The Conservation Status and Population Mapping of the Endangered Dracaena serrulata in the Dhofar Mountains, Oman
Abstract
1. Introduction
2. Material and Methods
2.1. Population Size and Distribution
2.2. Health Status of the Population
2.3. National Threat Assessment of Dracaena serrulata according to International Union for Conservation of Nature (IUCN)
3. Results
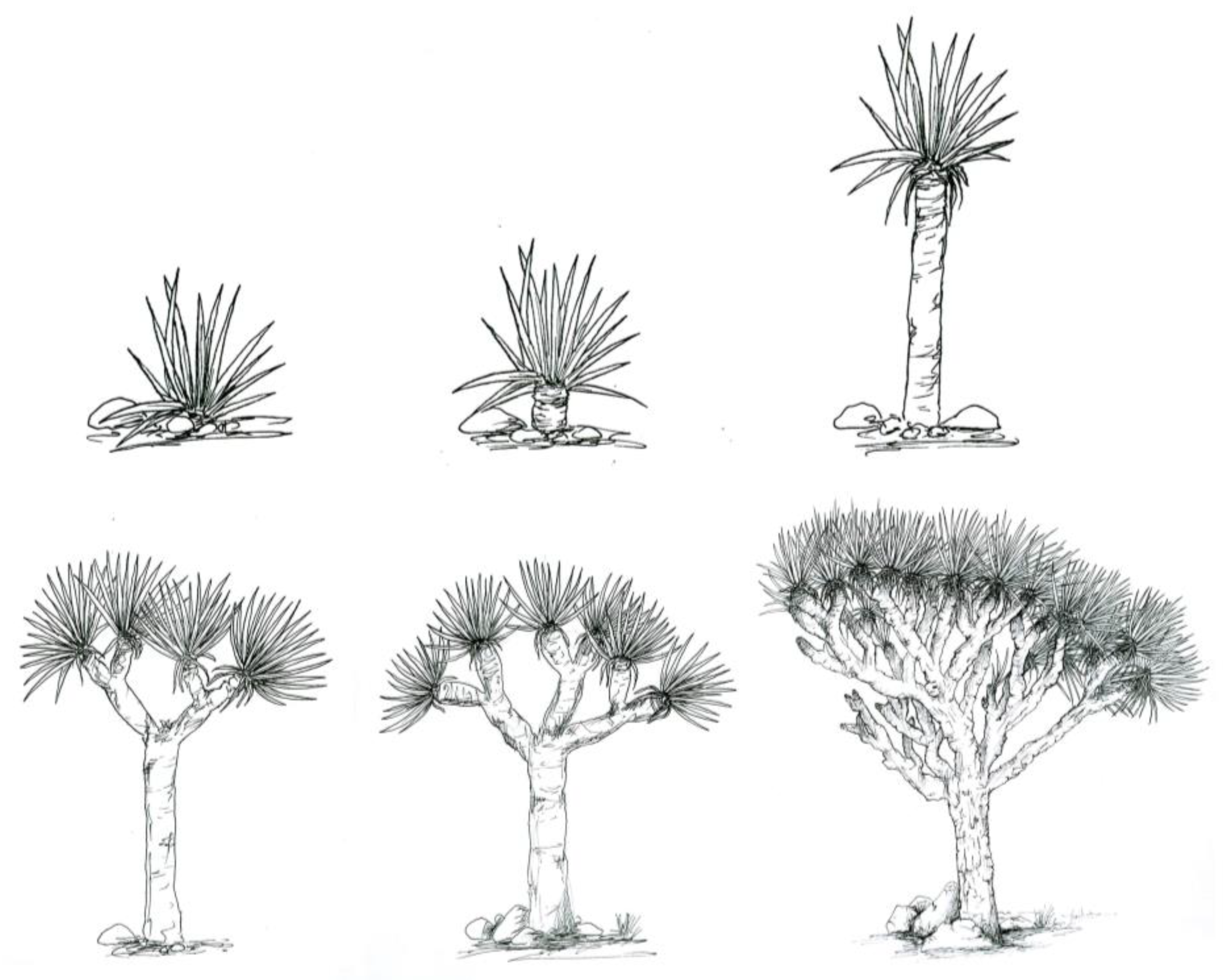
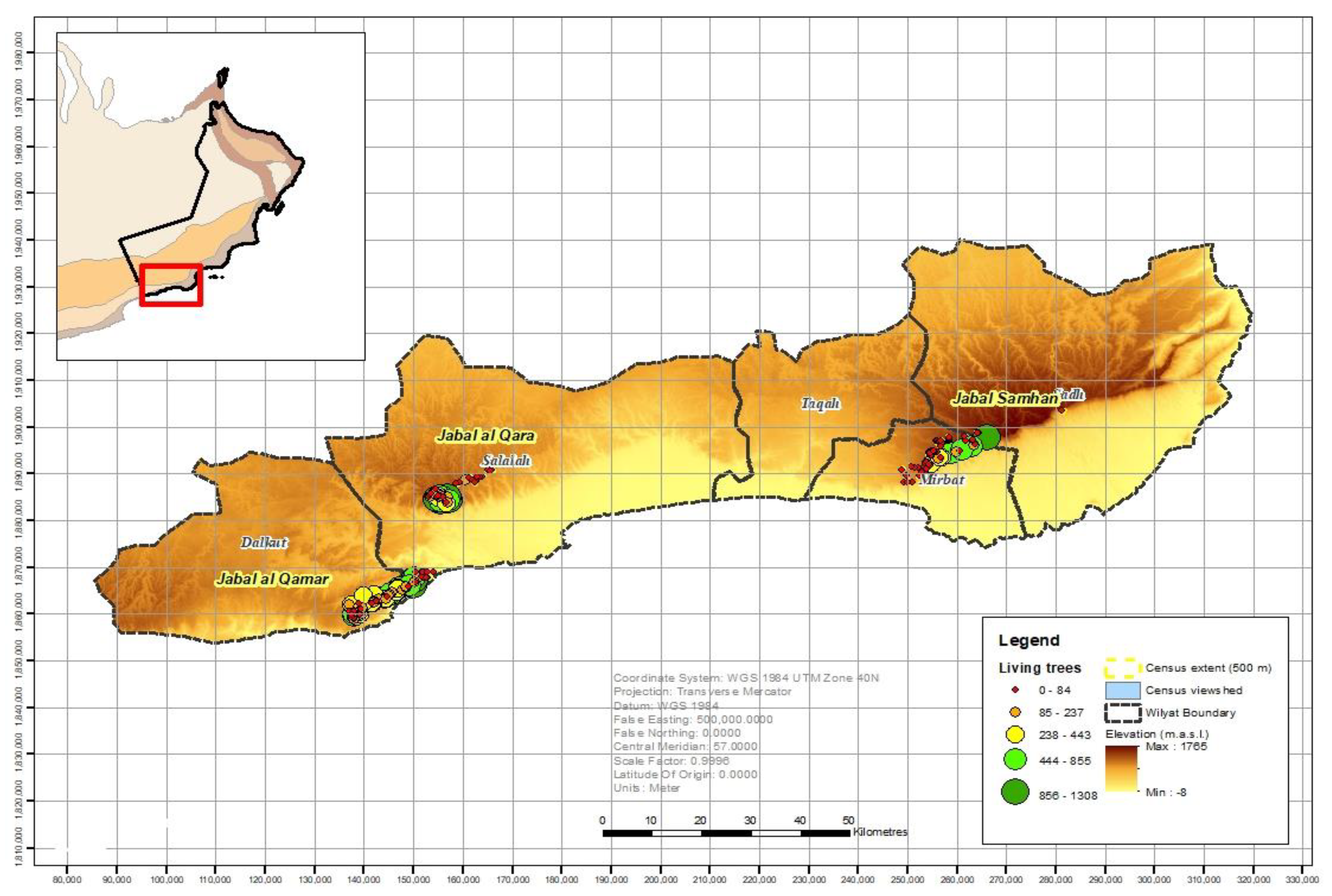
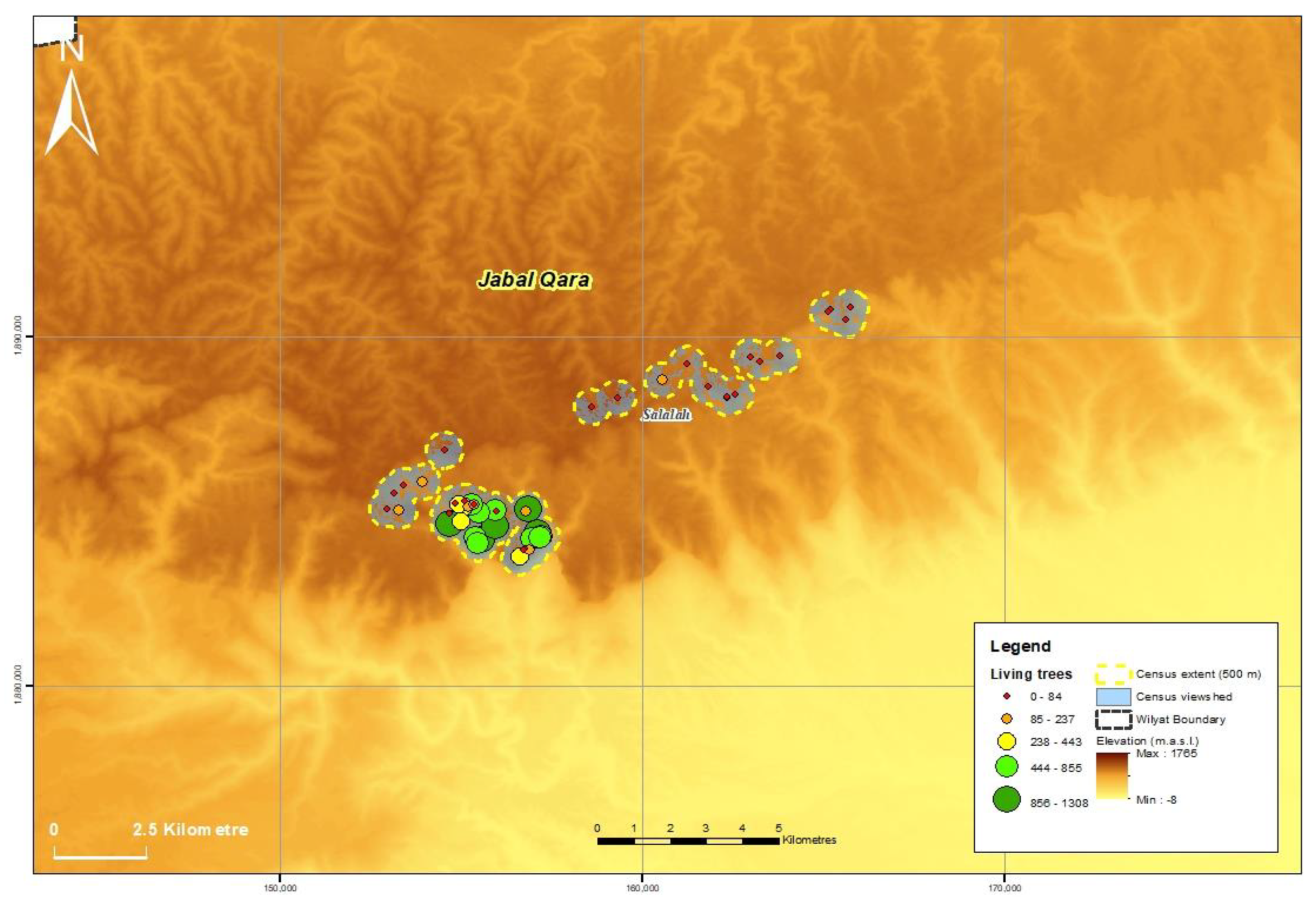
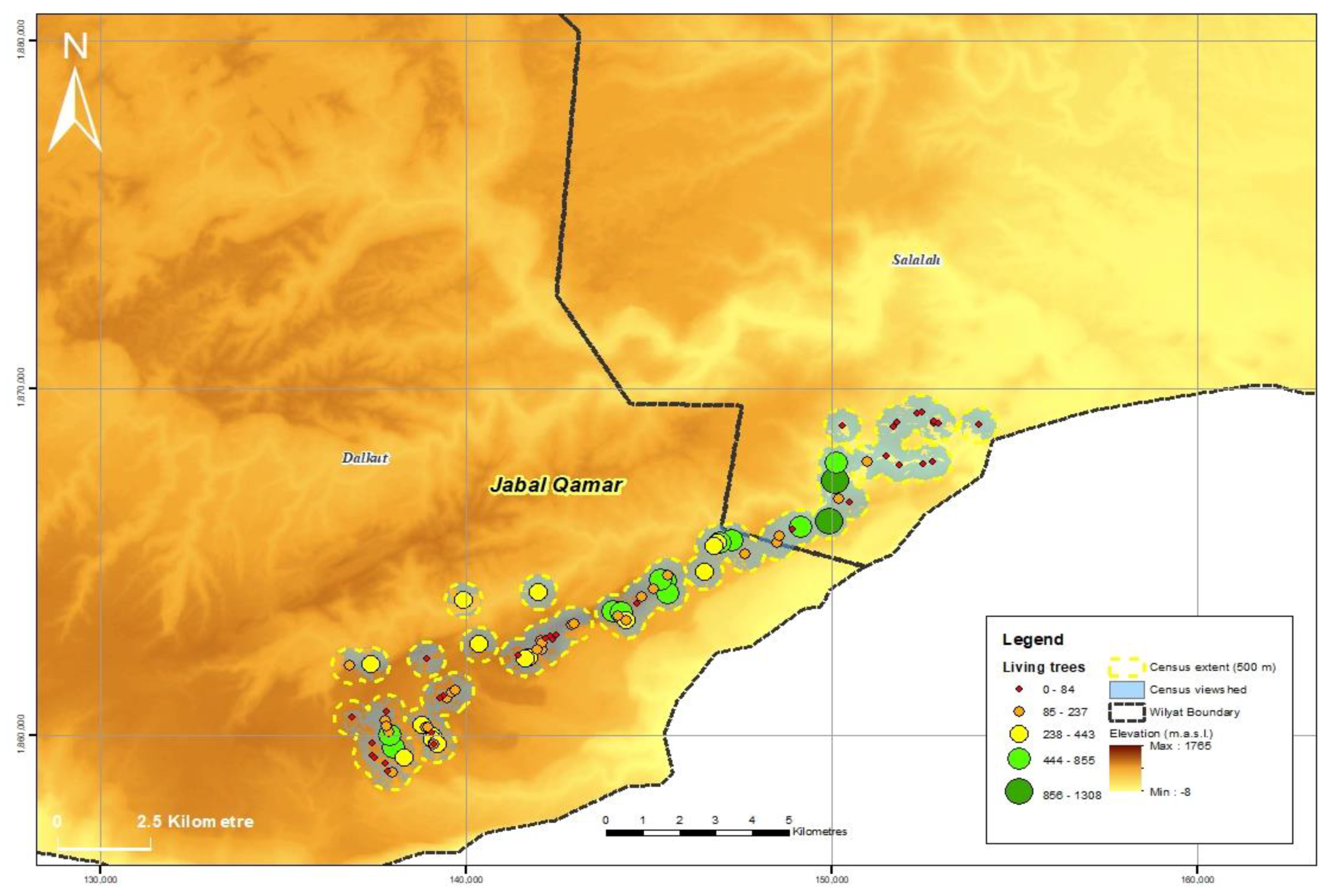
3.1. Population Size and Distribution
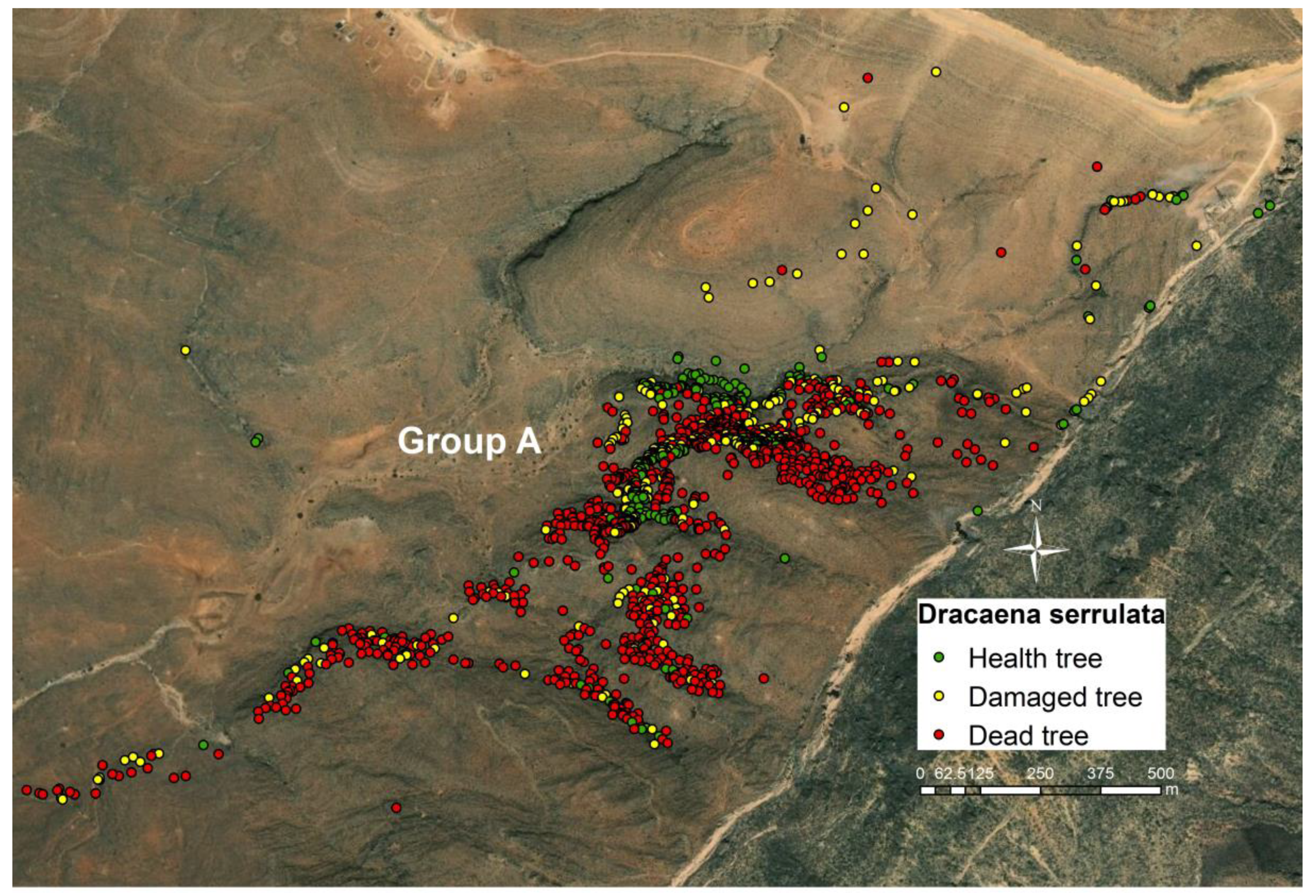
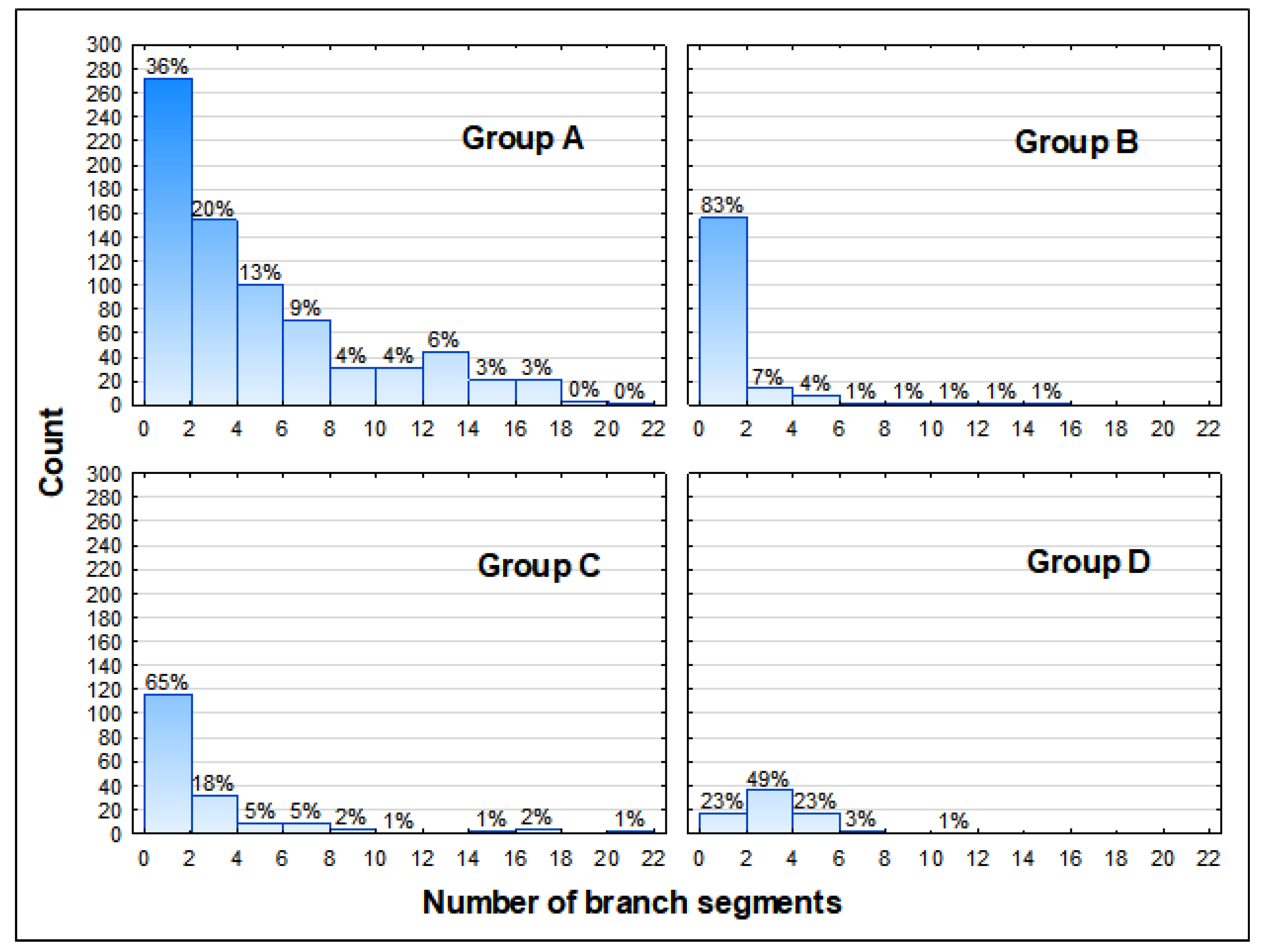
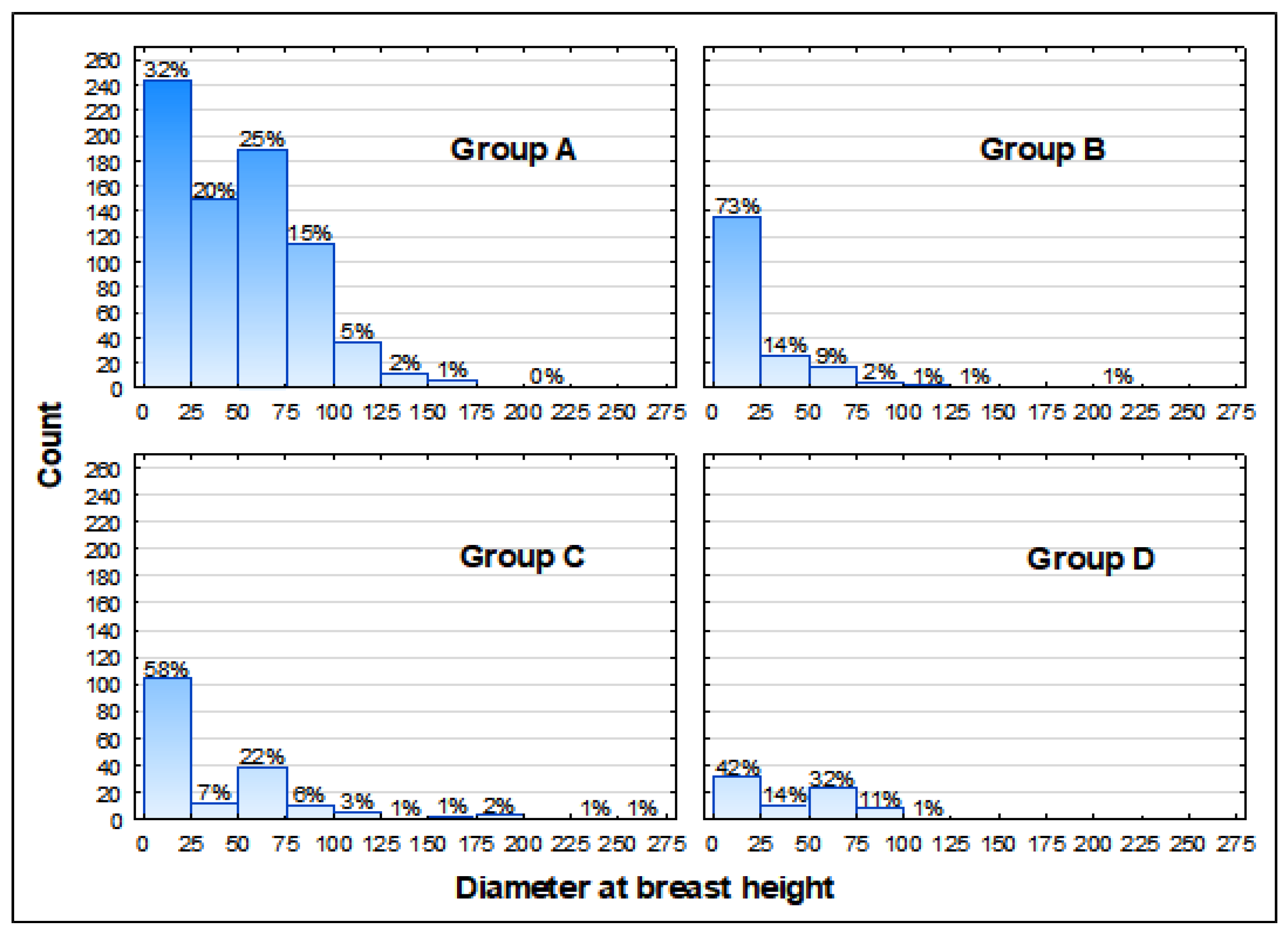
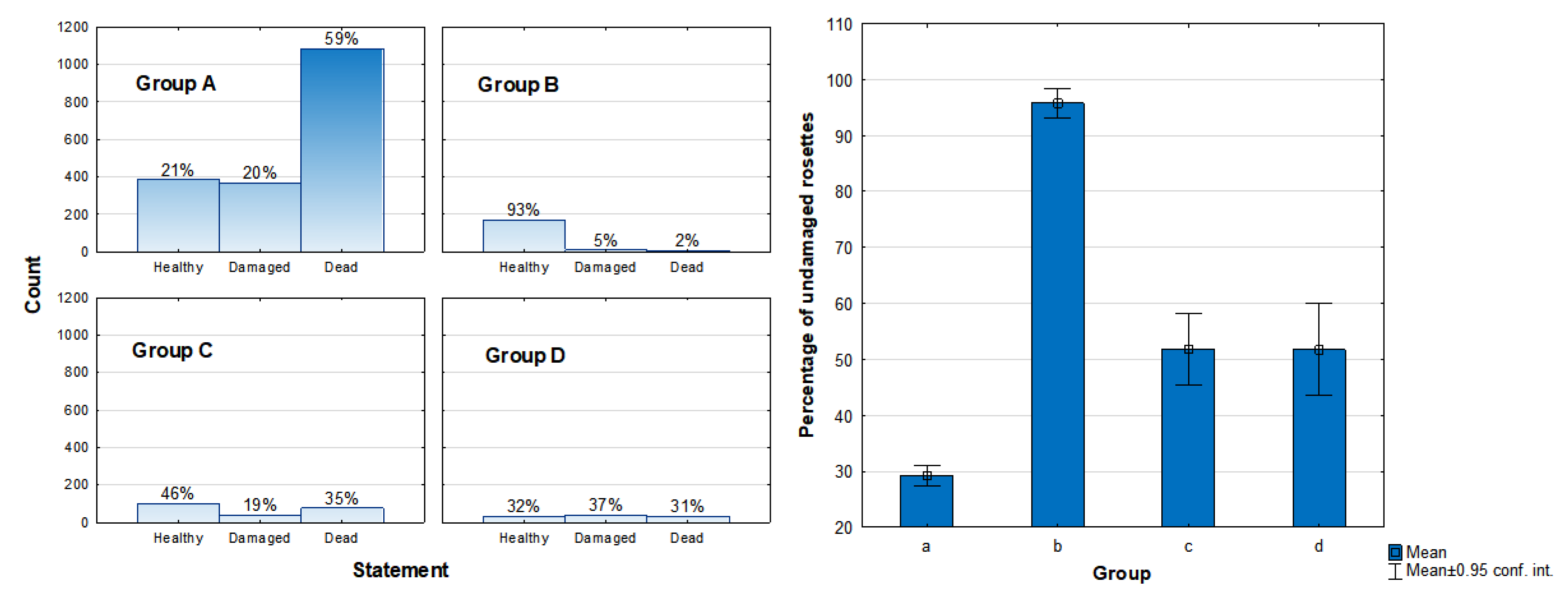
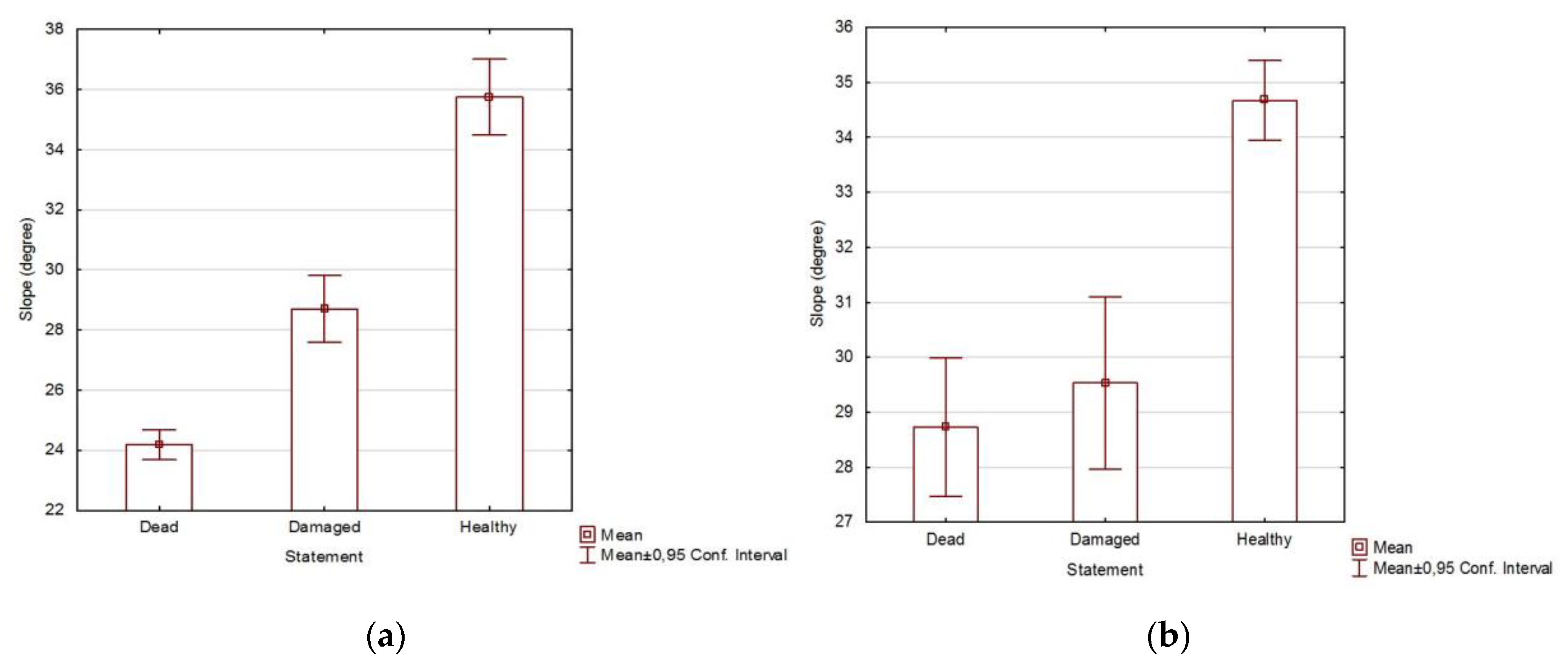
3.2. Health Status of the Population
3.3. IUCN National Assessment of Dracaena Serrulata
- (a)
- Number of locations ≤5 (locations the threats on the taxon).
- (b)
- Continuing decline inferred in (iii) extent and/or quality of habitat, (v) number of mature individuals.
- (a)
- Number of locations ≤5 (locations the threats on the taxon).
- (b)
- Continuing decline inferred in (iii) extent and/or quality of habitat, (v) number of mature individuals.
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brown, G.; Mies, B.A. Vegetation Ecology of Socotra. In Plant and Vegetation 7; Springers: Berlin/Heidelberg, Germany, 2012; p. 379. [Google Scholar]
- Bos, J. Dracaena in west Africa. In The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin Heidelberg, Germany, 1998; Volume III, pp. 238–241. [Google Scholar]
- Habrová, H.; Maděra, P. Ekologie společenstev dračince rumělkového (Dracaena cinnabari) na Sokotře; Polehla, P., Ed.; Hodnocení stavu a vývoje lesních geobinocenóz.: Brno, Czech Republic, 2004; ISBN 80-7157-787-1. [Google Scholar]
- Al Hosni, A.; Oliver, I.; Al Jabri, Y.; Al Saidi, A.; Al Rawahi, A.; Al Hinai, H. Ex situ conservation of Dracaena serrulata in Dhofar province, southern Oman. Acta Horticulturae 2018, 1190, 9–14. [Google Scholar] [CrossRef]
- Marrero, A.; Almeida, R.S.; Gonzàlez-Martìn, M. A new species of the wild dragon tree, Dracaena (Dracaenaceae) from Gran Canaria and its taxonomic and biogeographic implications. Bot. J. Linnaean Soc. 1998, 128, 291–314. [Google Scholar]
- Wilkin, P.; Suksathan, P.; Keeratikiat, K.; Van Welzen, P.; Wiland-Szymańska, J. A new threatened endemic species from central and northeastern Thailand, Dracaena jayniana (Asparagaceae: Tribe Nolinoideae). Kew Bull. 2012, 67, 697–705. [Google Scholar] [CrossRef]
- Zona, S.; Álvarez De Zayas, A.; Orellana, R.; Oviedo, R.; Jestrow, B.; Francisco-Ortega, J.; Dracaena, L. (Asparagaceae) in the New World: Its history and Botany. Vieraea 2014, 42, 219–240. [Google Scholar]
- Kew Science. Plants of the World on-Line. 2018. Available online: http://www.emonocot.org/taxon/urn:lsid:ipni.org:names:77170340-1 (accessed on 1 November 2019).
- Adolt, R.; Pavliš, J. Age structure and growth of Dracaena cinnabari populations on Socotra. Trees-Struct. Funct. 2004, 18, 43–53. [Google Scholar] [CrossRef]
- Nadezhdina, N.; Plichta, R.; Nadezhdin, V.; Gebauer, R.; Jupa, R.; Habrová, H.; Maděra, P. A comparative structural and functional study of leaf traits and sap flow in Dracaena cinnabari and Dracaena draco seedlings. Funct. Plant Biol. 2015, 42, 1092–1105. [Google Scholar] [CrossRef]
- Nadezhdina, N.; Nadezhdin, V. Are Dracaena nebulophytes able to drink atmospheric water? Environ. Exp. Bot. 2017, 139, 57–66. [Google Scholar] [CrossRef]
- Nadezhdina, N.; Al-Okaishi, A.; Maděra, P. Sap Flow Measurements in a Socotra Dragon’s Blood Tree (Dracaena cinnabari) in its Area of Origin. Trop. Plant Biol. 2018, 11, 107–118. [Google Scholar] [CrossRef]
- Miller, A.G.; Morris, M.; Diccon, A.; Atkinson, R. Ethnoflora of the Soqotra Archipelago; Royal Botanic Garden: Edinburgh, UK, 2004; p. 759. [Google Scholar]
- Hildebrandt, A.; Eltahir, E.A.B. (2006). Forest on the edge: Seasonal cloud forest in Oman creates its own ecological niche. Geophysical Research Letters 33: L11401, 2006 June. [Google Scholar] [CrossRef]
- Kürschner, H.; Hein, P.; Kilian, N.; Hubaishan, M.A. Diversity and zonation of the forests and woodlands of the mountains of northern Socotra, Yemen. Englera 2006, 28, 11–55. [Google Scholar] [CrossRef]
- Aynekulu, E.; Aerts, R.; Moonen, P.; Denich, M.; Gebrehiwot, K.; Vagen, T.G.; Mekuria, W.; Boehmer, H.J. Altitudinal variation and conservation priorities of vegetation along the Great Rift Valley escarpment, northern Ethiopia. Biodivers. Conserv. 2012, 21, 2691–2707. [Google Scholar] [CrossRef]
- De Sanctis, M.; Adeeb, A.; Farcomeni, A.; Patriarca Ch Saed, A.; Attorre, F. Classification and distribution patterns of plant communities on Socotra Island, Yemen. Appl. Veg. Sci. 2013, 16, 148–165. [Google Scholar] [CrossRef]
- Habrová, H.; Buček, A. Overview of biotope types of Socotra Island. J. Landsc. Ecol. 2013, 6, 60–83. [Google Scholar] [CrossRef]
- Bramwell, D. Panbiogeography of the Canary Islands flora. In Proceedings of the International Symposium on Biogeographical Aspects of Insularity, Accademia Nazionale dei Lincei, Roma, Italy, 18–22 May 1990; pp. 157–166. [Google Scholar]
- Denk, T.; Güner, H.T.; Grimm, G.W. From mesic to arid: Leaf epidermal features suggest preadaptation in Miocene dragon´s blood trees (Dracaena). Rev. Paleobotany Palynol. 2014, 200, 211–228. [Google Scholar] [CrossRef]
- Attorre, F.; Francesconi, F.; Taleb, N.; Scholte, P.; Saed, A.; Alfo, M.; Bruno, F. Will dragonblood survive the next period of climate change? Current and future potential distribution of Dracaena cinnabari (Socotra, Yemen). Biol. Conserv. 2007, 138, 430–439. [Google Scholar] [CrossRef]
- Vahalík, P.; Drápela, K.; Procházková, A.; Patočka, Z.; Balková, M.; Šenfeldr, M.; Lengálová, K.; Kalivodová, H.; Vaníčková, L.; Ehrenbergerová, L.; et al. Metrics of Growth Habit Derived from the 3D Tree Point Cloud Used for Species Determination—A New Approach in Botanical Taxonomy Tested on Dragon Tree Group Example. Forests 2020, 11, 272. [Google Scholar] [CrossRef]
- Kamel, M.; Ghazaly, U.M.; Callmander, M.W. Conservation status of the Endangered Nubian dragon tree Dracaena ombet in Gebel Elba National Park, Egypt. Oryx 2015, 49, 704–709. [Google Scholar] [CrossRef]
- Almeida Pérez, R.S. Censo, distribución, habitat y estado de conservation de Dracaena tamaranae A.Marrero, R.S., González, M., Gran Canaria, Islas Canarias. Bot. Macarónesica 2003, 24, 39–56. [Google Scholar]
- Almeida Pérez, R.S. Sobre la presencia de Dracaena draco (l.) L. en Gran Canaria (Islas Canarias): Aportacióncorológica, estado actual y significaciónbiogeográfica. Bot. Macarónesica 2003, 24, 17–38. [Google Scholar]
- Zheng, D.J.; Xie, L.S.; Zhu, J.H.; Zhang, Z.L. Low genetic diversity and local adaptive divergence of Dracaena cambodiana (Liliaceae) populations associated with historical population bottlenecks and natural selection: An endangered long-lived tree endemic to Hainan Island, China. Plant Biol. 2012, 14, 828–838. [Google Scholar] [CrossRef]
- Marrero, A.; Almeida Pérez, S.R. A new subspecies, Dracaena draco (L.) L. subsp. caboverdeana Marrero Rodr. & Almeida, R. (Dracaenacea) from Cape Verde Islands. Int. J. Geobot. Res. 2012, 2, 35–40. [Google Scholar]
- Adolt, R.; Maděra, P.; Abraham, J.; Čupa, P.; Svátek, M.; Matula, R.; Šebesta, J.; Čermák, M.; Volařík, D.; Koutecký, T.; et al. Field survey of Dracaena cinnabari populations in Firmihin, Socotra island: Methodology and preliminary results. J. Landsc. Ecol. 2013, 6, 7–34. [Google Scholar] [CrossRef]
- Lu, P.L.; Morden, C.W. Phylogenetic Relationships among Dracaenoid Genera (Asparagaceae: Nolinoideae) Inferred from Chloroplast DNA Loci. Syst. Bot. 2014, 39, 90–104. [Google Scholar] [CrossRef]
- Lavranos, J.J. A new, arborescent subspecies of Dracaena from Saudi Arabia. Cactus Succul. J. 2017, 89, 148–152. [Google Scholar] [CrossRef]
- Stanley Price, S.; Al–Harthy, A.H.; Whitcombe, R.P. Fog moisture and its ecological effects on Oman. Arid Lands Today Tomorrow 1988, 69–88. [Google Scholar]
- Patzelt, A. Synopsis of the Flora and Vegetation of Oman, with Special Emphasis on Patterns of Plant Endemism, Abhandlungen der Braunschweigischen Wissenschaftlichen Gesellschaft; Digitale Bibliothek: Braunschweig, Germany, 2015; pp. 282–317. [Google Scholar]
- Miller, A.G. CPD Site SWA1. Dhofar Fog Oasis. Oman and Yemen; Centres of Plant Diversity; Davis, S.D., Heywood, V.H., Hamilton, A.G., Eds.; IUCN publication Unit: Cambridge, UK, 1994; Volume 1, pp. 143–155. [Google Scholar]
- IUCN. IUCN Red List of Threatened Species; Version 2017.2; IUCN Global Species Programme Red List Unit: Cambridge, UK; Available online: www.iucnredlist.org (accessed on 25 October 2017).
- Maděra, P.; Habrová, H.; Šenfeldr, M.; Kholová, I.; Lvončík, S.; Ehrenbergerová, L.; Roth, M.; Nadezhdina, N.; Němec, P.; Rosenthal, J.; et al. Growth dynamics of endemic Dracaena cinnabari Balf. f. of Socotra Island suggest essential elements for a conservation strategy. Biológia (Bratislava) 2018. [Google Scholar] [CrossRef]
- Scholz, F.W.; Stephens, M.A. K-sample Anderson-Darling Tests. J. Am. Stat. Assoc. 1987, 82, 918–924. [Google Scholar]
- Gardenfors, U.; Hilton-Taylor, C.; Mace, G.M.; Rodriguez, J.P. The Application of IUCN Red List Criteria at Regional Levels. Conserv. Biol. 2001, 15, 1206–1212. [Google Scholar] [CrossRef]
- Král, K.; Pavliš, J. The first detailed land cover map of Socotra Island by Landsat /ETM+ data. Int. J. Remote Sens. 2006, 27, 3239–3250. [Google Scholar] [CrossRef]
- Maděra, P.; Volařík, D.; Patočka, Z.; Kalivodová, H.; Divín, J.; Rejžek, M.; Vybíral, J.; Lvončík, S.; Jeník, D.; Hanáček, P.; et al. Sustainable land use management needed to conserve the dragon´s blood tree of Socotra Island, a vulnerable endemic umbrella species. Sustainability 2019, 11, 3557. [Google Scholar] [CrossRef]
- Habrová, H.; Čermák, Z.; Pavliš, J. Dragon's blood tree-Threatened by overmaturity, not by extinction: Dynamics of a Dracaena cinnabari woodland in the mountains of Soqotra. Biol. Conserv. 2009, 142, 772–778. [Google Scholar] [CrossRef]
- Miller, A.G.; Morris, M. Plants of Dhofar. In The office of the Advisor for Conservation of the Environment Diwan of Royal Court Sultanate of Oman; Cambridge University Press: Cambridge, UK, 1998; pp. 324–325. [Google Scholar]
- Van Rampellbergh, M.; Fleitmann, D.; Verheyden, S.; Cheng, H.; Edwards, L.; De Geest, P.; De Vleeschouwer, D.; Burns, J.S.; Matter, A.; Claeys, P.; et al. Mid-To late holocene Indian ocean monsoon variability recorded in four speleothems from Socotra island, Yemen. Quat. Sci. Rev. 2013, 65, 129–142. [Google Scholar] [CrossRef]
- Hildebrand, A.; Al Aufi, M.; Amerjeed, M.; Shammas, M.; Eltahir, E. Ecohydrology of a Seasonal Cloud Forest in Dhofar. In Water Resources Research 43; American Geophysical Union: Washington, DC, USA, 2007. [Google Scholar]
- Meister, J.; Kilian, N.; Oberplielr, C. Genetic Structure of Eucleaschimperi (Ebenaceae) populations in monsoonal fog oases of the southern Arabian Peninsula. Nord. J. Bot. 2007, 25, 217–226. [Google Scholar] [CrossRef]
- Oberpreilr, C.; Meister, J.; Schneider, C.; Kilian, N. Genetic Structure of Anogeissusdhofarica (Combretatceae) populations endemic to the fog oases of the southern Arabian Peninsula. Biol. J.Linn. Soc. 2009, 97, 40–51. [Google Scholar] [CrossRef][Green Version]
- Hubálková, I. Prediction of Dragon's Blood Tree (Dracaena cinnabari Balf.) stand sample density on Soqotra Island. J. Landsc. Ecol. 2011, 4, 5–17. [Google Scholar] [CrossRef][Green Version]
- Shaffer, M.L.; Stein, B.A. Safeguarding our Precious Heritage. In Precious Heritage: The status of biodiversity in the United States; Stein, B.A., Kutner, L.S., Adams, J.S., Eds.; Oxford University Press: Oxford, UK.
- Moreno, J.C. (Ed.) Lista Roja 2008 de la flora vascular Española; Dirección General de Medio Natural y Política Forestal: Madrid, Español, 2008; p. 86. [Google Scholar]
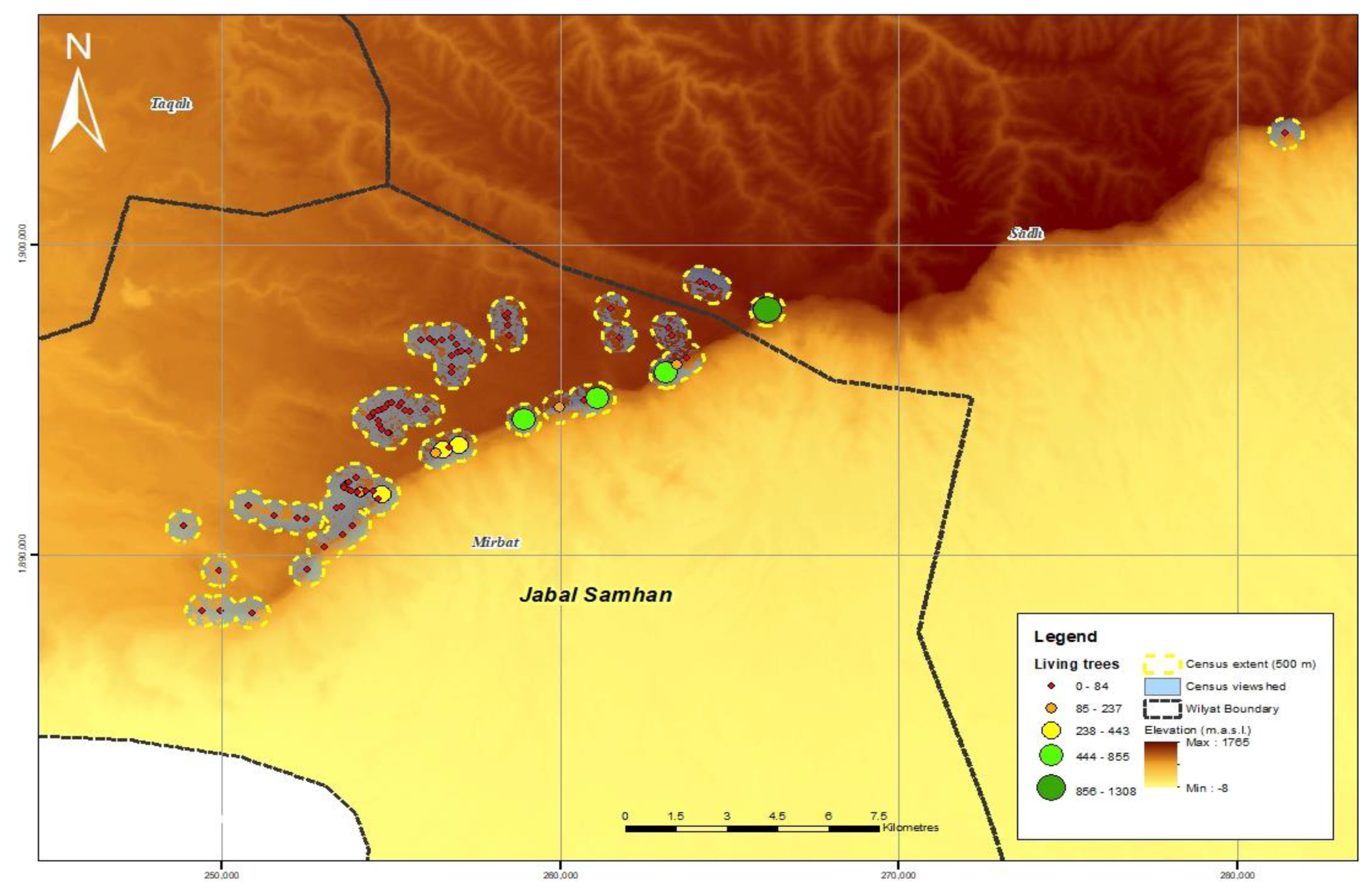

| Total Survey Points | Total Survey Area (km2) | Total Living Trees | Total Dead Trees | Total Juvenile Trees | Total | |
|---|---|---|---|---|---|---|
| Jabal al Qamar | 89 | 42 | 18,077 | 1021 | 1102 | 20,200 |
| Jabal al Qara | 50 | 24 | 14,502 | 1050 | 1475 | 17,027 |
| Jabal Samhan | 86 | 66 | 6041 | 261 | 154 | 6456 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vahalík, P.; Patočka, Z.; Drápela, K.; Habrová, H.; Ehrenbergerová, L.; Lengálová, K.; Kalivodová, H.; Pompeiano Vaníčková, L.; Al-Shamahi, E.; Lupton, D.; et al. The Conservation Status and Population Mapping of the Endangered Dracaena serrulata in the Dhofar Mountains, Oman. Forests 2020, 11, 322. https://doi.org/10.3390/f11030322
Vahalík P, Patočka Z, Drápela K, Habrová H, Ehrenbergerová L, Lengálová K, Kalivodová H, Pompeiano Vaníčková L, Al-Shamahi E, Lupton D, et al. The Conservation Status and Population Mapping of the Endangered Dracaena serrulata in the Dhofar Mountains, Oman. Forests. 2020; 11(3):322. https://doi.org/10.3390/f11030322
Chicago/Turabian StyleVahalík, Petr, Zdeněk Patočka, Karel Drápela, Hana Habrová, Lenka Ehrenbergerová, Klára Lengálová, Hana Kalivodová, Lucie Pompeiano Vaníčková, Ella Al-Shamahi, Darach Lupton, and et al. 2020. "The Conservation Status and Population Mapping of the Endangered Dracaena serrulata in the Dhofar Mountains, Oman" Forests 11, no. 3: 322. https://doi.org/10.3390/f11030322
APA StyleVahalík, P., Patočka, Z., Drápela, K., Habrová, H., Ehrenbergerová, L., Lengálová, K., Kalivodová, H., Pompeiano Vaníčková, L., Al-Shamahi, E., Lupton, D., Al Issai, G., Al Hinai, A., Al Hatmi, S., Starnes, T., & Maděra, P. (2020). The Conservation Status and Population Mapping of the Endangered Dracaena serrulata in the Dhofar Mountains, Oman. Forests, 11(3), 322. https://doi.org/10.3390/f11030322






