. Adaptability to climate change in forestry species drought effects on growth and wood anatomy of ponderosa pines growing at different competition levels. For. Syst. 2012. [Google Scholar] [CrossRef]
- Kerhoulas, L.P.; Kolb, T.E.; Hurteau, M.D.; Koch, G.W. Managing climate change adaptation in forests a case study from the U.S. Southwest. J. Appl. Ecol. 2013, 50, 1311–1320. [Google Scholar] [CrossRef]
- McDowell, N.G.; Adams, H.D.; Bailey, J.D.; Hess, M.; Kolb, T.E. Homeostatic Maintenance Of Ponderosa Pine Gas Exchange In Response To Stand Density Changes. Ecol. Appl. 2006, 16, 1164–1182. [Google Scholar] [CrossRef]
- Alton, A.L.; James, D.; Barton, J.R.; Miles, S.R. Field Efficiencies of Forest Sampling Methods. Ecology 1958, 39, 428–444. [Google Scholar] [CrossRef]
- Johann, K. Eine neue Jahrringmeßanlage für Bohrkerne und Stammscheiben. Forstarchiv 1977, 48, 24–26. [Google Scholar]
- Schweingruber, F.H.; Eckstein, D.; Serre-Bachet, F.; Bräker, O.U. Identification, presentation and interpretation of event years and pointer years in dendrochronology. Dendrochronologia 1990, 8, 9–38. [Google Scholar]
- Biondi, F.; Qeadan, F. A Theory-Driven Approach to Tree-Ring Standardization Defining the Biological Trend from Expected Basal Area Increment. Tree-Ring Res. 2008, 64, 81–96. [Google Scholar] [CrossRef]
- Holmes, R.L.; Adams, R.K.; Fritts, H.C. Tree-Ring Chronologies of Western North America California, Eastern Oregon and Northern Great Basin with Procedures Used in the Chronology Development Work Including Users Manuals for Computer Programs COFECHA and ARSTAN; Laboratory of Tree-Ring Research, University of Arizona Tucson: Tucson, AZ, USA, 1986. [Google Scholar]
- Hugershoff, R. Die Mathematischen Hilfsmittel der Kulturingenierurs und Biologen Herleitung von Gesetzmäßigen Zusammenhängen als Manuskript Veröffentlicht. 1936. Available online: https://books.google.com/books/about/Die_mathematischen_Hilfsmittel_der_Kultu.html?id=c_4YGwAACAAJ (accessed on 30 May 2020).
- Cook, E.R.; Peters, K. The smoothing spline a new approach to standardizing forest interior tree-ring width series for dendroclimatic studies. Tree-Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Fritts, H.C. Tree Rings and Climate, Reprint of second printing 1976; Blackburn Press: Caldwell, NJ, USA, 2001; ISBN 978-1930665392. [Google Scholar]
- Esper, J.; Cook, E.R.; Schweingruber, F.H. Low-Frequency Signals in Long Tree-Ring Chronologies for Reconstructing Past Temperature Variability. Science 2002, 295, 2250. [Google Scholar] [CrossRef] [PubMed]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the Average Value of Correlated Time Series, with Applications in Dendroclimatology and Hydrometeorology. J. Appl. Meteorol. Clim. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- R Core Team. R A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: www.R-project.org (accessed on 5 July 2019).
- Deutscher Wetterdienst. Grids of Monthly Averaged Daily Air Temperature (2m) over Germany Version v1.0. Available online: https://opendata.dwd.de/climate_environment/CDC/grids_germany/monthly/air_temperature_mean (accessed on 6 March 2020).
- Deutscher Wetterdienst. Grids of Monthly Total Precipitation over GERMANY Version v1.0. Available online: https://opendata.dwd.de/climate_environment/CDC/grids_germany/monthly/precipitation (accessed on 6 March 2020).
- PRISM Climate Group. PRISM Data Time Series Values for Individual Locations Monthly Ppt, Tmin, Tmean, Tmax. Available online: http://www.prism.oregonstate.edu/explorer (accessed on 6 March 2020).
- Middleton, N.; Thomas, D. World Atlas of Desertification, Repr; Arnold: London, UK, 1993; ISBN 0340555122. [Google Scholar]
- Thornthwaite, C.W. An Approach toward a Rational Classification of Climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- Walter, H.; Lieth, H. Klimadiagramm-Weltatlas; Gustav Fischer Verlag Jena: Stuttgart, Germany, 1976. [Google Scholar]
- Kerhoulas, L.P.; Kolb, T.E.; Koch, G.W. Tree size, stand density, and the source of water used across seasons by ponderosa pine in northern Arizona. For. Ecol. Manag. 2013, 289, 425–433. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Rebetez, M.; Mayer, H.; Dupont, O.; Schindler, D.; Gartner, K.; Kropp, J.P.; Menzel, A. Heat and drought 2003 in Europe A climate synthesis. Ann. For. Sci. 2006, 63, 569–577. [Google Scholar] [CrossRef]
- Ionita, M.; Tallaksen, L.M.; Kingston, D.G.; Stagge, J.H.; Laaha, G.; van Lanen, H.A.J.; Scholz, P.; Chelcea, S.M.; Haslinger, K. The European 2015 drought from a climatological perspective. Hydrol. Earth Syst. Sci. 2017, 21, 1397–1419. [Google Scholar] [CrossRef]
- Pretzsch, H.; Grams, T.; Häberle, K.H.; Pritsch, K.; Bauerle, T.; Rötzer, T. Growth and mortality of Norway spruce and European beech in monospecific and mixed-species stands under natural episodic and experimentally extended drought. Results of the KROOF throughfall exclusion experiment. Trees 2020. [Google Scholar] [CrossRef]
- Wardlow, B.D.; Anderson, M.C.; Verdin, J.P. Remote Sensing of Drought. Innovative Monitoring Approaches; CRC Press: Boca Raton, FL, USA, 2012; ISBN 1439835578. [Google Scholar]
- Fuchs, L.; Stevens, L.E.; Fulé, P.Z. Dendrochronological assessment of springs effects on ponderosa pine growth, Arizona, USA. For. Ecol. Manag. 2019, 435, 89–96. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming The Standardized Precipitation Evapotranspiration Index. J. Clim. 2009, 23, 1696–1718. [Google Scholar] [CrossRef]
- Winkler, S. Einführung in Die Pflanzenökologie, 2nd ed.; Fischer: Stuttgart, Germany, 1980; ISBN 3437202189. [Google Scholar]
- Potop, V.; Boroneanţ, C.; Možný, M.; Štěpánek, P.; Skalák, P. Observed spatiotemporal characteristics of drought on various time scales over the Czech Republic. Theor. Appl. Clim. 2014, 115, 563–581. [Google Scholar] [CrossRef]
- Assmann, E. The Principles of Forest Yield Study; Pergamon Press Oxford: Oxford, UK, 1970. [Google Scholar]
- Johann, K. DESER-Norm 1993. Normen der Sektion Ertragskunde im Deutschen Verband Forstlicher Forschungsanstalten zur Aufbereitung von waldwachstumskundlichen Dauerversuchen. In Deutscher Verband Forstlicher Forschungsanstalten Sektion Ertragskunde; Fundamentals of Tree-Ring Research: Tucson, AZ, USA, 1993; pp. 96–104. [Google Scholar]
- Thurm, E.A.; Uhl, E.; Pretzsch, H. Mixture reduces climate sensitivity of Douglas-fir stem growth. For. Ecol. Manag. 2016, 376, 205–220. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Crawley, M.J. The R Book, 2nd ed.; Wiley: Chichester, UK, 2013; ISBN 978-0-470-97392-9. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-141. Available online: https://CRAN.R-project.org/package=nlme (accessed on 10 December 2018).
- Lenth, R.V. Least-Squares Means The R Package lsmeans. J. Stat. Soft. 2016, 69. [Google Scholar] [CrossRef]
- Akaike, H. Likelihood of a model and information criteria. J. Econom. 1981, 16, 3–14. [Google Scholar] [CrossRef]
- Barton, K. MuMIn Multi-Model Inference. R Package Version 1.43.6. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 10 December 2018).
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010; ISBN 0816526842. [Google Scholar]
- Mäkinen, H.; Isomäki, A. Thinning intensity and growth of Scots pine stands in Finland. For. Ecol. Manag. 2004, 201, 311–325. [Google Scholar] [CrossRef]
- Mäkinen, H.; Isomäki, A. Thinning intensity and long-term changes in increment and stem form of Scots pine trees. For. Ecol. Manag. 2004, 203, 21–34. [Google Scholar] [CrossRef]
- Nickel, M.; Klemmt, H.J.; Uhl, E.; Pretzsch, H. Der Kiefern Standraum und Durchforstungsversuch Weiden 611. AFZ-Der Wald 2007, 24, 1316–1319. [Google Scholar]
- Sánchez-Salguero, R.; Linares, J.C.; Camarero, J.J.; Madrigal-González, J.; Hevia, A.; Sánchez-Miranda, Á.; Ballesteros-Cánovas, J.A.; Alfaro-Sánchez, R.; García-Cervigón, A.I.; Bigler, C.; et al. Disentangling the effects of competition and climate on individual tree growth A retrospective and dynamic approach in Scots pine. For. Ecol. Manag. 2015, 358, 12–25. [Google Scholar] [CrossRef]
- Kerr, G. The effect of heavy or ‘free growth’ thinning on oak (Quercus petraea and Q. robur). Forestry 1996, 69, 303–317. [Google Scholar] [CrossRef]
- Štefančík, I. Growth characteristics of oak (Quercus petraea [Mattusch.] Liebl.) stand under different thinning regimes. J. For. Sci. 2012, 58, 67–78. [Google Scholar] [CrossRef]
- Gaines, E.M.; Kotok, E.S. Thinning Ponderosa Pine in the Southwest. Sta. Pap. 17; Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1954.
- Myers, C.A. Growing Stock Levels in Even-Aged Ponderosa Pine, Res. Pap. RM-33; Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1967.
- Barrett, J.W. Twenty-Year Growth of Ponderosa Pine Saplings Thinned to Five Spacings in Central Oregon, Res. Pap. PNW-RP-301; Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1982.
- McDowell, N.; Brooks, J.R.; Fitzgerald, S.A.; Bond, B.J. Carbon isotope discrimination and growth response of old Pinus ponderosa trees to stand density reductions. Plant Cell Environ. 2003, 26, 631–644. [Google Scholar] [CrossRef]
- Skov, K.R.; Kolb, T.E.; Wallin, K.F. Tree Size and Drought Affect Ponderosa Pine Physiological Response to Thinning and Burning Treatments. For. Sci. 2004, 50, 81–91. [Google Scholar] [CrossRef]
- Miesel, J.R. Differential responses of Pinus ponderosa and Abies concolor foliar characteristics and diameter growth to thinning and prescribed fire treatments. For. Ecol. Manag. 2012, 284, 163–173. [Google Scholar] [CrossRef]
- Pretzsch, H. Forest Dynamics, Growth and Yield; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-88306-7. [Google Scholar]
- Hara, T. Dynamics of size structure in plant populations. Trends Ecol. Evol. 1988, 3, 129–133. [Google Scholar] [CrossRef]
- Schwinning, S.; Weiner, J. Mechanisms determining the degree of size asymmetry in competition among plants. Oecologia 1998, 113, 447–455. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P. Size-symmetric versus size-asymmetric competition and growth partitioning among trees in forest stands along an ecological gradient in central Europe. Can. J. For. Res. 2010, 40, 370–384. [Google Scholar] [CrossRef]
- Pérez-de-Lis, G.; García-González, I.; Rozas, V.; Arévalo, J.R. Effects of thinning intensity on radial growth patterns and temperature sensitivity in Pinus canariensis afforestations on Tenerife Island, Spain. Ann. For. Sci. 2011, 68, 1093. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Cochard, H.; Mencuccini, M.; Sterck, F.; Herrero, A.; Korhonen, J.F.J.; Llorens, P.; Nikinmaa, E.; Nolè, A.; Poyatos, R.; et al. Hydraulic adjustment of Scots pine across Europe. New Phytol. 2009, 184, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.; Annighöfer, P.; Schall, P.; Zimmermann, J.; Kahl, T.; Schulze, E.-D.; Ammer, C. Site-adapted admixed tree species reduce drought susceptibility of mature European beech. Glob. Chang. Biol. 2016, 22, 903–920. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.; Pretzsch, H.; Rothe, A. Size-dependent responses to summer drought in Scots pine, Norway spruce and common oak. Trees 2012, 26, 557–569. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought. New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Voelker, S.L.; DeRose, R.J.; Bekker, M.F.; Sriladda, C.; Leksungnoen, N.; Kjelgren, R.K. Anisohydric water use behavior links growing season evaporative demand to ring-width increment in conifers from summer-dry environments. Trees 2018, 32, 735–749. [Google Scholar] [CrossRef]
- Hartmann, H. Will a 385 million year-struggle for light become a struggle for water and for carbon-How trees may cope with more frequent climate change-type drought events. Glob. Chang. Biol. 2011, 17, 642–655. [Google Scholar] [CrossRef]
- Whitehead, D.; Jarvis, P.G.; Waring, R.H. Stomatal conductance, transpiration, and resistance to water uptake in a Pinussylvestris spacing experiment. Can. J. For. Res. 1984, 14, 692–700. [Google Scholar] [CrossRef]
- Fajardo, A.; McIntire, E.J.B. Under strong niche overlap conspecifics do not compete but help each other to survive facilitation at the intraspecific level. J. Ecol. 2010. [Google Scholar] [CrossRef]
- McIntire, E.J.B.; Fajardo, A. Facilitation within species a possible origin of group-selected superorganisms. Am. Nat. 2011, 178, 88–97. [Google Scholar] [CrossRef]
- Owen, S.M. Tree Regeneration Following Large Wildfires in Southwestern Ponderosa Pine Forests, Dissertation; Northern Arizona University: Flagstaff, AZ, USA, 2019. [Google Scholar]
- Calama, R.; Conde, M.; de-Dios-García, J.; Madrigal, G.; Vázquez-Piqué, J.; Gordo, F.J.; Pardos, M. Linking climate, annual growth and competition in a Mediterranean forest Pinus pinea in the Spanish Northern Plateau. Agric. For. Meteorol. 2019, 264, 309–321. [Google Scholar] [CrossRef]
- Del Rio, M.; Schutze, G.; Pretzsch, H. Temporal variation of competition and facilitation in mixed species forests in Central Europe. Plant Biol. 2014, 16, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; López, B.C.; Loepfe, L.; Lloret, F. Stand- and tree-level determinants of the drought response of Scots pine radial growth. Oecologia 2012, 168, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Pasho, E.; Camarero, J.J.; de Luis, M.; Vicente-Serrano, S.M. Impacts of drought at different time scales on forest growth across a wide climatic gradient in north-eastern Spain. Agric. For. Meteorol. 2011, 151, 1800–1811. [Google Scholar] [CrossRef]
- Serra-Maluquer, X.; Mencuccini, M.; Martínez-Vilalta, J. Changes in tree resistance, recovery and resilience across three successive extreme droughts in the northeast Iberian Peninsula. Oecologia 2018, 187, 343–354. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Anderegg, W.R.L.; Vicente-Serrano, S.M. Impacts of droughts on the growth resilience of Northern Hemisphere forests. Glob. Ecol. Biogeogr. 2017, 26, 166–176. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Schwalm, C.; Biondi, F.; Camarero, J.J.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Shevliakova, E.; Williams, A.P.; et al. FOREST ECOLOGY. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 2015, 349, 528–532. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
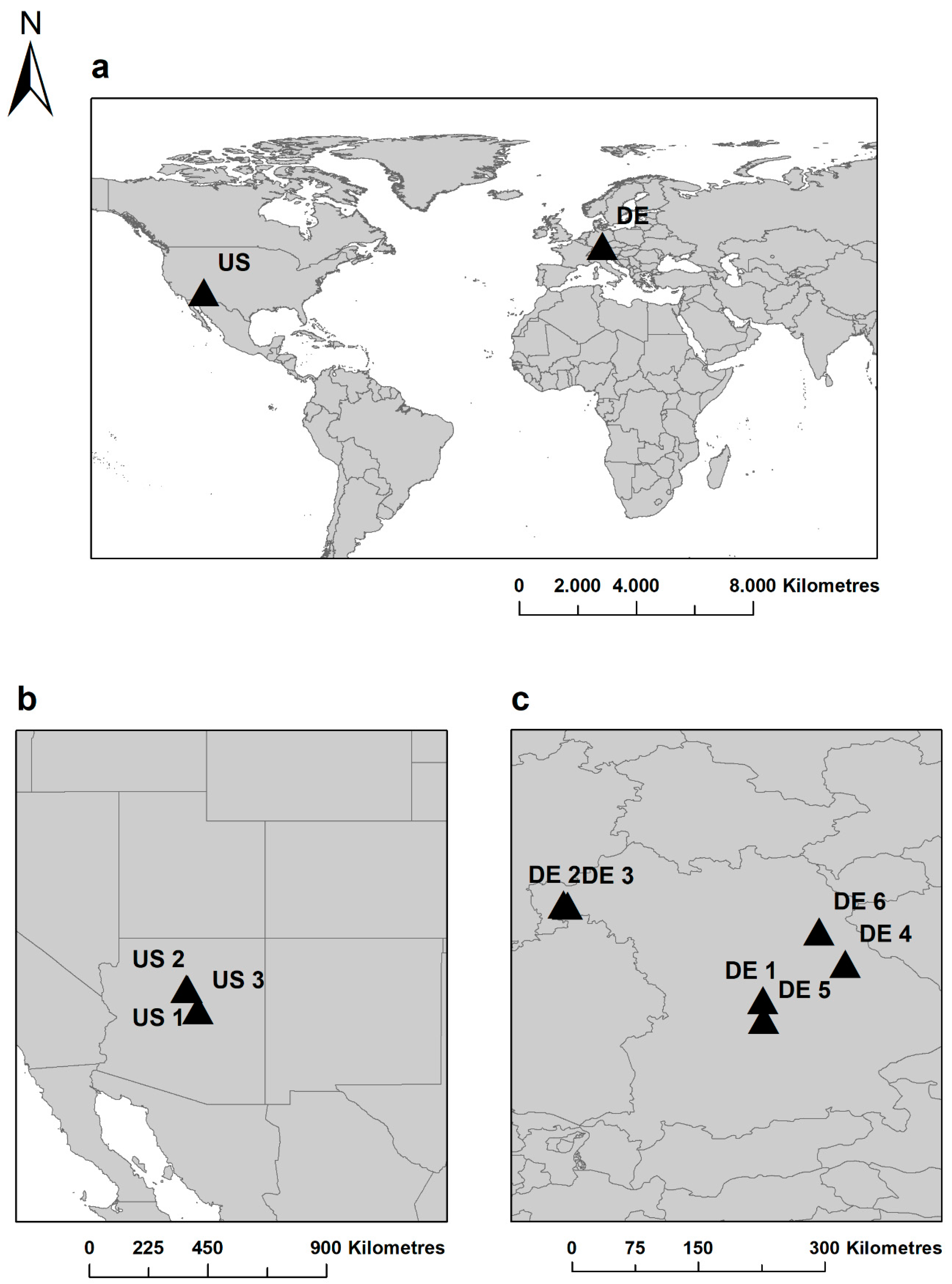
| Site | ID | Country | Species | Age | E | T | P | IA | PM |
|---|---|---|---|---|---|---|---|---|---|
| yrs | m a.s.l | °C | mm | ||||||
| DE 1 | GEI 649 | Germany | sessile oak | 34 | 495 | 8.3 | 767 | 1.26 | sand, loess |
| DE 2 | ROB 620 | Germany | sessile oak | 91 | 440 | 8.1 | 1021 | 1.70 | sand |
| DE 3 | ROB 90 | Germany | sessile oak | 153 | 470 | 8.3 | 981 | 1.62 | sand |
| DE 4 | BOD 610 | Germany | Scots pine | 65 | 400 | 8.5 | 722 | 1.17 | sand |
| DE 5 | GEI 335 | Germany | Scots pine | 68 | 380 | 8.9 | 756 | 1.20 | sand |
| DE 6 | WEI 611 | Germany | Scots pine | 47 | 410 | 8.3 | 714 | 1.16 | sand |
| US 1 | FV U1 | USA | ponderosa pine | 105 | 2250 | 7.1 | 575 | 1.07 | basalt |
| US 2 | FV U2 | USA | ponderosa pine | 100 | 2280 | 7.1 | 612 | 1.14 | basalt |
| US 3 | LV | USA | ponderosa pine | 100 | 2079 | 9.0 | 728 | 1.24 | basalt |
| Species | n | Statistic | Rt | Rc | Rs |
|---|---|---|---|---|---|
| Scots pine | 229 | Mean | 0.82 | 1.34 | 1.07 |
| sd | 0.16 | 0.34 | 0.27 | ||
| sessile oak | 225 | Mean | 0.99 | 1.04 | 1.02 |
| sd | 0.20 | 0.28 | 0.32 | ||
| ponderosa pine | 359 | Mean | 0.89 | 1.41 | 1.10 |
| sd | 0.37 | 0.68 | 0.35 |
| Species | Response Variable | Statistic | Intercept | ba | MRBA | IA | Age | MRBA * IA | MRBA * Age |
|---|---|---|---|---|---|---|---|---|---|
| a0 | a1 | a2 | a3 | a4 | a5 | a6 | |||
| Scots pine | Rt—Resistance | Estimate | 5.47 *** | - | - | −4.34 ** | 0.01 ** | - | - |
| SE | 1.44 | 1.33 | 0.00 | ||||||
| Rc—Recovery | Estimate | 16.09 *** | - | −15.29 ** | −12.42 *** | - | 12.85 * | - | |
| SE | 4.29 | 5.86 | 3.62 | 4.95 | |||||
| Rs—Resilience | Estimate | 12.31 *** | - | −9.92 * | −9.45 ** | - | 8.30 * | - | |
| SE | 3.39 | 4.62 | 2.86 | 3.91 | |||||
| sessile oak | Rt—Resistance | Estimate | 0.74 *** | - | - | 0.16 . | - | - | - |
| SE | 0.13 | 0.08 | |||||||
| Rc—Recovery | Estimate | 3.41 *** | - | −1.29 * | −1.38 *** | −0.00 *** | 0.82 * | - | |
| SE | 0.45 | 0.60 | 0.30 | 0.00 | 0.39 | ||||
| Rs—Resilience | Estimate | 2.33 *** | - | - | −0.82 *** | - | - | - | |
| SE | 0.19 | 0.12 | |||||||
| ponderosa pine | Rt—Resistance | Estimate | 0.71 *** | - | 0.30 *** | - | - | - | - |
| SE | 0.04 | 0.07 | |||||||
| Rc—Recovery | Estimate | 4.76 *** | - | −4.32 * | −2.72 * | - | 3.45 * | - | |
| SE | 1.37 | 2.01 | 1.18 | 1.73 | |||||
| Rs—Resilience | Estimate | 0.97 *** | - | 0.20 ** | - | - | - | - | |
| SE | 0.04 | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steckel, M.; Moser, W.K.; del Río, M.; Pretzsch, H. Implications of Reduced Stand Density on Tree Growth and Drought Susceptibility: A Study of Three Species under Varying Climate. Forests 2020, 11, 627. https://doi.org/10.3390/f11060627
Steckel M, Moser WK, del Río M, Pretzsch H. Implications of Reduced Stand Density on Tree Growth and Drought Susceptibility: A Study of Three Species under Varying Climate. Forests. 2020; 11(6):627. https://doi.org/10.3390/f11060627
Chicago/Turabian StyleSteckel, Mathias, W. Keith Moser, Miren del Río, and Hans Pretzsch. 2020. "Implications of Reduced Stand Density on Tree Growth and Drought Susceptibility: A Study of Three Species under Varying Climate" Forests 11, no. 6: 627. https://doi.org/10.3390/f11060627
APA StyleSteckel, M., Moser, W. K., del Río, M., & Pretzsch, H. (2020). Implications of Reduced Stand Density on Tree Growth and Drought Susceptibility: A Study of Three Species under Varying Climate. Forests, 11(6), 627. https://doi.org/10.3390/f11060627





