Patterns of Carbon Sequestration in a Young Forest Ecosystem after Clear-Cutting
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. EC Measurements
2.3. Data Processing and Analysis
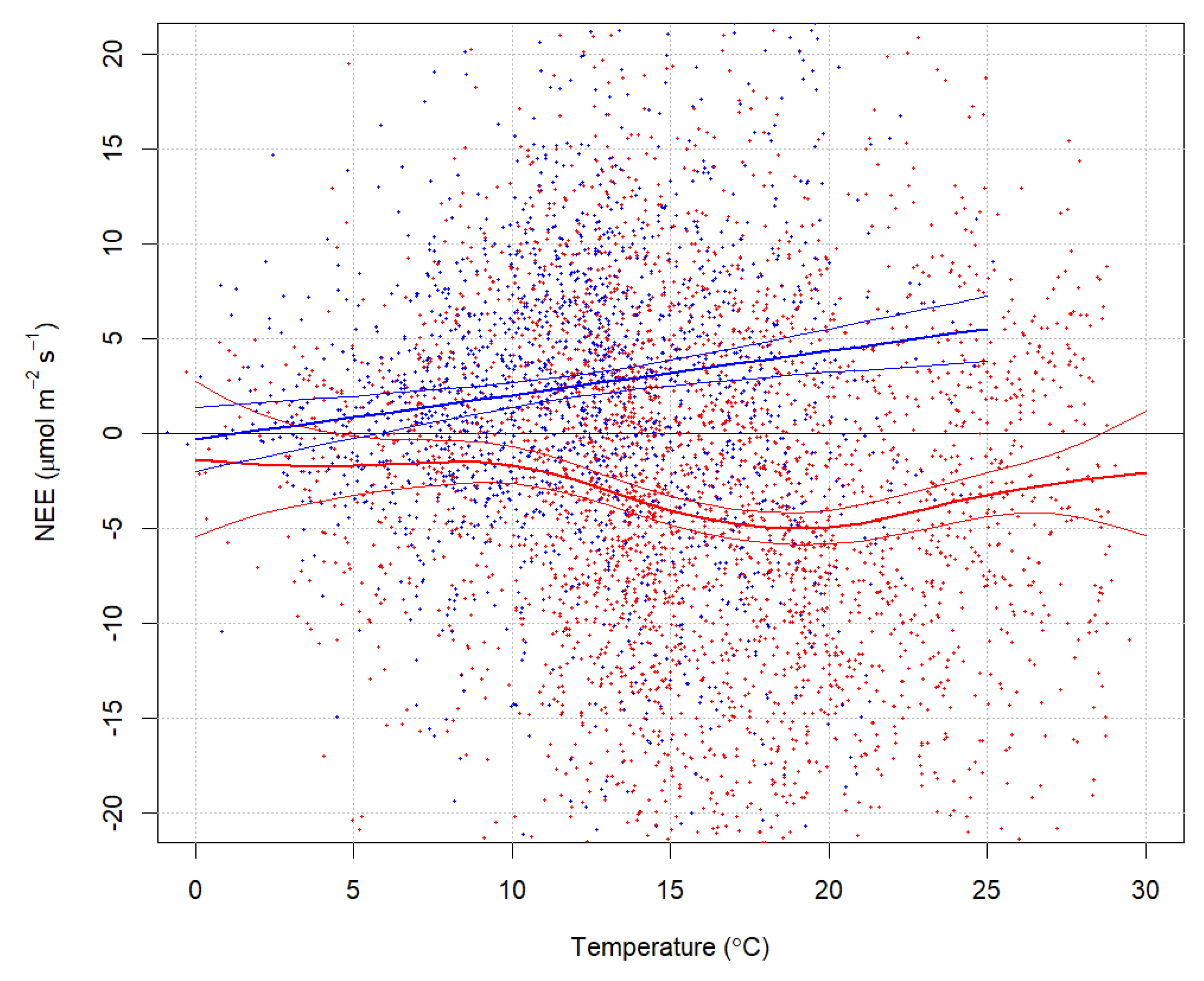
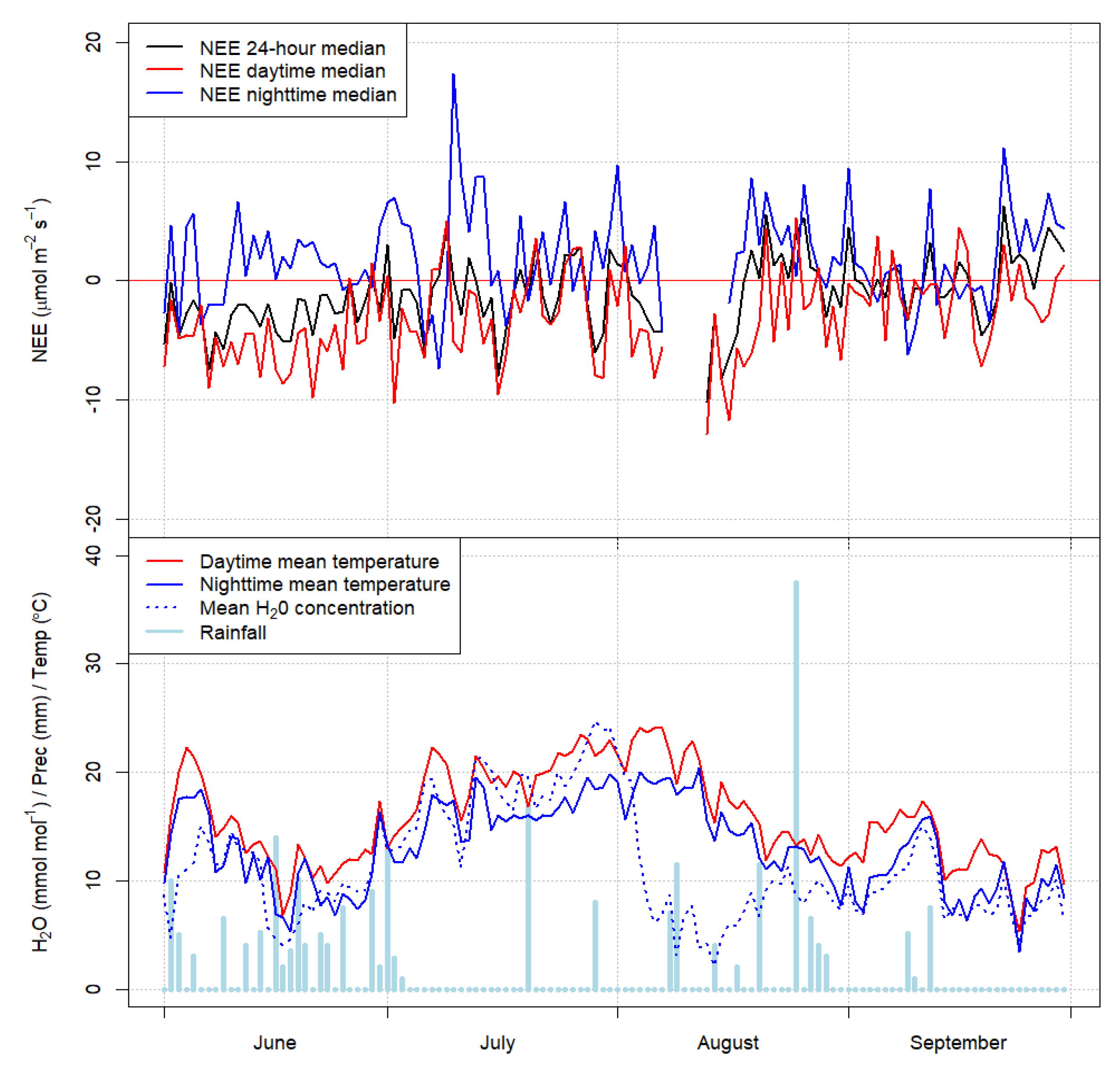
3. Results
Forest Ecosystem Carbon Balance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Franklin, J.F.; Spies, T.A.; Pelt, R.V.; Carey, A.B.; Thornburgh, D.A.; Berg, D.R.; Lindenmayer, D.B.; Harmon, M.E.; Keeton, W.S.; Shaw, D.C.; et al. Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. For. Ecol. Manag. 2002, 155, 399–423. [Google Scholar] [CrossRef]
- D’Andrea, E.; Micali, M.; Sicuriello, F.; Cammarano, M.; Ferlan, M.; Skudnik, M.; Mali, B.; Čater, M.; Simončič, P.; Cinti, B.D.; et al. Improving carbon sequestration and stocking as a function of forestry. Ital. J. Agron. 2016, 11, 56–60. [Google Scholar]
- IGBP Terrestrial Carbon Working Group. CLIMATE: The Terrestrial Carbon Cycle: Implications for the Kyoto Protocol. Science 1998, 280, 1393–1394. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Froelich, N.; Croft, H.; Chen, J.M.; Gonsamo, A.; Staebler, R.M. Trends of carbon fluxes and climate over a mixed temperate–boreal transition forest in southern Ontario, Canada. Agric. For. Meteorol. 2015, 211–212, 72–84. [Google Scholar] [CrossRef]
- Kolari, P.; Pumpanen, J.; Rannik, U.; Ilvesniemi, H.; Hari, P.; Berninger, F. Carbon balance of different aged Scots pine forests in Southern Finland. Glob. Chang. Biol. 2004, 10, 1106–1119. [Google Scholar] [CrossRef]
- Amiro, B.D.; Barr, A.G.; Barr, J.G.; Black, T.A.; Bracho, R.; Brown, M.; Chen, J.; Clark, K.L.; Davis, K.J.; Desai, A.R.; et al. Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J. Geophys. Res. Biogeosci. 2010, 115, G00K02. [Google Scholar] [CrossRef]
- Tang, X.; Li, H.; Ma, M.; Yao, L.; Peichl, M.; Arain, A.; Xu, X.; Goulden, M. How do disturbances and climate effects on carbon and water fluxes differ between multi-aged and even-aged coniferous forests? Sci. Total Environ. 2017, 599–600, 1583–1597. [Google Scholar] [CrossRef]
- Teets, A.; Fraver, S.; Hollinger, D.Y.; Weiskittel, A.R.; Seymour, R.S.; Richardson, A.D. Linking annual tree growth with eddy-flux measures of net ecosystem productivity across twenty years of observation in a mixed conifer forest. Agric. For. Meteorol. 2018, 249, 479–487. [Google Scholar] [CrossRef]
- Grant, R.F.; Barr, A.G.; Black, T.A.; Gaumont-Guay, D.; Iwashita, H.; Kidson, J.; McCAUGHEY, H.; Morgenstern, K.; Murayama, S.; Nesic, Z.; et al. Net ecosystem productivity of boreal jack pine stands regenerating from clearcutting under current and future climates. Glob. Chang. Biol. 2007, 13, 1423–1440. [Google Scholar] [CrossRef]
- Köster, K.; Köster, E.; Orumaa, A.; Parro, K.; Jõgiste, K.; Berninger, F.; Pumpanen, J.; Metslaid, M. How Time since Forest Fire Affects Stand Structure, Soil Physical-Chemical Properties and Soil CO2 Efflux in Hemiboreal Scots Pine Forest Fire Chronosequence? Forests 2016, 7, 201. [Google Scholar] [CrossRef]
- Parro, K.; Koster, K.; Jogiste, K.; Seglins, K.; Sims, A.; Stanturf, J.A.; Metslaid, M. Impact of post-fire management on soil respiration, carbon and nitrogen content in a managed hemiboreal forest. J. Environ. Manag. 2019, 233, 371–377. [Google Scholar] [CrossRef]
- Odum, E.P. An understanding of ecological succession provides a basis for resolving man’s conflict with nature. Sustainability 2005, 164, 9. [Google Scholar]
- Peltzer, D.A.; Bast, M.L.; Wilson, S.D.; Gerry, A.K. Plant diversity and tree responses following contrasting disturbances in boreal forest. For. Ecol. Manag. 2000, 127, 191–203. [Google Scholar] [CrossRef]
- Zha, T.; Barr, A.G.; Black, T.A.; McCaughey, J.H.; Bhatti, J.; Hawthorne, I.; Krishnan, P.; Kidston, J.; Saigusa, N.; Shashkov, A.; et al. Carbon sequestration in boreal jack pine stands following harvesting. Glob. Chang. Biol. 2009, 15, 1475–1487. [Google Scholar] [CrossRef]
- Aguilos, M.; Takagi, K.; Liang, N.; Ueyama, M.; Fukuzawa, K.; Nomura, M.; Kishida, O.; Fukazawa, T.; Takahashi, H.; Kotsuka, C.; et al. Dynamics of ecosystem carbon balance recovering from a clear-cutting in a cool-temperate forest. Agric. For. Meteorol. 2014, 197, 26–39. [Google Scholar] [CrossRef]
- Frank, D.; Reichstein, M.; Bahn, M.; Thonicke, K.; Frank, D.; Mahecha, M.D.; Smith, P.; van der Velde, M.; Vicca, S.; Babst, F.; et al. Effects of climate extremes on the terrestrial carbon cycle: Concepts, processes and potential future impacts. Glob. Chang. Biol. 2015, 21, 2861–2880. [Google Scholar] [CrossRef] [PubMed]
- Baldocchi, D.; Chu, H.; Reichstein, M. Inter-annual variability of net and gross ecosystem carbon fluxes: A review. Agric. For. Meteorol. 2018, 249, 520–533. [Google Scholar] [CrossRef]
- Humphreys, E.R.; Andrew Black, T.; Morgenstern, K.; Li, Z.; Nesic, Z. Net ecosystem production of a Douglas-fir stand for 3 years following clearcut harvesting. Glob. Chang. Biol. 2005, 11, 450–464. [Google Scholar] [CrossRef]
- Tang, J.; Baldocchi, D.D.; Xu, L. Tree photosynthesis modulates soil respiration on a diurnal time scale. Glob. Chang. Biol. 2005, 11, 1298–1304. [Google Scholar] [CrossRef]
- Grace, J. Understanding and managing the global carbon cycle. J. Ecol. 2004, 92, 189–202. [Google Scholar] [CrossRef]
- Wharton, S.; Schroeder, M.; Paw, U.K.T.; Falk, M.; Bible, K. Turbulence considerations for comparing ecosystem exchange over old-growth and clear-cut stands for limited fetch and complex canopy flow conditions. Agric. For. Meteorol. 2009, 149, 1477–1490. [Google Scholar] [CrossRef]
- Baldocchi, D.D. Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: Past, present and future. Glob. Chang. Biol. 2003, 9, 479–492. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Y.; Yu, G.; Zhou, G.; Zhang, L.; Li, K.; Tan, Z.; Sha, L. Seasonal and inter-annual variations in net ecosystem exchange of two old-growth forests in southern China. Agric. For. Meteorol. 2013, 182–183, 257–265. [Google Scholar] [CrossRef]
- Nicolini, G.; Aubinet, M.; Feigenwinter, C.; Heinesch, B.; Lindroth, A.; Mamadou, O.; Moderow, U.; Mölder, M.; Montagnani, L.; Rebmann, C.; et al. Impact of CO2 storage flux sampling uncertainty on net ecosystem exchange measured by eddy covariance. Agric. For. Meteorol. 2018, 248, 228–239. [Google Scholar] [CrossRef]
- Carrara, A.; Kowalski, A.S.; Neirynck, J.; Janssens, I.A.; Yuste, J.C.; Ceulemans, R. Net ecosystem CO2 exchange of mixed forest in Belgium over 5 years. Agric. For. Meteorol. 2003, 119, 209–227. [Google Scholar] [CrossRef]
- Sulman, B.N.; Roman, D.T.; Scanlon, T.M.; Wang, L.; Novick, K.A. Comparing methods for partitioning a decade of carbon dioxide and water vapor fluxes in a temperate forest. Agric. For. Meteorol. 2016, 226–227, 229–245. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Olson, R.; Anthoni, P.; Aubinet, M.; Bernhofer, C.; Burba, G.; Ceulemans, R.; Clement, R.; Dolman, H.; et al. Gap filling strategies for defensible annual sums of net ecosystem exchange. Agric. For. Meteorol. 2001, 107, 43–69. [Google Scholar] [CrossRef]
- Jensen, R.; Herbst, M.; Friborg, T. Direct and indirect controls of the interannual variability in atmospheric CO2 exchange of three contrasting ecosystems in Denmark. Agric. For. Meteorol. 2017, 233, 12–31. [Google Scholar] [CrossRef]
- Ilvesniemi, H.; Forsius, M.; Finér, L.; Holmberg, M.; Lepistö, A.; Piirainen, S.; Pumpanen, J.; Rankinen, K.; Starr, M.; Tamminen, P.; et al. Carbon and nitrogen storages and fluxes in Finnish forest ecosystems. Underst. Glob. Syst. 2002, 14, 69–82. [Google Scholar]
- Oren, R.; Hsieh, C.-I.; Stoy, P.; Albertson, J.; Mccarthy, H.R.; Harrell, P.; Katul, G.G. Estimating the uncertainty in annual net ecosystem carbon exchange: Spatial variation in turbulent fluxes and sampling errors in eddy-covariance measurements. Glob. Chang. Biol. 2006, 12, 883–896. [Google Scholar] [CrossRef]
- Lõhmus, E. Eesti Metsakasvukohatüübid; Eesti NSV Metsamajanduse ja Looduskaitse Ministeerium, Eesti NSV Agrotööstuskoondise Info-ja Juurutusvalitsus: Tallinn, Estonia, 1984; pp. 43–44. (In Estonian) [Google Scholar]
- Burba, G.; Madsen, R.; Feese, K. Eddy Covariance Method for CO2 Emission Measurements in CCUS Applications: Principles, Instrumentation and Software. Energy Procedia 2013, 40, 329–336. [Google Scholar] [CrossRef]
- Humphreys, E.R.; Black, T.A.; Morgenstern, K.; Cai, T.; Drewitt, G.B.; Nesic, Z.; Trofymow, J.A. Carbon dioxide fluxes in coastal Douglas-fir stands at different stages of development after clearcut harvesting. Agric. For. Meteorol. 2006, 140, 6–22. [Google Scholar] [CrossRef]
- Vickers, D.; Mahrt, L. Quality Control and Flux Sampling Problems for Tower and Aircraft Data. J. Atmos. Ocean. Technol. 1997, 14, 15. [Google Scholar] [CrossRef]
- Moncrieff, J.; Valentini, R.; Greco, S.; Guenther, S.; Ciccioli, P. Trace gas exchange over terrestrial ecosystems: Methods and perspectives in micrometeorology. J. Exp. Bot. 1997, 48, 1133–1142. [Google Scholar] [CrossRef]
- Jaksic, V.; Kiely, G.; Albertson, J.; Oren, R.; Katul, G.; Leahy, P.; Byrne, K.A. Net ecosystem exchange of grassland in contrasting wet and dry years. Agric. For. Meteorol. 2006, 139, 323–334. [Google Scholar] [CrossRef]
- Foken, T.; Gröockede, M.; Mauder, M. Post-field data quality control. In Handbook of Micrometeorology: A Guide for Surface Flux Measurements and Analysis; Lee, X., Ed.; Kluwer: Dordrecht, The Netherlands, 2004; pp. 181–208. [Google Scholar]
- Iglewicz, B.; Hoaglin, D.C. How to Detect and Handle Outliers; ASQC basic references in quality control; ASQC Quality Press: Milwaukee, WI, USA, 1993; ISBN 978-0-87389-247-6. [Google Scholar]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Chang. Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Kljun, N.; Calanca, P.; Rotach, M.W.; Schmid, H.P. A Simple Parameterisation for Flux Footprint Predictions. Bound. Layer Meteorol. 2004, 112, 503–523. [Google Scholar] [CrossRef]
- Kljun, N.; Calanca, P.; Rotach, M.W.; Schmid, H.P. A simple two-dimensional parameterisation for Flux Footprint Prediction (FFP). Geosci. Model Dev. 2015, 8, 3695–3713. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R.; Texts in statistical science; Chapman & Hall/CRC: Boca Raton, FL, USA, 2006; ISBN 978-1-58488-474-3. [Google Scholar]
- Uri, V.; Kukumägi, M.; Aosaar, J.; Varik, M.; Becker, H.; Aun, K.; Krasnova, A.; Morozov, G.; Ostonen, I.; Mander, Ü.; et al. The carbon balance of a six-year-old Scots pine (Pinus sylvestris L.) ecosystem estimated by different methods. For. Ecol. Manag. 2019, 433, 248–262. [Google Scholar] [CrossRef]
- Chen, B.; Arain, M.A.; Khomik, M.; Trofymow, J.A.; Grant, R.F.; Kurz, W.A.; Yeluripati, J.; Wang, Z. Evaluating the impacts of climate variability and disturbance regimes on the historic carbon budget of a forest landscape. Agric. For. Meteorol. 2013, 180, 265–280. [Google Scholar] [CrossRef]
- Amiro, B.D.; Barr, A.G.; Black, T.A.; Iwashita, H.; Kljun, N.; McCaughey, J.H.; Morgenstern, K.; Murayama, S.; Nesic, Z.; Orchansky, A.L.; et al. Carbon, energy and water fluxes at mature and disturbed forest sites, Saskatchewan, Canada. Agric. For. Meteorol. 2006, 136, 237–251. [Google Scholar] [CrossRef]
- Payeur-Poirier, J.-L.; Coursolle, C.; Margolis, H.A.; Giasson, M.-A. CO2 fluxes of a boreal black spruce chronosequence in eastern North America. Agric. For. Meteorol. 2012, 153, 94–105. [Google Scholar] [CrossRef]
- Coursolle, C.; Giasson, M.-A.; Margolis, H.A.; Bernier, P.Y. Moving towards carbon neutrality: CO2 exchange of a black spruce forest ecosystem during the first 10 years of recovery after harvest. Can. J. For. Res. 2012, 42, 1908–1918. [Google Scholar] [CrossRef]
- Ney, P.; Graf, A.; Bogena, H.; Diekkrueger, B.; Druee, C.; Esser, O.; Heinemann, G.; Klosterhalfen, A.; Pick, K.; Puetz, T.; et al. CO2 fluxes before and after partial deforestation of a Central European spruce forest. Agric. For. Meteorol. 2019, 274, 61–74. [Google Scholar] [CrossRef]
- Rannik, Ü.; Altimir, N.; Raittila, J.; Suni, T.; Gaman, A.; Hussein, T.; Hölttä, T.; Lassila, H.; Latokartano, M.; Lauri, A.; et al. Fluxes of carbon dioxide and water vapour over Scots pine forest and clearing. Agric. For. Meteorol. 2002, 111, 187–202. [Google Scholar] [CrossRef]
- Law, B.E.; Ryan, M.G.; Anthoni, P.M. Seasonal and annual respiration of a ponderosa pine ecosystem. Glob. Chang. Biol. 1999, 5, 169–182. [Google Scholar] [CrossRef]
- Harmon, M.E.; Bond-Lamberty, B.; Tang, J.; Vargas, R. Heterotrophic respiration in disturbed forests: A review with examples from North America. J. Geophys. Res. 2011, 116, G00K04. [Google Scholar] [CrossRef]
- Goulden, M.L.; McMillan, A.M.S.; Winston, G.C.; Rocha, A.V.; Manies, K.L.; Harden, J.W.; Bond-Lamberty, B.P. Patterns of NPP, GPP, respiration, and NEP during boreal forest succession. Glob. Chang. Biol. 2011, 17, 855–871. [Google Scholar] [CrossRef]
- Niu, S.; Fu, Z.; Luo, Y.; Stoy, P.C.; Keenan, T.F.; Poulter, B.; Zhang, L.; Piao, S.; Zhou, X.; Zheng, H.; et al. Interannual variability of ecosystem carbon exchange: From observation to prediction. Glob. Ecol. Biogeogr. 2017, 26, 1225–1237. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Tenhunen, J.; Aubinet, M.; Bakwin, P.; Berbigier, P.; Bernhofer, C.; Burba, G.; Clement, R.; Davis, K.J.; et al. Seasonality of ecosystem respiration and gross primary production as derived from FLUXNET measurements. Agric. For. Meteorol. 2002, 113, 53–74. [Google Scholar] [CrossRef]
- Schulze, E.D.; Lloyd, J.; Kelliher, F.M.; Wirth, C.; Rebmann, C.; Lühker, B.; Mund, M.; Knohl, A.; Milyukova, I.M.; Schulze, W.; et al. Productivity of forests in the Eurosiberian boreal region and their potential to act as a carbon sink-a synthesis. Glob. Chang. Biol. 1999, 5, 703–722. [Google Scholar] [CrossRef]
- Krasnova, A.; Kukumägi, M.; Mander, Ü.; Torga, R.; Krasnov, D.; Noe, S.M.; Ostonen, I.; Püttsepp, Ü.; Killian, H.; Uri, V.; et al. Carbon exchange in a hemiboreal mixed forest in relation to tree species composition. Agric. For. Meteorol. 2019, 275, 11–23. [Google Scholar] [CrossRef]
- Chi, J.; Nilsson, M.B.; Kljun, N.; Wallerman, J.; Fransson, J.E.S.; Laudon, H.; Lundmark, T.; Peichl, M. The carbon balance of a managed boreal landscape measured from a tall tower in northern Sweden. Agric. For. Meteorol. 2019, 274, 29–41. [Google Scholar] [CrossRef]
- Jurán, S.; Edwards-Jonášová, M.; Cudlín, P.; Zapletal, M.; Šigut, L.; Grace, J.; Urban, O. Prediction of ozone effects on net ecosystem production of Norway spruce forest. iForest 2018, 11, 743–750. [Google Scholar] [CrossRef]
- Juráň, S.; Šigut, L.; Holub, P.; Fares, S.; Klem, K.; Grace, J.; Urban, O. Ozone flux and ozone deposition in a mountain spruce forest are modulated by sky conditions. Sci. Total Environ. 2019, 672, 296–304. [Google Scholar] [CrossRef]
- Oishi, A.C.; Miniat, C.F.; Novick, K.A.; Brantley, S.T.; Vose, J.M.; Walker, J.T. Warmer temperatures reduce net carbon uptake, but do not affect water use, in a mature southern Appalachian forest. Agric. For. Meteorol. 2018, 252, 269–282. [Google Scholar] [CrossRef]
- Jaagus, J.; Mändla, K. Climate change scenarios for Estonia based on climate models from the IPCC Fourth Assessment Report. Est. J. Earth Sci. 2014, 63, 166. [Google Scholar] [CrossRef]
- Jaagus, J.; Sepp, M.; Tamm, T.; Järvet, A.; Mõisja, K. Trends and regime shifts in climatic conditions and river runoff in Estonia during 1951–2015. Earth Syst. Dynam. 2017, 8, 963–976. [Google Scholar] [CrossRef]
- Kulmala, L.; Aaltonen, H.; Berninger, F.; Kieloaho, A.-J.; Levula, J.; Bäck, J.; Hari, P.; Kolari, P.; Korhonen, J.F.J.; Kulmala, M.; et al. Changes in biogeochemistry and carbon fluxes in a boreal forest after the clear-cutting and partial burning of slash. Agric. For. Meteorol. 2014, 188, 33–44. [Google Scholar] [CrossRef]
- Johnson, D.W.; Curtis, P.S. Effects of forest management on soil C and N storage: Meta analysis. For. Ecol. Manag. 2001, 140, 227–238. [Google Scholar] [CrossRef]
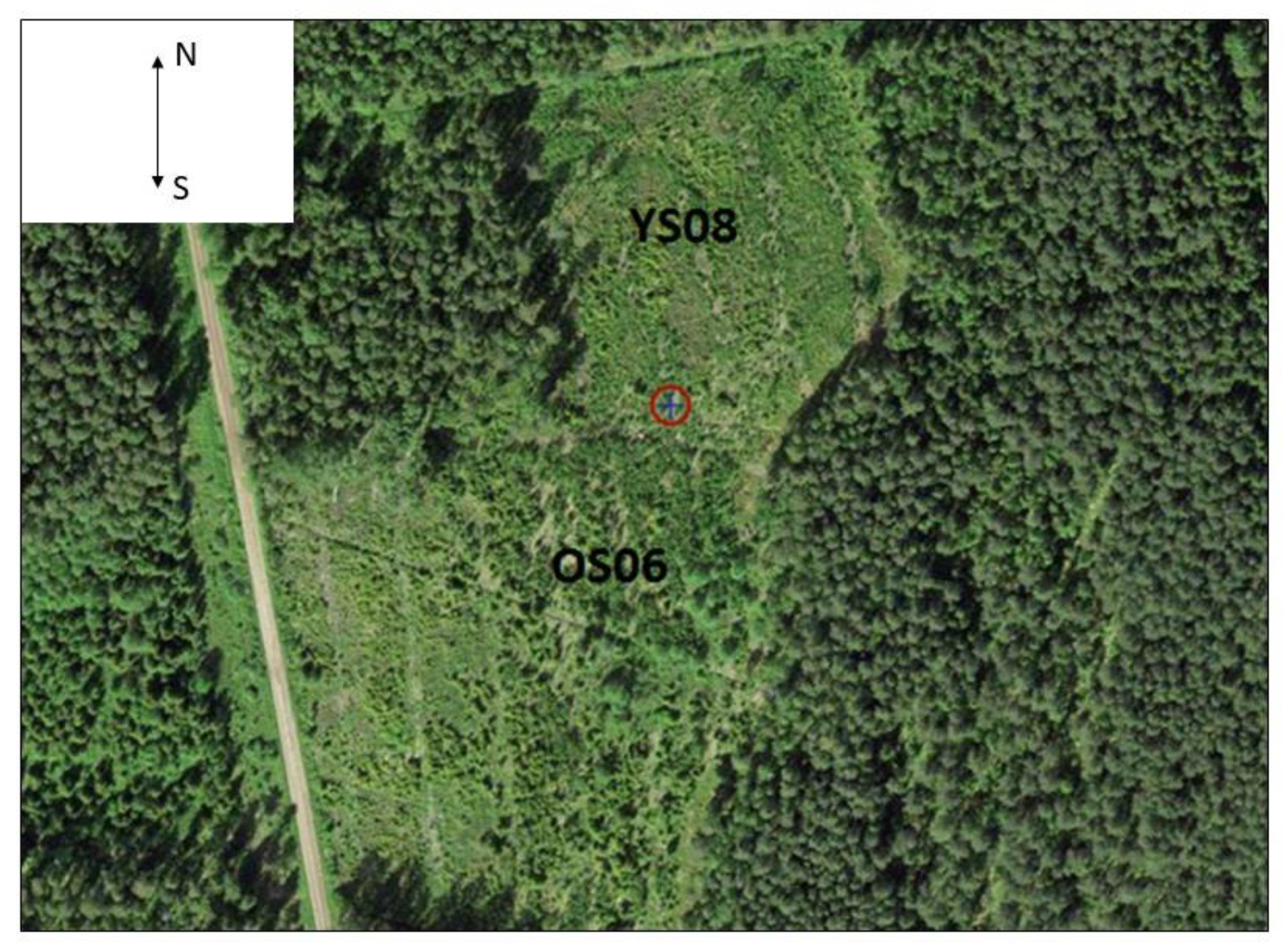


| YS08 | OS06 | |
|---|---|---|
| Stand age (years since clear-cutting) | 6 | 8 |
| Trees average height (m) | 1.3 | 2.1 |
| Area of the stand (ha) | 0.9 | 1.4 |
| Open wind directions (°) | 335–50 | 135–285 |
| Growing season length (days) | 121 (May–September) | 121 (May–September) |
| Dominant tree species after harvest (percent): | ||
| Silver birch (Betula pendula) | 58 | 29 |
| Norway spruce (Picea abies) | 36 | 53 |
| European aspen (Populus tremula) | 0 | 12 |
| Scots pine (Pinus sylvestris) | 6 | 6 |
| YS08 | OS06 | Average * | ||
|---|---|---|---|---|
| June | NEE | −4.89 | −4.45 | −4.60 |
| SD | 13.61 | 11.27 | 12.12 | |
| Data points | 372 | 708 | ||
| July | NEE | 0.96 | −3.55 | −1.17 |
| SD | 16.41 | 12.86 | 15.00 | |
| Data points | 613 | 547 | ||
| August | NEE | −0.66 | −0.81 | −0.77 |
| SD | 12.96 | 10.62 | 11.27 | |
| Data points | 231 | 658 | ||
| September | NEE | 0.23 | −0.45 | −0.25 |
| SD | 13.15 | 12.37 | 12.60 | |
| Data points | 323 | 783 | ||
| Seasonal average | NEE | −0.85 | −2.22 | −1.72 |
| SD | 14.78 | 11.90 | 13.04 | |
| Sum of data points | 1539 | 2696 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebane, S.; Jõgiste, K.; Kiviste, A.; Stanturf, J.A.; Metslaid, M. Patterns of Carbon Sequestration in a Young Forest Ecosystem after Clear-Cutting. Forests 2020, 11, 126. https://doi.org/10.3390/f11020126
Rebane S, Jõgiste K, Kiviste A, Stanturf JA, Metslaid M. Patterns of Carbon Sequestration in a Young Forest Ecosystem after Clear-Cutting. Forests. 2020; 11(2):126. https://doi.org/10.3390/f11020126
Chicago/Turabian StyleRebane, Sille, Kalev Jõgiste, Andres Kiviste, John A. Stanturf, and Marek Metslaid. 2020. "Patterns of Carbon Sequestration in a Young Forest Ecosystem after Clear-Cutting" Forests 11, no. 2: 126. https://doi.org/10.3390/f11020126
APA StyleRebane, S., Jõgiste, K., Kiviste, A., Stanturf, J. A., & Metslaid, M. (2020). Patterns of Carbon Sequestration in a Young Forest Ecosystem after Clear-Cutting. Forests, 11(2), 126. https://doi.org/10.3390/f11020126






