Phylogenetic Relationships between Phlebiopsis gigantea and Selected Basidiomycota Species Inferred from Partial DNA Sequence of Elongation Factor 1-Alpha Gene
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Isolates
2.2. DNA Extraction
2.3. Primers and PCR Conditions
2.4. DNA Sequence Analysis
3. Results
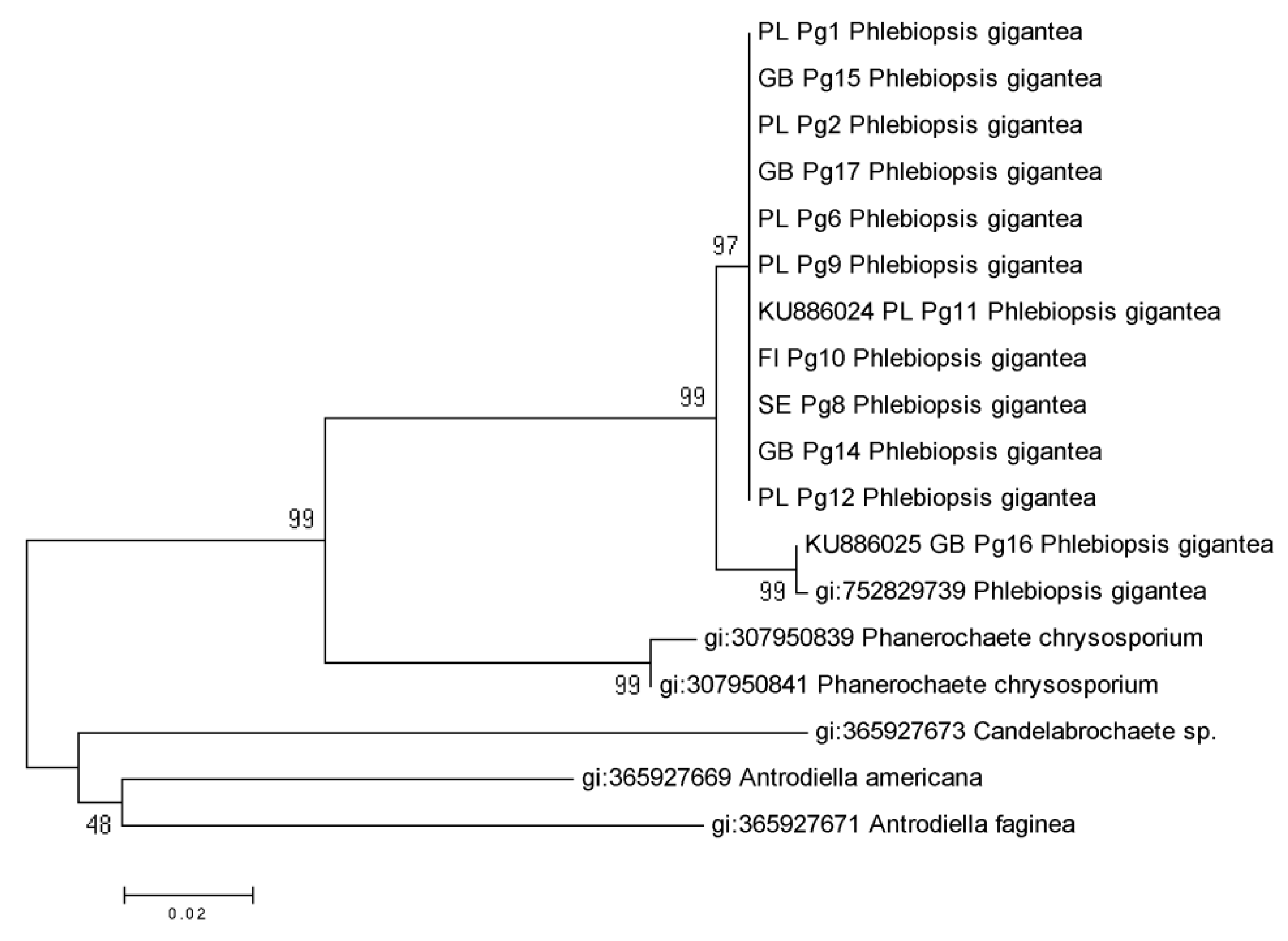
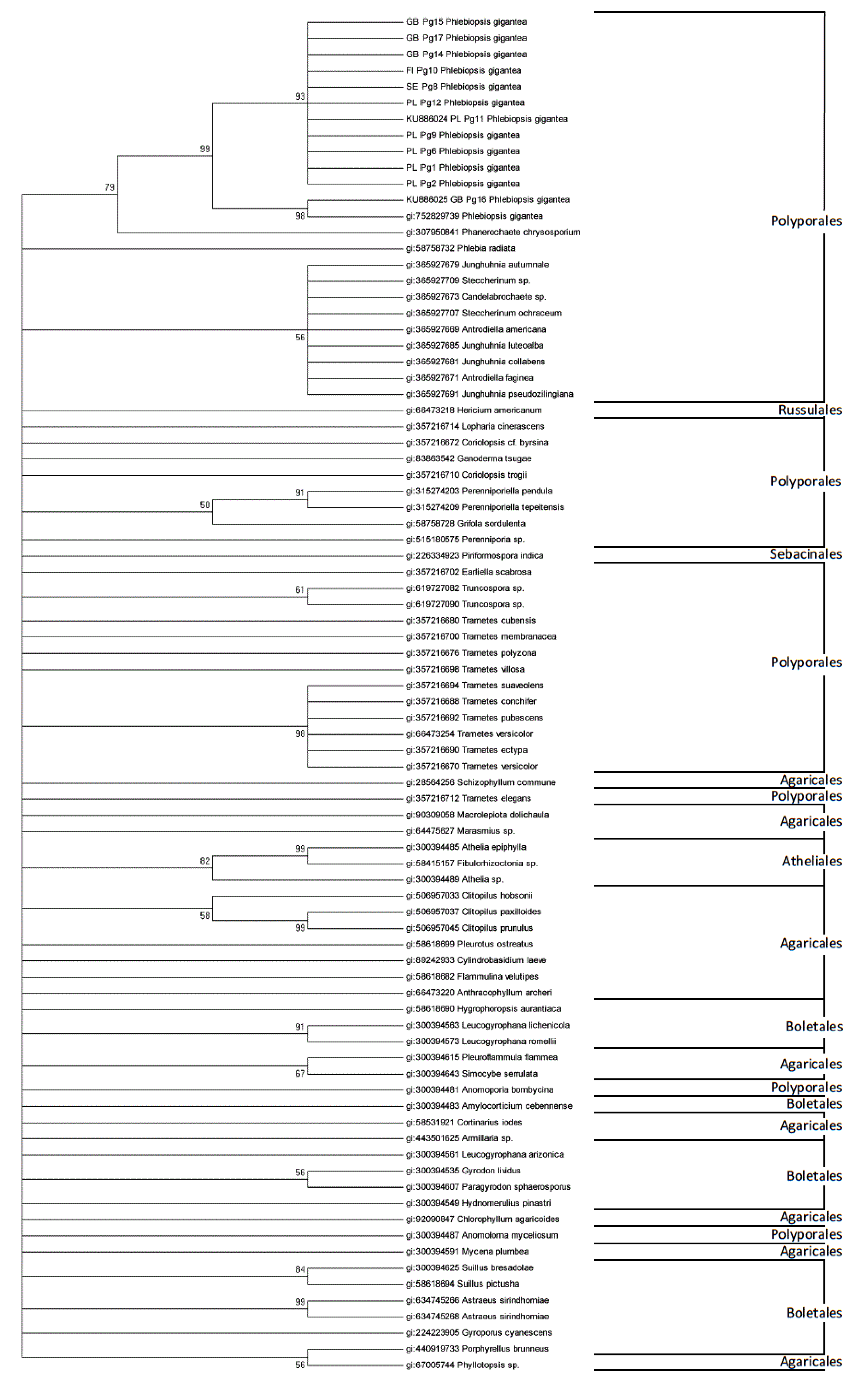
EF1α Partial Gene of P. gigantea Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sierota, Z. Heterobasidion root rot in forests on former agricultural lands in Poland: Scale of threat and prevention. Sci. Res. Essays 2013, 8, 2298–2305. [Google Scholar]
- Żółciak, A.; Sikora, K.; Nowakowska, J.A.; Małecka, M.; Borys, M.; Tereba, T.; Sierota, Z. Antrodia gossypium, Phlebiopsis gigantea and Heterobasidion parviporum: In vitro growth and Norway spruce wood block decay. Biocontrol Sci. Technol. 2016, 26, 1706–1718. [Google Scholar] [CrossRef]
- Żółciak, A.; Sierota, Z.; Małecka, M. Characterization of some Phlebiopsis gigantea isolates with respect to enzymatic activity and decay of Norway spruce wood. Biocontrol Sci. Technol. 2012, 22, 777–790. [Google Scholar] [CrossRef]
- Żółciak, A.; Sikora, K.; Wrzosek, M.; Damszel, M.; Sierota, Z. Why Does Phlebiopsis gigantea not Always Inhibit Root and Butt Rot in Conifers? Forests 2020, 11, 129. [Google Scholar] [CrossRef]
- Sun, H.; Paulin, L.; Alatalo, E.; Asiegbu, F.O. Response of living tissues of Pinus sylvestris to the saprotrophic biocontrol fungus Phlebiopsis gigantea. Tree Physiol. 2011, 31, 438–451. [Google Scholar] [CrossRef]
- Sun, H.; Terhonen, E.; Koskinen, K.; Paulin, L.; Kasanen, R.; Asiegbu, F.O. The impacts of treatment with biocontrol fungus (Phlebiopsis gigantea) on bacterial diversity of Norway spruce stumps. Biological Control 2013, 64, 238–246. [Google Scholar] [CrossRef]
- Mgbeahuruike, A.C.; Karlsson, M.; Asiegbu, F.O. Differential expression of two hydrophobin genes (Pgh1 and Pgh2) from the biological control agent Phlebiopsis gigantea. Fungal Biol. 2012, 116, 620–629. [Google Scholar] [CrossRef]
- Mgbeahuruike, A.C.; Kohler, A.; Asiegbu, F.O. Expression analysis of the impact of culture filtrates from the biocontrol agent, Phlebiopsis gigantea on the conifer pathogen, Heterobasidion annosum s.s. Transcriptome. Microb. Ecol. 2013, 66, 669–681. [Google Scholar] [CrossRef]
- Garbelotto, M.; Gonthier, P. Biology, epidemiology, and control of Heterobasidion species worldwide. Annu. Rev. Phytopathol. 2013, 51, 39–59. [Google Scholar] [CrossRef]
- Sierota, Z.; Nowakowska, J.A.; Sikora, K.; Wrzosek, M.; Żółciak, A.; Małecka, M. What is important in selecting Phlebiopsis gigantea strain for commercial use? J. Agric. Sci. Technol. B 2015, 5, 55–64. [Google Scholar]
- Sierota, Z.; Nowakowska, J.A.; Sikora, K.; Wrzosek, M.; Żółciak, A.; Małecka, M. Genetic variation among Phlebiopsis gigantea strains determined by Random Amplified Microsatellite Markers. Balt. For. 2015, 21, 178–183. [Google Scholar]
- Blomquist, M.; Herrera, S.L.; Hofmann, J.; Beram, R.C.; Cleary, M.; Rönnberg, J. Size matters but is big always better? Effectiveness of urea and Phlebiopsis gigantea as treatment against Heterobasidion on Picea abies stumps of variable size. For. Ecol. Manag. 2020, 462, 117998. [Google Scholar] [CrossRef]
- Parmasto, E.; Hallenberg, N. A taxonomic study of phlebioid fungi (Basidiomycota). Nord. J. Bot. 2000, 20, 105–118. [Google Scholar] [CrossRef]
- Grillo, R.; Hantula, J.; Korhonen, K. Interfertility between North American and European strains of Phlebiopsis gigantea. For. Pathol. 2005, 35, 173–182. [Google Scholar] [CrossRef]
- Żółciak, A. Inokulacja pniaków sosnowych preparatami biologicznymi z Phlebiopsis gigantea/Scots pine stumps inoculation with Phlebiopsis gigantea biological preparations. Leśne Pr. Badaw. 2007, 2, 77–94. (In Polish) [Google Scholar]
- Kozlakidis, Z.; Hacker, C.V.; Bradley, D.; Jamal, A.; Phoon, X.; Webber, J.; Brasier, C.M.; Buck, K.W.; Coutts, R.H.A. Molecular characterization of two novel double-stranded RNA elements from Phlebiopsis gigantea. Virus Genes 2009, 39, 132–136. [Google Scholar] [CrossRef]
- Menkis, A.; Burokienė, D.; Gaitnieks, T.; Uotila, A.; Johannesson, H.; Rosling, A.; Finlay, R.D.; Stenlid, J.; Vasaitis, R. Occurrence and impact of the root-rot biocontrol agent Phlebiopsis gigantea on soil fungal communities in Picea abies forests of Northern Europe. FEMS Microbiol. Ecol. 2012, 81, 438–445. [Google Scholar] [CrossRef]
- Rehner, S. Primers for Elongation Factor 1-α (EF1-α); Insect Biocontrol Laboratory USDA, ARS, PSI: Beltsville, MD, USA, 2001; p. 4. 1p, Available online: http://ocid.NACSE.ORG/research/deephyphae/EF1primer.pdf (accessed on 20 May 2020).
- Stępień, Ł.; Koczyk, G.; Waśkiewicz, A. Genetic and phenotypic variation of Fusarium proliferatum isolates from different host species. J. Appl. Genet. 2011, 52, 487–496. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Vainio, E.J.; Hantula, J. Genetic differentiation between European and North American populations of Phlebiopsis gigantea. Mycologia 2000, 92, 436–446. [Google Scholar] [CrossRef]
- Liu, A.Z.; Samils, N.; Higgins, B.; Stenlid, J.; Slippers, B.; Nairn, C.J.; Covert, S.F. Microsatellite markers for the wood decay fungus Phlebiopsis gigantea. Conserv. Genet. 2009, 10, 1529–1532. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E.A. Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Matheny, P.B.; Wang, Z.; Binder, M.; Curtis, J.M.; Lim, Y.W.; Nilsson, R.H.; Hughes, K.W.; Hofstetter, V.; Ammirati, J.F.; Schoch, C.L.; et al. Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol. Phylogenet. Evol. 2007, 43, 430–451. [Google Scholar] [CrossRef] [PubMed]
- Roger, A.J.; Sandblom, O.; Doolittle, W.F.; Philippe, H. An evaluation of elongation factor 1 alpha as a phylogenetic marker for Eukaryotes. Mol. Biol. Evol. 1999, 16, 218–233. [Google Scholar] [CrossRef]
- Hori, C.; Ishida, T.; Igarashi, K.; Samejima, M.; Suzuki, H.; Master, E.; Ferreira, P.; Ruiz-Dueñas, F.J.; Held, B.; Canessa, P.; et al. Analysis of the Phlebiopsis gigantea genome, transcriptome and secretome provides insight into its pioneer colonization strategies of wood. PLoS Genet. 2014, 10, e1004759. [Google Scholar] [CrossRef]
- Sierota, Z.; Wrzosek, M.; Małecka, M.; Zółciak, A. Decay indices for evaluating wood decomposition activity. Biocontrol Sci. Technol. 2016, 26, 163–173. [Google Scholar] [CrossRef]
- Grossbard, E. Antibiotic Production by Fungi on Organic Manures and in Soil. J. Gen. Microbiol. 1952, 6, 295–310. [Google Scholar] [CrossRef][Green Version]
- Schardl, C.L.; Craven, K.D. Interspecific hybridization in plant-associated Fungi and Oomycetes: A review. Mol. Ecol. 2003, 12, 2861–2873. [Google Scholar] [CrossRef]
- Kwaśna, H.; Chełkowski, J.; Zajkowski, P. Grzyby (Mycota), Grzyby niedoskonałe (Deuteromycetes), Strzępczakowe (Hyphomycetales), Gruzełkowate (Tuberculariaceae), Sierpik (Fusarium); Instytut Botaniki PAN: Warszawa–Kraków, Poland, 1991; Volume T.22, p. 136. (In Polish) [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Yu, J.; Hu, S.; Wang, J.; Wong, G.K.; Li, S.; Liu, B.; Deng, Y.; Dai, L.; Zhou, Y.; Zhang, X.; et al. A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 2002, 296, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, V.; Kosarev, P.; Seledsov, I.; Vorobyev, D. Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol. 2006, 7 (Suppl. 1), 10.1–1012. [Google Scholar] [CrossRef] [PubMed]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000; p. 333. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Roy, S.W.; Gilbert, W. The evolution of spliceosomal introns: Patterns, puzzles and progress. Nat. Rev. Genet. 2006, 7, 211–221. [Google Scholar]
- Loftus, B.J.; Fung, E.; Roncaglia, P.; Rowley, D.; Amedeo, P.; Bruno, D.; Vamathevan, J.; Miranda, M.; Anderson, I.J.; Fraser, J.A.; et al. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 2005, 307, 1321–1324. [Google Scholar] [CrossRef]
- Deutsch, M.; Long, M. Intron-exon structures of eukaryotic model organisms. Nucleic Acids Res. 1999, 27, 3219–3228. [Google Scholar]
- Wakuliński, W. Ogólna charakterystyka grzybów i ich cechy jako patogenów roślin/General characteristics of fungi and their traits as plant pathogens. In Fitopatologia. T. 1, Podstawy fitopatologii; Kryczyński, S., Weber, Z., Eds.; Wyd. zbiorowe. PWRiL: Poznań, Poland, 2010; pp. 206–244. (In Polish) [Google Scholar]
- Stajich, J.E.; Dietrich, F.S.; Roy, S.W. Comparative genomic analysis of fungal genomes reveals intron-rich ancestors. Genome Biol. 2007, 8, R223. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Silva, J.; Burstein, D.; Pupko, T.; Eyras, E.; Ast, G. Large-scale comparative analysis of splicing signals and their corresponding splicing factors in eukaryotes. Genome Res. 2008, 18, 88–103. [Google Scholar] [CrossRef]
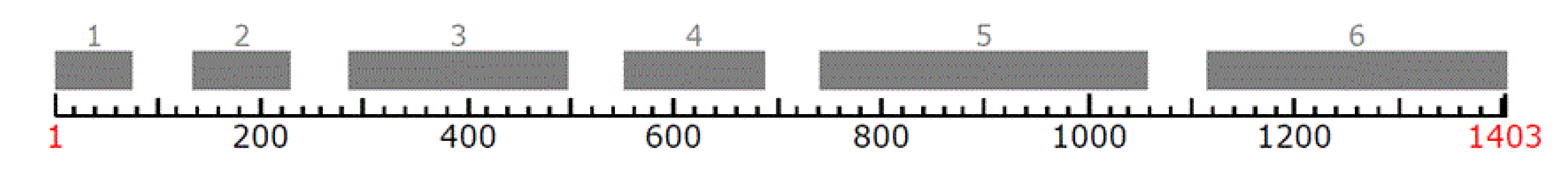
| Polish Isolate Codes | Location Coordinates/Forest District/Country | Host | Substrate | Collector | Collection (Institution Name, Isolate Code, Country) |
|---|---|---|---|---|---|
| PL Pg1 | x:52.09859 y: 20.85479 Chojnów FD, Poland | Pinus sylvestris L. | Stump wood | A. Żółciak | Forest Research Institute, 01.06.08.1.5; 02.10.23.1.2; 03.11.04.1.1; 03.11.13.1.3; 99.09.10.1.1; 04.11.19.1.2 Poland |
| PL Pg2 | |||||
| PL Pg6 | |||||
| PL Pg9 | |||||
| PL Pg11 (Accession number: KU886024) | x:51.22000 y: 20.33157 Barycz FD, Poland | Fruitbody on stump | Z. Sierota | ||
| PL Pg12 | x:53.42508 y: 20.59593 Nidzica FD, Poland | Stump wood | A. Żółciak | ||
| SE Pg8 | Råberg near Uppsala, Sweden | Picea abies(L.) Karst. | K. Korhonen | Lallemand Plant Care - Verdera Oy, VRA 1984 *, Finland | |
| FI Pg10 | Loppi, Finland | Log wood | Lallemand Plant Care - Verdera Oy, VRA 1835 *, Finland | ||
| GB Pg14 | Mull, Great Britain | Pinus contorta Dougl. ex Loud. | No data | No data | Forestry Commission, FOC PG 410.3 *, Great Britain |
| GB Pg15 | Roslin, GB | No data | Forestry Commission, FOC PG SP log 5 *, Great Britain | ||
| GB Pg16 (Accession number: KU886025) | NRS, GB | Pinus sylvestris L. | Forestry Commission, FOC PG B 20/5 *, Great Britain | ||
| GB Pg17 | Buchan, GB | Forestry Commission, FOC PG BU 3 *, Great Britain |
| Region | Sequence | bp | m | s | Ps | Θ | π | D |
|---|---|---|---|---|---|---|---|---|
| Intron | I1 | 58 | 13 | 1 | 0.017241 | 0.005556 | 0.004863 | −0.274290 |
| I2 | 55 | 13 | 1 | 0.018182 | 0.005859 | 0.005128 | −0.274290 | |
| I3 | 53 | 13 | 3 | 0.056604 | 0.018240 | 0.013546 | −0.813698 | |
| I4 | 52 | 13 | 3 | 0.057692 | 0.018591 | 0.013807 | −0.813698 | |
| I5 | 56 | 13 | 6 | 0.107143 | 0.034526 | 0.025641 | −0.950320 | |
| Exon | Partial E1 | 75 | 13 | 0 | 0.000000 | 0.00000E+000 | 0.000000 | n/c |
| E2 | 95 | 13 | 1 | 0.010526 | 0.003392 | 0.002969 | −0.274290 | |
| E3 | 213 | 13 | 4 | 0.018779 | 0.006052 | 0.004093 | −1.099317 | |
| E4 | 137 | 13 | 3 | 0.021898 | 0.007057 | 0.006176 | −0.394391 | |
| E5 | 318 | 13 | 3 | 0.009434 | 0.003040 | 0.002661 | −0.394391 | |
| E6 | 299 | 13 | 7 | 0.023411 | 0.007544 | 0.006174 | −0.688629 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wit, M.; Sierota, Z.; Żółciak, A.; Mirzwa-Mróz, E.; Jabłońska, E.; Wakuliński, W. Phylogenetic Relationships between Phlebiopsis gigantea and Selected Basidiomycota Species Inferred from Partial DNA Sequence of Elongation Factor 1-Alpha Gene. Forests 2020, 11, 592. https://doi.org/10.3390/f11050592
Wit M, Sierota Z, Żółciak A, Mirzwa-Mróz E, Jabłońska E, Wakuliński W. Phylogenetic Relationships between Phlebiopsis gigantea and Selected Basidiomycota Species Inferred from Partial DNA Sequence of Elongation Factor 1-Alpha Gene. Forests. 2020; 11(5):592. https://doi.org/10.3390/f11050592
Chicago/Turabian StyleWit, Marcin, Zbigniew Sierota, Anna Żółciak, Ewa Mirzwa-Mróz, Emilia Jabłońska, and Wojciech Wakuliński. 2020. "Phylogenetic Relationships between Phlebiopsis gigantea and Selected Basidiomycota Species Inferred from Partial DNA Sequence of Elongation Factor 1-Alpha Gene" Forests 11, no. 5: 592. https://doi.org/10.3390/f11050592
APA StyleWit, M., Sierota, Z., Żółciak, A., Mirzwa-Mróz, E., Jabłońska, E., & Wakuliński, W. (2020). Phylogenetic Relationships between Phlebiopsis gigantea and Selected Basidiomycota Species Inferred from Partial DNA Sequence of Elongation Factor 1-Alpha Gene. Forests, 11(5), 592. https://doi.org/10.3390/f11050592





