Abstract
Pinus sylvestris var. mongolica, a widely planted tree species, is facing long-lasting, unresolved degradation in desertified Northern China. Ectomycorrhizal fungi (EMF) are closely related to the stand status, because they substantially participate in ecological processes of terrestrial forest ecosystems. EMF may be key to solving the introduction recession. Therefore, we performed DNA sequencing of P. sylvestris root samples from plantations and natural forests as control to characterize the EMF from semi-arid and dry sub-humid regions, using ITS Illumina sequencing and conventional soil physicochemical index determination. The results indicated that (1) the dominant EMF genera were Suillus, Rhizopogon, and Wilcoxina in the Hulunbuir, Mu Us, and Horqin Sandy Lands, respectively. Their dominance retained with stand ageing. (2) Plantation EM fungal diversity differs significantly among the three sandy lands and was significantly lower than in natural forest. The diversity varied with stand age, showing distinct trends at the local scale. (3) At the regional scale, the mean annual sunshine times and the soil organic carbon content affect EMF diversity. The community composition and structure were more characterized by temperature and precipitation. At the local scale, besides the soil organic carbon content, the EM fungal community composition and structure were correlated with total nitrogen and phosphorus content (Hulunbuir), the total phosphorus content (Mu Us), and the pH and total soil porosity (Horqin). The EM fungal community composition and structure have the obvious geographical distribution variation; they were strongly correlated with the meteorological elements and soil nutrients at the regional scale. At the local scale, they were jointly driven by stand age and soil properties. This improved information contributes to increasing the understanding of the interaction between EMF and forest ecosystems and guides sustainable forest management of degraded P. sylvestris plantations.
1. Introduction
Ectomycorrhizal fungi (EMF) are widely found in forest ecosystems [1]. The EMF mycelium infects vegetative roots of trees and forms symbiotic structures [2]. Through this symbiosis, EMF obtain carbon from host plants for growth, and host plants increase the absorption and utilization of soil moisture and mineral nutrients [3]. Ectomycorrhizae (EM) can improve host plant resistance to environmental changes and stress, thus maintaining forest ecosystem stability [4].
Pineaceae was the oldest known EM plant taxon in the word and is widely distributed in the boreal forests [5]. Many EMF were identified in pine forests [6,7,8]. In China, more than 30% of the reported EMF were pine-associated [9]. Pinus sylvestris var. mongolica (P. sylvestris for short below) is native to the Hulunbuir Sandy Land. It is the most widely planted evergreen arbor in desertified Northern China [10]. At first, plantations were generally successful, but many introduction areas experienced large-scale decline and death over time. Currently, this is a critically unresolved issue hindering development and ecological restoration in China.
P. sylvestris is a typical ectomycorrhizal-dependent species [11]. EMF have a mutually beneficial relationship with P. sylvestris [12]. Some scholars believe that EMF loss is a major reason for the decline of P. sylvestris plantations as their crucial function throughout the pine life cycle [13,14], including the role of promoting host resistance to drought, diseases, and other poor surroundings [15,16]. Moreover, EMF also play an irreplaceable role in the natural regeneration and seedling establishment and survival [17]. The positive role of EM in the tree physiology and ecosystem function is widely known. Conversely, the EM fungal community variation will also retroact to plant health and ecosystem stability [18]. Consequently, EMF should not be neglected in studies of the sustainable management of P. sylvestris plantations, because the disrupted EMF symbiosis may have a significant effect on the P. sylvestris growth and metabolism.
Soil EMF have numerous species and geospatial variabilities [19], which are driven by the host, environment, and geographical distance. In Northern China, all P. sylvestris were introduced from the Hulunbuir Sandy Land; hence, genetic variation among trees is small [20]. Therefore, environmental elements, rather than the host species, likely influence the EM fungal community. Generally, the geographical climate has directly and indirectly shaped and regulated EMF [19]. Soil physical and chemical factors alter the community composition and EMF structure [21]. At the regional scale, the fungal dispersal process which was limited by geographical distance played an important role in the EM fungal distribution [22]. At the same time, EM fungal community succession occurs with aging of the host plant [23]. In addition, mycorrhizal functional traits themselves modulate and stabilize EMF geographic pattern and ecosystem functions. For example, the role of nutrient capture may regulate the community distribution along the biogeochemical pattern [24]. Consequently, differences in ectomycorrhizal fungal populations between introduced and native areas may be key factors affecting the vigorous growth of P. sylvestris. However, the connection between EMF and P. sylvestris decline remains largely unknown.
We investigated how P. sylvestris-associated EM fungal communities respond to host ageing and bioclimatic zones. We tried to find the key impact factors of EM fungal communities on different scales during the introduction and forest development. It contributes to the further study of identifying relevant functional relationships between EM fungal communities and P. sylvestris plantation degradation. We hypothesized that 1) the natural forest has rich EM fungal diversity which should also be higher in the original habitat among plantation, and the EM fungal diversity increased with the plantation stand ageing. 2) Climate factors drive EMF communities at the regional scale, with variable EMF composition driven by soil environmental factors at the local scale.
2. Material and Methods
2.1. Study Site
This study was conducted in P. sylvestris origin and introduction areas (Figure 1). The natural forest plot was located in Honghuaerji Forest Park (Inner Mongolia Autonomous Region). The introduction areas included Hailar Forest Park (Inner Mongolia Autonomous Region), Hongshixia Sandy Botanical Park (Shaanxi Province), and Zhanggutai Sandy Land Forest Park (Liaoning Province). The selected plantations were afforested by the same method, and there was no manual management after planting. Hulunbuir Sandy Land (6400 km2) is the origin area of P. sylvestris. Honghuaerji and Hailar Forest Parks are located in the Hulunbuir Sandy Land, which has a semi-arid continental climate (Table 1). The dominant shrub and herb vegetation mainly include Artemisia desertorum, Salix kochiana, and Saussurea japonica. Hongshixia Sandy Botanical Park is located in the Mu Us Sandy Land and has a semi-arid continental monsoon climate (Table 1). The Mu Us Sandy Land is the westernmost sandy land in China (42,200 km2), and it is one of the most important P. sylvestris introduction areas. The dominant shrub and herb vegetation types mainly include A. desertorum, S. cheilophila, and Agropyron cristatum. Zhanggutai Sandy Land Forest Park is located in the Horqin Sandy Land, which has a dry sub-humid continental monsoon climate (Table 1). The Horqin Sandy Land is the largest sandy land in China (63,600 km2). It is the first P. sylvestris introduction area in China. The P. sylvestris recession was first found here. The dominant shrub and herb vegetation mainly includes A. desertorum, Lespedeza bicolor, and A. argyi.
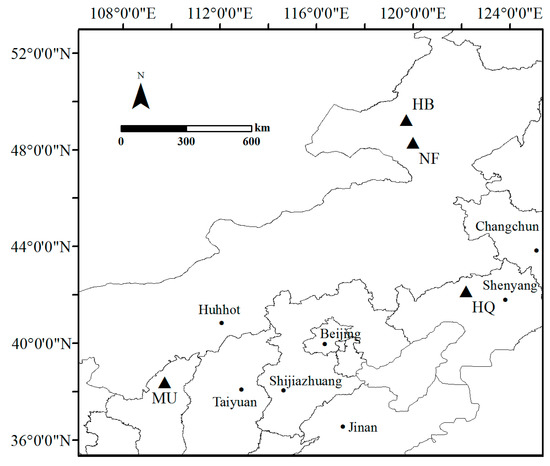
Figure 1.
The location of study sites. NF: natural forest; HB: the Hulunbuir Sandy Land; MU: the Mu Us Sandy Land; HQ: the Horqin Sandy Land.

Table 1.
General information of study and sampling sites
2.2. Sample Collection
Soil-roots samples were collected in July–August 2017 during the peak of plant biomass production and soil microbial activity. Plantations of three age groups (half-mature, nearly mature, and mature forest) without manual management in each sandy land area were selected for study [25]. The natural P. sylvestris origin forest was used as a control (Table 1). Fine P. sylvestris roots were excavated along the base of the standard tree trunk. Roots and accompanying soil were pooled into a plastic bag, but litter, herbs roots, and undergrowth humus layers were excluded. There was at least 10 m apart between each sampling tree. Five composite samples (consisting of three replicate root samples) were collected from each stand. All the composite samples ((3 sandy lands × 3 stand ages + NF) × 5 = 50 composite samples) were stored at 4 °C for transport to the lab for analysis. The general soil samples from 0–20 cm were collected to analyze physical and chemical soil properties. The undisturbed soil samples were additionally collected with cutting rings.
2.3. DNA Extraction and PCR Amplification
The roots composite samples were thoroughly mixed before DNA extraction. The sampled EM roots were cleaned under running water prior to DNA extraction. 2g roots were used to extract DNA. DNA was extracted with ground tissue homogenated by using a Powersoil DNA Isolation Kit (MoBio, USA). Extracted DNA concentration was quantified on a NanoDrop spectrophotometer (Thermo Scientific, USA). EMF ITS regions were amplified using the common fungal primers ITS1F (5-GGAAGTAAAAGTCGTAACAAGG-3) and ITS2 (5-TCCTCCGCTTATTGATATGC-3). The PCR mixtures were as follows: 4 µL 5× FastPfu Buffer, 1 µL of each primer (5 µM), 2 µL of dNTP mixture (2.5 mM), 2 µL template DNA, and 10 µL H2O. The PCR amplification program consisted of an initial denaturation at 95 °C for 2 min, followed by 30 cycles of 95 °C for 30 secs, 55 °C for 30 secs, 72 °C for 30 secs, and a final extension at 72 °C for 5 min. PCR amplification was performed in triplicate to account for potentially heterogeneous amplification from the environmental template for each sample. PCR products were purified using an AXYGEN Gel Extraction Kit (QIAGEN, Germany). DNA extracted from one sample can be used several times. In order to reduce the error of amplification, one sample was amplified three times (equivalent to three experimental replicates), and then the three amplification products were mixed equimolarly into one amplification product for subsequent library construction. The amplified products of all samples were mixed uniformly according to the pooling ratio. The mixed products were sequenced using NEB Next Ultra II DNA Library Prep Kit for sequencing library construction. Illumina sequencing adapters were completed in the step of library building. An equimolar mix of all three amplicon libraries was used for sequencing at Allwegene Technology Inc. China, using an Illumina MiSeq sequencing system (Illumina, USA).
2.4. Sequencing Data Analysis
The original sequences were processed using the Mothur (v1.30.1, https://www.mothur.org) Sequences with quality scores > 30 and longer than 200 bp were used for analysis. Chimeric sequences were removed using the software package Usearch (Version 8.1.1861, http://www.drive5.com/usearch/). The remaining high-quality sequences were classified as an operational classification unit (OTU) with more than 97% similarity using Uclust [26]. In order to ensure that the coverage of all samples was as high as possible and to reduce the error caused by the different data size, the data size of all samples was homogenized to 120,204 sequences. Singleton tags were removed before data processing. Taxonomic assignment was performed using the Ribosomal Database Project (RDP) classifier. Assigned taxa were verified by NCBI BLAST (https://www.ncbi.nlm.nih.gov/). The non-EM fungal sequences were removed based on the guilds output using FUNGuild v1.0 (http://www.stbates.org/guilds/app.php) [27], the EM fungal OTU sequences were submitted to the NCBI GenBank (Accession number in Table S1). EM fungal OTU richness (Chao1), Shannon, Simpson, and Pielou indices were computed using the vegan package in R (version 3.4.3).
2.5. Soil Properties and Climate Data Analysis
The general soil samples were air-dried for 2 days. Roots and stone debris were removed, and soil pH was measured using 1:1 soil and distilled water mixture by a PHS-3E pH meter (INESA, China) [28]. The total soil porosity (TSP) was gravimetrically determined after the undisturbed soil samples soaking in water for 8 h [29].
The total soil organic carbon (SOC) was determined using the dichromate oxidation method [30]. Total nitrogen (TN) and total phosphorus (TP) content were analyzed with a Smartchem Discrete Auto Analyzer (AMS, Italy) with the indophenol-blue spectrophotometric method and Mo-Sh anti-colorimetric analysis methods [31,32], respectively.
Climate data from 2007 to 2016 for each site were obtained from the China Meteorological Data Service Center (CMDC, http://data.cma.cn/en). The mean annual temperature (Ta), mean annual precipitation (Pa), and the mean annual sunshine time (St) were calculated by averaging the annual value (the sum of average monthly value) over ten years. The average maximum and minimum temperature (T+ and T-) was calculated using monthly average maximum/minimum temperature over ten years.
2.6. Statistical Analysis
The relative abundance was calculated by the number of OTU divided by the total number of OTUs in this sample. The relative frequency was calculated by the times of OTU occurrences divided by the total times of all OTUs occurrences in this sample.
In order to compare the soil properties (pH, SOC, TN, and TP), fungal richness, and diversity indices among the three age groups within the same sandy land, one-way analysis of variance (ANOVA) and post-hoc Tukey tests after the normality test and the homogeneity test of variance (data conforms to a normal distribution with uniform variance) were conducted. The effects of different sandy lands and stand age on EM fungal diversity indices were tested by two-way ANOVA with post-hoc Tukey tests. EM fungal diversity and soil properties/meteorological factor correlations were evaluated using the Pearson method. All statistical analyses were performed using IBM SPSS 20.0 (IBM, Armonk, NY, USA), with p < 0.05 considered significant. The statistical analyses were performed within sandy land (local scale) and over all sandy lands (regional scale).
RDA, NMDS, ANOSIM, and Mantel test were used to visualize the fungal community intergroup similarity and explain the correlation between the soil properties/climate factors and fungal community composition. Redundancy analysis (RDA) of EM fungal communities and environmental factors (meteorological and edaphic) was performed using Canoco for Windows 5.0 [33]. EM fungal composition similarity between different samples was analyzed using non-metric multidimensional scaling (NMDS) and one-way analysis of similarity (ANOSIM). Bray–Curtis distances of EM fungal communities and Euclidean distances of soil variables and geographical distance were used to construct dissimilarity matrices for Mantel tests. NMDS, ANOSIM, and Mantel tests were performed with the vegan package in R (version 3.4.3).
Cluster heatmaps and NMDS figures were generated with R (version 3.4.3, https://www.r-project.org/). Boxplots and histograms were generated with Origin 2016 (OriginLab Corporation, Northampton, MA, USA). Network maps were drawn using Cytoscape v3.6.0.
3. Results
3.1. Diversity of EMF Communities
A total of 645,186 high-quality sequences were obtained from the samples. The sequences were grouped into 2024 OTUs. We identified 104 OTUs by filtering for ECM fungal taxa using the FUNGuild v1.0.
The diversity indices based on identified OTUs (Figure 2) showed the EMF of natural forests (NF) were more diverse than plantations. Diversity measures differed significantly among the plantations in different sandy lands (p < 0.05), with the highest values in the Mu Us Sandy Land and the lowest in the Hulunbuir Sandy Land. The changing laws of the EM fungal diversity variation with stand age were different among the sandy lands. In the Hulunbir Sandy Land, EM fungal alpha diversity first increased, and then decreased with stand age. However, alpha diversity decreased in the Mu Us Sandy Land and increased continuously in the Horqin Sandy Land. The EM fungal richness in the natural forest (Chao1 = 46.70 ± 4.66) was higher than those in plantations. The EM fungal richness in plantations were HB (44.32 ± 3.39) > MU (41.67 ± 7.11) > HQ (30.64 ± 3.49) at a regional scale.
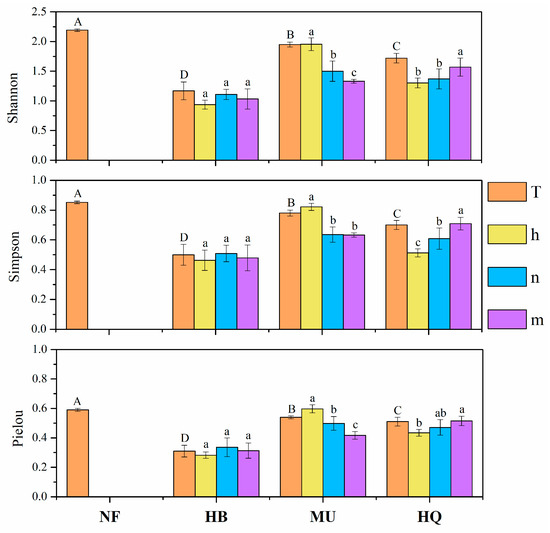
Figure 2.
Diversity indices (±SE) for Ectomycorrhizae (EM) fungal communities in P. sylvestris natural forest and plantations in three sandy lands (n = 5). T for the composed value (included five mixed replicates by adding one of five samples in each age group in the same sandy land together) of each sandy land. h for half-mature, n for nearly mature, m for mature. The capital letters indicate significant differences among sandy lands (columns in orange), the lowercase letters indicate significant differences among age groups within the same sandy land, p < 0.05.
Stand age and sandy land have a significant interaction effect on EM fungal diversity indices (p < 0.05, Table 2). There were significant differences in EMF alpha diversity indices associated with P. sylvestris plantations among different sandy lands (p < 0.05). The age main effect on EM fungal diversity was not significant (p > 0.05) at the regional scale. While in nested analysis, stand age had a significant effect on diversity indices (p < 0.05). This represented that stand age’s alpha diversity difference was depending on sandy lands.

Table 2.
Two-factor variance analysis of effect of stand age and sandy land on EM fungal diversity.
3.2. EM Fungal Community Composition and Structure
In total, 104 EM fungal OTUs were successfully identified (Table S1). Of these, 88 belonged to Basidiomycota and 16 to Ascomycota, representing 30 EMF genera. Natural forest samples contained 50 OTUs, while the plantation samples from three sandy lands included 64 (HB), 55 (MU), and 38 (HQ) OTUs. Only 8 OTUs were shared among all groups (Figure 3). Among them, the Hulunbuir Sandy Land plantations had the most OTUs similar to natural forest. Relative frequency analysis revealed Basidiomycota was the most abundant phylum (84%), followed by Ascomycota (16%) (Figure 4). P. sylvestris-associated EMF in the Hulunbuir (both natural forest and plantations) and Mu Us Sandy land were mainly Basidiomycota. The relative abundance of Basidiomycota initially decreased and then increased with increasing stand age in these two sandy lands. But in the Horqin Sandy Land, EMF was mainly Ascomycota, with significantly decreasing relative abundance (p < 0.05, Figure 4).
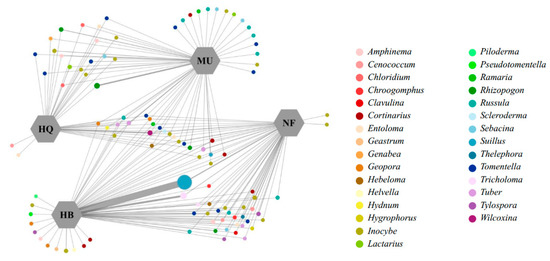
Figure 3.
Network of EM fungal operational classification units (OTUs) in each P. sylvestris stand across three sandy lands. Circle size represents absolute OTU abundance. Line thickness represents relative OTU abundance in sandy land.
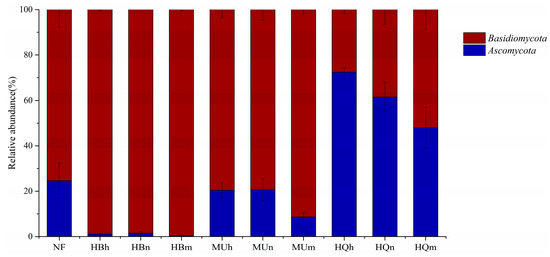
Figure 4.
Relative abundance of EM fungal phyla in each P. sylvestris stand across three sandy lands.
The relative abundances were calculated on the OTUs numbers. Of these, among Basidiomycota, Suillus was the OTU-richest genus, followed by Tricholoma. Among Ascomycota, Wilcoxina was the most abundant genus. A detailed analysis of the EM fungal genera in each P. sylvestris stand (Figure 5) revealed that the relative fungal abundance was more balanced in natural forest and was predominantly Suillus and Inocybe. The dominant genera of the Hulunbuir Sandy Land plantations were Suillus and Tricholoma. Suillus accumulated as stand age increased, but Tricholoma did the opposite. In the Mu Us Sandy Land, Rhizopogon and Tuber were highly abundant. Relative Rhizopogon abundance rose with stand age, while Tuber decreased. In the Horqin Sandy Land, Wilcoxina was the major genus, and abundance decreased with stand age. Finally, Lactarius enrichment initially decreased and then increased as stand age increased.
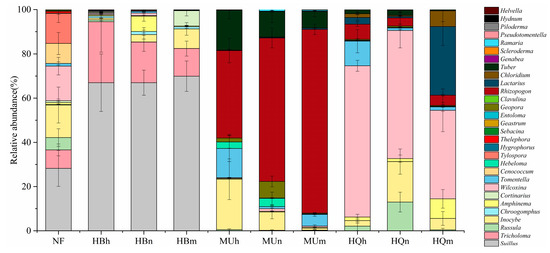
Figure 5.
Relative abundances of EM fungal genera in each P. sylvestris stand across three sandy lands. The relative abundances were calculated as an average of 5 replicates in each stand.
The EMF community composition differed among sampling locations. Inocybe, Russula, Amphinema, Wilcoxina, Lactarius, Tomentella, Cenococcum, Hebeloma, Sebacina, Geopora, Rhizopogon, and Ramaria were detected in samples from all three sandy land areas. Tricholoma, Chroogomphus, Hygrophorus, Tylospora, Thelephora, Helvella, Clavulina, Geastrum, Piloderma, and Pseudotomentella were absent in the Mu Us and Horqin Sandy Lands, while Chloridium and Genabea were not found in the Hulunbuir Sandy Land.
The EM fungal communities of different age groups in the same sandy land clustered well (Figure 6), as did natural forest samples and samples from the Hulunbuir Sandy Land plantations. The EM fungal communities from the Hulunbuir Sandy Land were the most different from the other two. The nearly mature and mature forests showed greater similarity in the Hulunbuir and Mu Us Sandy Land. However, half mature and nearly mature forests were more similar in the Horqin Sandy Land. NMDS analysis showed clear area separation (Figure 7). The ANOSIM results indicated that location has significant effects on the EM fungal community structure (r = 0.977, p = 0.001).
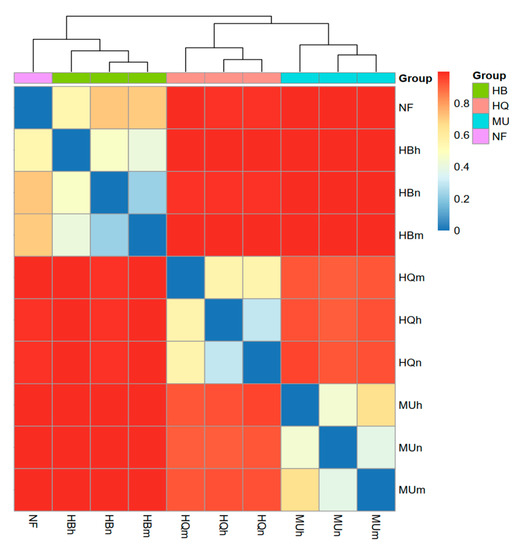
Figure 6.
Bray–Curtis distance heatmap of each P. sylvestris stand across three sandy lands. Color varies from blue to red, representing dissimilarities in EM fungal community composition from low (blue) to high (red).
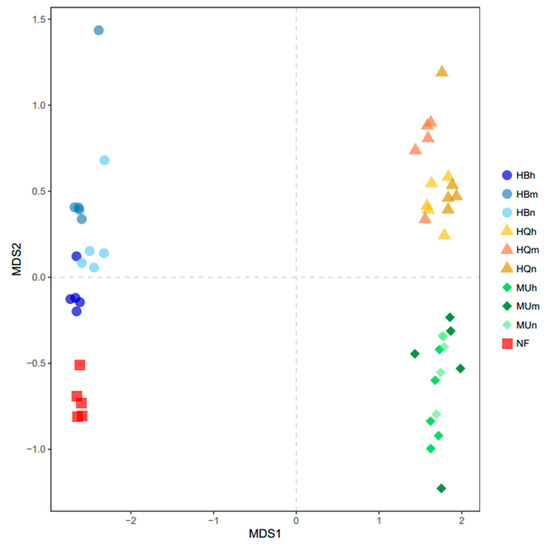
Figure 7.
EM fungal community structure based on operational taxonomic units (OTUs), as determined by non-metric multidimensional scaling (NMDS), stress = 0.078.
3.3. The Response of EM Fungal Communities to Environmental Variation
From Table 3, the Shannon and Simpson diversity and Pielou evenness indices had the strongest positive correlation with the mean annual sunshine times (0.592 and 0.573) and the mean annual temperature (0.506). They also had the strongest negative correlation with the total soil organic carbon content (−0.601, −0.578, and −0.742, respectively). The Shannon index was significantly correlated with the mean annual sunshine times, soil organic carbon, and total nitrogen content (p < 0.05). The Simpson index was significantly correlated with the mean annual temperature, sunshine, minimum temperature, soil organic carbon, and total nitrogen content (p < 0.05). The factors that were most significantly associated with the evenness index included meteorological factors and soil organic carbon content (p < 0.05).

Table 3.
Pearson correlation coefficients for EM fungal diversity and climate factor/soil properties.
Based on the Mantel test (Table 4), the geographical distance, the selected climate factors, and soil properties (Table S2) were significantly correlated with EM fungal community composition (p = 0.001). On the regional scale, the EM fungal communities are strongly significantly affected by geographical distance (r = 0.777, p = 0.001). The effect of the mean minimum temperature (T-) and the mean annual temperature on EM fungal community composition was the most obvious (r = 0.763 and 0.733, respectively) among the climate factors. Soil nutrients had a more pronounced effect than other soil factors. The correlation with stand age was significant (r = 0.103, p = 0.010), though relatively weak at the regional scale. At the local scale, increasing stand age had a significant impact on EMF community structure (p < 0.01, Table S3), as did SOC. Moreover, the EMF community structures were strongly significantly associated with TP and TN content in different age groups in the Hulunbuir Sandy Land (p < 0.01). The EMF community structures were extremely significantly associated with TP content in the Mu Us Sandy Land, and they were extremely significantly associated with TSP and pH in the Horqin Sandy Land (p < 0.01).

Table 4.
Correlations between EM fungal community composition and climate factors/soil properties/stand age/geographical distance assessed by Mantel test.
From the redundancy analysis (Figure 8), climatic variables and SOC content were the main factors in explaining the differences in community composition between NF+HB and MU+HQ. The differences between NF and HB as well as MU and HQ were mainly caused by differences in TN, TP content, TSP, and soil pH. The difference in EMF community composition between HB and the other types (especially MU and HQ) was caused by the much higher SOC in HB. The difference in EMF community composition between MU and the other types was caused by the much higher TP in MU. Similarly, the difference between HQ and others was caused by TN.
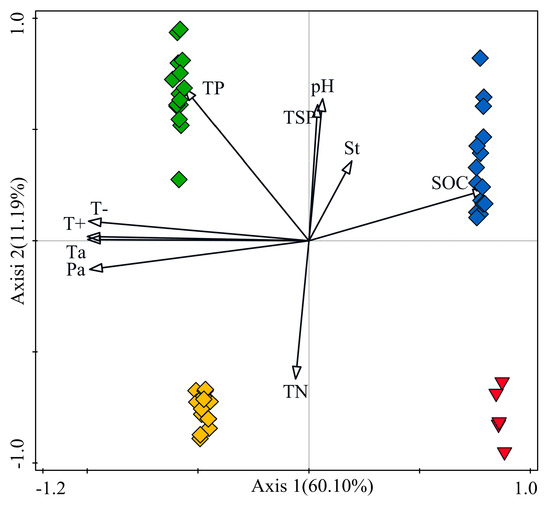
Figure 8.
EM fungal community dissimilarity in each P. sylvestris stand across three sandy lands was tested by redundancy analysis (RDA). Red triangles represent NF (natural forest), blue diamonds represent HB (the Hulunbuir Sandy Land), green diamonds represent MU (the Mu Us Sandy Land), and yellow diamonds represent HQ (the Horqin Sandy Land). Arrows represent environment variables.
4. Discussion
4.1. Variation in Ectomycorrhizal Fungal Community Composition with Introduction
EM fungal diversity can affect the hosts growth and nitrogen uptake [34]. The highest EM fungal diversity in the natural forest partly supported our first hypothesis. Earlier observations showed that EM fungi were more adapted to the prevailing conditions in the natural forests, with higher richness and more even distribution [35]. The natural forest was highly ecologically stable, with a diverse age group and rich understory vegetation [36]. The average diversity indices of plantations among the three sandy lands were significantly different (p < 0.05) as MU>HQ>HB. Prior studies noted EMF biogeographic and ecological patterns were affected by host plant identity [37], while our study objects were the same species without notable genetic variation, but under various growth status in different sandy lands. Contrary to our expectations, the EM fungal diversity of plantations in the Hulunbuir Sandy Land was not higher than that in the Mu Us and Horqin Sandy Land, suggesting that EM fungal diversity is not positively correlated with host status (as mentioned in Table 1, normal growth or decline). No clear relationship between EM fungal diversity and host status has been reported to date. Variation in the diversity indices according to stand age was inconsistent across the tested sandy lands. Results from previous studies on the relationship between EMF diversity and stand age varied widely, with clear differences even within one species across different regions [23,38,39]. However, whether stand age influences fungal diversity remains controversial, this could be known through analyses of long-term soil chronosequences.
Among the 50 samples we obtained, the relative frequency of Basidiomyceta was higher than Ascomycota, which is consistent with general EM fungal distribution [40]. The relative abundance of Basidiomyceta was also higher except in the Horqin Sandy Land, where the dominant genus belonged to Ascomycota.
The present genera have a wide geographical distribution, and they are commonly associated with pine [41]. During the stand ageing, some fungi appeared in the early stages, and some occur in late stages [39,42]. In our study, the most dominant genera were consistent in all age groups within one sandy land area. This result is in consistent with previous data that Atheliaceae was always dominant in the EM fungal community of P. sylvestris stands at the 10- to 80-year-old stage [43].
In native areas, both natural and planted forests, the dominant taxon is Suillus. Suilloid EMF (specifically, the genera Suillus and Rhizopogon) are considered to be “pioneers” in human-introduced pine stands and are key to understanding plantation success or failure [44]. These EMF facilitate pine seedling establishment and are important for later stage growth [45]. Suillus was predominant in the three age groups in the Hulunbuir Sandy Land, with no significant fluctuation with age. In the Mu Us and Horqin Sandy Lands, the dominant genera changed with stand development, which corresponded to decreased and increased diversity. The dominant genus of the Mu Us Sand Land was Rhizopogon, which is a common EMF genus often observed at great distance from the host source area [46]. Wilcoxina was dominant in the Horqin Sandy Land. In China, Wilcoxina species was first discovered in the northeastern region [47], which is also the location of the Horqin Sandy Land. With the stand aging, its relative abundance was gradually reduced.
4.2. Interactions between Environmental Change, Ectomycorrhizal Fungal Community Composition, and Stand Ageing
P. sylvestris from one source was introduced into different regions, producing different stand structures under the differing hydrothermal conditions, including the EM fungal communities. At the regional scale, significant differences in geographical distribution of EM fungal community structure were detected. Among them, environmental heterogeneity mainly included climate and soil composition. Climate factors markedly impact the EM fungal diversity and community structure [48]. The biological distribution of terrestrial ecosystems is primary and mostly regulated by temperature and moisture [49]. Mantel tests showed that temperature had the strongest influence on EM fungal communities. Increased EMF diversity occurs with elevated temperature based on the promoted plant productivity [50,51], as the EM fungal alpha diversity indices of plantations are correlated with the mean annual or extreme temperatures. Long-term warming studies in the Artic found an increased abundance and richness of EMF with increasing temperature [50,52]. In a boreal forest, however, warming in combination with drying was found to negatively affect fungal abundance [53]. The response of the below-ground ecosystem to temperature changes requires more in-depth and comprehensive research. Solar radiation is an important heat source, so the mean annual sunshine time was used as a characterization index. Energy from the sun directly affects soil temperature and plant metabolic processes [54]. The mean annual sunshine time had significant impacts on all three indices. EMF obtain carbon from the host plant for growth. We posit that sunlight indirectly influences EMF by driving photosynthesis within the host plant. Fungal species react differently to changes in temperature and moisture. Therefore, large regional climate changes substantially affect the composition and regulation of EM fungal communities [55]. Furthermore, precipitation can also influence EM fungal communities, particularly in desert regions [56]. Generally speaking, drought decreases soil microbial diversity at a global scale [57]. However, the study on the EM colonization of pinyon pine in Northern Arizona found that EM colonization was significantly higher at the much drier cinder site for 5 of 12 months than that in moist sandy-loam soils sites [58]. Drought influenced the EMF community composition in a water-exclusion experiment on beech, and the EMF responded to drought differently in terms of their abundance [59]. In our study, although the precipitation was HQ>MU>HB, evaporation within HQ and MU was double the evaporation of HB. We found that MU and HQ experienced greater drought stress than HB base on the aridity index (potential evaporation/precipitation). This could probably explain why fungi with large fruiting bodies (such as Suillus) did not appear in the Horqin and Mu Us Sandy Land. Hypogeous fungi, like Wilcoxina and Rhizopogon, were thought to be adapted to water scarcity [6] and are dominant in those sandy lands.
Soil conditions are external abiotic factors directly affecting EM fungal communities. In our study, the total soil porosity, pH, and total phosphorus content drove EM fungal community composition, but they had no significant correlation with diversity. This was also reported in previous studies [60]. Most EM fungi were more suitable for slightly acidic or neutral pH, and soil pH exhibited a significant effect on both EM diversity and community structures in Beech, Pine, and Spruce Forests at both a continental and global scale [5,61]. At the same time, EMF had a large pH adaptation range, EMF could grow in the pH range of 3–8 in both culture media and peat associated with P. sylvestris seedlings [62]. The contribution of pH to the variation of EM fungal communities was not highlighted due to the minor differences among our samples.
At the regional scale, soil organic carbon was significantly correlated with EM fungal community composition among the soil nutrient elements. Soil organic carbon in forests is mainly derived from litter, root, and dead microbial cells in the soil decomposition and is an important part of the carbon cycle [63]. EMF mediate carbon cycling, while carbon productivity is also a driver of ectomycorrhizal abundance and diversity [64]. The Mantel test revealed that the total nitrogen content was a major factor influencing the EM fungal community structure in our study. EM fungal communities are affected by available nitrogen and form large-scale patterns by nitrogen variation based on a European biomonitoring network of pine forest plots [65]. Fungal diversity and total soil nitrogen content were negatively correlated in our results. Diverse fungal communities are observed by field experiment in a mixed boreal P. sylvestris and Picea abies forests in Sweden, where the organic layer is relatively nitrogen poor [66]. Soil phosphorus content has selective influence on EM fungal community composition and ectomycorrhizal role of increasing water absorption [67]. The relationship between nutrient content and EMF diversity are contrary to some studies. This may be due to low soil nutrients in sandy lands (Table S2). The nutrient content was less variable, and our forest stand conditions were distinct from other terrestrial forest ecosystems.
At the local scale, EM fungal richness in the three sandy lands was very different according to stand age. Previous studies showed that EM fungal richness increased, decreased, or did not significantly change with the increased stand age [23,68,69]. There was no clear pattern for the change of EM fungal diversity with stand aging, either among the different tree species or the same tree species in different regions. At the local scale, stand age was the major factor driving the composition and structure of the EMF community (Table S3). It was demonstrated that aging of forest soil determines the EM fungal composition in secondary stands of P. sylvestris in the Netherlands [70]. The change in soil properties resulting from stand ageing were considerable factors in EM fungal community composition. The EMF communities were mainly regulated by SOC, TN, and TP content in the Hulunbuir Sandy Land. The SOC and TN content increased with stand ageing, showing consistent homogeneity. Filtration and influence of soil nutrient and organic matter on EMF communities have also been reported in pine forest of California [71]. The EM fungal community composition in Mu Us Sandy Land plantations was also affected by SOC and TP content. In this group, the TP content had a larger effect. The increase in soil phosphorus content promotes the growth of most EMF species based on the field investigation on the production of EM mycelium in the Norway spruce forests in Southern Sweden [72], particularly Rhizopogon, which was consistent with our data. However, soil phosphorus has no effect on Suillus [73]. Moreover, there is no strong evidence for the impact of phosphorus on EM fungal diversity. In the Horqin Sandy Land, the total soil porosity, pH, and SOC regulated EM fungal community composition. Soil acidity is a crucial factor affecting soil fungi [5]. Our data indicate that the EMF community in the Horqin Sandy Land may be sensitive to soil pH. Soil porosity controls root and fungal mycelial growth [74]. Several hypogeous fungi (Wilcoxina) which sporulated mitotically within the soil [6], exist in the Horqin Sandy Land, making TSP a vital factor.
4.3. Ectomycorrhizal Fungi and P. sylvestris Plantation Degradation: a Supposition
The EM fungal composition and community structure are filtered and shaped by climate and soil conditions in different sandy lands. Changes in the community structure under introduction of host plant can have consequences for ecosystem function. Community composition and structure of ectomycorrhizal fungi in declined and non-declined forests were significantly different [75]. Considering the benefits from EMF to tree health and ecosystem service, the EM association feedback may be a causal factor of trees degradation [76]. In the declining Phytophthora cinnamomic-infected Quercus ilex forest, the changes of the EM fungal diversity and abundance caused by human impact were involved in the Q. ilex decline, this counteracted the positive symbiosis effect and might lead to further tree death [77].
Variation in EM fungal community composition can result from nutrient cycle efficiency [78]. At the same time, because of their extremely important role in the nutrient cycle, they would affect the host tree productivity and health in turn [79]. In addition, the loss of fungi which have strong pathogen antagonism and drought tolerance may lead to stand degradation. Host plants suffer from withering dead leaves and low pathogen resistance, likely due to the lack of essential trace elements such as manganese, zinc, and copper [80,81]. The introduction of EMF which have these elements’ transporters can help the host plant absorb and transport the elements [82,83]. This could alter trace element shortage and the low absorption rate in desert ecosystems. Simultaneously, the local reduction in the relative abundance of dominant genera may be an overlooked cause of stand decline. The dominant genus in the Hulunbuir Sandy Land was in a relatively stable state during stand evolution, while the other two had larger fluctuations. A population competition mechanism could have a non-negligible influence on EM fungal ecosystem function [84]. The regeneration barriers—“able to germinate, unable to survive”—are important phenomena in plantation degradation. EM may be a key point to alleviate this problem because they have critical benefits to seedling growth and survival [85], especially considering that Suilloid fungi play an essential role in P. sylvestris invasion and seedling establishment [44].
In the strong mutual feedback relationship between EMF, host plant, and the environment, the composition and structure of ectomycorrhizal fungi community could be used as a crucial indicator of plant health and ecosystem function. Further, this research could inform an answer to plantation degradation.
5. Conclusions
EMF associated with P. sylvestris are diverse with various genera and have clear regional differences. The environmental changes caused by the large-scale introduction of P. sylvestris strongly affected the diversity and composition of the EM fungal community. We propose that this introduction reduced the EM fungal diversity and richness of P. sylvestris. The original plantations were not superior to other introduced areas regarding EM fungal diversity. The variety of EM fungal diversity and richness with the age gradient had no consensus across three sandy lands.
At the regional scale, the mean annual sunshine times and the soil organic carbon content affect EMF species diversity. The EM fungal community composition and structure were more characterized by temperature and precipitation. EM fungal changes were expected to differ among the sandy lands. At the local scale, the factors which drive and modulate the EM fungal communities during stand development were significantly different among three sandy lands. In addition to the soil organic carbon content, the EM fungal community structures were closely correlated with total nitrogen and phosphorus content (Hulunbuir), the total phosphorus content (Mu Us), and the pH and total soil porosity (Horqin).
The primary relationship between EM fungal community and plantation introduction is supported by our results. The EM fungal community changes and related nutrient cycles could reflect stand status. Therefore, we highly considered that study on fungal dynamics of multi-spatial EMF could alleviate the P. sylvestris decline and solve the regeneration problem. Our results have the potential to guide sustainable forest management, such as cultivating specific functional mycorrhizal seedlings and mycorrhizal fungal inocula configuration.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/11/3/323/s1, Table S1. BLAST results of the ITS region of DNA extracted from root tips of P. sylvestris, Table S2. Soil properties compared by ANOVA (mean values ± S.E., n = 5), Table S3. Correlations between local EM fungal community structure and soil properties/plantation stand age as determined by Mantel test.
Author Contributions
Data curation, M.G.; Funding acquisition, G.G.; Methodology, M.G., G.G. and Y.Z.; Project administration, G.G. and G.D.; Software, M.G.; Supervision, G.D. and Y.Z.; Validation, G.D.; Writing—original draft, M.G.; Writing—review & editing, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Fundamental Research Funds for the Central Universities (no. 2015ZCQ-SB-02 and 2017PT03), the National Natural Science Foundation of China (no. 31600583), and the National Key Research and Development Program of China (no. 2018YFC0507101).
Acknowledgments
We would like to thank Weilin Jin (Hulunbuir Forestry and Grassland Bureau), Guoping Zhao (Desert Control Research Institute of Shaanxi Province), Gang Lyu (Liaoning Technical University), and Hongda Chen (Liaoning Sand Land Amelioration and Utilization Research Institute) for their generous help with the field investigation. Special thanks to Yue Ren, Hongyu Cao, and Yuxuan Chen for their help with sampling and measurements in the field and laboratory.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Steidinger, B.; Bhatnagar, J.; Vilgalys, R.; Taylor, J.; Bruns, T.; Peay, K. Global climate changes will lead to regionally divergent trajectories for ectomycorrhizal communities in North American Pinaceae forests. bioRxiv 2018. [Google Scholar] [CrossRef]
- Anderson, I.C.; Cairney, J.W.G. Ectomycorrhizal fungi: Exploring the mycelial frontier. FEMS Microbiology Reviews 2010, 31, 388–406. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. 6-Structure and development of ectomycorrhizal roots. In Mycorrhizal Symbiosis, 3rd ed.; Smith, S.E., Read, D., Eds.; Academic Press: London, UK, 2008; pp. 191–268. [Google Scholar]
- Courty, P.E.; Buée, M.; Diedhiou, A.G.; Frey-Klett, P.; Tacon, F.L.; Rineau, F.; Turpault, M.P.; Uroz, S.; Garbaye, J. The role of ectomycorrhizal communities in forest ecosystem processes: New perspectives and emerging concepts. Soil Biol. Biochem. 2010, 42, 679–698. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Toots, M.; Diedhiou, A.G.; Henkel, T.W.; Kjøller, R.; Morris, M.H.; Nara, K.; Nouhra, E.R.; Peay, K.; et al. Towards global patterns in the diversity and community structure of ectomycorrhizal fungi. Mol. Ecol. 2012, 21, 4160–4170. [Google Scholar] [CrossRef] [PubMed]
- Glassman, S.I.; Peay, K.G.; Talbot, J.M.; Smith, D.P.; Chung, J.A.; Taylor, J.W.; Vilgalys, R.; Bruns, T.D. A continental view of pine-associated ectomycorrhizal fungal spore banks: A quiescent functional guild with a strong biogeographic pattern. New Phytol. 2015, 205, 1619–1631. [Google Scholar] [CrossRef]
- Reverchon, F.; del Pilar Ortega-Larrocea, M.; Bonilla-Rosso, G.; Pérez-Moreno, J. Structure and species composition of ectomycorrhizal fungal communities colonizing seedlings and adult trees of Pinus montezumae in Mexican neotropical forests. FEMS Microbiol. Ecol. 2012, 80, 479–487. [Google Scholar] [CrossRef]
- Murata, M.; Kanetani, S.; Nara, K. Ectomycorrhizal fungal communities in endangered Pinus amamiana forests. PLoS ONE 2017, 12, e0189957. [Google Scholar] [CrossRef]
- He, X.H.; Duan, Y.H.; Chen, Y.L.; Xu, M.G. A 60-year journey of mycorrhizal research in China: Past, present and future directions. Sci. China-life Sci. 2010, 53, 1374–1398. [Google Scholar] [CrossRef]
- Li, M.; Ding, G.; Gao, G.; Zhao, Y.; Yu, M.; Wang, D. Introduction suitability of Pinus sylvestris var. mongholica in 10 northern provinces of China. J. Desert Res. 2016, 36, 1021–1028. [Google Scholar] [CrossRef]
- Zhu, J.J.; Kang, H.Z.; Xu, M.L.; Wu, X.Y.; Wang, W. Effects of ectomycorrhizal fungi on alleviating the decline of Pinus sylvestris var. mongolica plantations on Keerqin sandy land. Chin. J. Appl. Ecol. 2007, 14, 232–235. [Google Scholar] [CrossRef]
- Alberton, O.; Kuyper, T.W. Ectomycorrhizal fungi associated with Pinus sylvestris seedlings respond differently to increased carbon and nitrogen availability: Implications for ecosystem responses to global change. Glob. Chang. Biol. 2010, 15, 166–175. [Google Scholar] [CrossRef]
- Hayward, J.; Horton, T.R.; Pauchard, A.; Nuñez, M.A. A single ectomycorrhizal fungal species can enable a Pinus invasion. Ecology 2015, 96, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Li, F.Q.; Xu, M.L.; Kang, H.Z.; Wu, X.Y. The role of ectomycorrhizal fungi in alleviating pine decline in semiarid sandy soil of northern China: An experimental approach. Ann. For. Sci. 2008, 65, 304. [Google Scholar] [CrossRef]
- Lehto, T.; Zwiazek, J.J. Ectomycorrhizas and water relations of trees: A review. Mycorrhiza 2011, 21, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, P.; Messmer, M.M. Breeding for mycorrhizal symbiosis: Focus on disease resistance. Euphytica 2017, 213, 113. [Google Scholar] [CrossRef]
- Jonsson, L.; Dahlberg, A.; Nilsson, M.C.; Karen, O.; Zackrisson, O. Continuity of ectomycorrhizal fungi in self-regenerating boreal Pinus sylvestris forests studied by comparing mycobiont diversity on seedlings and mature trees. New Phytol. 1999, 142, 151–162. [Google Scholar] [CrossRef]
- Scott, P.; Shearer, B.; Barber, P.; St, G.; Hardy, G. Relationships between the crown health, fine root and ectomycorrhizae density of declining Eucalyptus gomphocephala. Australas. Plant Pathol. 2012, 42. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Miao, Y.B.; Zhu, X.M.; Li, Z.J.; Jia, F.L.; Li, W. Genetic evaluation of breeding resources of Pinus sylvestris var. mongolica from different improved generations. J. Beijing For. Univ. 2017, 39, 71–78. [Google Scholar] [CrossRef]
- Treseder, K.K.; Bent, E.; Borneman, J.; Mcguire, K.L. Shifts in fungal communities during decomposition of boreal forest litter. Fungal Ecol. 2014, 10, 58–69. [Google Scholar] [CrossRef]
- Peay, K.G.; Schubert, M.G.; Nguyen, N.H.; Bruns, T.D. Measuring ectomycorrhizal fungal dispersal: Macroecological patterns driven by microscopic propagules. Mol. Ecol. 2012, 21, 4122–4136. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, Y.; Shi, N.N.; Zheng, Y.; Chen, L.; Tesfaye, W.; Helge, B.; Sabine, B.; Francois, B.; Ding, Q. Community assembly of ectomycorrhizal fungi along a subtropical secondary forest succession. New Phytol. 2015, 205, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Wurzburger, N.; Brookshire, E.N.J.; Mccormack, M.L.; Lankau, R. Mycorrhizal fungi as drivers and modulators of terrestrial ecosystem processes. New Phytol. 2017, 213, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, X. Simulating effects of precipitation and initial planting density on population size of Mongolian pine in the Horqin Sandy Land, China. Agrofor. Syst. 2018, 92, 1–9. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Survey Field and Laboratory Methods Manual, Soil Survey Investigations Report, No. 51; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2009.
- Klute, A. Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; Agronomy Monograph, American Society of Agronomy: Madison, WI, USA, 1986. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Mason, C.; Edwards, M.; Riby, P.; Coe, G. The use of microwaves in the acceleration of digestion and colour development in the determination of total Kjeldahl nitrogen in soil. Analyst 1999, 124, 1719–1726. [Google Scholar] [CrossRef]
- John, M. Colorimetric determination of phosphorus in soil and plant materials with ascorbic acid. Soil Sci. 1970, 109, 214–220. [Google Scholar] [CrossRef]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Leake, J.R. Is diversity of ectomycorrhizal fungi important for ecosystem function? New Phytol. 2001, 152, 1–3. [Google Scholar] [CrossRef]
- Grebenc, T.; Christensen, M.; Vilhar, U.; Čater, M.; Martín, M.P.; Simončič, P.; Kraigher, H. Response of ectomycorrhizal community structure to gap opening in natural and managed temperate beech-dominated forests. Can. J. For. Res. 2009, 39, 1375–1386. [Google Scholar] [CrossRef]
- He, J.Z.; Li, J.; Zheng, Y.M. Thoughts on the microbial diversity-stability relationship in soil ecosystems. Biodivers. Sci. 2013, 4, 411–420. [Google Scholar] [CrossRef]
- Dickie, I.A.; Moyersoen, B. Towards a global view of ectomycorrhizal ecology. New Phytol. 2008, 180, 263–265. [Google Scholar] [CrossRef] [PubMed]
- North, M.; Trappe, J.; Franklin, J. Standing crop and animal consumption of fungal sporocarps in Pacific northwest forests. Ecology 1997, 78, 1543–1554. [Google Scholar] [CrossRef]
- Smith, J.E.; Molina, R.; Huso, M.M.; Luoma, D.L.; McKay, D.; Castellano, M.A.; Lebel, T.; Valachovic, Y. Species richness, abundance, and composition of hypogeous and epigeous ectomycorrhizal fungal sporocarps in young, rotation-age, and old-growth stands of Douglas-fir (Pseudotsuga menziesii) in the Cascade Range of Oregon, U.S.A. Can. J. Bot. 2002, 80, 186–204. [Google Scholar] [CrossRef]
- Vellinga, E.C.; Wolfe, B.E.; Anne, P. Global patterns of ectomycorrhizal introductions. New Phytol. 2009, 181, 960–973. [Google Scholar] [CrossRef]
- Chung, H.C.; Kim, D.H.; Cho, N.S.; Lee, S.S. Observation and distribution of ectomycorrhizal fungi in Pinus roots. Mycobiology 2003, 31, 1. [Google Scholar] [CrossRef]
- Martínez-Peña, F.; Ágreda, T.; Águeda, B.; Ortega-Martínez, P.; Fernández-Toirán, L.M. Edible sporocarp production by age class in a Scots pine stand in Northern Spain. Mycorrhiza 2012, 22, 167–174. [Google Scholar] [CrossRef]
- Kyaschenko, J.; Clemmensen, K.E.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. Isme J. 2017, 11, 863. [Google Scholar] [CrossRef]
- Nahuel, P.; Thomas, D.B.; Rytas, V.; Martin, A.N. Suilloid fungi as global drivers of pine invasions. New Phytol. 2019, 222, 714–725. [Google Scholar] [CrossRef]
- Hayward, J.; Thomas, R.H.; Martin, A.N. Ectomycorrhizal fungal communities coinvading with Pinaceae host plants in Argentina: Gringos bajo el bosque. New Phytol. 2015, 208, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Salgado, S.M.E.; Barroetaveña, C.; Rajchenberg, M. Do pine plantations provide mycorrhizal inocula for seedlings establishment in grasslands from Patagonia, Argentina? New For. 2011, 41, 191–205. [Google Scholar] [CrossRef]
- Shi, C.H.; Tolgor, B.; Li, Y. Newly recorded genus and species of Pezizales in China. Mycosystema 2016, 35, 1348–1356. [Google Scholar] [CrossRef]
- Drigo, B.; Kowalchuk, G.; van Veen, J.A. Climate change goes underground: Effects of elevated atmospheric CO2 on microbial community structure and activities in the rhizosphere. Biol. Fertil. Soils 2008, 44, 667–679. [Google Scholar] [CrossRef]
- Bennett, A.E.; Classen, A.T. Climate change influences mycorrhizal fungal–plant interactions, but conclusions are limited by geographical study bias. Ecology 2020, e02978. [Google Scholar] [CrossRef] [PubMed]
- Deslippe, J.R.; Hartmann, M.; MOHN, W.W.; Simard, S. Long-term experimental manipulation alters the ectomycorrhizal community of Betula nana in Arctic tundra. Glob. Chang. Biol. 2010, 17, 1625–1636. [Google Scholar] [CrossRef]
- Maclean, C.; Dickson, A.; Bell, G. Resource competition and adaptive radiation in a microbial microcosm. Ecol. Lett. 2004, 8, 38–46. [Google Scholar] [CrossRef]
- Clemmensen, K.; Michelsen, A.; Jonasson, S.; Shaver, G. Increased ectomycorrhizal fungal abundance after long-term fertilization and warming of two arctic tundra ecosystems. New Phytol. 2006, 171, 391–404. [Google Scholar] [CrossRef]
- Allison, S.; Treseder, K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Chang. Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, Q.; Nakajima, T.; Nakata, M.; Lu, P.; He, J. Influence of changes in solar radiation on changes of surface temperature in China. Acta Meteorol. Sin. 2013, 27, 87–97. [Google Scholar] [CrossRef]
- Pickles, B.J.; Egger, K.N.; Massicotte, H.B.; Green, D.S. Ectomycorrhizas and climate change. Fungal Ecol. 2012, 5, 73–84. [Google Scholar] [CrossRef]
- Jarvis, S.; Woodward, S.; Alexander, I.J.; Taylor, A.F.S. Regional scale gradients of climate and nitrogen deposition drive variation in ectomycorrhizal fungal communities associated with native Scots pine. Glob. Chang. Biol. 2013, 19, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef] [PubMed]
- Swaty, R.; Gehring, C.; Ert, M.; Theimer, T.A.D.; Keim, P.; Whitham, T. Temporal variation in temperature and rainfall differentially affects ectomycorrhizal colonization at two contrasting sites. New Phytol. 1998, 139, 733–739. [Google Scholar] [CrossRef]
- Shi, L.; Guttenberger, M.; Kottke, I.; Hampp, R. The effect of drought on mycorrhizas of beech (Fagus sylvatica L.): Changes in community structure, and the content of carbohydrates and nitrogen storage bodies of the fungi. Mycorrhiza 2002, 12, 303–311. [Google Scholar] [CrossRef]
- Dumbrell, A.J.; Nelson, M.; Helgason, T.; Dytham, C.; Fitter, A.H. Relative roles of niche and neutral process in structuring a soil microbial community. ISME J. 2010, 4, 337–345. [Google Scholar] [CrossRef]
- Rosinger, C.; Sandén, H.; Matthews, B.; Mayer, M.; Godbold, D. Patterns in Ectomycorrhizal Diversity, Community Composition, and Exploration Types in European Beech, Pine, and Spruce Forests. Forests 2018, 9, 445. [Google Scholar] [CrossRef]
- Erland, S.; Söderström, B.; Andersson, S. Effects of liming on ectomycorrhizal fungi infecting Pinus sylvestris L. 2. Growth rates in pure culture at different ph values compared to growth-rates in symbiosis with the host plant. New Phytol. 1990, 115, 683–688. [Google Scholar] [CrossRef]
- Richter, D.D.; Markewitz, D.; Trumbore, S.E.; Wells, C.G. Rapid accumulation and turnover of soil carbon in a re-establishing forest. Nature 1999, 400, 56–58. [Google Scholar] [CrossRef]
- Druebert, C.; Lang, C.; Valtanen, K.; Polle, A. Beech carbon productivity as driver of ectomycorrhizal abundance and diversity. Plant Cell Environ. 2009, 32, 992–1003. [Google Scholar] [CrossRef]
- Cox, F.; Barsoum, N.; Lilleskov, E.A.; Bidartondo, M.I. Nitrogen availability is a primary determinant of conifer mycorrhizas across complex environmental gradients. Ecol. Lett. 2010, 13, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Sterkenburg, E.; Clemmensen, K.; Ekblad, A.; Finlay, R.; Lindahl, B. Contrasting effects of ectomycorrhizal fungi on early and late stage decomposition in a boreal forest. ISME J. 2018, 12, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Cairney, J.W.G. Ectomycorrhizal fungi: The symbiotic route to the root for phosphorus in forest soils. Plant Soil 2011, 344, 51–71. [Google Scholar] [CrossRef]
- Rao, C.; Sharma, G.; Shukla, A. Distribution of ectomycorrhizal fungi in pure stands of different age groups of Pinus kesiya. Can. J. Microbiol. 2011, 43, 85–91. [Google Scholar] [CrossRef][Green Version]
- Zhang, T.T.; Wang, Q.; Du, C.; Zhang, D.N.; Zhang, F.; Ma, W.; Wang, Z.K.; Li, X.; Geng, Z.C. Diversity of ectomycorrhizal fungi associated with Betula albosinensis in Xinjiashan forest region of Qinling Mountains. Mycosystema 2017, 36, 851–860. [Google Scholar] [CrossRef]
- Baar, J.; Vries, A.H.M.d. Effects of manipulation of litter and humus layers on ectomycorrhizal colonization potential in Scots pine stands of different age. Mycorrhiza 1995, 5, 267–272. [Google Scholar] [CrossRef]
- Glassman, S.; Wang, I.; Bruns, T. Environmental filtering by pH and soil nutrients drives community assembly in fungi at fine spatial scales. Mol. Ecol. 2017, 26. [Google Scholar] [CrossRef]
- Hagerberg, D.; Thelin, G.; Wallander, H. The production of ectomycorrhizal mycelium in forests: Relation between forest nutrient status and local mineral sources. Plant Soil 2003, 252, 279–290. [Google Scholar] [CrossRef]
- Bidartondo, M.; Ek, H.; Wallander, H.; Soderstrom, B. Do nutrient additions alter carbon sink strength of ectomycorrhizal fungi? New Phytol. 2001, 151, 543–550. [Google Scholar] [CrossRef]
- Bauman, J.M.; Keiffer, C.H.; Hiremath, S.; Mccarthy, B.C.; Kardol, P. Soil preparation methods promoting ectomycorrhizal colonization and American chestnut Castanea dentata establishment in coal mine restoration. J. Appl. Ecol. 2013, 50, 721–729. [Google Scholar] [CrossRef]
- Horton, B.M.; Glen, M.; Davidson, N.J.; Ratkowsky, D.; Close, D.C.; Wardlaw, T.J.; Mohammed, C. Temperate eucalypt forest decline is linked to altered ectomycorrhizal communities mediated by soil chemistry. For. Ecol. Manag. 2013, 302, 329–337. [Google Scholar] [CrossRef]
- Close, D.C.; Davidson, N.J.; Johnson, D.W.; Abrams, M.D.; Hart, S.C.; Lunt, I.D.; Archibald, R.D.; Horton, B.; Adams, M.A. Premature decline of Eucalyptus and altered ecosystem processes in the absence of fire in some Australian forests. Bot. Rev. 2009, 75, 191–202. [Google Scholar] [CrossRef]
- Corcobado, T.; Moreno, G.; Azul, A.; Solla, A. Seasonal variations of ectomycorrhizal communities in declining Quercus ilex forests: Interactions with topography, tree health status and Phytophthora cinnamomi infections. Forestry 2015, 88, 257–266. [Google Scholar] [CrossRef]
- Berner, C.; Johansson, T.; Wallander, H. Long-term effect of apatite on ectomycorrhizal growth and community structure. Mycorrhiza 2012, 22, 615–621. [Google Scholar] [CrossRef]
- Twieg, B.D.; Durall, D.M.; Simard, S.W. Ectomycorrhizal fungal succession in mixed temperate forests. New Phytol. 2007, 176, 437–447. [Google Scholar] [CrossRef]
- Manoharachary, C.; Reddy, P. Plant Mineral Nutrition Through Ectomycorrhiza. In Mycorrhizal Biology; Mukerji, K.G., Chamola, B.P., Singh, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Fränzle, S. Autocatalytic processes and the role of essential elements in plant growth. In Chemical Elements in Plant and Soil: Parameters Controlling Essentiality; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Blaudez, D.; Chalot, M. Characterization of the ER-located zinc transporter ZnT1 and identification of a vesicular zinc storage compartment in Hebeloma cylindrosporum. Fungal Genet. Biol. 2011, 48, 496–503. [Google Scholar] [CrossRef]
- Migeon, A.; Blaudez, D.; Wilkins, O.; Montanini, B.; Campbell, M.M.; Richaud, P.; Thomine, S.; Chalot, M. Genome-wide analysis of plant metal transporters, with an emphasis on poplar. Cell. Mol. Life Sci. 2010, 67, 3763–3784. [Google Scholar] [CrossRef]
- Kennedy, P. Ectomycorrhizal fungi and interspecific competition: Species interactions, community structure, coexistence mechanisms, and future research directions. New Phytol. 2010, 187, 895–910. [Google Scholar] [CrossRef]
- Teste, F.; Simard, S.; Durall, D.M.; Guy, R.D.; Jones, M.D.; Schoonmaker, A.L. Access to mycorrhizal networks and roots of trees: Importance for seedling survival and resource transfer. Ecology 2009, 90, 2808–2822. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).