Influence of Heat Treatment and Tannin Impregnation on Boron Depletion and Wood Durability
Abstract
1. Introduction
- (1)
- Test boron fixation through heat treatment and tannin impregnation.
- (2)
- Investigate wood decay after leaching against brown-rot fungus Gloeophyllum trabeum in southern yellow pine and white-rot fungus Trametes versicolor in yellow-poplar;
- (3)
- Determine eastern subterranean termite (Reticulitermes flavipes) attack on heat-treated southern yellow pine modified with tannins/borates.
2. Materials and Methods
2.1. Wood Samples
2.2. Tannin Characterization
2.3. Thermal Modification
- m1 = initial oven-dry mass after impregnation, g;
- m2 = weight of samples after thermal modification, g.
2.4. Leaching Method
- Wafter imp. = weight after impregnation, g;
- Wod = oven-dry weight before impregnation, g;
- C = concentration of the solution;
- 0.8 = volume of the solution, L.
- m2 = oven-dry weight before leaching, g.
- m3 = oven-dry weight after leaching, g.
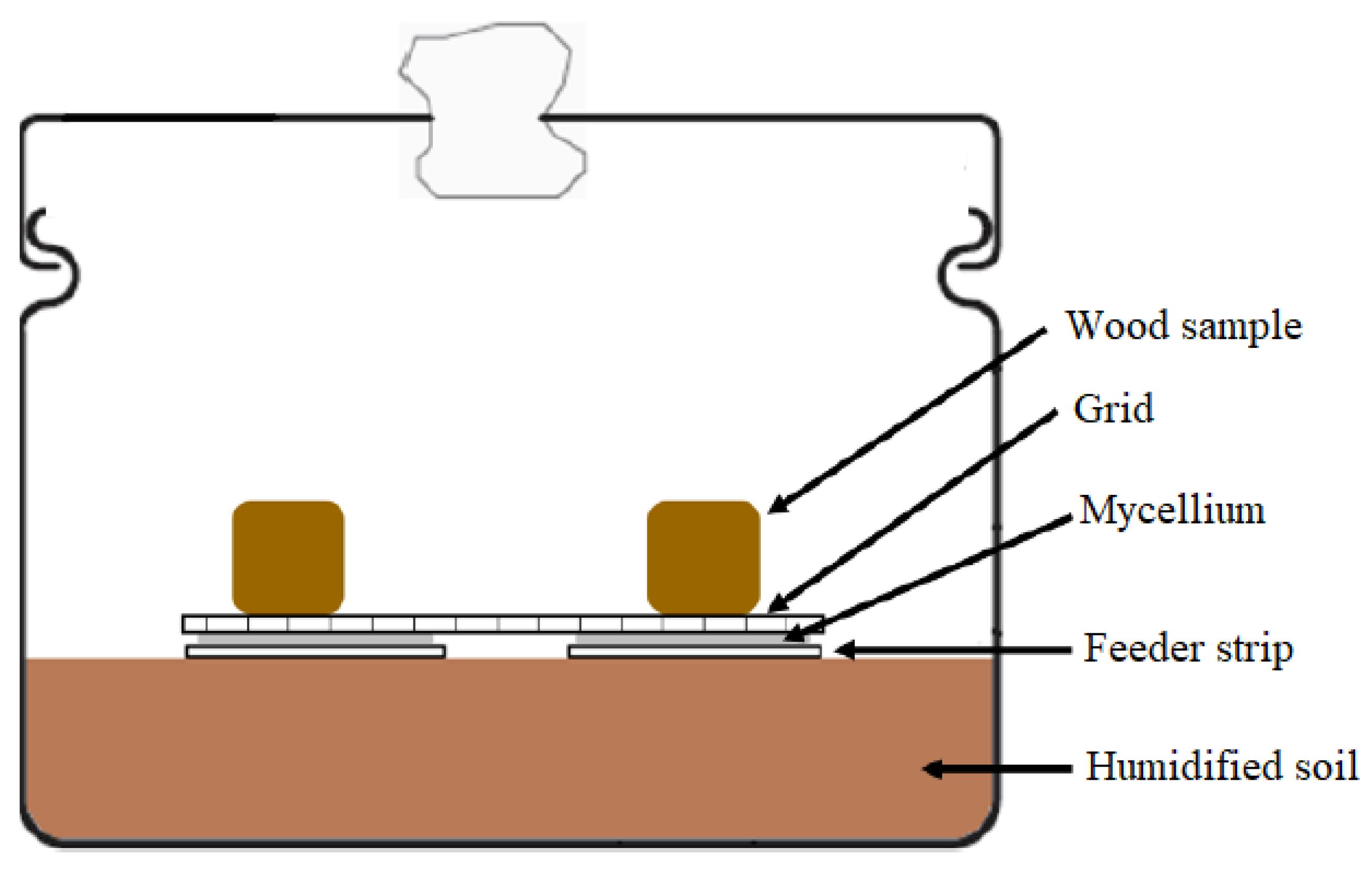
2.5. Fungal Resistance
2.6. Termite Resistance
- Omitted AWPA E1sections 6.4.2 and 8.1.4–8.16 pertaining to termite mortality following testing. Tunneling presence, majority termite position, and termite mortality were approximated as per American Society for Testing and Materials—ASTMD3345 [27] sections 12.2.2–12.2.4.
- Omitted the moisture content portion of AWPA E1 6.3.1 and 6.3.2 as bottles maintained in the chamber do not require this procedure. This allowed us to avoid disrupting termite activity during the course of the study.
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preservative Retention
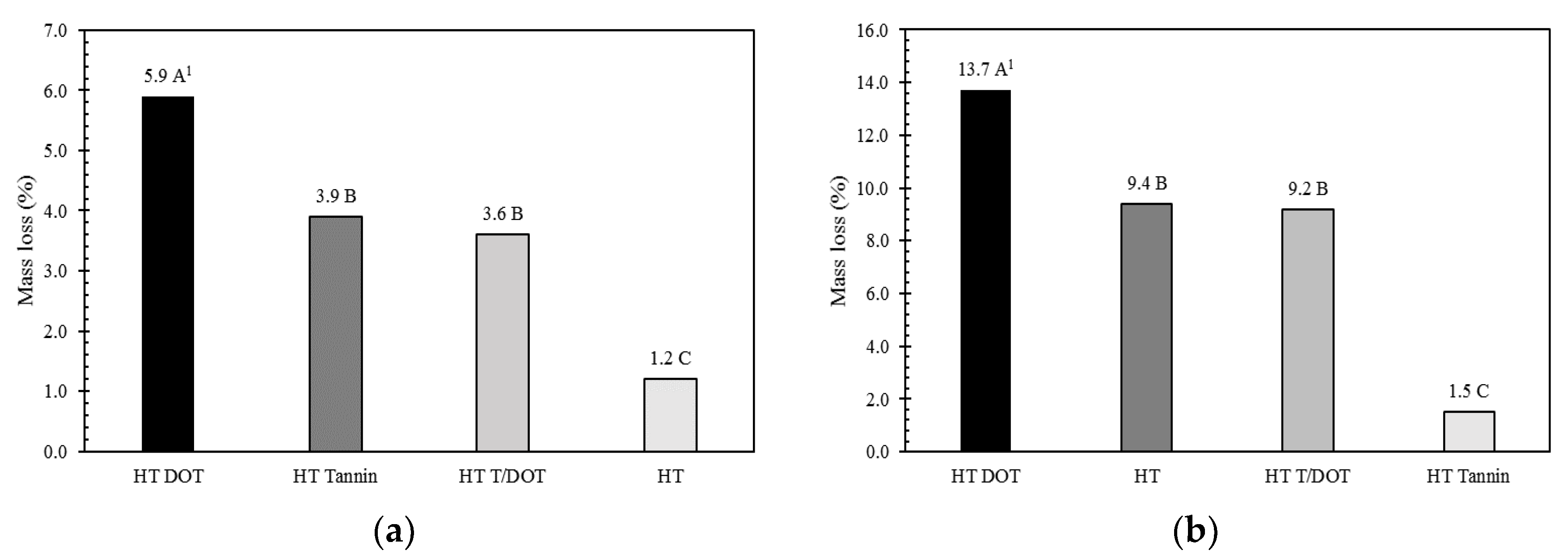
3.2. Mass Loss
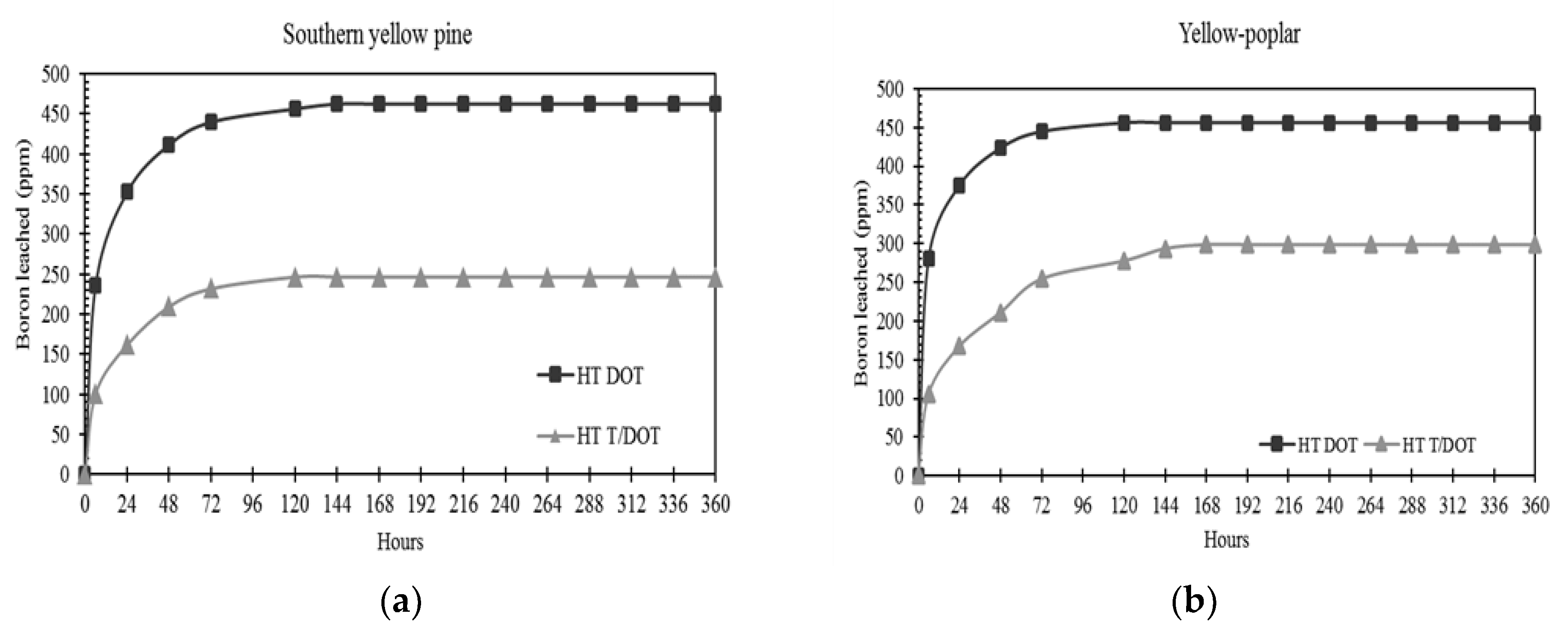
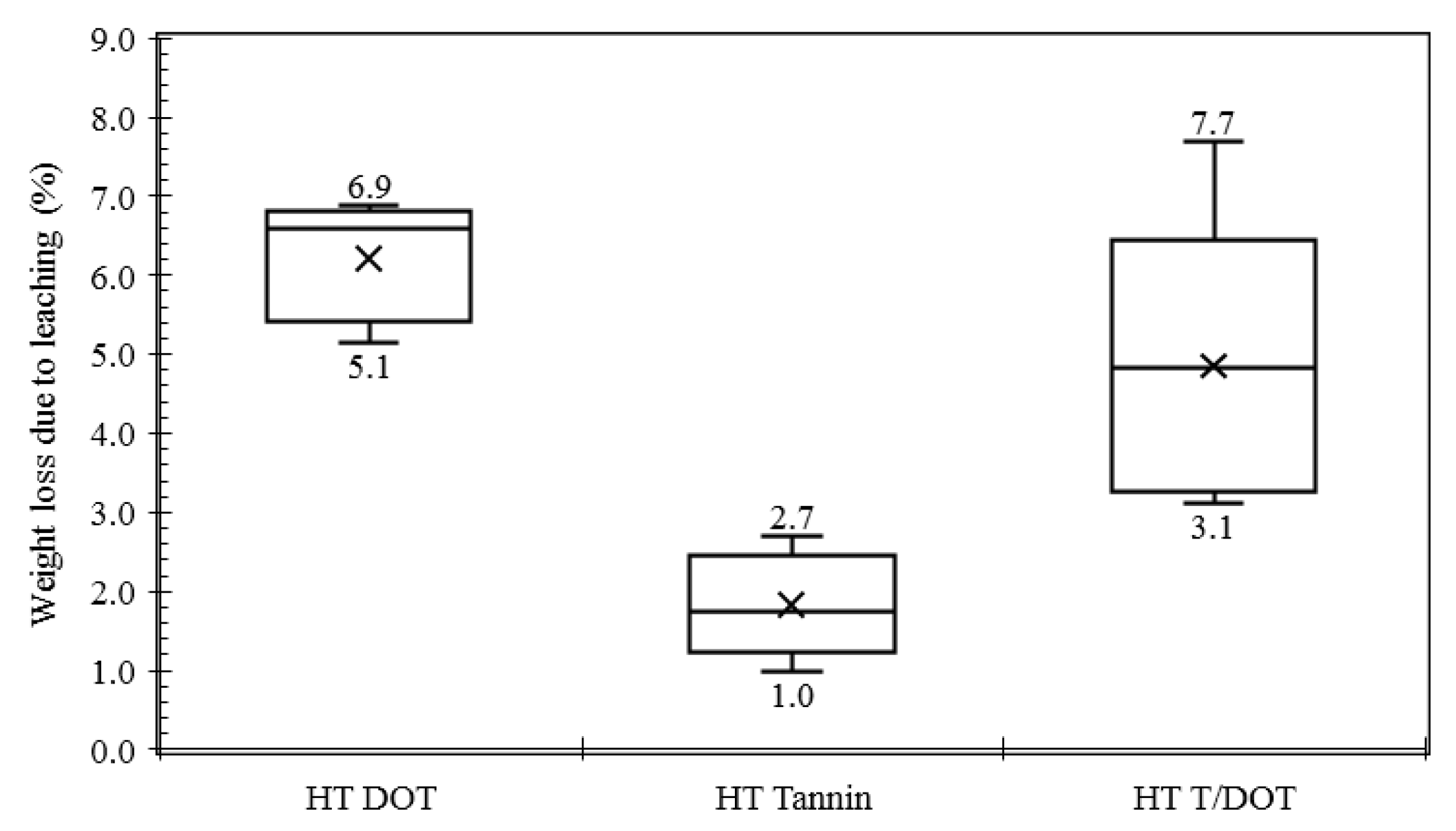
3.3. Boron Leaching Control
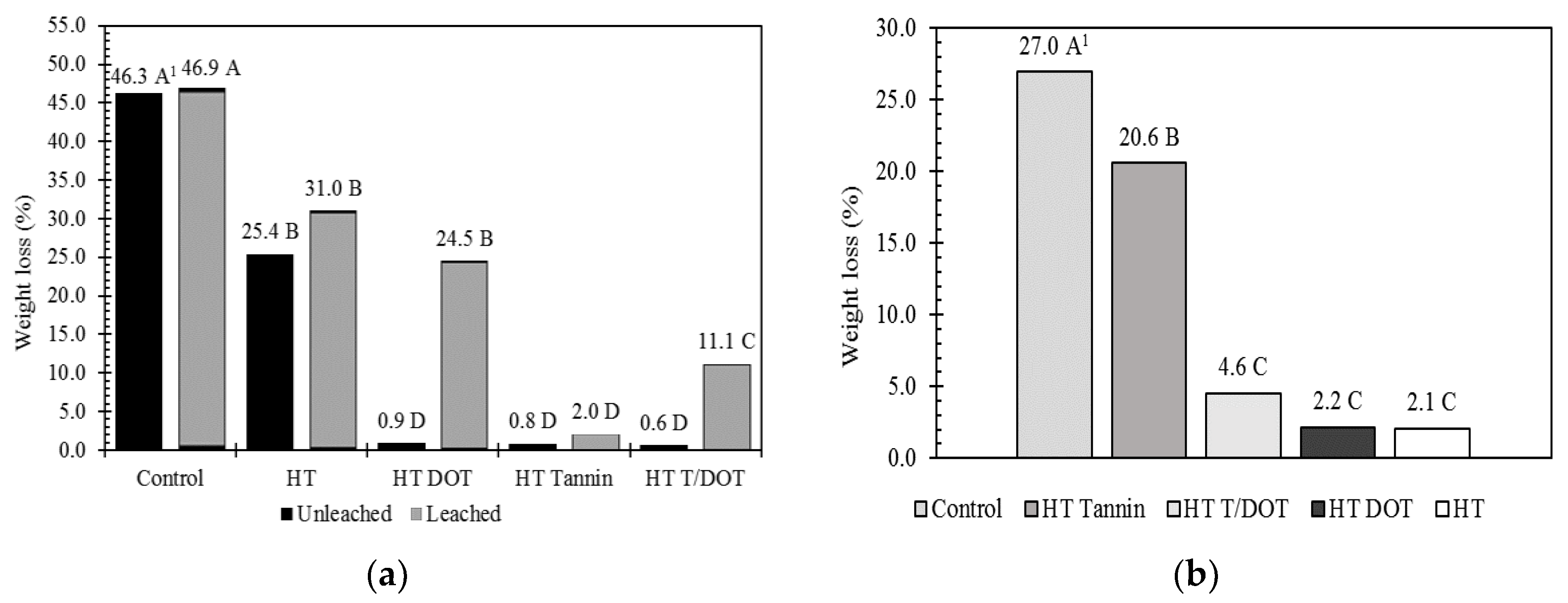
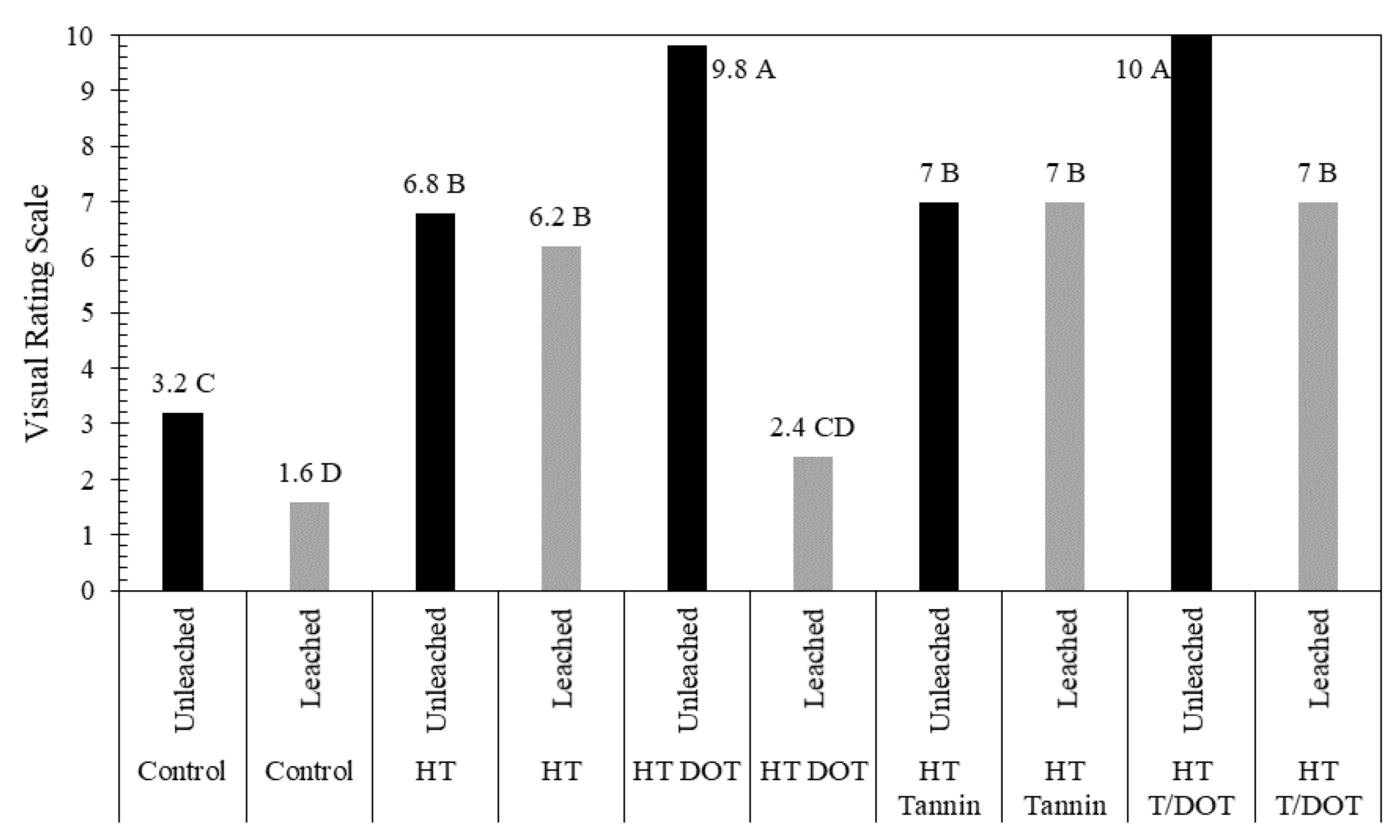
3.4. Fungi Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reinprecht, L. Wood Deterioration, Protection and Maintenance; Wiley Blackwell: Zvolen, Slovakia, 2016; 376p. [Google Scholar]
- Baysal, E.; Degirmentepe, S.; Simsek, H. Some surface properties of thermally modified Scots pine after artificial weathering. Maderas Cienc. Tecnol. 2014, 16, 355–364. [Google Scholar] [CrossRef]
- Esteves, B.M.; Pereira, H.M. Wood modification by heat treatment: A review. Bioresources 2009, 4, 370–404. [Google Scholar]
- Metsä-Kortelainen, S.; Paajanen, L.; Viitanen, H. Durability of thermally modified Norway spruce and Scots pine in above-ground conditions. Wood Mater. Sci. Eng. 2011, 6, 163–169. [Google Scholar] [CrossRef]
- Sivrikaya, H.; Ekinci, E.; Can, A.; Tasdelen, M.; Gokmen, K. Effect of heat treatment on the weathering and hardness properties of some wood species. In Proceedings of the 11th Meeting of the Northern European Network for Wood Sciences and Engineering (WSE), Poznan, Poland, 14–15 September 2015. [Google Scholar]
- Salman, S.; Petrissans, A.; Thevenon, M.F.; Dumarçay, S.; Gerardin, P. Decay and termites resistance of pine blocks impregnated with different additives and subjected to heat treatment. Eur. J. Wood Prod. 2016, 74, 37–42. [Google Scholar] [CrossRef]
- Morrell, J.J.; Love, C.S.; Freitas, C.M. Integrated Remedial Protection of Wood in Bridges. In Proceedings of the National Conference on Wood Transportation Structures, Madison, WI, USA, 23–25 October 1996. [Google Scholar]
- Ra, J.B.; Barnes, H.M.; Conners, T.E. Determination of boron diffusion coefficients in wood. Wood Fiber Sci. 2001, 33, 90–103. [Google Scholar]
- Obanda, D.N.; Shupe, T.F.; Barnes, H.M. Reducing leaching of boron-based wood preservatives—A review of research. Bioresour. Technol. 2008, 99, 7312–7322. [Google Scholar] [CrossRef]
- Caldeira, F. Boron in wood preservation a review in its physico-chemical aspects. Silva Lusit. 2010, 18, 179–196. [Google Scholar]
- Lebow, S.; Lebow, P.; Halverson, S. Penetration of Boron from Topically Applied Borate Solutions. For. Prod. J. 2010, 60, 13–22. [Google Scholar] [CrossRef]
- Salman, S.; Petrissans, A.; Thevenon, M.F.; Dumarçay, S.; Perrin, D.; Pollier, B.; Gerardin, P. Development of new wood treatments combining boron impregnation and thermo modification—Effect of additives on boron leachability. Eur. J. Wood Prod. 2014, 72, 355–365. [Google Scholar] [CrossRef]
- Hedley, M.; Page, D. Performance of boron treated radiata pine in above ground field tests in New Zealand. In Proceedings of the International Research Group on Wood Protection (IRG/WP 2006), Stockholm, Sweden, 18–22 June 2006. [Google Scholar]
- Hutter, F.G.; Lake, M.A.; Smith, D.L.; Robinson, P.H.; Bishop, F.E. Method for Treating Wood. U.S. Patent 2,194,827, 29 November 2004. [Google Scholar]
- Kartal, N.S.; Green, F. Leachability of boron from wood treated with natural and semi-synthetic polymers and calcium precipitating agent. Holz Roh Werkst. 2003, 61, 388–389. [Google Scholar] [CrossRef]
- Amburgey, T.L.; Freeman, M.H. An update on the efficacy and performance of a copper naphthenate—Borax preservative paste. In Proceedings of the International Conference on Utility Line Structures, FortCollins, CO, USA, 20–22 March 2000; pp. 33–37. [Google Scholar]
- Carr, J.M.; Duggan, P.J.; Humphrey, D.G.; Tyndall, E.M. Enhanced anti-fungal activity of the organo-soluble borate ester,tetra-n-butylammonium bis (ortho-hydroxymethylphenolato) borate. Aust. J. Chem. 2005, 58, 21–25. [Google Scholar] [CrossRef]
- Yalinkilic, M.K.; Gezer, E.D.; Takahashi, M.; Demirci, R.; Imamura, Y. Boron addition to non- or low-formaldehyde cross-linking reagents to enhance biological resistance and dimensional stability of wood. Holz Roh Werkst. 1999, 57, 351–357. [Google Scholar] [CrossRef]
- Yamaguchi, H. Silicic acid/boric acid complexes as ecologically friendly wood preservatives. For. Prod. J. 2005, 55, 88–92. [Google Scholar]
- Pizzi, A.; Baecker, A.W. A new boron fixation mechanism for non-toxic wood preservatives. Holzforschung 1996, 50, 507–510. [Google Scholar] [CrossRef]
- Tondi, G.; Wieland, S.; Wimmer, T.; Thevenon, M.F.; Pizzi, A.; Petutschnigg, A. Tannin-boron preservatives for wood buildings: Mechanical and fire properties. Eur. J. Wood Prod. 2012, 70, 689–696. [Google Scholar] [CrossRef]
- Zobel, B.J.; Van Buijtenen, J.P. Wood Variation—Its Causes and Control; Springer: Berlin, Germany, 1989; 363p. [Google Scholar]
- Peterson, C.; Wagner, T.; Mulrooney, J.; Shelton, T. Subterranean Termites; USDA Forest Service Home and Garden Bulletin; USDA: Starkville, MS, USA, 2006. [Google Scholar]
- Clausen, C.A. Biodeterioration of wood. In Wood Handbook: Wood As an Engineering Material; Ross, R., Ed.; Dept. of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2010; pp. 14.1–14.16. [Google Scholar]
- AWPA. Laboratory Method for Evaluating the Decay Resistance of Wood-Based Materials against Pure Basidiomycete Cultures: Soil/Block Test; Book of Standards, E10; American Wood Protection Association: Birmingham, AL, USA, 2017; 624p. [Google Scholar]
- AWPA. Laboratory Methods for Evaluating the Termite Resistance of Wood-Based Materials: Choice and No-Choice Tests; Book of Standards, E1; American Wood Protection Association: Birmingham, AL, USA, 2017; 624p. [Google Scholar]
- ASTM. Standard Test Method for Laboratory Evaluation of Wood and Other Cellulosic Materials for Resistance to Termites; ASTM D3345; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- AWPA. Commodity Section A: Sawn Products; Book of Standards, U1; American Wood Protection Association: Birmingham, AL, USA, 2017; 624p. [Google Scholar]
- Lebow, S.; Shupe, T.; Woodward, B.; Crawford, D.; Via, B. Formosan and nativesubterranean termite attack of pressure-treated SPF wood species exposed in Louisiana. Wood Fiber Sci. 2006, 38, 609–620. [Google Scholar]
- Siau, J.F. Transport process in wood. In Springer Series in Wood Science; Timell, T.E., Ed.; Springer: New York, NY, USA, 1984. [Google Scholar]
- Gérardin, P. New alternatives for wood preservation based on thermal and chemical modification of wood a review. Ann. For. Sci. 2016, 73, 559–570. [Google Scholar] [CrossRef]
- Bourgois, J.; Bartholin, M.; Guyonnet, R. Thermal treatment of wood: Analysis of the obtained product. Wood Sci. Technol. 1989, 23, 303–310. [Google Scholar] [CrossRef]
- Mohareb, A.; Sirmah, P.; Desharnais, L.; Dumarçay, S.; Pétrissans, M.; Gérardin, P. Effect of extractives on conferred and natural durability of Cupressus lusitanica heartwood. Ann. For. Sci. 2010, 67, 504. [Google Scholar] [CrossRef][Green Version]
- Özgenç, Ö.; Durmaz, S.; Boyaci, I.H.; Eksikocak, H. Determination of chemical changes in heat-treated wood using ATR-FTIR and FT-Raman spectrometry. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2017, 171, 395–400. [Google Scholar] [CrossRef]
- Lisperguer, J.; Saravia, Y.; Vergara, E. Structure and Thermal Behavior of Tannins from Acacia Dealbata Bark and Their Reactivity toward Formaldehyde. J. Chil. Chem. Soc. 2016, 61, 3188–3190. [Google Scholar] [CrossRef]
- Lebow, S.; Lebow, P.; Hirth, K.C. Technical note: Comparison of accelerated methods for evaluating leaching from preservative-treated wood. Wood Fiber Sci. 2017, 49, 93–104. [Google Scholar]
- Lebow, S. Leaching of Wood Preservative Components and Their Mobility in the Environment. Summary of Pertinent Literature; Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 1996; 36p. [Google Scholar]
- Slabbert, N. Plant polyphenols: Synthesis, properties, significance. In Plant Polyphenols: Synthesis, Properties; Hemingway, R.W., Laks, P.E., Eds.; Plenum Press: New York, NY, USA, 1992; pp. 421–436. [Google Scholar]
- Lekounougou, S.; Pétrissans, M.; Jacquot, J.P.; Gelhaye, E.; Gérardin, P. Effect of heat treatment on extracellular enzymatic activities involved in beech wood degradation by Trametes versicolor. Wood Sci. Technol. 2009, 43, 331–341. [Google Scholar] [CrossRef]
- Lekounougou, S.; Kocaefe, D. Bioresistance of thermally modified Populus tremuloides (North American Aspen) wood against four decay fungi. Int. Wood Prod. J. 2013, 4, 46–51. [Google Scholar] [CrossRef]
- Kapich, A.N.; Prior, B.A.; Botha, A.; Galkin, S.; Lundell, T.; Hatakka, A. Effect of lignocellulose-containing substrates on production of ligninolytic peroxidades in submerged cultures of Phanerochaete chrysosporium. Enzym. Microb. Technol. 2004, 34, 187–195. [Google Scholar] [CrossRef]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Deactivation of cellulases by phenols. Enzym. Microb. Technol. 2011, 48, 54–60. [Google Scholar] [CrossRef]
- Blanchette, R.A. Manganese accumulation in wood decayed by rot fungi. Phytopathology 1984, 74, 725–730. [Google Scholar] [CrossRef]
- Hatakka, A. Lignin-modifying enzymes from selected white-rot fungi: Production and role from in lignin degradation. FEMS Microbiol. Rev. 1994, 13, 125–135. [Google Scholar] [CrossRef]
- Tuor, U.; Winterhalter, K.; Fiechter, A. Enzymes of white–rot fungi involved in lignin degradation and ecological determinants for wood decay. J. Biotechnol. 1995, 41, 1–17. [Google Scholar] [CrossRef]
- Bianchi, S.; Kroslakova, I.; Janzon, R.; Mayer, I.; Saake, B.; Pichelin, F. Characterization of condensed tannins and carbohydrates in hot water bark extracts of European softwood species. Phytochemistry 2015, 120, 53–61. [Google Scholar] [CrossRef]
- Salman, S.; Thévenom, M.F.; Pétrissans, A.; Dumarçay, S.; Candelier, K.; Gérardin, P. Improvement of the durability of heat-treated wood against termites. Maderas. Cienc. Tecnol. 2017, 19, 317–328. [Google Scholar] [CrossRef]
- Shi, J.L.; Kocaefe, D.; Amburgey, T.; Zhang, J.L. A comparative study on brown-rot fungus decay and subterranean termite resistance of thermally-modified and ACQ-C-treated wood. Holz Roh Werkst. 2007, 65, 353–358. [Google Scholar] [CrossRef]
- Bignell, D.E.; Roisin, Y.; Lo, N. Biology of Termites: A Modern Synthesis; Springer: Dordrecht, The Netherlands, 2011; 576p. [Google Scholar]
- Yamaguchi, H.; Yoshino, K.; Kido, A. Termite resistance and wood-penetrability of chemically modified tannin and tannin-copper complexes as wood preservatives. J. Wood Sci. 2002, 48, 331–337. [Google Scholar] [CrossRef]
| Treatment | pH | Viscosity (cp) |
|---|---|---|
| HT Tannin | 5.83 | 2.8 |
| HT T/DOT | 6.14 | 2.6 |
| Southern Yellow Pine | |||||
| Treatment | Leaching Period | Initial Retention(kg m−3) | Remaining Retention(kg m−3) | ||
| DOT | Tannin | DOT | Tannin | ||
| Heat-treated DOT | 15 days | 7.4 | - | 0.2 | - |
| Heat-treated Tannin | 15 days | - | 39.7 | - | 38.3 |
| Heat-treated T/DOT | 15 days | 8.1 | 42.1 | 4.6 | 39.2 |
| Yellow-Poplar | |||||
| Treatment | Leaching Period | Initial Retention(kg m−3) | Remaining Retention(kg m−3) | ||
| DOT | Tannin | DOT | Tannin | ||
| Heat-treated DOT | 15 days | 8.6 | - | 1.7 | - |
| Heat-treated Tannin | 15 days | - | 42.6 | - | 41.2 |
| Heat-treated T/DOT | 15 days | 8.3 | 42.9 | 4.0 | 40.1 |
| Weight Loss (%) Mean | Treatment | Time (Days) | Mortality 2 | Tunneling 3 | Positioning 4 |
|---|---|---|---|---|---|
| 26.76 A 1 | Control | 15 | s | + | u |
| 25.84 A | Control | 0 | s | + | u/d |
| 20.62 B | HT DOT | 15 | s | + | u |
| 13.08 C | HT T/DOT | 15 | x | + | N/A |
| 10.95 C | Heat-treated | 15 | x | + | N/A |
| 10.41 CD | Heat-treated | 0 | x | + | N/A |
| 6.20 ED | HT Tannin | 15 | x | + | N/A |
| 3.02 E | HT Tannin | 0 | x | + | N/A |
| 2.66 E | HT DOT | 0 | x | − | N/A |
| 1.55 E | HT T/DOT | 0 | x | − | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verly Lopes, D.J.; Barnes, H.M.; dos Santos Bobadilha, G. Influence of Heat Treatment and Tannin Impregnation on Boron Depletion and Wood Durability. Forests 2020, 11, 201. https://doi.org/10.3390/f11020201
Verly Lopes DJ, Barnes HM, dos Santos Bobadilha G. Influence of Heat Treatment and Tannin Impregnation on Boron Depletion and Wood Durability. Forests. 2020; 11(2):201. https://doi.org/10.3390/f11020201
Chicago/Turabian StyleVerly Lopes, Dercilio Junior, H. Michael Barnes, and Gabrielly dos Santos Bobadilha. 2020. "Influence of Heat Treatment and Tannin Impregnation on Boron Depletion and Wood Durability" Forests 11, no. 2: 201. https://doi.org/10.3390/f11020201
APA StyleVerly Lopes, D. J., Barnes, H. M., & dos Santos Bobadilha, G. (2020). Influence of Heat Treatment and Tannin Impregnation on Boron Depletion and Wood Durability. Forests, 11(2), 201. https://doi.org/10.3390/f11020201




