Advance Regeneration of Norway Spruce and Scots Pine in Hemiboreal Forests in Latvia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Design
2.2. Data Analysis
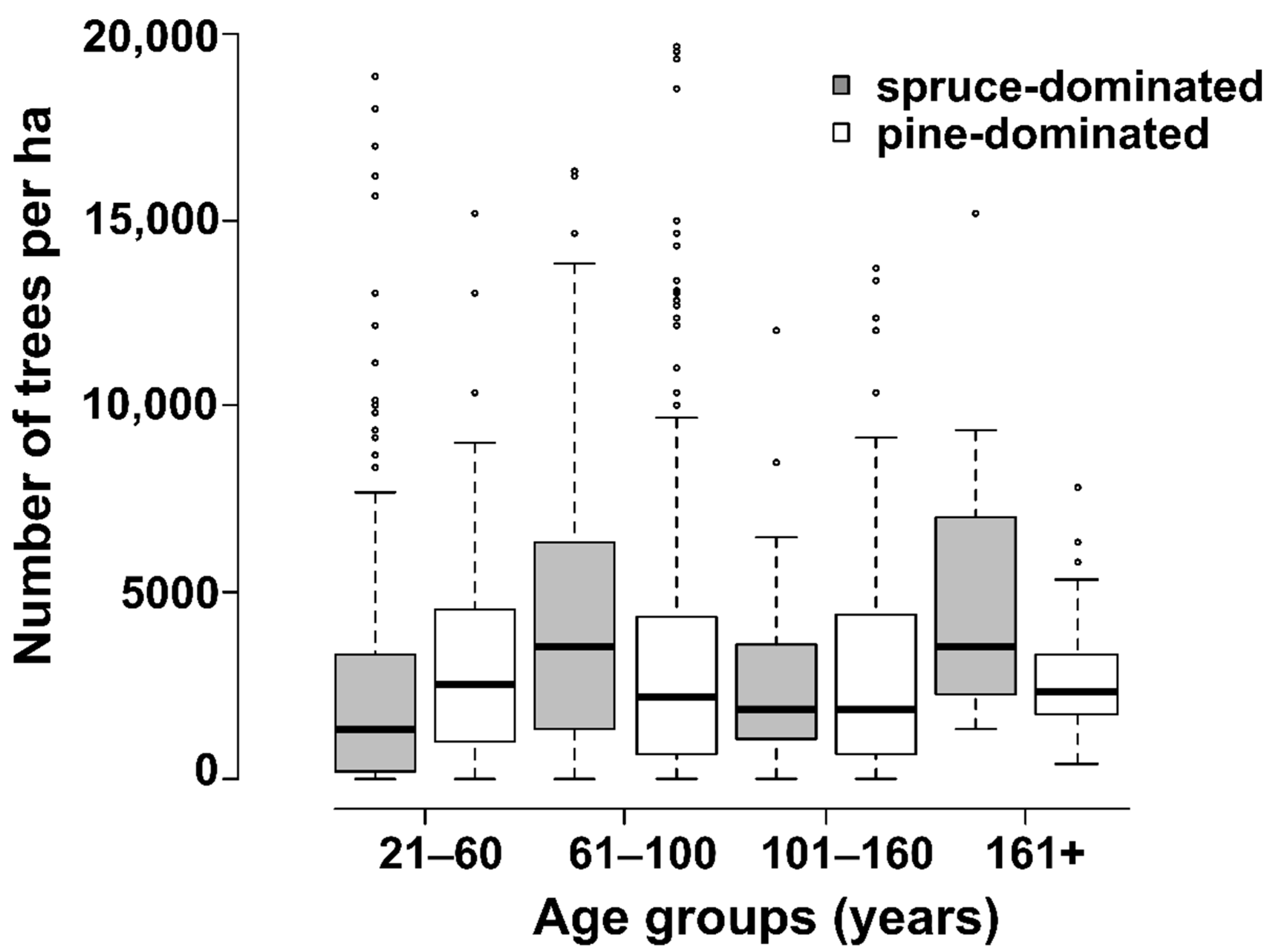
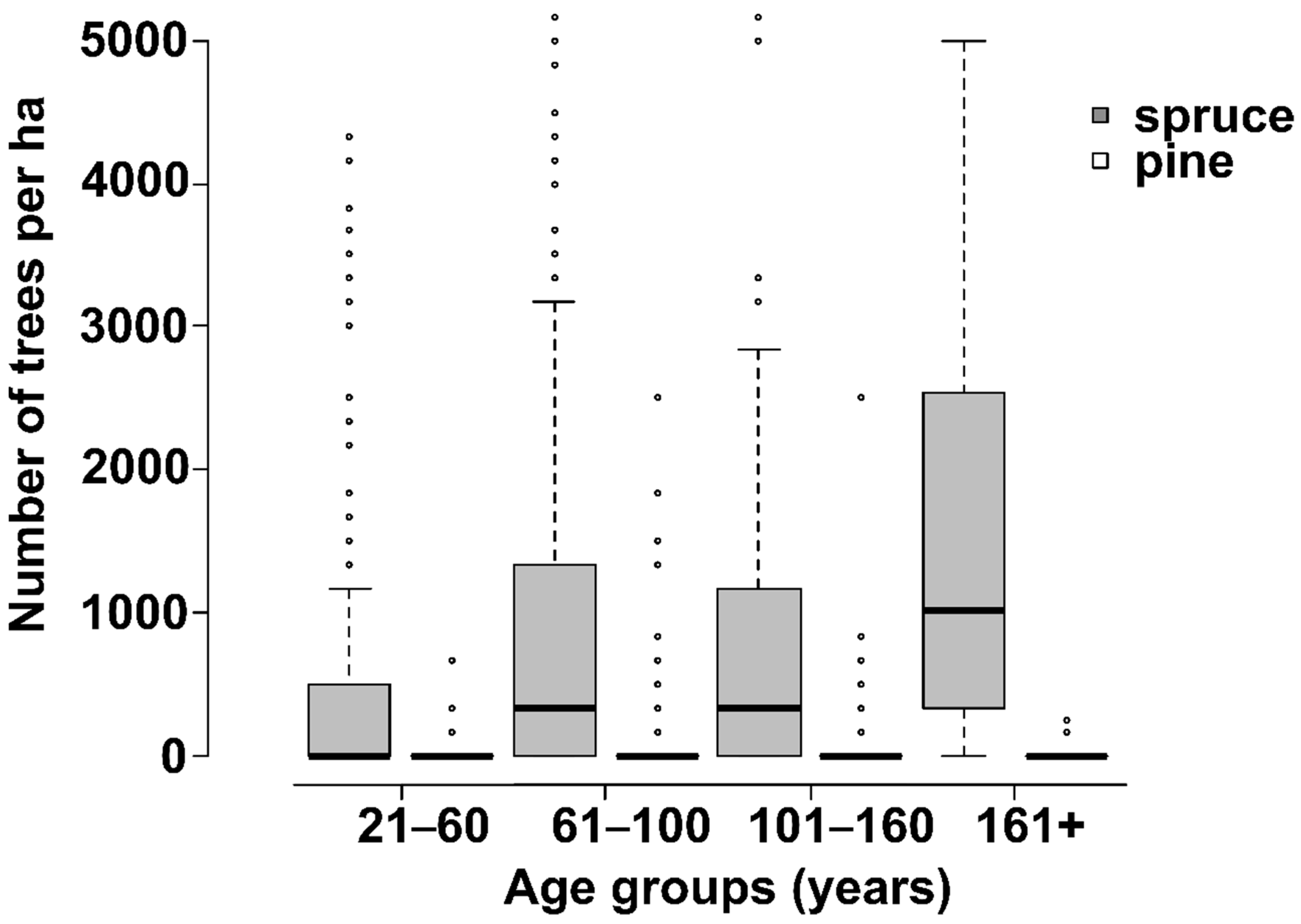
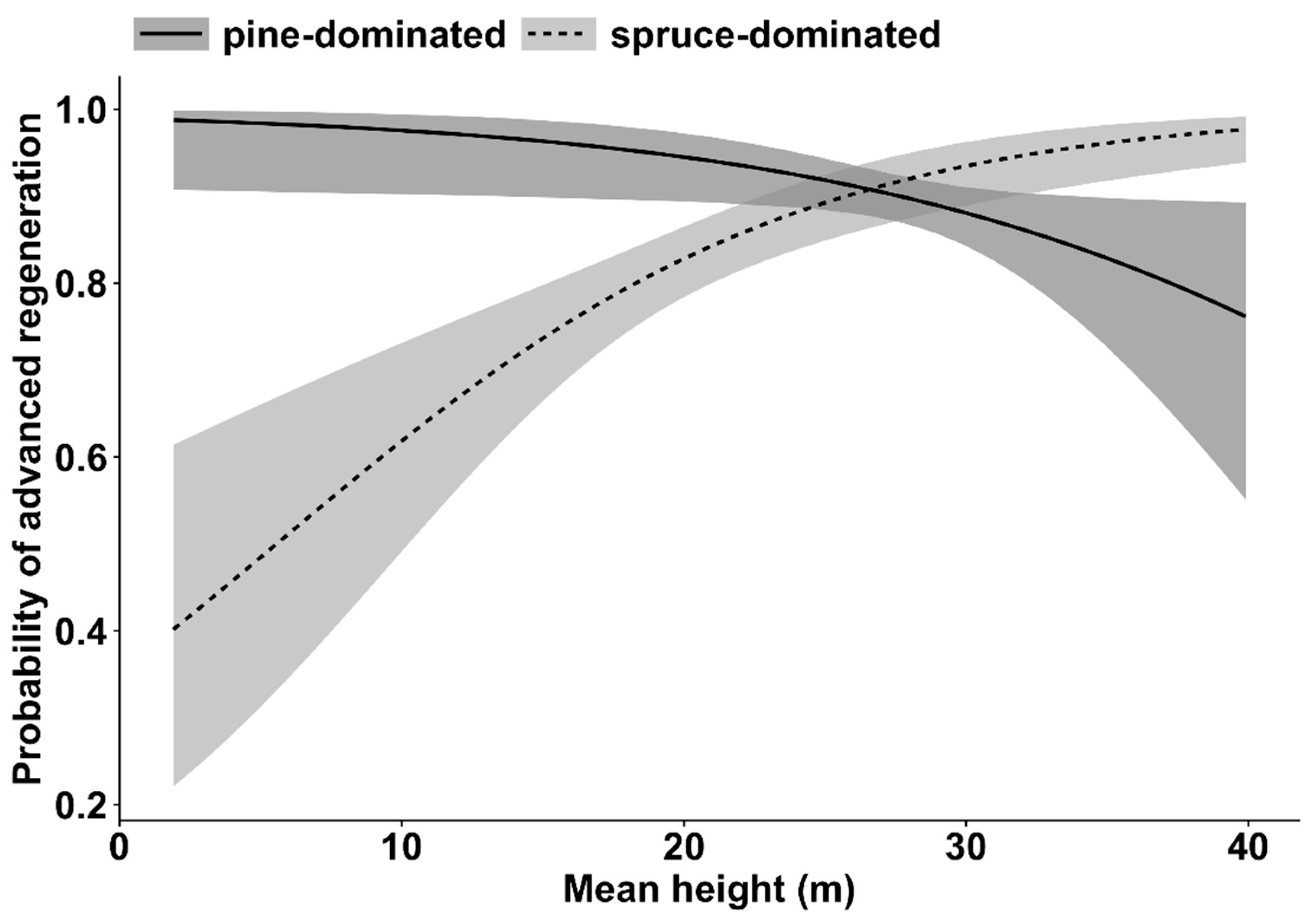
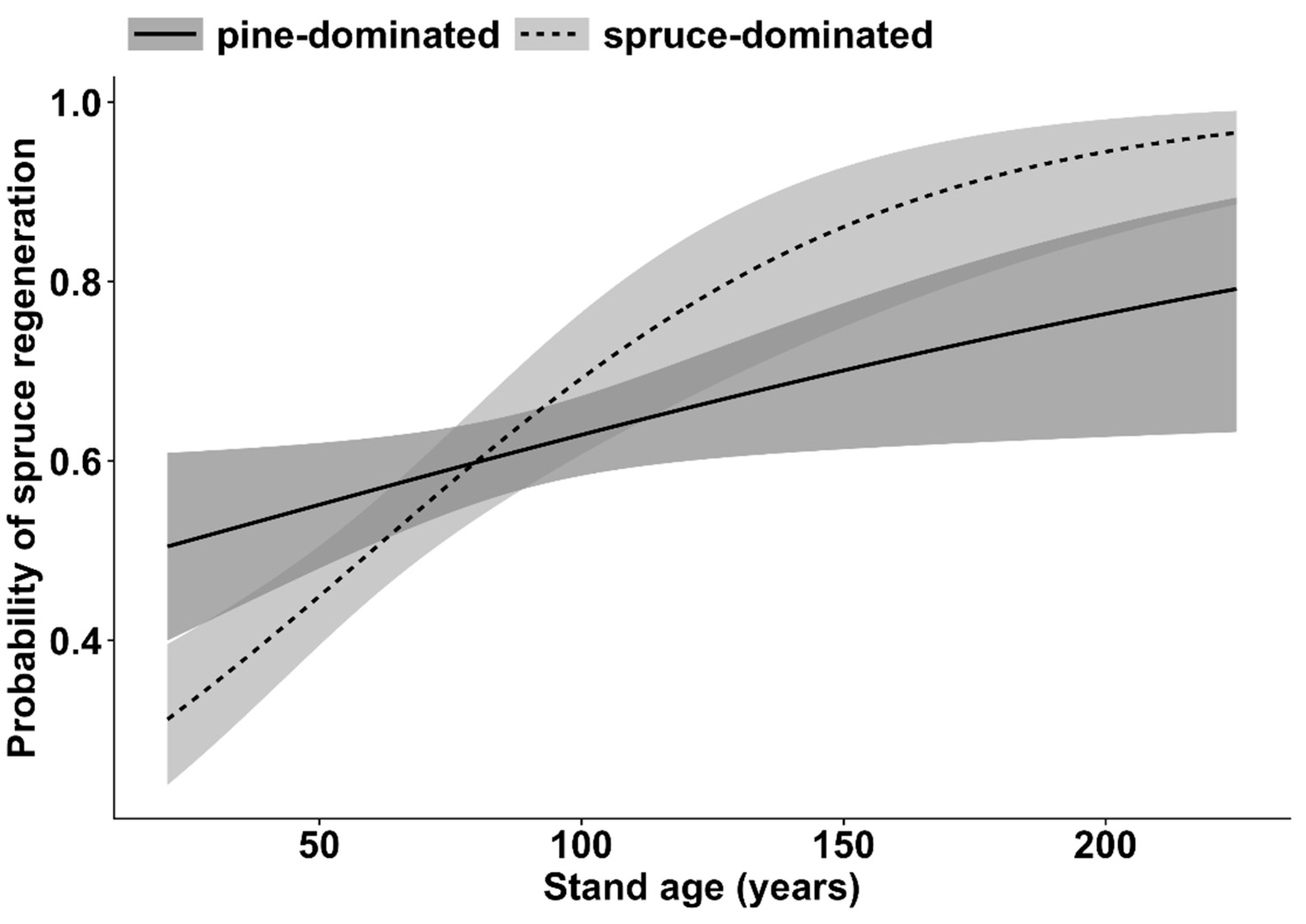
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puettmann, K.J.; Coates, K.D.; Messier, C.C. A Critique of Silviculture: Managing for Complexity; Island Press: Washington, DC, USA, 2009; p. 206. [Google Scholar]
- Messier, C.; Puettmann, K.J.; Coates, K.D. The complex adaptive system: A new integrative framework for understanding and managing the world forest. In Managing Forests as Complex Adaptive Systems: Building Resilience to the Challenge of Global Change; Messier, C., Puettmann, K.J., Coates, K.D., Eds.; Routledge: London, UK, 2013; pp. 327–341. [Google Scholar]
- Fahey, R.T.; Alveshere, B.C.; Burton, J.I.; D’Amato, A.W.; Dickinson, Y.L.; Keeton, W.S.; Kern, C.C.; Larson, A.J.; Palik, B.J.; Puettmann, K.J.; et al. Shifting conceptions of complexity in forest management and silviculture. For. Ecol. Manag. 2018, 421, 59–71. [Google Scholar] [CrossRef]
- Messier, C.; Puettmann, K.; Chazdon, R.; Andersson, K.P.; Angers, V.A.; Brotons, L.; Filotas, E.; Tittler, R.; Parrott, L.; Levin, S.A. From Management to Stewardship: Viewing Forests as Complex Adaptive Systems in an Uncertain World. Conserv. Lett. 2015, 8, 368–377. [Google Scholar] [CrossRef]
- Jacobsen, M.K. History and Principles of Close to Nature Forest Management: A Central European Perspective. In Textbook 2—Tools for Preserving Woodland Biodiversity; Read, H., Forfang, A.S., Marciau, R., Paltto, H., Andersson, L., Tardy, B., Eds.; Nature Conservation Exchange Experience, NACONEX, Pro-Natura: Göteborg, Sweden, 2001; pp. 56–60. Available online: http://www.pro-natura.net/naconex/news5/E2_11.pdf (accessed on 15 December 2019).
- Gayer, K. Der Gemischte Wald; Verlag von Paul Parey: Berlin, Germany, 1886; p. 168. [Google Scholar]
- Engler, A. Aus der Theorie und Praxis des Femelschlagbetriebes. Schweiz. Z. Forstwes. 1905, 56, 29–35. [Google Scholar]
- Möller, A. Der Dauerwaldgedanke, sein Sinn und seine Bedeutung; Springer: Berlin, Germany, 1922; p. 84. [Google Scholar]
- Mlinšek, D. PRO SILVA. 1989. Available online: https://www.prosilva.org (accessed on 15 December 2019).
- Schütz, J. Der Plenterwald und Weitere Formen Strukturierter und Gemischter Wälder; Parey Buchverlag: Berlin, Germany, 2001; p. 207. [Google Scholar]
- Schütz, J. Close-to-nature silviculture: Is this concept compatible with species diversity? Forestry 1999, 72, 359–366. [Google Scholar] [CrossRef]
- Schütz, J.P. Charakterisierung des naturnahen Waldbaus und Bedarf an wissenschaftlichen Grundlagen. Schweiz. Z. Forstwes. 1986, 137, 747–760. [Google Scholar]
- Brang, P.; Spathelf, P.; Larsen, J.B.; Bauhus, J.; Boncčìna, A.; Chauvin, C.; Drössler, L.; García-Güemes, C.; Heiri, C.; Kerr, G.; et al. Suitability of close-to-nature silviculture for adapting temperate European forests to climate change. Forestry 2014, 87, 492–503. [Google Scholar] [CrossRef]
- Fady, B.; Cottrell, J.; Ackzell, L.; Alía, R.; Muys, B.; Prada, A.; González-Martínez, S.C. Forests and global change: What can genetics contribute to the major forest management and policy challenges of the twenty-first century? Reg. Environ. Chang. 2016, 16, 927–939. [Google Scholar] [CrossRef]
- Rungis, D.; Luguza, S.; Bāders, E.; Šķipars, V.; Jansons, Ā. Comparison of Genetic Diversity in Naturally Regenerated Norway Spruce Stands and Seed Orchard Progeny Trials. Forests 2019, 10, 926. [Google Scholar] [CrossRef]
- Pothier, D. Ten-year results of strip clear-cutting in Quebec black spruce stands. Can. J. For. Res. 2000, 30. [Google Scholar] [CrossRef]
- Prèvost, M.; Dumais, D. Long-term growth response of black spruce advance regeneration (layers), natural seedlings and planted seedlings to scarification: 25th year update. Scand. J. For. Res. 2018, 33, 583–593. [Google Scholar] [CrossRef]
- Metslaid, M.; Jõgiste, K.; Nikinmaa, E.; Moser, W.K.; Porcar-Castell, A. Tree variables related to growth response and acclimation of advance regeneration of Norway spruce and other coniferous species after release. For. Ecol. Manag. 2007, 250, 56–63. [Google Scholar] [CrossRef]
- Kellomäki, S.; Strandman, H.; Peltola, H. Effects of even-aged and uneven-aged management on carbon dynamics and timber yield in boreal Norway spruce stands: A forest ecosystem model approach. Forestry 2019, 92, 635–647. [Google Scholar] [CrossRef]
- Pineda-García, F.; Paz, H.; Tinoco-ojanguren, C. Morphological and physiological differentiation of seedlings between dry and wet habitats in a tropical dry forest. Plant Cell Environ. 2011, 34, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Silava, Methodology of National Forest Inventory. Available online: http://www.silava.lv/userfiles/file/Nacionalais%20meza%20monitorings/Me%C5%BEa%20resursu%20monitoringa%20metodika%2026_04_2013.pdf (accessed on 12 December 2019). (In Latvian).
- Ahti, T.; Hämet-ahti, L.; Jalas, J. Vegetation zones and their sections in north-western Europe. Ann. Bot. Fenn. 1968, 5, 169–211. [Google Scholar]
- Keeley, J.E. Ecology and evolution of pine life histories. Ann. For. Sci. 2012, 69, 445–453. [Google Scholar] [CrossRef]
- Béland, M.; Agestam, E.; Ekö, P.M.; Gemmel, P.; Nilsson, U.; Agestam, E.; Eko, P.M. Scarification and Seedfall affects Natural Regeneration of Scots Pine Under Two Shelterwood Densities and a Clear-cut in Southern Sweden. Scand. J. For. Res. 2000, 15, 247–255. [Google Scholar] [CrossRef]
- Hille, M.; den Ouden, J. Improved recruitment and early growth of Scots pine (Pinus sylvestris L.) seedlings after fire and soil scarification. Eur. J. For. Res. 2004, 123, 213–218. [Google Scholar] [CrossRef]
- Beghin, R.; Lingua, E.; Garbarino, M.; Lonati, M.; Bovio, G.; Motta, R.; Marzano, R. Pinus sylvestris forest regeneration under different post-fire restoration practices in the northwestern Italian. Alps. Ecol. Eng. 2010, 36, 1365–1372. [Google Scholar] [CrossRef]
- Rouvinen, S.; Kouki, J. Tree Regeneration in Artificial Canopy Gaps Established for Restoring Natural Structural Variability in a Scots Pine Stand. Silva Fenn. 2011, 45, 1079–1091. [Google Scholar] [CrossRef]
- Parro, K.; Metslaid, M.; Renel, G.; Sims, A.; Stanturf, J.A.; Jõgiste, K.; Köster, K. Impact of postfire management on forest regeneration in a managed hemiboreal forest, Estonia. Can. J. For. Res. 2015, 45, 1192–1197. [Google Scholar] [CrossRef]
- Vickers, A.D.; Palmer, S.C.F. The influence of canopy cover and other factors upon the regeneration of Scots pine and its associated ground flora within Glen Tanar National Nature Reserve. Forestry 2000, 73, 37–49. [Google Scholar] [CrossRef]
- Lundqvist, L.; Ahlström, M.A.; Axelsson, E.P.; Mörling, T.; Valinger, E. Multi-layered Scots pine forests in boreal Sweden result from mass regeneration and size stratification. For. Ecol. Manag. 2019, 441, 176–181. [Google Scholar] [CrossRef]
- De Chantal, M.; Leinonen, K.; Kuuluvainen, T.; Cescatti, A. Early response of Pinus sylvestris and Picea abies seedlings to an experimental canopy gap in a boreal spruce forest. For. Ecol. Manag. 2003, 176, 321–336. [Google Scholar] [CrossRef]
- Messier, C.; Doucet, R.; Ruel, J.; Claveau, Y.; Kelly, C.; Lechowicz, M.J. Functional ecology of advance regeneration in relation to light in boreal forests. Can. J. For. Res. 1999, 29, 812–823. [Google Scholar] [CrossRef]
- Örlander, G.; Karlsson, C. Influence of Shelterwood Density on Survival and Height Increment of Picea abies Advance Growth. Scand. J. For. Res. 2000, 15, 20–29. [Google Scholar]
- Kuuluvainen, T.; Kalmari, R. Regeneration microsites of Picea abies seedlings in a windthrow area of a boreal old-growth. Ann. Bot. Fenn. 2003, 40, 401–413. [Google Scholar]
- Chrimes, D.; Nilson, K. Overstorey density influence on the height of Picea abies regeneration in northern Sweden. Forestry 2005, 78, 433–442. [Google Scholar] [CrossRef]
- Lundqvist, L.; Fridman, E. Influence of local stand basal area on density and growth of regeneration in uneven-aged Picea abies stands. Scand. J. For. Res. 1996, 11, 364–369. [Google Scholar] [CrossRef]
- Čater, M.; Diaci, J. Divergent response of European beech, silver fir and Norway spruce advance regeneration to increased light levels following natural disturbance. For. Ecol. Manag. 2017, 399, 206–212. [Google Scholar] [CrossRef]
- Lundqvist, L.; Valinger, E. Stem diameter growth of scots pine trees after increased mechanical load in the crown during dormancy and (or) growth. Ann. Bot. 1996, 77, 59–62. [Google Scholar] [CrossRef]
- Löf, M.; Karlsson, M.; Sonesson, K.; Welander, T.N.; Collet, C. Growth and mortality in underplanted tree seedlings in response to variations in canopy closure of Norway spruce stands. Forestry 2007, 80, 371–384. [Google Scholar] [CrossRef]
- Brandeis, T.J.; Newton, M.; Cole, E. A comparison of overstory density measures for describing understorey conifer growth. For. Ecol. Manag. 2001, 152, 149–157. [Google Scholar] [CrossRef]
- Mitchell, J.E.; Popowich, S.J. Effectiveness of basal area for estimating canopy cover of ponderosa pine. For. Ecol. Manag. 1997, 95, 45–51. [Google Scholar] [CrossRef]
- Petriţan, I.C.; von Lüpke, B.; Petriţan, A.M. Effects of root trenching of overstorey Norway spruce (Picea abies) on growth and biomass of underplanted beech (Fagus sylvatica) and Douglas fir (Pseudotsuga menziesii) saplings. Eur. J. Forest Res. 2011, 130, 813–828. [Google Scholar] [CrossRef]
- Nilson, K.; Lundqvist, L. Effect of Stand Structure and Density on Development of Natural Regeneration in Two Picea abies Stands in Sweden. Scand. J. For. Res. 2001, 16, 253–259. [Google Scholar] [CrossRef]
- Paluch, L.; Bartkowicz, J.; Moser, W.K. Interspecific effects between overstorey and regeneration in small - scale mixtures of three late-successional species in the Western Carpathians (southern Poland). Eur. J. For. Res. 2019, 138, 889–905. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Rouvinen, S. Post-fire understorey regeneration in boreal Pinus sylvestris forest sites with different fire histories. J. Veg. Sci. 2000, 11, 801–812. [Google Scholar] [CrossRef]
- Vodde, F.; Jõgiste, K.; Engelhart, J.; Frelich, L.E.; Moser, W.K.; Sims, A.; Metslaid, M. Impact of wind-induced microsites and disturbance severity on tree regeneration patterns: Results from the first post-storm decade. For. Ecol. Manag. 2015, 348, 174–185. [Google Scholar] [CrossRef]
- Meigs, G.W.; Morrissey, R.C.; Ba, R.; Chaskovskyy, O.; Després, T.; Donato, D.C.; Janda, P.; Lábusová, J.; Seedre, M.; Mikolá, M.; et al. Mixed-severity disturbance regimes foster structural complexity via multiple developmental pathways. For. Ecol. Manag. 2017, 406, 410–426. [Google Scholar] [CrossRef]
- Nilsson, U.; Gemmel, P.; Johansson, U.; Karlsson, M.; Welander, T.N. Natural regeneration of Norway spruce, Scots pine and birch under Norway spruce shelterwoods of varying densities on a mesic-dry site in southern Sweden. For. Ecol. Manag. 2002, 161, 133–145. [Google Scholar] [CrossRef]
- Ligot, G.; Ameztegui, A.; Courbaud, B.; Coll, L.; Kneeshaw, D.D. Tree light capture and spatial variability of understory light increase with species mixing and tree size heterogeneity. Can. J. For. Res. 2016, 46, 968–977. [Google Scholar] [CrossRef]
- Levers, C.; Verkerk, P.J.; Müller, D.; Verburg, P.H.; Butsic, V.; Leitão, P.J.; Lindner, M.; Kuemmerle, T. Drivers of forest harvesting intensity patterns in Europe. For. Ecol. Manag. 2014, 315, 160–172. [Google Scholar] [CrossRef]
- Rendenieks, Z.; Nikodemus, O.; Brūmelis, G. The implications of stand composition, age and spatial patterns of forest regions with different ownership type for management optimisation in northern Latvia. For. Ecol. Manag. 2015, 335, 216–244. [Google Scholar] [CrossRef]
- Latvian State Forest Service, Forest Statistics in Latvia. State Forest Service. 2018. Available online: http://www.vmd.gov.lv/valsts-meza-dienests/statiskas-lapas/publikacijas-un-statistika/meza-statistikas-cd?nid=1809#jump (accessed on 10 December 2019). (In Latvian)
- Greene, D.F.; Kneeshaw, D.D.; Messier, C.; Lieffers, V.; Cormier, D.; Doucet, R.; Coates, K.D.; Groot, A.; Grover, G.; Calogeropoulos, C. Modelling silvicultural alternatives for conifer regeneration in boreal mixedwood stands (aspen/white spruce/balsam fir). For.Chron. 2002, 78, 281–295. [Google Scholar] [CrossRef]
- Zdors, L.; Šepsts, G.; Donis, J. Stem volume increment after group shelterwood cutting in Scots pine stands in Myrtillosa forest type. Balt. For. 2017, 23, 463–470. [Google Scholar]
- Vodde, F.; Jõgiste, K.; Gruson, L.; Ilisson, T.; Köster, K.; Stanturf, J.A. Regeneration in windthrow areas in hemiboreal forests: The influence of microsite on the height growths of different tree species. J. For. Res. 2010, 15, 55–64. [Google Scholar] [CrossRef]
- Baders, E.; Senhofa, S.; Purina, L.; Jansons, A. Natural Succession of Norway Spruce Stands in Hemiboreal Forests: Case Study in Slitere National Park, Latvia. Balt. For. 2017, 23, 522–528. [Google Scholar]
- Metslaid, M.; Ilisson, T.; Nikinmaa, E.; Kusmin, J.; Jõgiste, K. Recovery of advance regeneration after disturbances: Acclimation of needle characteristics in Picea abies. Scand. J. For. Res. 2005, 20, 112–121. [Google Scholar] [CrossRef]
- Metslaid, M.; Ilisson, T.; Vicente, M.; Nikinmaa, E.; Jõgiste, K. Growth of advance regeneration of Norway spruce after clear-cutting. Tree Physiol. 2005, 25, 793–801. [Google Scholar] [CrossRef]
- Bušs, K. Fundamentals of Forest Classification in Latvia SSR; LRZTIPI: Rīga, Latvia, 1976; Volume 24. (In Latvian) [Google Scholar]
- Buchwald, E. A hierarchical terminology for more or less natural forests in relation to sustainable management and biodiversity conservation. In Proceedings of the Third Expert Meeting on Harmonizing Forest-Related Definitions Food and Agriculture Organization of the United Nations, Rome, Italy, 17–19 January 2005; pp. 111–127. [Google Scholar]
- McGullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; CRC Press: New York, NY, USA, 1989; p. 532. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 12 December 2019).
- Granhus, A.; Metslaid, M.; von Lupke, N. Effects of nutrient supply on understory Picea abies seedling growth in partially cut stands in south-east Norway. Forestry 2016, 89, 182–190. [Google Scholar] [CrossRef]
- Bergh, J.; Linder, S.; Lundmark, T.; Elfving, B. The effect of water and nutrient availability on the productivity of Norway spruce in northern and southern Sweden. For. Ecol. Manag. 1999, 119, 51–62. [Google Scholar] [CrossRef]
- Zviedris, A. Norway Spruce and Spruce Forests in Latvian SSR; Latvijas PSR Zinātņu Akadēmijas Izdevniecība: Rīga, Latvia, 1960; p. 239. (In Latvian) [Google Scholar]
- Żywiec, M.; Ledwoń, M. Spatial and temporal patterns of rowan (Sorbus aucuparia L.) regeneration in West Carpathian subalpine spruce forest. Plant Ecol. 2008, 194, 283–291. [Google Scholar] [CrossRef]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics, Update Edition; John Wiley: New York, NY, USA, 1996; p. 544. [Google Scholar]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved—A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Petersson, L.; Holmström, E.; Lindbladh, M.; Felton, A. Tree species impact on understory vegetation: Vascular plant communities of Scots pine and Norway spruce managed stands in northern Europe. For. Ecol. Manag. 2019, 448, 330–345. [Google Scholar] [CrossRef]
- Messier, C.; Puttonen, P. Growth, allocation, and morphological responses of Betula pubescens and Betula pendula to shade in developing Scots pine stands. Can. J. For. Res. 1995, 25, 629–637. [Google Scholar] [CrossRef]
- Lutz, J.A.; Halpern, C.B. Tree mortality during early forest development: A long-term study of rates, causes, and consequences. Ecol. Monogr. 2006, 76, 257–275. [Google Scholar] [CrossRef]
- Harmon, M.E.; Pabst, R.J. Testing predictions of forest succession using long-term measurements: 100 yrs of observations in the Oregon Cascades. J. Veg. Sci. 2015, 26, 722–732. [Google Scholar] [CrossRef]
- Lībiete, Z.; Donis, J.; Jansons, J.; Zālītis, P. Growth potential of even-aged spruce forest. In Even-Aged Spruce Stands in Latvia; Jansons, J., Ed.; Daugavpils Universitātes Akadēmiskais Apgāds: Saule, Latvia, 2019; pp. 11–55. (In Latvian) [Google Scholar]
- De Römer, A.H.; Kneeshaw, D.D.; Bergeron, Y. Small Gap Dynamics in the Southern Boreal Forest of Eastern Canada: Do Canopy Gaps Influence Stand Development? J. Veg. Sci. 2007, 18, 815–826. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Aakala, T. Natural Forest Dynamics in Boreal Fennoscandia: A Review and Classification. Silva Fenn. 2011, 45, 823–841. [Google Scholar] [CrossRef]
- Tishler, M.; Tullus, T.; Tullus, A.; Jäärats, A.; Lutter, R.; Lundmark, T.; Tullus, H. Effects of shelterwood method and plant stock type on the early growth and survival of pine seedlings in regeneration stands under hemiboreal conditions. Scand. J. For. Res. 2020. [Google Scholar] [CrossRef]
- Eerikäinen, K.; Valkonen, S.; Saksa, T. Ingrowth, survival and height growth of small trees in uneven-aged Picea abies stands in southern Finland. For. Ecosyst. 2014, 1, 1–10. [Google Scholar] [CrossRef]
- Sonohat, G.; Balandier, P.; Ruchaud, F. Predicting solar radiation transmittance in the understory of even-aged coniferous stands in temperate forests. Ann. For. Sci. 2004, 61, 629–641. [Google Scholar] [CrossRef]
- Hale, S.E.; Edwards, C.; Mason, W.L.; Price, M.; Peace, A. Relationships between canopy transmittance and stand parameters in Sitka spruce and Scots pine stands in Britain. Forestry 2009, 82, 503–513. [Google Scholar] [CrossRef]
- Wallenius, T.H.; Kauhanen, H.; Herva, H.; Pennanen, J. Long fire cycle in northern boreal Pinus forests in Finnish Lapland. Can. J. For. Res. 2010, 40, 2027–2035. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Hokkanen, T.; Jarvinen, E.; Pukkala, T. Factors related to seedling growth in boreal Scots pine stand: A spatial analysis of a vegetation-soil system. Can. J. For. Res. 1993, 23, 2101–2109. [Google Scholar] [CrossRef]
- Donis, J.; Kitenberga, M.; Snepsts, G.; Matisons, R.; Zarins, J.; Jansons, A. The forest fire regime in Latvia during 1922–2014. Silva Fenn. 2017, 51, 1–15. [Google Scholar] [CrossRef]
- Nilsson, O.; Hjelm, K.; Nilsson, U. Early growth of planted Norway spruce and Scots pine after site preparation in Sweden. Scand. J. For. Res. 2019, 34, 678–688. [Google Scholar] [CrossRef]

| Dependent Variable | Explanatory Variables | Chi-Squared | Df | p-Value |
|---|---|---|---|---|
| Regeneration in general (all tree species) | Mean height | 22.2 | 1 | <0.001 |
| Dominant tree species | 20.3 | 1 | <0.001 | |
| Mean height * Dominant tree species | 19.1 | 1 | <0.001 | |
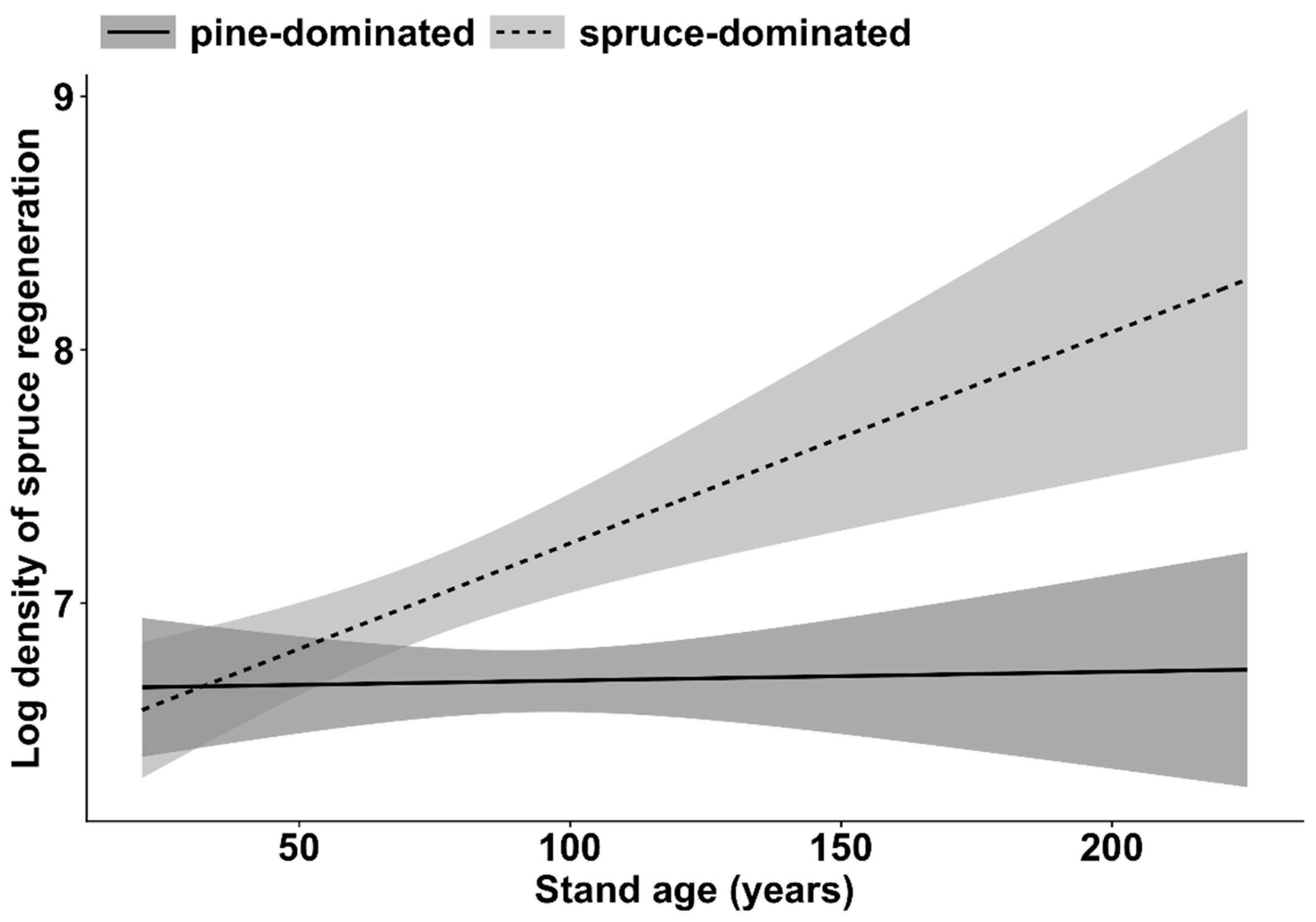
| Regeneration of Norway spruce | Stand age | 32.1 | 1 | <0.001 |
| Dominant tree species | 8.5 | 1 | <0.01 | |
| Stand age * Dominant tree species | 8.3 | 1 | <0.01 | |
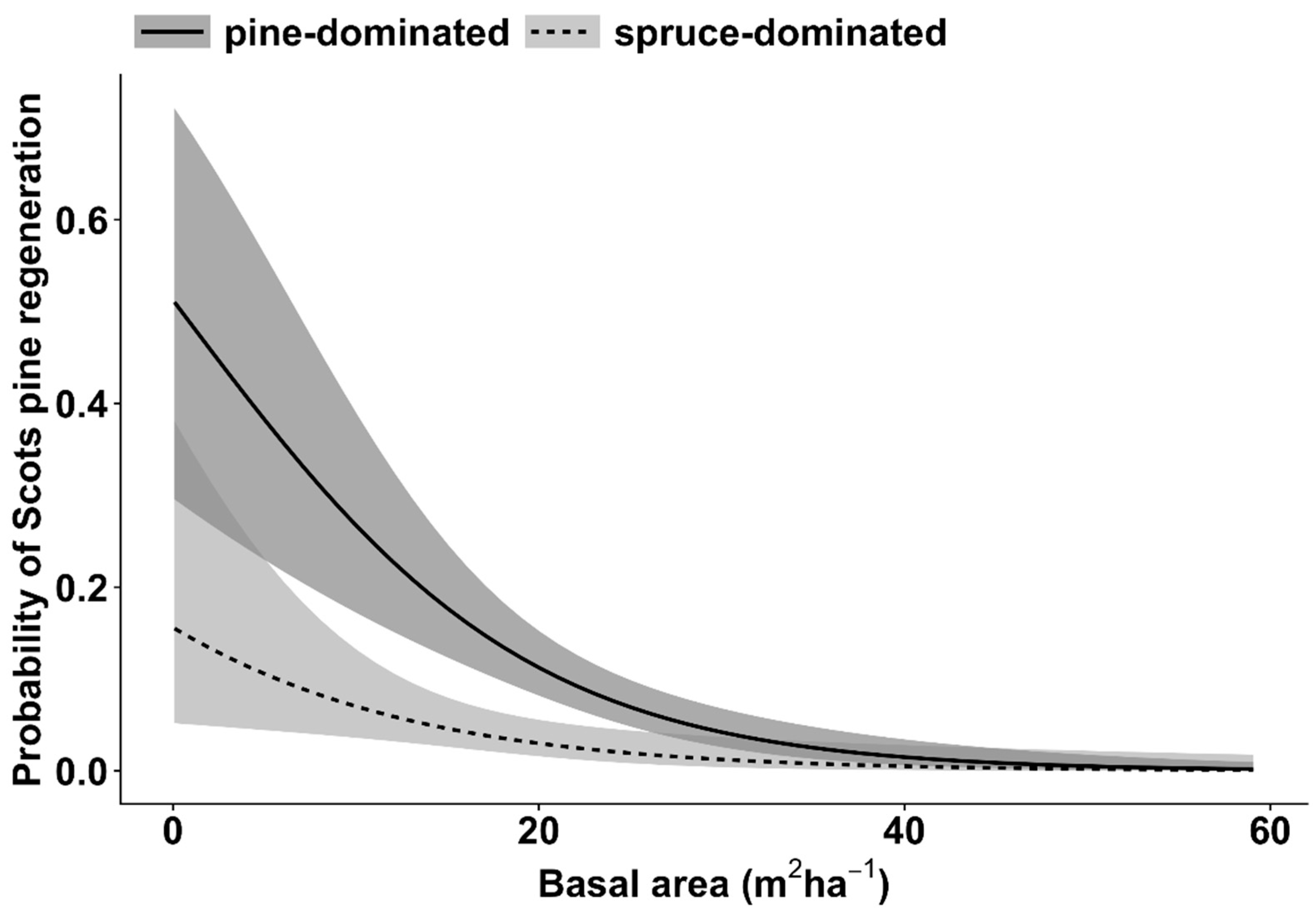
| Regeneration of Scots pine | Basal area | 7.6 | 1 | <0.01 |
| Dominant tree species | 5.3 | 1 | <0.05 | |
| Basal area * Dominant tree species | 0.1 | 1 | 0.6 |
| Dependent Variable | Explanatory Variables | Sum of Squares | Df | F-Value | p-Value |
|---|---|---|---|---|---|
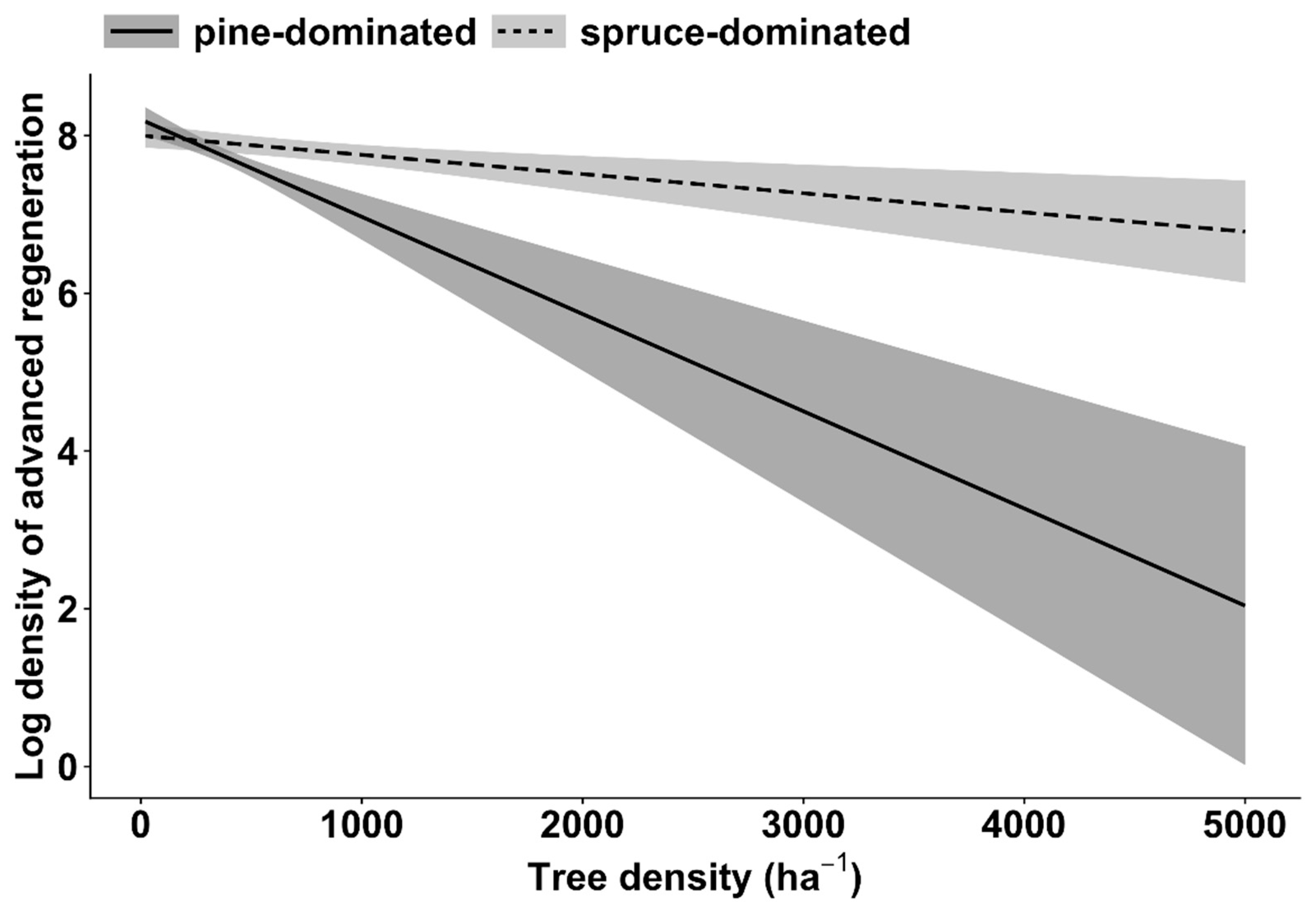
| Log regeneration density in general (all tree species) | Intercept | 11,945.1 | 1 | 10,827.8 | <0.001 |
| Stand density | 11.7 | 1 | 10.5 | <0.01 | |
| Dominant tree species | 3 | 1 | 2.7 | 0.09 | |
| Stand density * Dominant tree species | 19.7 | 1 | 17.8 | <0.001 | |
| Log regeneration density of Norway spruce | Intercept | 1600.13 | 1 | 1326.1 | <0.001 |
| Stand age | 17.77 | 1 | 14.7 | <0.001 | |
| Dominant tree species | 1.32 | 1 | 1.1 | 0.2 | |
| Stand age * Dominant tree species | 10.01 | 1 | 8.2 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luguza, S.; Snepsts, G.; Donis, J.; Desaine, I.; Baders, E.; Kitenberga, M.; Elferts, D.; Jansons, A. Advance Regeneration of Norway Spruce and Scots Pine in Hemiboreal Forests in Latvia. Forests 2020, 11, 215. https://doi.org/10.3390/f11020215
Luguza S, Snepsts G, Donis J, Desaine I, Baders E, Kitenberga M, Elferts D, Jansons A. Advance Regeneration of Norway Spruce and Scots Pine in Hemiboreal Forests in Latvia. Forests. 2020; 11(2):215. https://doi.org/10.3390/f11020215
Chicago/Turabian StyleLuguza, Solveiga, Guntars Snepsts, Janis Donis, Iveta Desaine, Endijs Baders, Mara Kitenberga, Didzis Elferts, and Aris Jansons. 2020. "Advance Regeneration of Norway Spruce and Scots Pine in Hemiboreal Forests in Latvia" Forests 11, no. 2: 215. https://doi.org/10.3390/f11020215
APA StyleLuguza, S., Snepsts, G., Donis, J., Desaine, I., Baders, E., Kitenberga, M., Elferts, D., & Jansons, A. (2020). Advance Regeneration of Norway Spruce and Scots Pine in Hemiboreal Forests in Latvia. Forests, 11(2), 215. https://doi.org/10.3390/f11020215







