Micro-Hotspots for Conservation: An Umbrella Tree Species for the Unique Socotran Reptile Fauna
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Data Analyses
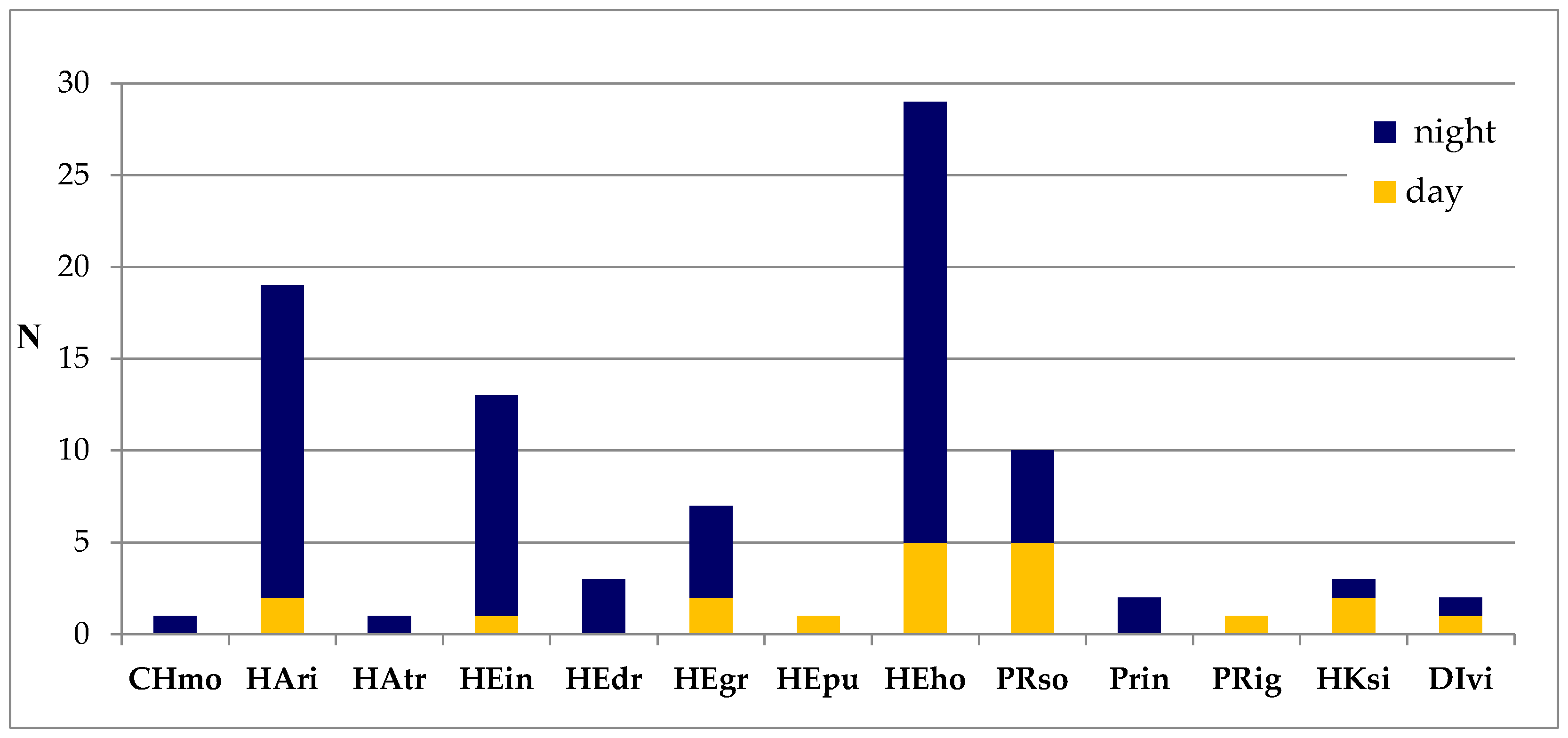
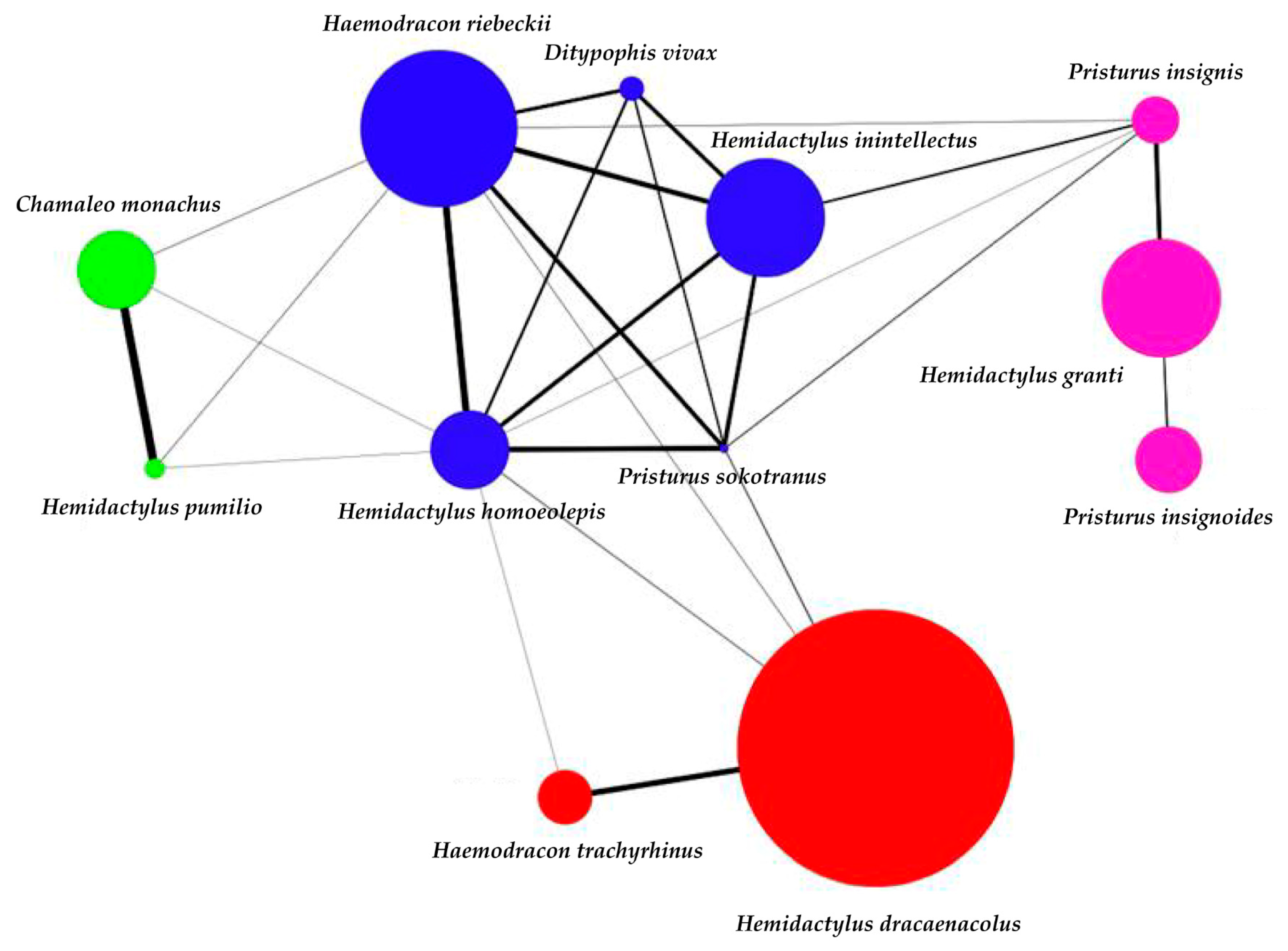
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Codes | Family | IUCN | Distribution | Sites |
|---|---|---|---|---|---|
| Chamaeleo monachus | CHmo | Chamaeleonidae | NT | Socotra Island | 1 |
| Haemodracon riebeckii | HAri | Phyllodactylidae | LC | Socotra, Samha | 8 |
| Haemodracon trachyrhinus | HAtr | Phyllodactylidae | LC | Socotra Island | 1 |
| Hemidactylus dracaenacolus | HEdr | Gekkonidae | CR | Socotra Island | 2 |
| Hemidactylus granti | HEgr | Gekkonidae | NT | Socotra Island | 2 |
| Hemidactylus homoeolepis | HEho | Gekkonidae | LC | Socotra, Samha & Darsa | 9 |
| Hemidactylus inintellectus | HEin | Gekkonidae | LC | Socotra Island | 5 |
| Hemidactylus pumilio | HEpu | Gekkonidae | LC | Socotra Island | 1 |
| Pristurus insignoides | PRig | Sphaerodactylidae | LC | Socotra Island | 2 |
| Pristurus insignis | PRin | Sphaerodactylidae | LC | Socotra Island | 1 |
| Pristurus sokotranus | PRso | Sphaerodactylidae | LC | Socotra Island | 7 |
| Ditypophis vivax | DIvi | Pseudoxyrhophiidae | LC | Socotra Island | 2 |
References
- Lambeck, R.J. Focal species: A multi-species umbrella for nature conservation. Conserv. Biol. 1997, 11, 849–856. [Google Scholar] [CrossRef]
- Fleishman, E.; Murphy, D.D.; Brussard, P.F. A new method for selection of umbrella species for conservation planning. Ecol. Appl. 2000, 10, 569–579. [Google Scholar] [CrossRef]
- Roberge, J.; Angelstam, P. Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 2004, 18, 76–85. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation?: The critical role of hotspots. In Biodiversity Hotspots Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer-Verlag: Basel, Switzerland, 2012; pp. 3–22. [Google Scholar]
- Courchamp, F.; Hoffmann, B.D.; Russell, J.C.; Leclerc, C.; Bellard, C. Climate change, sea-level rise, and conservation: Keeping island biodiversity afloat. Trends Ecol. Evol. 2014, 29, 127–130. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton Univ. Press: Princeton, NJ, USA, 1967. [Google Scholar]
- Losos, J.B.; Ricklefs, R.E. The Theory of Island Biogeography Revisited; Princeton Univ. Press: Princeton, NJ, USA, 2009. [Google Scholar]
- Autin, J.; Bellahsen, N.; Leroy, S.; Husson, L.; Beslier, M.-O.; d’Acremont, E. The role of structural inheritance in oblique rifting: Insights from analogue models and application to the Gulf of Aden. Tectonophysics 2013, 607, 51–64. [Google Scholar] [CrossRef]
- Miller, A.G.; Morris, M. Ethnoflora of the Soqotra Archipelago; The Royal Botanic Garden: Edinburgh, Scotland, 2004. [Google Scholar]
- Van Damme, K. Socotra Archipelago. In Encyclopedia of Islands; Gillespie, R., Clague, D., Eds.; Univ. California: Berkeley, CA, USA; Los Angeles, CA, USA, 2009; pp. 846–851. [Google Scholar]
- Razzetti, E.; Sindaco, R.; Griego, C.; Pella, F.; Ziliani, U.; Pupin, F.; Riservato, E.; Pelliteri-Rosa, D.; Butikofer, L.; Saeed Suleiman, A.; et al. Annotated checklist and distribution of the Socotran Archipelago Herpetofauna (Reptilia). Zootaxa 2011, 2826, 1–44. [Google Scholar] [CrossRef]
- Sindaco, R.; Metallinou, M.; Pupin, F.; Fasola, M.; Carranza, S. Forgotten in the ocean: Systematics, biogeography and evolution of the Trachylepis skinks of the Socotra Archipelago. Zool. Scr. 2012, 41, 346–362. [Google Scholar] [CrossRef]
- Vasconcelos, R.; Carranza, S. Systematics and biogeography of Hemidactylus homoeolepis Blanford, 1881, with the description of a new species from Arabia. Zootaxa 2014, 3835, 501–527. [Google Scholar] [CrossRef]
- Schätti, B.; Utiger, U. Hemerophis, a new genus for Zamenis socotrae Günther, and a contribution to the phylogeny of Old World racers, whip snakes, and related genera (Reptilia: Squamata: Colubrinae). Rev. Suisse Zool. 2001, 108, 919–948. [Google Scholar] [CrossRef]
- Nagy, Z.T.; Joger, H.; Wink, M.; Glaw, F.; Vences, M. Multiple colonization of Madagascar and Socotra by colubrid snakes: Evidence from nuclear and mitochondrial gene phylogenies. Proc. R. Soc. B 2003, 270, 2613–2621. [Google Scholar] [CrossRef]
- Gómez-Díaz, E.; Sindaco, R.; Pupin, F.; Fasola, M.; Carranza, S. Origin and in situ diversification in Hemidactylus geckos of the Socotra Archipelago. Mol. Ecol. 2012, 21, 4074–4092. [Google Scholar] [CrossRef] [PubMed]
- Miller, A. Dracaena Cinnabari. The IUCN Red List of Threatened Species 2004: E.T30428A9548491. 2004. Available online: https://dx.doi.org/10.2305/IUCN.UK.2004.RLTS.T30428A9548491.en (accessed on 17 January 2020).
- Adolt, R.; Habrova, H.; Madera, P. Crown age estimation of a monocotyledonous tree species Dracaena cinnabari using logistic regression. Trees 2012, 26, 1287–1298. [Google Scholar] [CrossRef]
- Maděra, P.; Volařík, D.; Patočka, Z.; Kalivodová, H.; Divín, J.; Rejžek, M.; Vybíral, J.; Lvončík, S.; Jeník, D.; Hanáček, P.; et al. Sustainable Land Use Management Needed to Conserve the Dragon’s Blood Tree of Socotra Island, a Vulnerable Endemic Umbrella Species. Sustainability 2019, 11, 3557. [Google Scholar] [CrossRef]
- Stone, L.; Roberts, A. The checkerboard score and species distributions. Oecologia 1990, 85, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.J.; Entsminger, G.L. EcoSim: Null models Software for Ecology; Version 7.0; Acquired Intelligence Inc.; Kesey-Bear, 2001; Available online: http://homepages.together.net/~gentsmin/ecosim.htm (accessed on 17 January 2018).
- Blondel, V.D.; Guillaume, J.-L.; Lambiotte, R.; Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, 10, P10008. [Google Scholar] [CrossRef]
- Batagelj, V.; Mrvar, A. Pajek 2.05. Program for Analysis and Visualization of Large Networks. 2001. Available online: http://mrvar.fdv.uni-lj.si/pajek/be2.htm (accessed on 17 January 2018).
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. 2009. Available online: https://gephi.org/publications/gephi-bastian-feb09.pdf (accessed on 17 January 2018).
- De la Cruz-Francisco, V. Rhizophora mangle Linnaeus, 1753 as umbrella species and biological reason for the protection and restoration of Tampamachoco lagoon, Veracruz, Mexico. BIOCYT 2012, 5, 341–352. [Google Scholar]
- Vasconcelos, R.; Montero-Mendieta, S.; Simó-Riudalbas, M.; Sindaco, R.; Santos, X.; Fasola, M.; Llorente, G.; Razzetti, E.; Carranza, S. Unexpectedly high levels of cryptic diversity uncovered by a complete DNA barcoding of reptiles of the Socotra Archipelago. PLoS ONE 2016, 11, e0149985. [Google Scholar] [CrossRef]
- Vasconcelos, R.; Razgour, O.; Tarroso, P.; Fasola, M.; Carranza, S.; Alves, P.C. Combining molecular and landscape tools for targeting evolutionary processes in reserve design: An approach for islands. PLoS ONE 2018, 13, e0200830. [Google Scholar] [CrossRef]
- García, C.; Vasconcelos, R. The beauty and the beast: Endemic mutualistic interactions promote community-based conservation on Socotra Island (Yemen). J. Nat. Conserv. 2017, 35, 20–23. [Google Scholar] [CrossRef]
- Dormann, C.F.; Bobrowski, M.; Dehling, D.M.; Harris, D.J.; Hartig, F.; Lischke, H.; Moretti, M.D.; Pagel, J.; Pinkert, S.; Schleuning, M.; et al. Biotic interactions in species distribution modelling: 10 questions to guide interpretation and avoid false conclusions. Glob. Ecol. Biogeogr. 2018, 27, 1004–1016. [Google Scholar] [CrossRef]
- Adolt, R.; Pavlis, J. Age structure and growth of Dracaena cinnabari populations on Socotra. Trees 2004, 18, 43–53. [Google Scholar] [CrossRef]
- Attorre, F.; Francesconi, F.; Taleb, N.; Scholte, P.; Saeed, A.; Alfo, M.; Bruno, F. Will dragonblood survive the next period of climate change? Current and future potential distribution of Dracaena cinnabari (Socotra, Yemen). Biol. Conserv. 2007, 138, 430–439. [Google Scholar] [CrossRef]
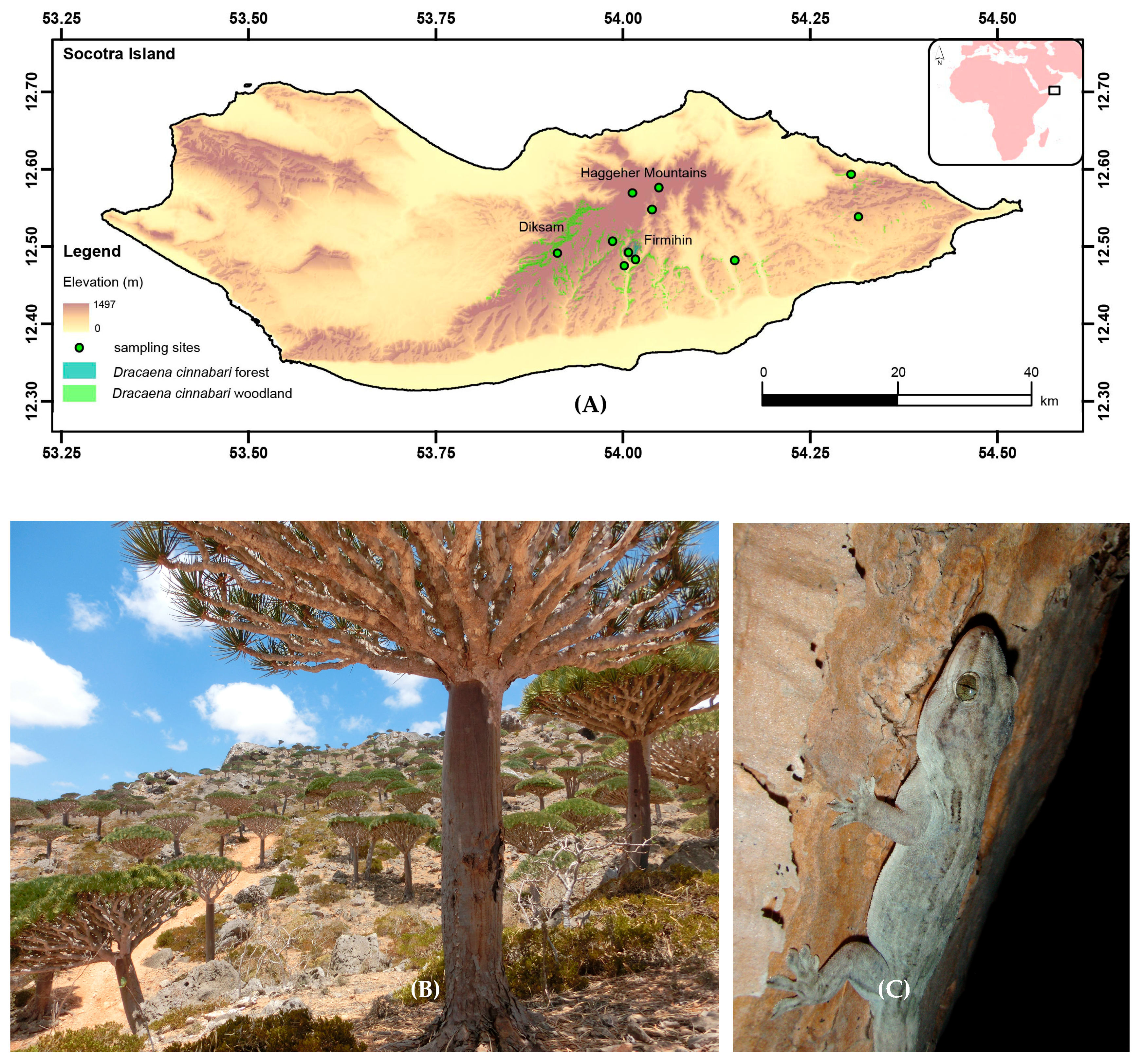
| S | Lat | Long | Location | N | MD1 | XD3 | G | SH | BE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12.572 | 54.048 | Adho Di Meleh, Haggeher | 6 | 1500 | 1500 | 117 | 272 | 4.6 |
| 2 | 12.572 | 54.049 | Ba’a, 2 km NE of Diksam | 7 | 125 | 1360 | 158 | 281 | 4.8 |
| 3 | 12.467 | 54.002 | Qafshifo, 3 km SE Firmihin | 18 | 410 | 1186 | 246 | 288 | 7.2 |
| 4 | 12.476 | 54.017 | Firmihin Protected area | 12 | 1500 | 1500 | 226 | 336 | 5.4 |
| 5 | 12.485 | 54.008 | Haif, Firmihin | 4 | 345 | 1112 | 160 | 306 | 4.8 |
| 6 | 12.484 | 53.913 | Shibehon plateau | 4 | 262 | 1414 | 175 | 246 | 5.4 |
| 7 | 12.589 | 54.305 | Homhil, Hallah | 9 | 140 | 1342 | 225 | 290 | 5.6 |
| 8 | 12.475 | 54.149 | Di Gisfo, Wadi Di-Fa’rhroh | 8 | 192 | 1156 | 173 | 275 | 5.9 |
| 9 | 12.533 | 54.315 | Killisan, 3km NW Qademinoh | 17 | 150 | 1286 | 207 | 268 | 5.1 |
| 10 | 12.500 | 53.987 | Diksam, Qafshifo | 4 | 65 | 1269 | 160 | 333 | 5.6 |
| 11 | 12.565 | 54.013 | Skand, Haggeher | 2 | 233 | 1191 | 114 | 260 | 4.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos, R.; Pujol-Buxó, E.; Llorente, G.A.; Saeed, A.; Carranza, S. Micro-Hotspots for Conservation: An Umbrella Tree Species for the Unique Socotran Reptile Fauna. Forests 2020, 11, 353. https://doi.org/10.3390/f11030353
Vasconcelos R, Pujol-Buxó E, Llorente GA, Saeed A, Carranza S. Micro-Hotspots for Conservation: An Umbrella Tree Species for the Unique Socotran Reptile Fauna. Forests. 2020; 11(3):353. https://doi.org/10.3390/f11030353
Chicago/Turabian StyleVasconcelos, Raquel, Eudald Pujol-Buxó, Gustavo A. Llorente, Ahmed Saeed, and Salvador Carranza. 2020. "Micro-Hotspots for Conservation: An Umbrella Tree Species for the Unique Socotran Reptile Fauna" Forests 11, no. 3: 353. https://doi.org/10.3390/f11030353
APA StyleVasconcelos, R., Pujol-Buxó, E., Llorente, G. A., Saeed, A., & Carranza, S. (2020). Micro-Hotspots for Conservation: An Umbrella Tree Species for the Unique Socotran Reptile Fauna. Forests, 11(3), 353. https://doi.org/10.3390/f11030353






