Role of the Dominant Species on the Distributions of Neighbor Species in a Subtropical Forest
Abstract
1. Introduction
2. Methods
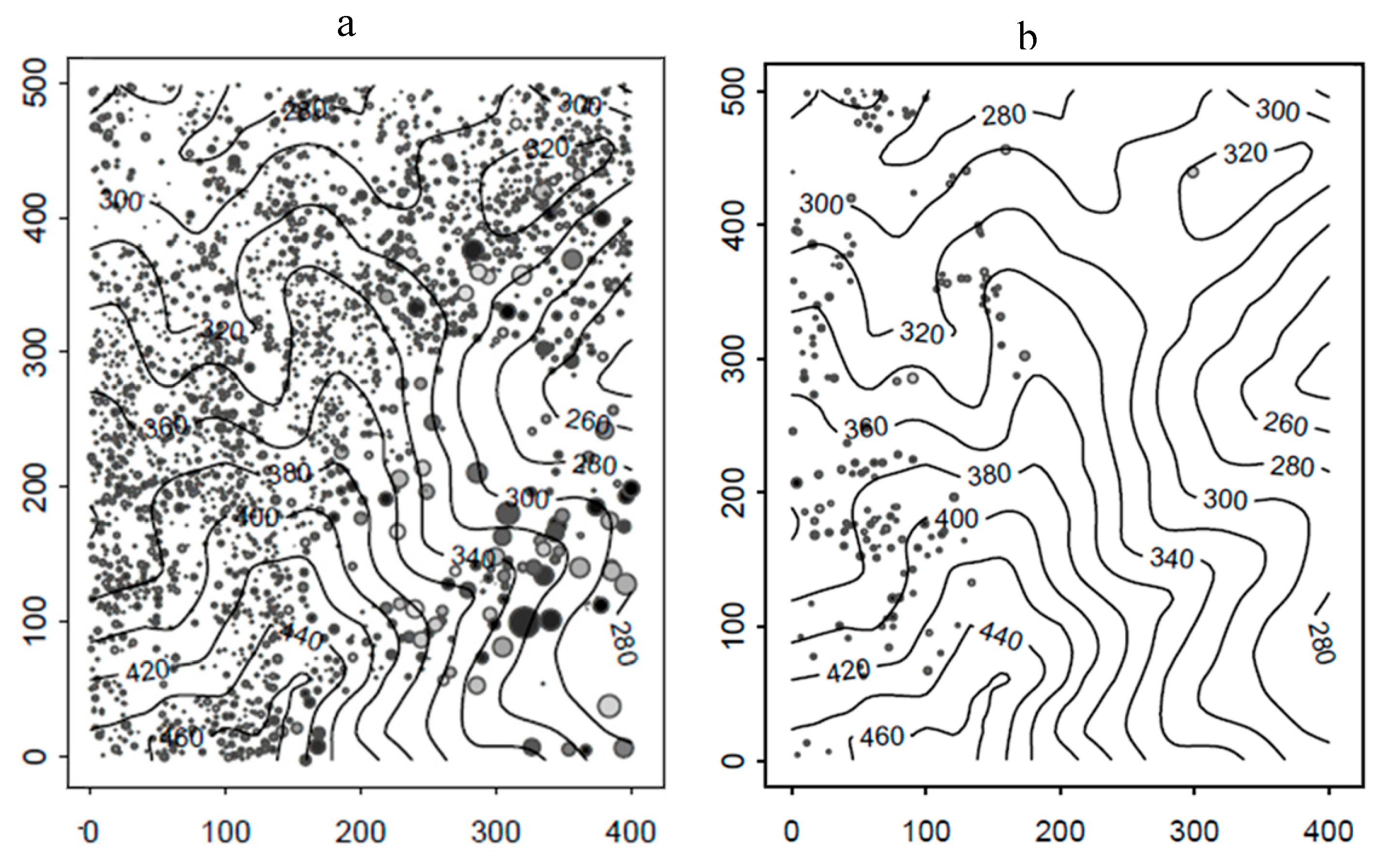
2.1. Study Area
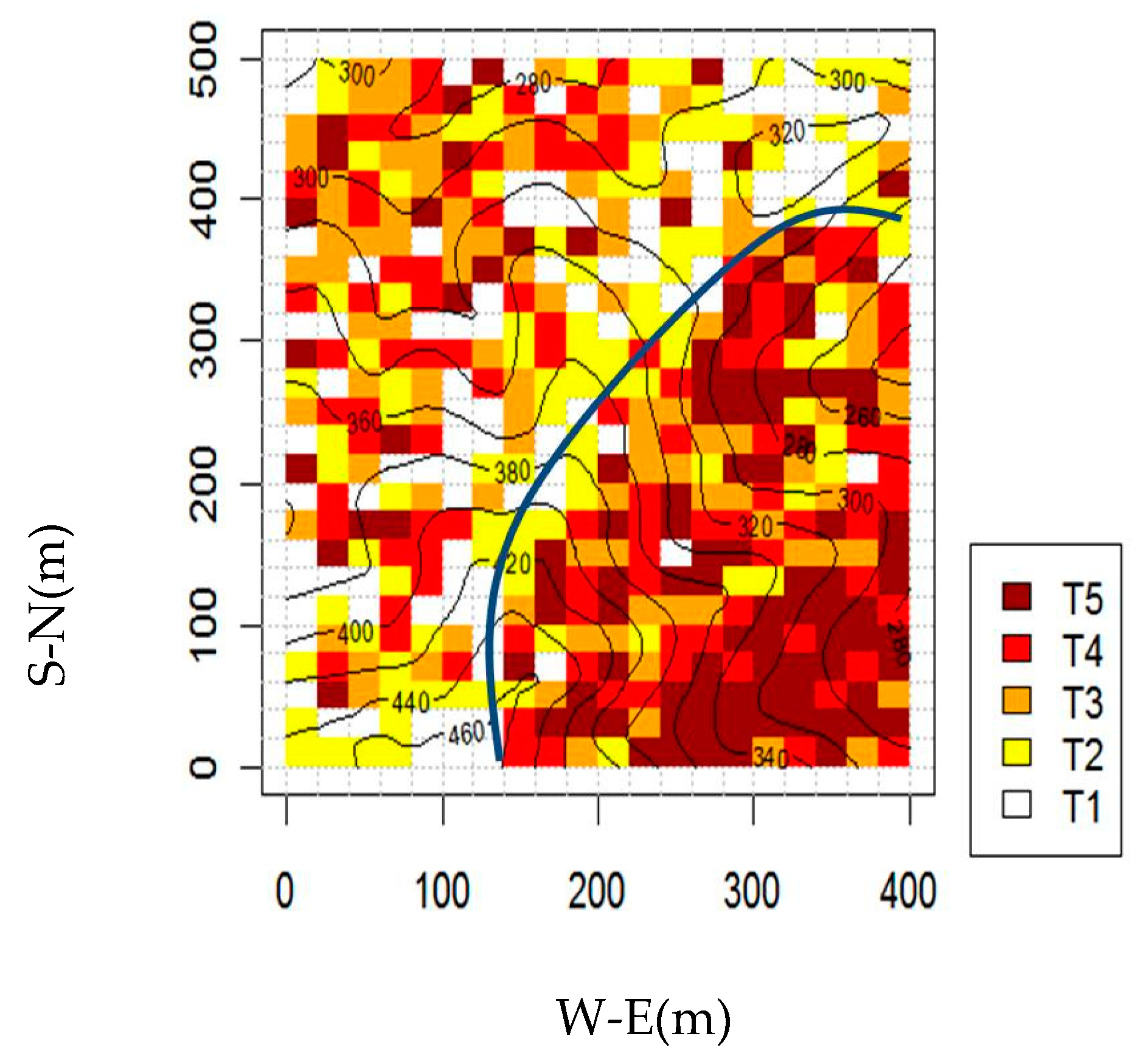
2.2. Successional Stages
2.3. The Relatedness between Castanopsis chinensis and Other Species
2.4. Data Analysis
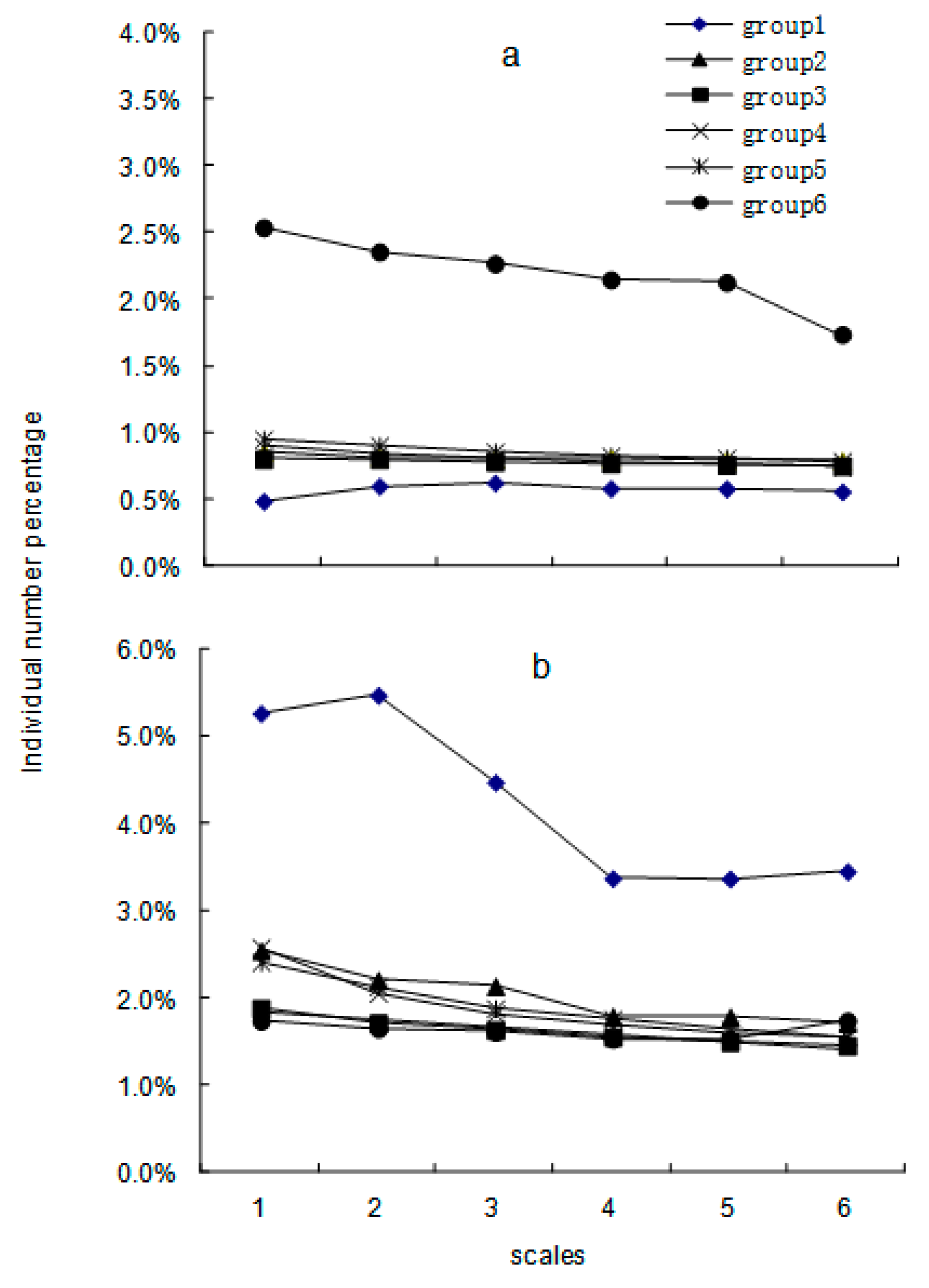
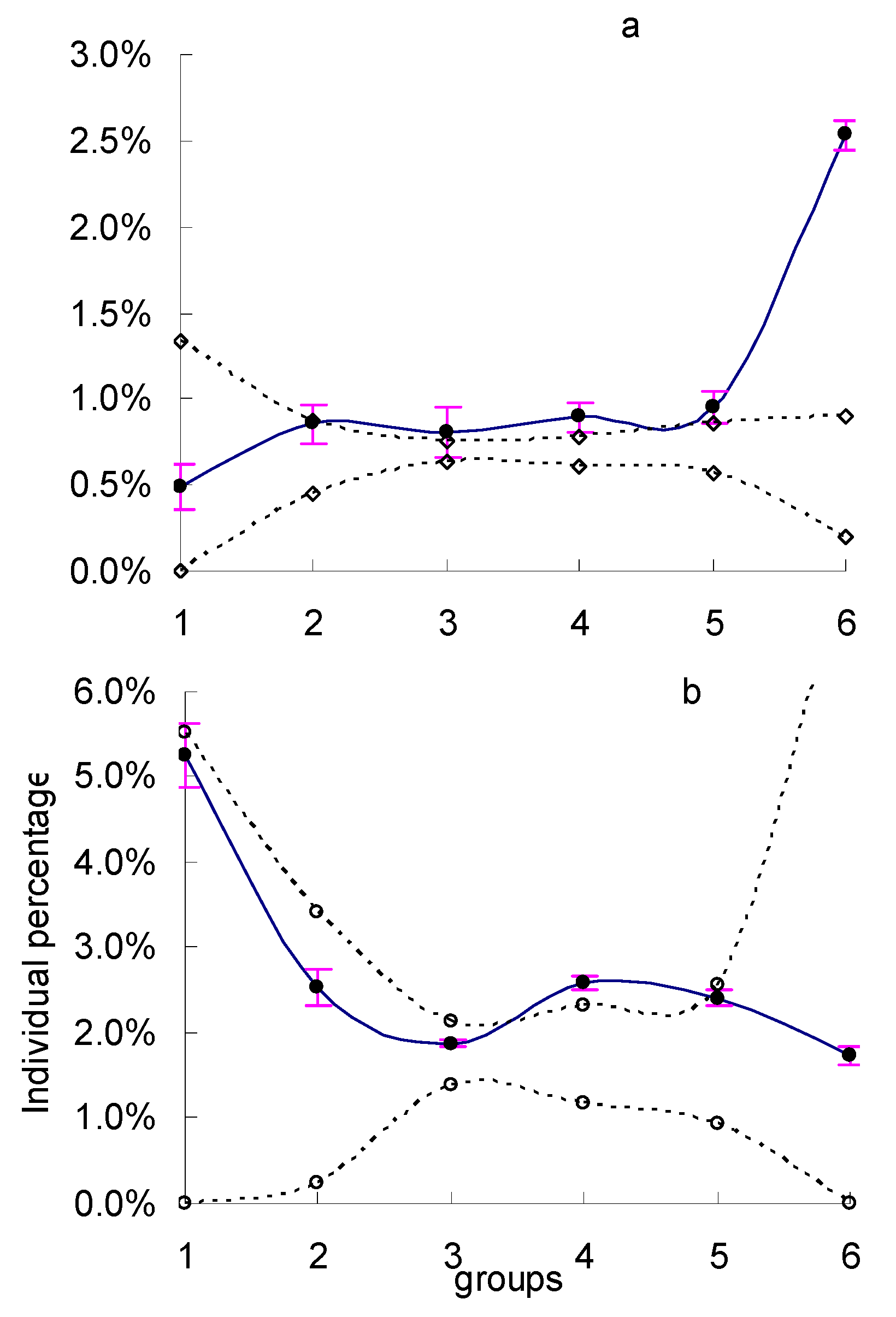
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Legendre, P.; Mi, X.; Ren, H.; Ma, K.; Yu, M.; Sun, I.F.; He, F. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 2009, 90, 663–674. [Google Scholar] [CrossRef]
- He, F.L.; Duncan, P.R. Density-Dependent Effects on Tree Survival in an Old-Growth Douglas Fir Forest. J. Ecol. 2000, 88, 676–688. [Google Scholar] [CrossRef]
- Swenson, N.G.; Enquist, B.J.; Thompson, J.; Zimmerman, J.K. The influence of spatial and size scale on phylogenetic relatedness in tropical forest communities. Ecology 2007, 88, 1770–1780. [Google Scholar] [CrossRef]
- Wang, Z.F.; Lian, J.Y.; Huang, G.M.; Ye, W.H.; Cao, H.L.; Wang, Z.M. Genetic groups in the common plant species Castanopsis chinensis and their associations with topographic habitats. Oikos 2012, 121, 2044–2051. [Google Scholar] [CrossRef]
- Martorell, C.; Freckleton, R.P. Testing the roles of competition, facilitation and stochasticity on community structure in a species-rich assemblage. J. Ecol. 2014, 102, 74–85. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Kozak, K.H.; Fine, P.V.A.; Kembel, S.W. The merging of community ecology and phylogenetic biology. Ecol. Lett. 2009, 12, 693–715. [Google Scholar] [CrossRef]
- Peterson, A.T. Ecological niche conservatism: A time-structured review of evidence. J. Biogeogr. 2011, 38, 817–827. [Google Scholar] [CrossRef]
- Gerhold, P.; Cahill, J.F.; Winter, M.; Bartish, I.V.; Prinzing, A. Phylogenetic patterns are not proxies of community assembly mechanisms (they are far better). Funct. Ecol. 2015, 29, 600–614. [Google Scholar] [CrossRef]
- Blomberg, S.P.; Garland, T.; Ives, A.R. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar] [CrossRef]
- Pei, N.C.; Lian, J.Y.; Erickson, D.L.; Swenson, N.G.; Kress, W.J.; Ye, W.H.; Ge, X.J. Exploring Tree-Habitat Associations in a Chinese Subtropical Forest Plot Using a Molecular Phylogeny Generated from DNA Barcode Loci. PLoS ONE 2011, 6, e21273. [Google Scholar] [CrossRef]
- Mooney, K.A.; Jones, P.; Agrawal, A.A. Coexisting congeners: demography, competition, and interactions with cardenolides for two milkweed-feeding aphids. Oikos 2008, 117, 450–458. [Google Scholar] [CrossRef]
- Uriarte, M.; Canham, C.D.; Thompson, J.; Zimmerman, J.K. A neighborhood analysis of tree growth and survival in a hurricane-driven tropical forest. Ecol. Monogr. 2004, 74, 591–614. [Google Scholar] [CrossRef]
- Réjou-Méchain, M.; Flores, O.; Pélissier, R.; Fayolle, A.; Fauvet, N.; Gourlet-Fleury, S. Tropical tree assembly depends on the interactions between successional and soil filtering processes. Glob. Ecol. Biogeogr. 2014, 23, 1440–1449. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Letcher, S.G.; Chazdon, R.L.; Andrade, A.C.S.; Bongers, F.; Breugel, M.V.; Finegan, B.; Laurance, S.G.; Mesquita, R.; Martínez Ramos, M.; Williamson, G.B. Phylogenetic community structure during succession: Evidence from three Neotropical forest sites. Perspect. Plant. Eco. 2012, 14, 79–87. [Google Scholar] [CrossRef]
- Paine, R.T. Intertidal community structure: experimental studies on the relationship between a dominant competitor and its principal predator. Oecologia 1974, 15, 93–120. [Google Scholar] [CrossRef]
- Vamosi, S.; Heard, S.; Vamosi, J.; Webb, C. Emerging patterns in the comparative analysis of phylogenetic community structure. Mol. Ecol. 2009, 18, 572–592. [Google Scholar] [CrossRef]
- Ren, H.; Yang, L.; Liu, N. Nurse plant theory and its application in ecological restoration in lower subtropics of China. Prog. Nat. Sci. 2008, 18, 137–142. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.L.; Ye, W.H.; Cao, H.L.; Wei, S.G.; Wang, Z.G.; Lian, J.Y.; Sun, Y.F.; Ma, K.P.; He, F.L. Spatial distributions of tree species in a subtropical forest of China. Oikos 2009, 118, 495–502. [Google Scholar] [CrossRef]
- Ye, W.H.; Cao, H.L.; Huang, Z.L.; Lian, J.Y.; Wang, Z.G.; Li, L.; Wei, S.G.; Wang, Z.M. Community structure of a 20 hm2 lower subtropical evergreen broadleaved forest plot in Dinghushan, China. J. Plant. Ecol.-China 2008, 32, 274–286. [Google Scholar]
- Wei, S.G.; Li, L.; Walther, B.A.; Ye, W.H.; Huang, Z.L.; Cao, H.L.; Lian, J.Y.; Wang, Z.G.; Chen, Y.Y. Comparative performance of species-richness estimators using data from a subtropical forest tree community. Ecol. Res. 2010, 25, 93–101. [Google Scholar] [CrossRef]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Li, L.; Wei, S.-G.; Huang, Z.-L.; Ye, W.-H.; Cao, H.-L. Spatial patterns and interspecific associations of three canopy species at different life stages in a subtropical forest, China. J. Integr. Plant Biol. 2008, 50, 1140–1150. [Google Scholar] [CrossRef]
- Zhang, H.D.; Wang, B.S.; Zhang, C.C.; Qiu, H.X. A study of plant community of Dinghushan in Gaoyao, Guangdong. Acta Sci. Nat. Univ. Sunyatseni 1955, 159–225. [Google Scholar]
- Lian, J.Y.; Chen, C.; Huang, Z.L.; Cao, H.L.; Ye, W.H. Community composition and stand age in a subtropical forest, southern China. Biodivers. Sci. 2015, 23, 174–182. [Google Scholar] [CrossRef]
- Kress, W.J.; Erickson, D.L.; Jones, F.A.; Swenson, N.G.; Perez, R.; Sanjur, O.; Bermingham, E. Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama. Proc. Natl. Acad. Sci. USA 2009, 106, 18621–18626. [Google Scholar] [CrossRef]
- Doyle, J.; Doyle, J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1986, 19, 11–15. [Google Scholar]
- Chessel, D.; Dufour, A.B.; Thioulouse, J. The ade4 package-I- One-table methods. R News 2004, 4, 5–10. [Google Scholar]
- Cadotte, M.W.; Dinnage, R.; Tilman, D. Phylogenetic diversity promotes ecosystem stability. Ecology 2012, 93, 223–233. [Google Scholar] [CrossRef]
- Hughes, A.R.; Inouye, B.D.; Johnson, M.T.; Underwood, N.; Vellend, M. Ecological consequences of genetic diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef]
- Beltrán, E.; Valiente-Banuet, A.; VerdúVerd, M. Trait divergence and indirect interactions allow facilitation of congeneric species. Ann. Bot. 2012, 110, 1369–1376. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, W.; Cao, H.; Huang, Z.; Lian, J.; Li, L.; Wei, S.; Sun, I.F. Species-topography association in a species-rich subtropical forest of China. Basic Appl. Ecol. 2009, 10, 648–655. [Google Scholar] [CrossRef]
- Guillot, G.; Rousset, F. Dismantling the Mantel tests. Methods Ecol. Evol. 2013, 4, 336–344. [Google Scholar] [CrossRef]
- Bin, Y.; Lian, J.; Wang, Z.; Ye, W.; Cao, H. Tree Mortality and Recruitment in a Subtropical Broadleaved Monsoon Forest in South China. J. Trop. For. Sci. 2011, 23, 57–66. [Google Scholar]
- Shen, Y.; Santiago, L.S.; Ma, L.; Lin, G.J.; Lian, J.Y.; Cao, H.L.; Ye, W.H. Forest dynamics of a subtropical monsoon forest in Dinghushan, China: recruitment, mortality and the pace of community change. J. Trop. Ecol. 2013, 29, 131–145. [Google Scholar] [CrossRef]
- Cornwell, W.; Ackerly, D. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol. Monogr. 2009, 79, 109–126. [Google Scholar] [CrossRef]
- Kembel, S.W.; Hubbell, S.P. The phylogenetic structure of a neotropical forest tree community. Ecology 2006, 87, S86–S99. [Google Scholar] [CrossRef]
- Darwin, C. On The Origin of Species by Means of Natural Selection. Am. Anthropol. 1963, 61, 176–177. [Google Scholar]
| Ecological Process (Hypothesis) | Predicted Relationship |
|---|---|
| Environmental filter hypothesis | Positive relationship between dominant species and phylogenetic distance of neighbor species due to close relatives with similar phenotypic utilizing analogous resources. |
| Negative density-dependence hypothesis | Negative relationship between dominant species and phylogenetic distance of neighbor species due to resource competition and predation or disease limiting coexistence of conspecific individuals. |
| Neutrality hypothesis | No relationship between dominant and phylogenetic distance of neighbor species (null/neutral model) |
| Phylogenetic Distance (Group No.) | Whole Plot | Succession Forest | Mature Forest | |||
|---|---|---|---|---|---|---|
| Sp. No. | Ind. No. | Sp. No. | Ind. No. | Sp. No. | Ind. No. | |
| 1 | 1 | 273 | 1 | 130 | 1 | 143 |
| 2 | 9 | 1743 | 9 | 1296 | 6 | 447 |
| 3 | 99 | 29811 | 81 | 14258 | 87 | 15553 |
| 4 | 54 | 22871 | 45 | 14932 | 44 | 7939 |
| 5 | 27 | 14212 | 22 | 10892 | 24 | 3320 |
| 6 | 4 | 230 | 4 | 196 | 2 | 34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Li, L.; Lian, J.; Nielsen, S.E.; Wang, Z.; Mao, L.; Ouyang, X.; Cao, H.; Ye, W. Role of the Dominant Species on the Distributions of Neighbor Species in a Subtropical Forest. Forests 2020, 11, 352. https://doi.org/10.3390/f11030352
Wei S, Li L, Lian J, Nielsen SE, Wang Z, Mao L, Ouyang X, Cao H, Ye W. Role of the Dominant Species on the Distributions of Neighbor Species in a Subtropical Forest. Forests. 2020; 11(3):352. https://doi.org/10.3390/f11030352
Chicago/Turabian StyleWei, Shiguang, Lin Li, Juyu Lian, Scott E. Nielsen, Zhigao Wang, Lingfeng Mao, Xuejun Ouyang, Honglin Cao, and Wanhui Ye. 2020. "Role of the Dominant Species on the Distributions of Neighbor Species in a Subtropical Forest" Forests 11, no. 3: 352. https://doi.org/10.3390/f11030352
APA StyleWei, S., Li, L., Lian, J., Nielsen, S. E., Wang, Z., Mao, L., Ouyang, X., Cao, H., & Ye, W. (2020). Role of the Dominant Species on the Distributions of Neighbor Species in a Subtropical Forest. Forests, 11(3), 352. https://doi.org/10.3390/f11030352




