Native Bamboo Invasions into Subtropical Forests Alter Microbial Communities in Litter and Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling Design
2.3. Chemical Analyses
2.4. Microbial Analysis
2.5. Bioinformatics and Statistical Analysis
3. Results
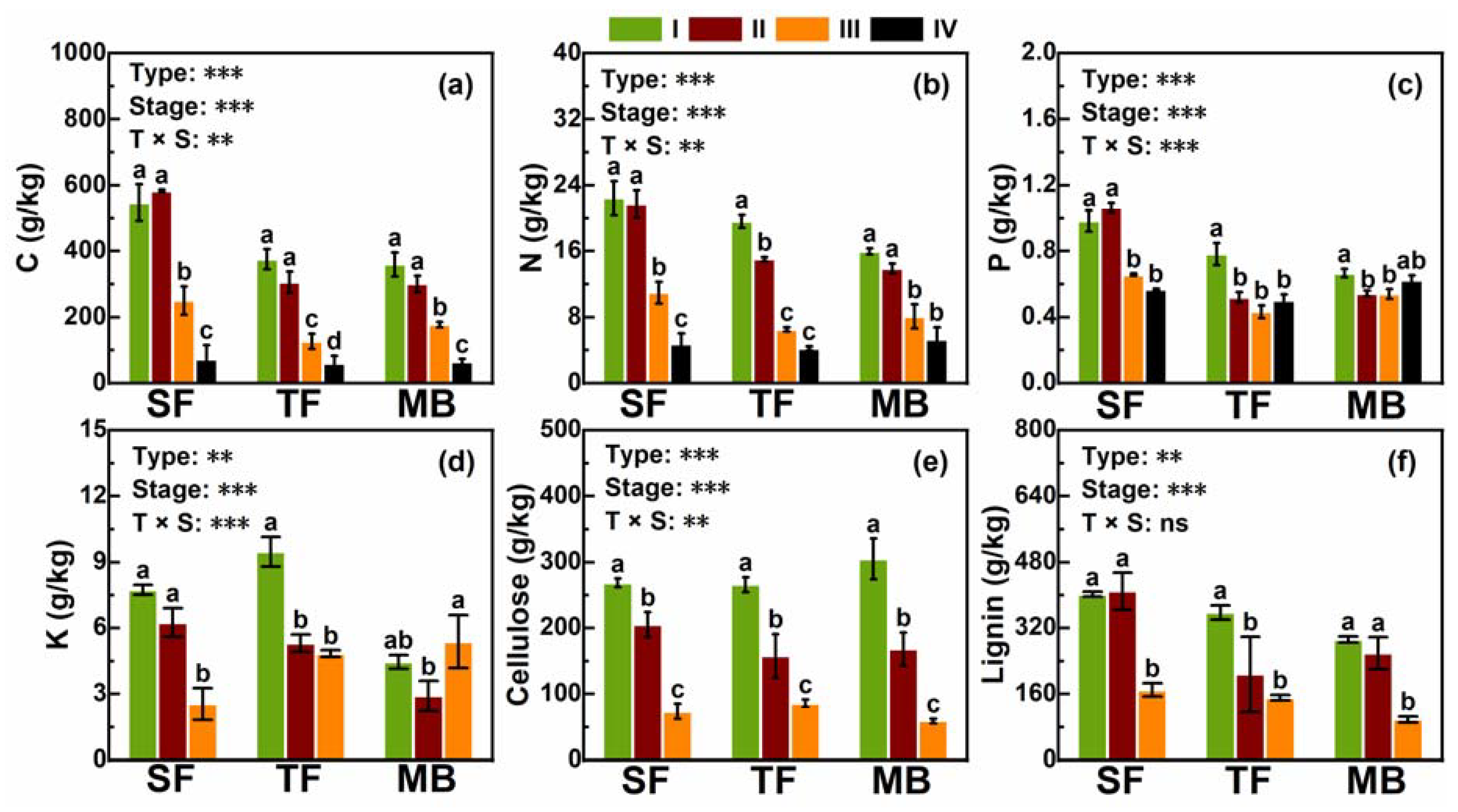
3.1. Macro-Elements, Cellulose, and Lignin
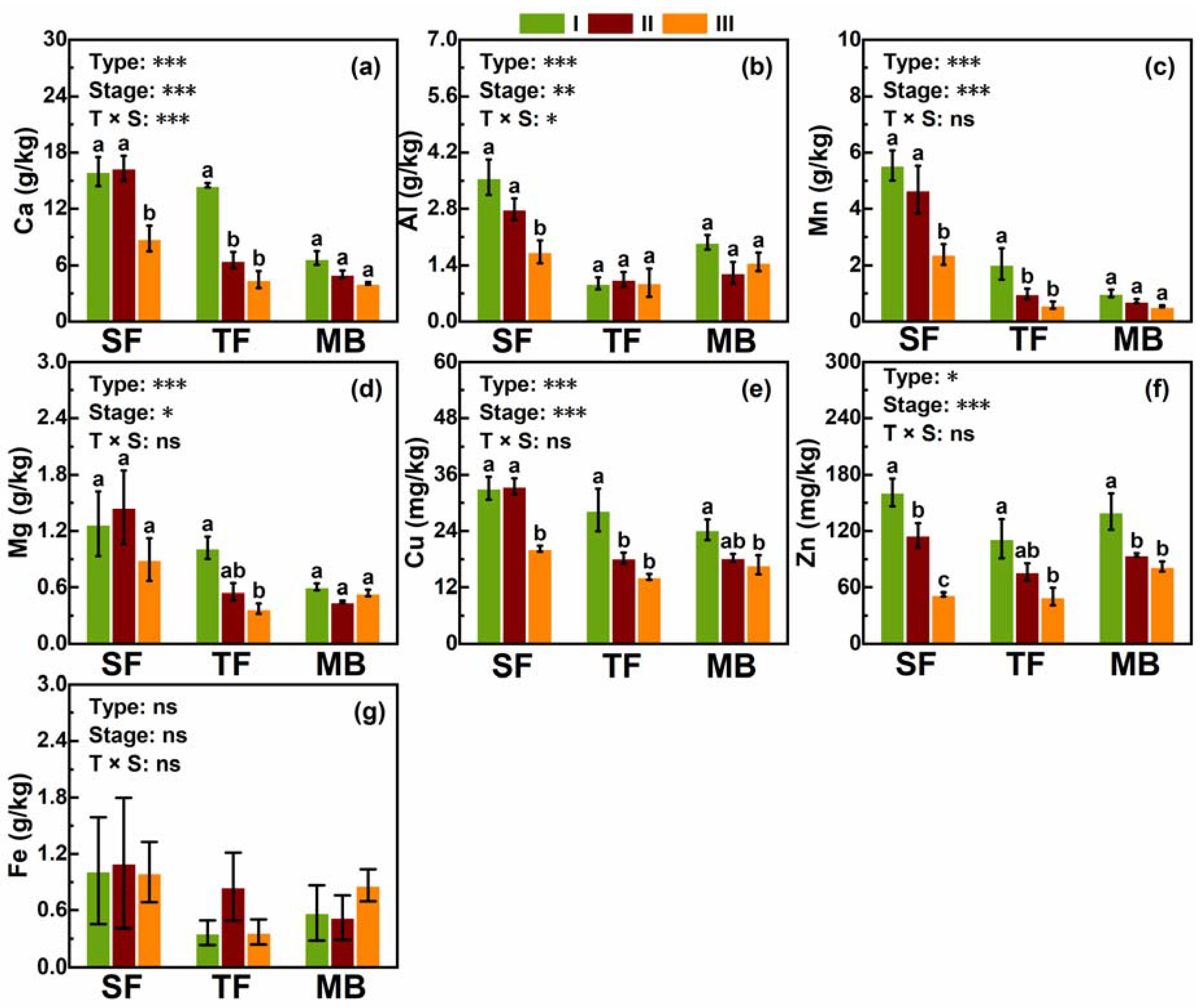
3.2. Meso- and Micro-Elements
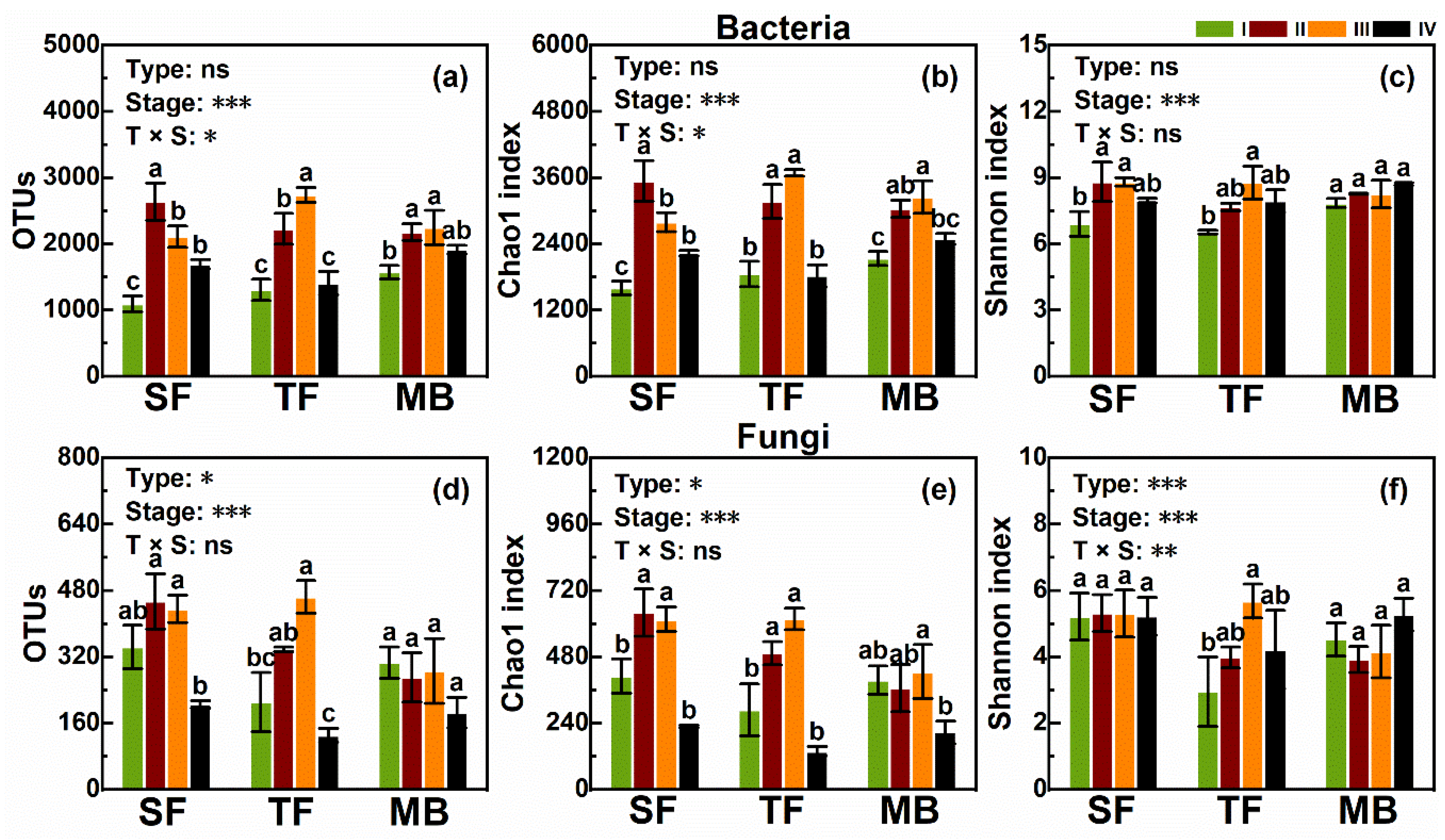
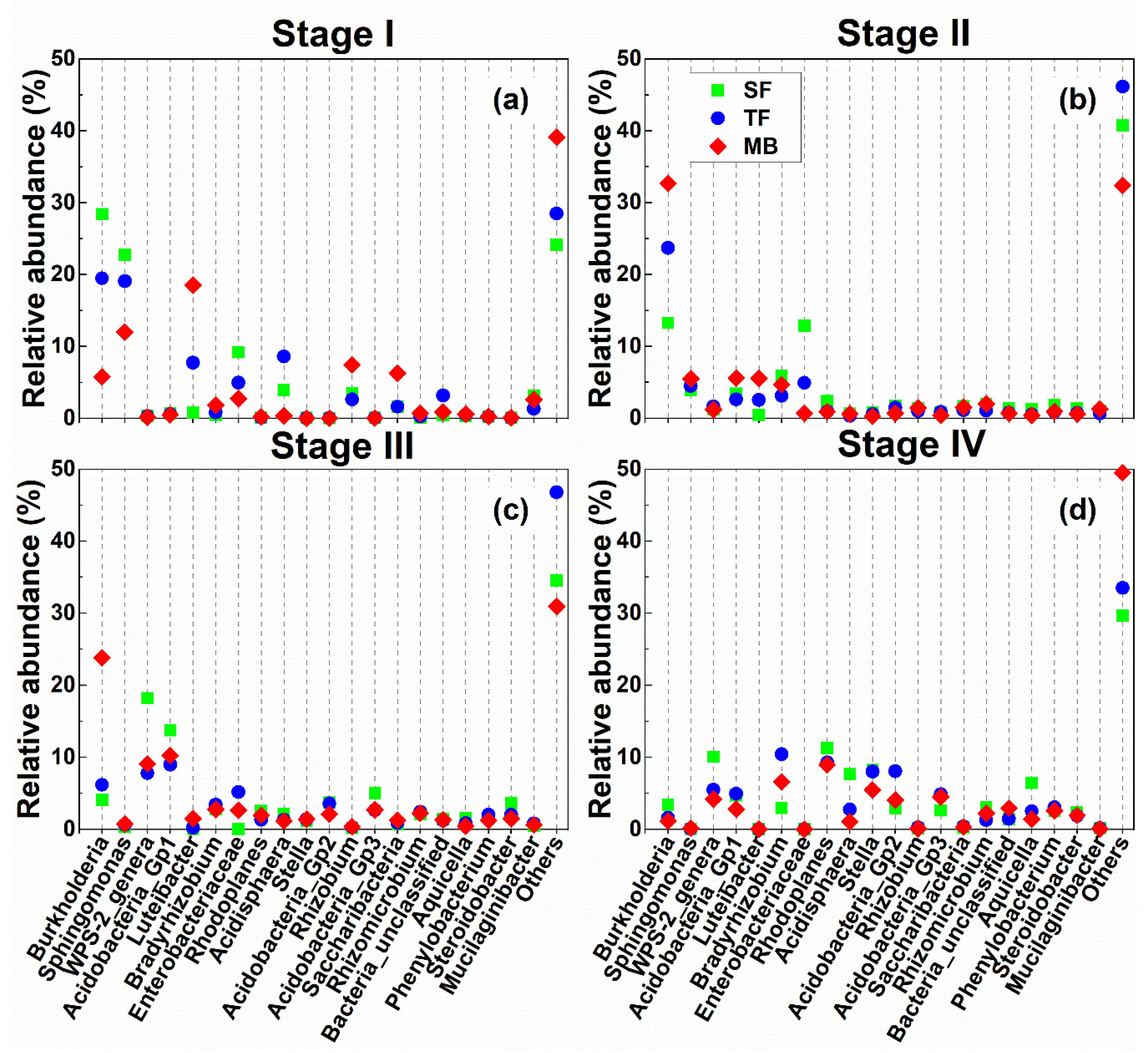
3.3. Bacteria
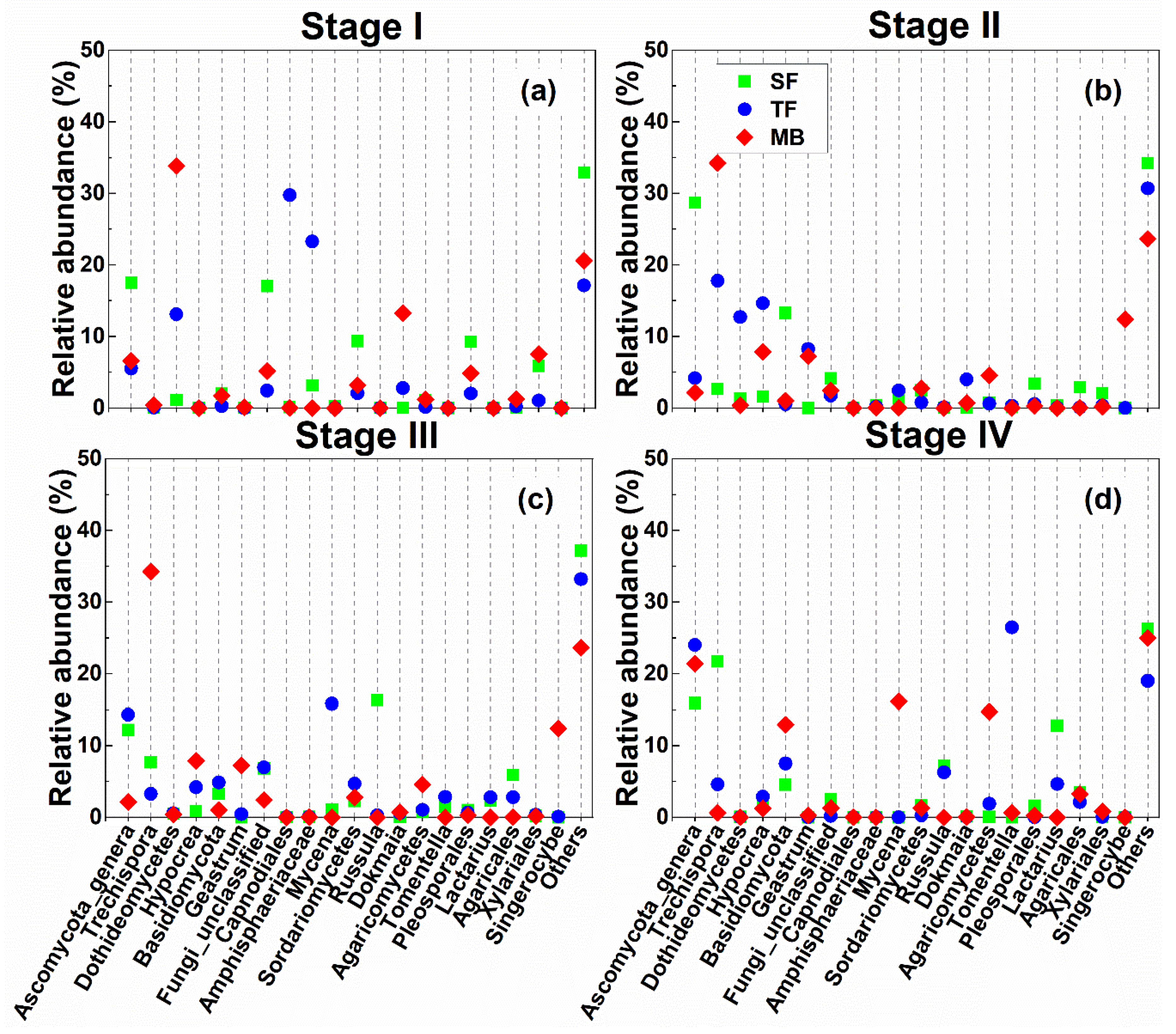
3.4. Fungi
4. Discussion
4.1. Effects of Moso Bamboo Invasions on Nutrients in Litter and Soil
4.2. Effects of Bamboo Invasion on Bacteria and Fungi in Litter and Soil
4.3. Litter Microbial Communities in Relation to Litter Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marbuah, G.; Gren, I.-M.; McKie, B. Economics of Harmful Invasive Species: A Review. Diversity 2014, 6, 500–523. [Google Scholar] [CrossRef]
- Engelkes, T.; Meisner, A.; Morrien, E.; Kostenko, O.; Van Der Putten, W.H.; Macel, M. Herbivory and dominance shifts among exotic and congeneric native plant species during plant community establishment. Oecologia 2016, 180, 507–517. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Müller-Schärer, H.; Van Kleunen, M.; Cai, A.-M.; Zhang, P.; Yan, R.; Dong, B.-C.; Yu, F.-H. Invasive alien plants benefit more from clonal integration in heterogeneous environments than natives. New Phytol. 2017, 216, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Chen, D.; Yan, R.; Yu, F.-H.; Van Kleunen, M. Invasive alien clonal plants are competitively superior over co-occurring native clonal plants. Perspect. Plant Ecol. Evol. Syst. 2019, 40, 125484. [Google Scholar] [CrossRef]
- Zenni, R.D.; Ziller, S.R.; Pauchard, A.; Rodriguez-Cabal, M.; Nuñez, M.A. Invasion science in the developing world: a response to ricciardi et al. Trends Ecol. Evol. 2017, 32, 807–808. [Google Scholar] [CrossRef] [PubMed]
- Prevéy, J.S.; Germino, M.J.; Huntly, N.; Inouye, R. Exotic plants increase and native plants decrease with loss of foundation species in sagebrush steppe. Plant Ecology 2009, 207, 39–51. [Google Scholar] [CrossRef]
- Davies, K.W. Plant community diversity and native plant abundance decline with increasing abundance of an exotic annual grass. Oecologia 2011, 167, 481–491. [Google Scholar] [CrossRef]
- A Driscoll, D. Disturbance maintains native and exotic plant species richness in invaded grassy woodlands. J. Veg. Sci. 2017, 28, 573–584. [Google Scholar] [CrossRef]
- Cleland, E.E.; Larios, L.; Suding, K. Strengthening invasion filters to reassemble native plant communities: soil resources and phenological overlap. Restor. Ecol. 2012, 21, 390–398. [Google Scholar] [CrossRef]
- Jackson, R.B.; Banner, J.L.; Jobbágy, E.G.; Pockman, W.; Wall, D.H. Ecosystem carbon loss with woody plant invasion of grasslands. Nature 2002, 418, 623–626. [Google Scholar] [CrossRef]
- Sardans, J.; Bartrons, M.; Margalef, O.; Gargallo-Garriga, A.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Sigurdsson, B.D.; Chen, H.Y.H.; Penuelas, J. Plant invasion is associated with higher plant-soil nutrient concentrations in nutrient poor-environments. Glob. Chang. Boil. 2016, 23, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sardans, J.; Wang, C.; Zeng, C.; Tong, C.; Chen, G.; Huang, J.; Pan, H.; Peguero, G.; Vallicrosa, H.; et al. The response of stocks of C, N, and P to plant invasion in the coastal wetlands of China. Glob. Chang. Boil. 2018, 25, 733–743. [Google Scholar] [CrossRef]
- Aragón, R.; Montti, L.; Ayup, M.M.; Fernandez, R.D. Exotic species as modifiers of ecosystem processes: Litter decomposition in native and invaded secondary forests of NW Argentina. Acta Oecologica 2014, 54, 21–28. [Google Scholar] [CrossRef]
- Braun, K.; Collantes, M.B.; Yahdjian, L.; Escartin, C.; Anchorena, J.A. Increased litter decomposition rates of exotic invasive species Hieracium pilosella (Asteraceae) in Southern Patagonia, Argentina. Plant Ecology 2019, 220, 393–403. [Google Scholar] [CrossRef]
- Castro-Diez, P.; Álvaro, A.; Romero-Blanco, A. Effects of litter mixing on litter decomposition and soil properties along simulated invasion gradients of non-native trees. Plant Soil 2019, 442, 79–96. [Google Scholar] [CrossRef]
- Inderjit; Van Der Putten, W.H. Impacts of soil microbial communities on exotic plant invasions. Trends Ecol. Evol. 2010, 25, 512–519. [Google Scholar] [CrossRef]
- Hynson, N.A.; Merckx, V.S.; Perry, B.A.; Treseder, K. Identities and distributions of the co-invading ectomycorrhizal fungal symbionts of exotic pines in the Hawaiian Islands. Boil. Invasions 2013, 15, 2373–2385. [Google Scholar] [CrossRef]
- McLeod, M.L.; Cleveland, C.C.; Lekberg, Y.; Maron, J.L.; Philippot, L.; Bru, D.; Callaway, R.M. Exotic invasive plants increase productivity, abundance of ammonia-oxidizing bacteria and nitrogen availability in intermountain grasslands. J. Ecol. 2016, 104, 994–1002. [Google Scholar] [CrossRef]
- Perkins, L.; Johnson, D.W.; Nowak, R.S. Plant-induced changes in soil nutrient dynamics by native and invasive grass species. Plant Soil 2011, 345, 365–374. [Google Scholar] [CrossRef]
- Wickramathilake, B.A.K.; Weerasinghe, T.K.; Ranwala, S.M.W. Impacts of woody invader Dillenia suffruticosa (Griff.) Martelli on physio-chemical properties of soil and, below and above ground flora. J. Trop. Forest. Environ. 2013, 3, 66–75. [Google Scholar] [CrossRef]
- Feng, J.; Zhou, J.; Wang, L.; Cui, X.; Ning, C.; Wu, H.; Zhu, X.; Lin, G. Effects of short-term invasion of Spartina alterniflora and the subsequent restoration of native mangroves on the soil organic carbon, nitrogen and phosphorus stock. Chemosphere 2017, 184, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Zhang, L.; Wang, W.; Gauci, V.; Marrs, R.; Liu, B.; Jia, R.; Zeng, C. Contrasting nutrient stocks and litter decomposition in stands of native and invasive species in a sub-tropical estuarine marsh. Environ. Res. 2011, 111, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Mincheva, T.; Barni, E.; Varese, G.C.; Brusa, G.; Cerabolini, B.E.L.; Siniscalco, C. Litter quality, decomposition rates and saprotrophic mycoflora in Fallopia japonica (Houtt.) RonseDecraene and in adjacent native grassland vegetation. Acta Oecologica 2014, 54, 29–35. [Google Scholar] [CrossRef]
- Niu, H.-B.; Liu, W.-X.; Wan, F.-H.; Liu, B. An invasive aster (Ageratina adenophora) invades and dominates forest understories in China: altered soil microbial communities facilitate the invader and inhibit natives. Plant Soil 2007, 294, 73–85. [Google Scholar] [CrossRef]
- Perkins, L.; Nowak, R.S. Native and non-native grasses generate common types of plant-soil feedbacks by altering soil nutrients and microbial communities. Oikos 2012, 122, 199–208. [Google Scholar] [CrossRef]
- Mamet, S.D.; Lamb, E.G.; Piper, C.L.; Winsley, T.; Siciliano, S.D. Archaea and bacteria mediate the effects of native species root loss on fungi during plant invasion. ISME J. 2017, 11, 1261–1275. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Chauhan, B.S.; Farooq, M.; Shabbir, A.; Adkins, S.W. What do we really know about alien plant invasion? A review of the invasion mechanism of one of the world’s worst weeds. Planta 2016, 244, 39–57. [Google Scholar] [CrossRef]
- Warren, R.J.; King, J.R.; Tarsa, C.; Haas, B.; Henderson, J. A systematic review of context bias in invasion biology. PLoS One 2017, 12, e0182502. [Google Scholar] [CrossRef]
- Nackley, L.; West, A.; Skowno, A.L.; Bond, W.J. The nebulous ecology of native invasions. Trends Ecol. Evol. 2017, 32, 814–824. [Google Scholar] [CrossRef]
- Ying, W.; Jin, J.; Jiang, H.; Zhang, X.; Lu, X.; Chen, X.; Zhang, J. Satellite-based detection of bamboo expansion over the past 30 years in Mount Tianmushan, China. Int. J. Remote. Sens. 2016, 37, 2908–2922. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Yao, X.M.; Tian, X.M. The regeress analysis between fertilization and the output, the economic benefit in Phyllostachys pubescens for culm-producing. Chin. Agr. Sci. Bull. 2009, 25, 166–169. [Google Scholar]
- Li, X.X.; Liu, P.; Rong, J.D.; Li, S.K.; Chen, L.Y.; He, T.Y.; Zheng, Y.S. Research state and development trend of moso bamboo forest management. World Bamboo Ratt. 2018, 16, 58–61. [Google Scholar]
- Xu, Q.-F.; Liang, C.-F.; Chen, J.-H.; Li, Y.-C.; Qin, H.; Fuhrmann, J. Rapid bamboo invasion (expansion) and its effects on biodiversity and soil processes +. Glob. Ecol. Conserv. 2020, 21, e00787. [Google Scholar] [CrossRef]
- Bai, S.B.; Zhou, G.M.; Wang, Y.X.; Liang, Q.Q.; Chen, J.; Cheng, Y.Y.; Shen, R. Plant species diversity and dynamics in forests invaded by moso bamboo (Phyllostachys edulis) in tianmu mountain nature reserve. Biodiversity Sci. 2013, 21, 288–295. [Google Scholar]
- Fukushima, K.; Usui, N.; Ogawa, R.; Tokuchi, N. Impacts of moso bamboo (Phyllostachys pubescens) invasion on dry matter and carbon and nitrogen stocks in a broad-leaved secondary forest located in K yoto, western Japan. Plant. Spec. Biol. 2015, 30, 81–95. [Google Scholar] [CrossRef]
- Wang, H.-C.; Tian, G.; Chiu, C.-Y. Invasion of moso bamboo into a Japanese cedar plantation affects the chemical composition and humification of soil organic matter. Sci. Rep. 2016, 6, 32211. [Google Scholar] [CrossRef] [PubMed]
- Shiau, Y.-J.; Chiu, C.-Y. Changes in Soil Biochemical Properties in a Cedar Plantation Invaded by Moso Bamboo. Forests 2017, 8, 222. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Deng, B.; Liu, Y.; Kong, F.; Huang, G.; Zou, Q.; Liu, Q.; Guo, X.; Fu, Y.; et al. Effects of moso bamboo (Phyllostachys edulis) invasions on soil nitrogen cycles depend on invasion stage and warming. Environ. Sci. Pollut. Res. 2017, 24, 24989–24999. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Stanek, M.; Nobis, M.; Zubek, S. Species-specific effects of plant invasions on activity, biomass, and composition of soil microbial communities. Boil. Fertil. Soils 2016, 52, 841–852. [Google Scholar] [CrossRef]
- Dawson, W.; Schrama, M. Identifying the role of soil microbes in plant invasions. J. Ecol. 2016, 104, 1211–1218. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, P.-K.; Wu, J.-S.; Zhou, G.-M.; Shen, R.-F.; Fuhrmann, J.J. Bamboo invasion of native broadleaf forest modified soil microbial communities and diversity. Boil. Invasions 2014, 17, 433–444. [Google Scholar] [CrossRef]
- Bani, A.; Pioli, S.; Ventura, M.; Panzacchi, P.; Borruso, L.; Tognetti, R.; Tonon, G.; Brusetti, L. The role of microbial community in the decomposition of leaf litter and deadwood. Appl. Soil Ecol. 2018, 126, 75–84. [Google Scholar] [CrossRef]
- Purahong, W.; Wubet, T.; Lentendu, G.; Schloter, M.; Pecyna, M.J.; Kapturska, D.; Hofrichter, M.; Krüger, D.; Buscot, F. Life in leaf litter: novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol. Ecol. 2016, 25, 4059–4074. [Google Scholar] [CrossRef] [PubMed]
- Hobara, S.; Osono, T.; Hirose, D.; Noro, K.; Hirota, M.; Benner, R. The roles of microorganisms in litter decomposition and soil formation. Biogeochem. 2013, 118, 471–486. [Google Scholar] [CrossRef]
- Keiser, A.D.; Knoepp, J.D.; Bradford, M.A. Microbial communities may modify how litter quality affects potential decomposition rates as tree species migrate. Plant Soil 2013, 372, 167–176. [Google Scholar] [CrossRef]
- Elgersma, K.J.; Ehrenfeld, J.G.; Yu, S.; Vor, T. Legacy effects overwhelm the short-term effects of exotic plant invasion and restoration on soil microbial community structure, enzyme activities, and nitrogen cycling. Oecologia 2011, 167, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Zhou, B.; Ge, X.G.; Wang, X.-M.; Li, Q. Leaf-litter decomposition dynamic, carbon loss and nutrient return for Moso Bamboo forest with different litter mass accumulation. Adv. Mater. Res. 2013, 726, 4222–4225. [Google Scholar] [CrossRef]
- Kanerva, S.M.; Smolander, A. Microbial activities in forest floor layers under silver birch, Norway spruce and Scots pine. Soil Boil. Biochem. 2007, 39, 1459–1467. [Google Scholar] [CrossRef]
- Bao, S.D. Agro-Chemical Analysis of Soil; Agricultural Publish House of China: Beijing, China, 2000; pp. 146–336. [Google Scholar]
- Rowland, A.P.; Roberts, J.D. Lignin and cellulose fractionation in decomposition studies using acid?detergentfibre methods. Commun. Soil Sci. Plant Anal. 1994, 25, 269–277. [Google Scholar] [CrossRef]
- Blaxter, M.; Mann, J.; Chapman, T.; Thomas, F.; Whitton, C.; Floyd, R.; Abebe, E. Defining operational taxonomic units using DNA barcode data. Philos. Trans. R. Soc. B Boil. Sci. 2005, 360, 1935–1943. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Drenovsky, R.E.; Grewell, B.J.; D’Antonio, C.M.; Funk, J.L.; James, J.J.; Molinari, N.; Parker, I.M.; Richards, C. A functional trait perspective on plant invasion. Ann. Bot. 2012, 110, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Tlaskal, V.; Baldrian, P.; Voriskova, J.; Boer, W. Bacterial succession on decomposing leaf litter exhibits a specific occurrence pattern of cellulolytic taxa and potential decomposers of fungal mycelia. FEMS Microbiol. Ecol. 2016, 92, 177. [Google Scholar] [CrossRef] [PubMed]
- Grayston, S.J.; Prescott, C.E. Microbial communities in forest floors under four tree species in coastal British Columbia. Soil Boil. Biochem. 2005, 37, 1157–1167. [Google Scholar] [CrossRef]
- Ushio, M.; Kitayama, K.; Balser, T.C. Tree species-mediated spatial patchiness of the composition of microbial community and physicochemical properties in the topsoils of a tropical montane forest. Soil Boil. Biochem. 2010, 42, 1588–1595. [Google Scholar] [CrossRef]
- Sodhi, D.; Livingstone, S.W.; Carboni, M.; Cadotte, M.W. Plant invasion alters trait composition and diversity across habitats. Ecol. Evol. 2019, 9, 6199–6210. [Google Scholar] [CrossRef]
- Wu, C.; Mo, Q.; Wang, H.; Zhang, Z.; Huang, G.; Ye, Q.; Zou, Q.; Kong, F.; Liu, Y.; Wang, G.G. Moso bamboo (Phyllostachys edulis (Carriere) J. Houzeau) invasion affects soil phosphorus dynamics in adjacent coniferous forests in subtropical China. Ann. For. Sci. 2018, 75, 24. [Google Scholar] [CrossRef]
- Umemura, M.; Takenaka, C. Changes in chemical characteristics of surface soils in hinoki cypress (Chamaecyparis obtusa) forests induced by the invasion of exotic Moso bamboo (Phyllostachys pubescens) in central Japan. Plant Species Boil. 2014, 30, 72–79. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Ke, P.; Miki, T.; Ding, T.-S. The soil microbial community predicts the importance of plant traits in plant-soil feedback. New Phytol. 2014, 206, 329–341. [Google Scholar] [CrossRef]
- Day, N.; Dunfield, K.E.; Antunes, P.M. Fungi from a non-native invasive plant increase its growth but have different growth effects on native plants. Boil. Invasions 2015, 18, 231–243. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Liu, J.; Jiang, K.; Xiao, H.; Du, D. Responses of the soil fungal communities to the co-invasion of two invasive species with different cover classes. Plant Boil. 2017, 20, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Fitter, A. Microbial mediation of plant competition and community structure. Funct. Ecol. 2012, 27, 865–875. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, J.-T.; Ma, K. Soil biota reduce allelopathic effects of the invasive Eupatorium adenophorum. PLoS ONE 2011, 6, e25393. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Boil. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Q.; Liu, N.; Li, L.; Zhang, C.-G.; Liu, Z.B.; Zhang, Y.-Y. Effects of different leaf litters on the physicochemical properties and bacterial communities in Panax ginseng -growing soil. Appl. Soil Ecol. 2017, 111, 17–24. [Google Scholar] [CrossRef]
- He, Z.; Yu, Z.; Huang, Z.; Davis, M.; Yang, Y. Litter decomposition, residue chemistry and microbial community structure under two subtropical forest plantations: A reciprocal litter transplant study. Appl. Soil Ecol. 2016, 101, 84–92. [Google Scholar] [CrossRef]
- Dukunde, A.; Schneider, D.; Schmidt, M.; Veldkamp, E.; Daniel, R. Tree species shape soil bacterial community structure and function in temperate deciduous forests. Front. Microbiol. 2019, 10, 1519. [Google Scholar] [CrossRef]
- Bissett, A.; Brown, M.V.; Siciliano, S.D.; Thrall, P.H. Microbial community responses to anthropogenically induced environmental change: towards a systems approach. Ecol. Lett. 2013, 16, 128–139. [Google Scholar] [CrossRef]
- Boberg, J.; Finlay, R.; Stenlid, J.; Ekblad, A.; Lindahl, B.D. Nitrogen and carbon reallocation in fungal Mycelia during decomposition of boreal forest litter. PLoS ONE 2014, 9, e92897. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest soil bacteria: diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Boil. Rev. 2017, 81, 00063-16. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.-K.; Wang, M.-Y.; Meng, P.; Zhang, J.-S.; Zhou, B.-Z.; Ge, X.-G.; Yu, F.-H.; Li, M.-H. Native Bamboo Invasions into Subtropical Forests Alter Microbial Communities in Litter and Soil. Forests 2020, 11, 314. https://doi.org/10.3390/f11030314
Tian X-K, Wang M-Y, Meng P, Zhang J-S, Zhou B-Z, Ge X-G, Yu F-H, Li M-H. Native Bamboo Invasions into Subtropical Forests Alter Microbial Communities in Litter and Soil. Forests. 2020; 11(3):314. https://doi.org/10.3390/f11030314
Chicago/Turabian StyleTian, Xiao-Kun, Min-Yan Wang, Ping Meng, Jin-Song Zhang, Ben-Zhi Zhou, Xiao-Gai Ge, Fei-Hai Yu, and Mai-He Li. 2020. "Native Bamboo Invasions into Subtropical Forests Alter Microbial Communities in Litter and Soil" Forests 11, no. 3: 314. https://doi.org/10.3390/f11030314
APA StyleTian, X.-K., Wang, M.-Y., Meng, P., Zhang, J.-S., Zhou, B.-Z., Ge, X.-G., Yu, F.-H., & Li, M.-H. (2020). Native Bamboo Invasions into Subtropical Forests Alter Microbial Communities in Litter and Soil. Forests, 11(3), 314. https://doi.org/10.3390/f11030314





