Half-Sib Lines of Pedunculate Oak (Quercus robur L.) Respond Differently to Drought Through Biometrical, Anatomical and Physiological Traits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Set-up
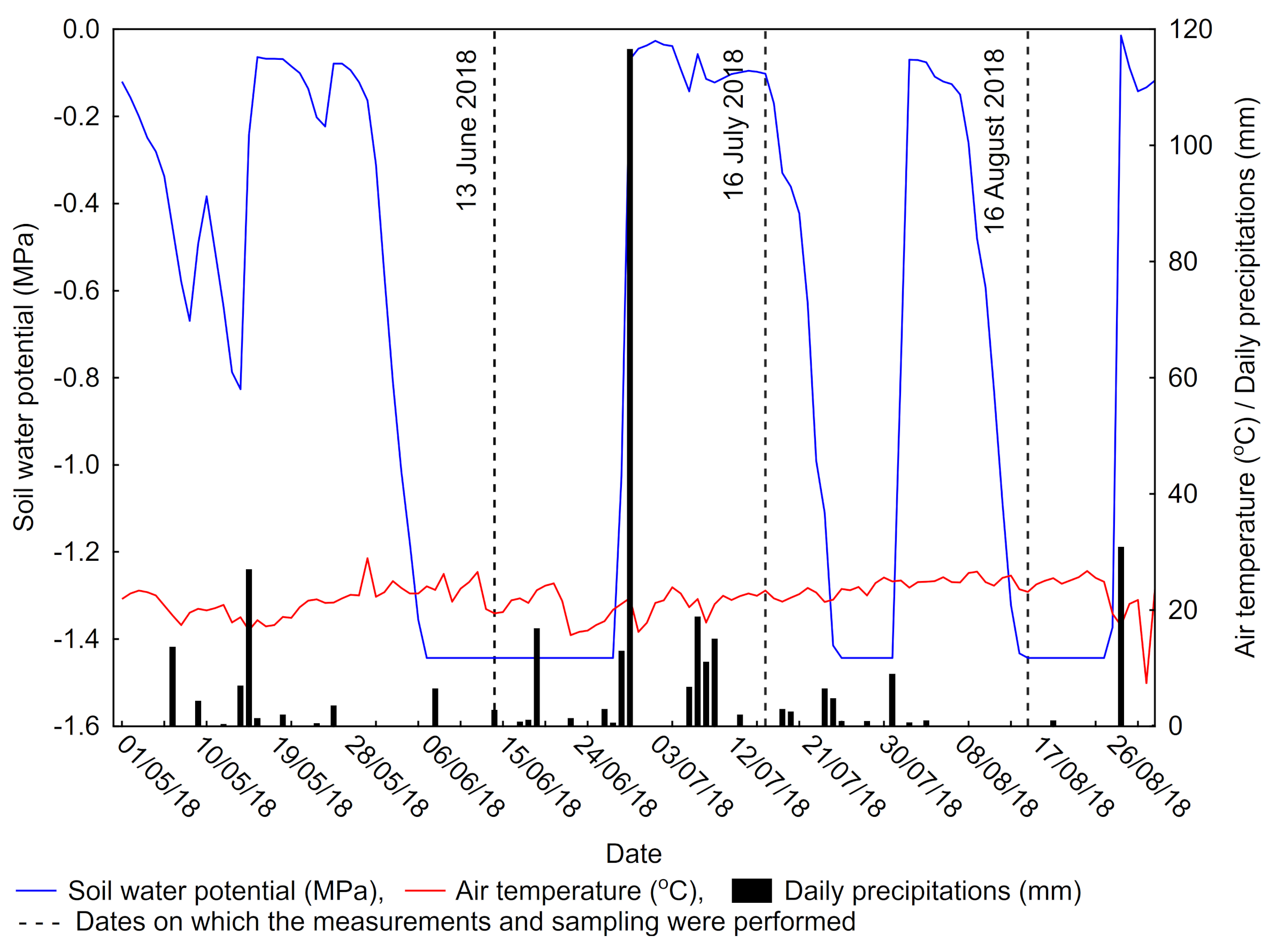
2.2. Meteorological Data
2.3. Plant Biometric Characterisation
2.4. Leaf Anatomical Traits Measurements
2.5. Chlorophyll a Fluorescence Measurements
2.6. Statistical Analyses
3. Results
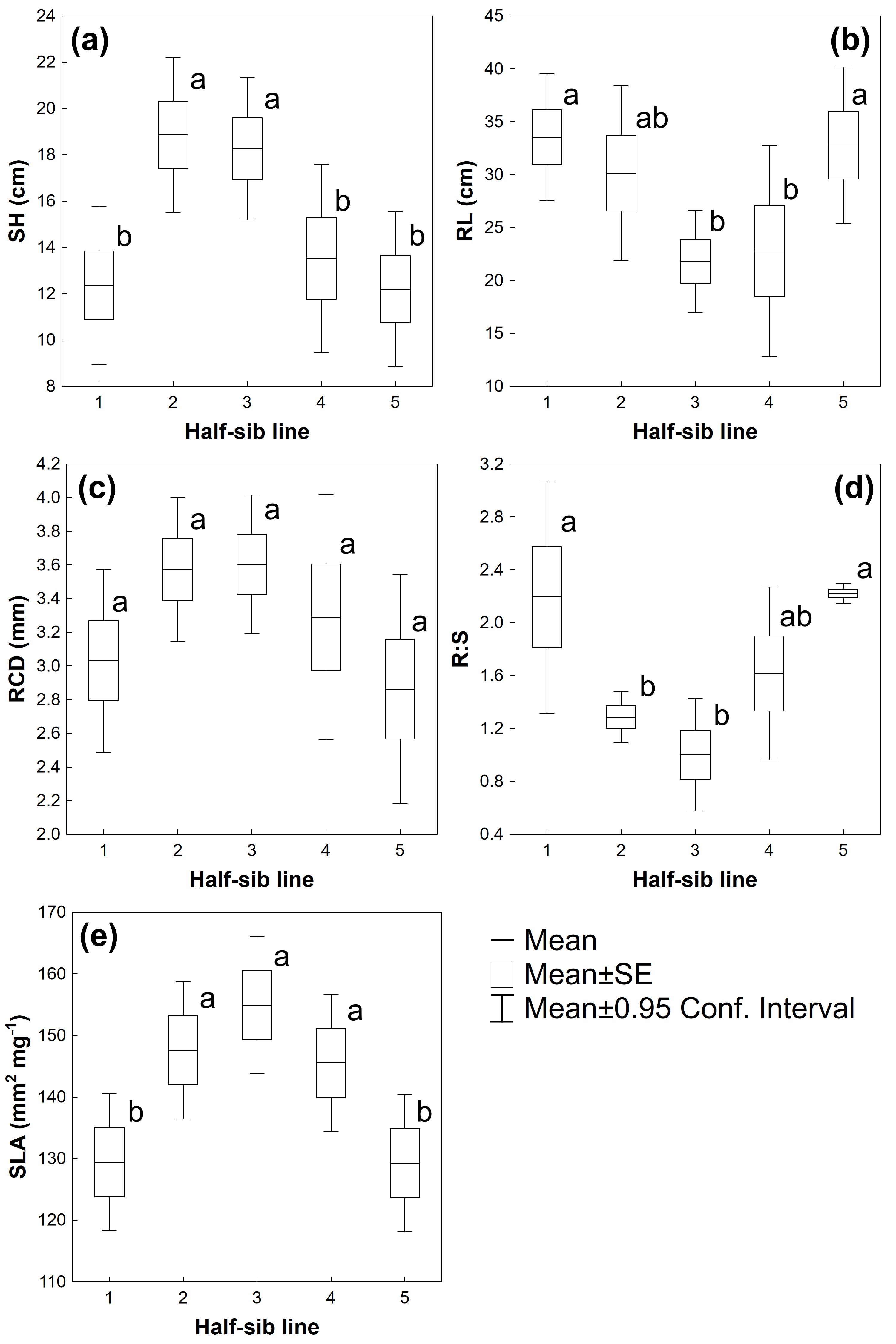
3.1. Biometrical Traits
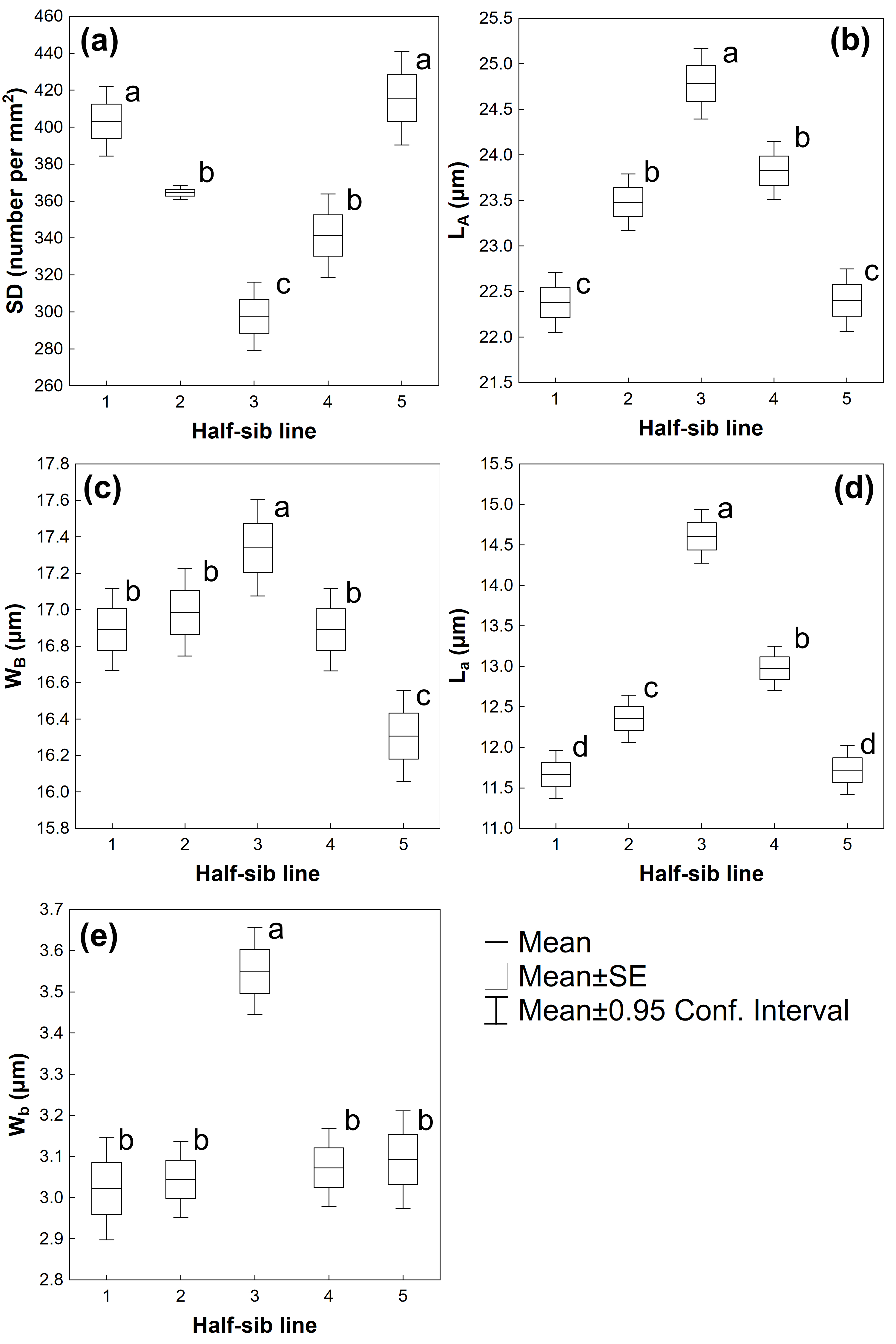
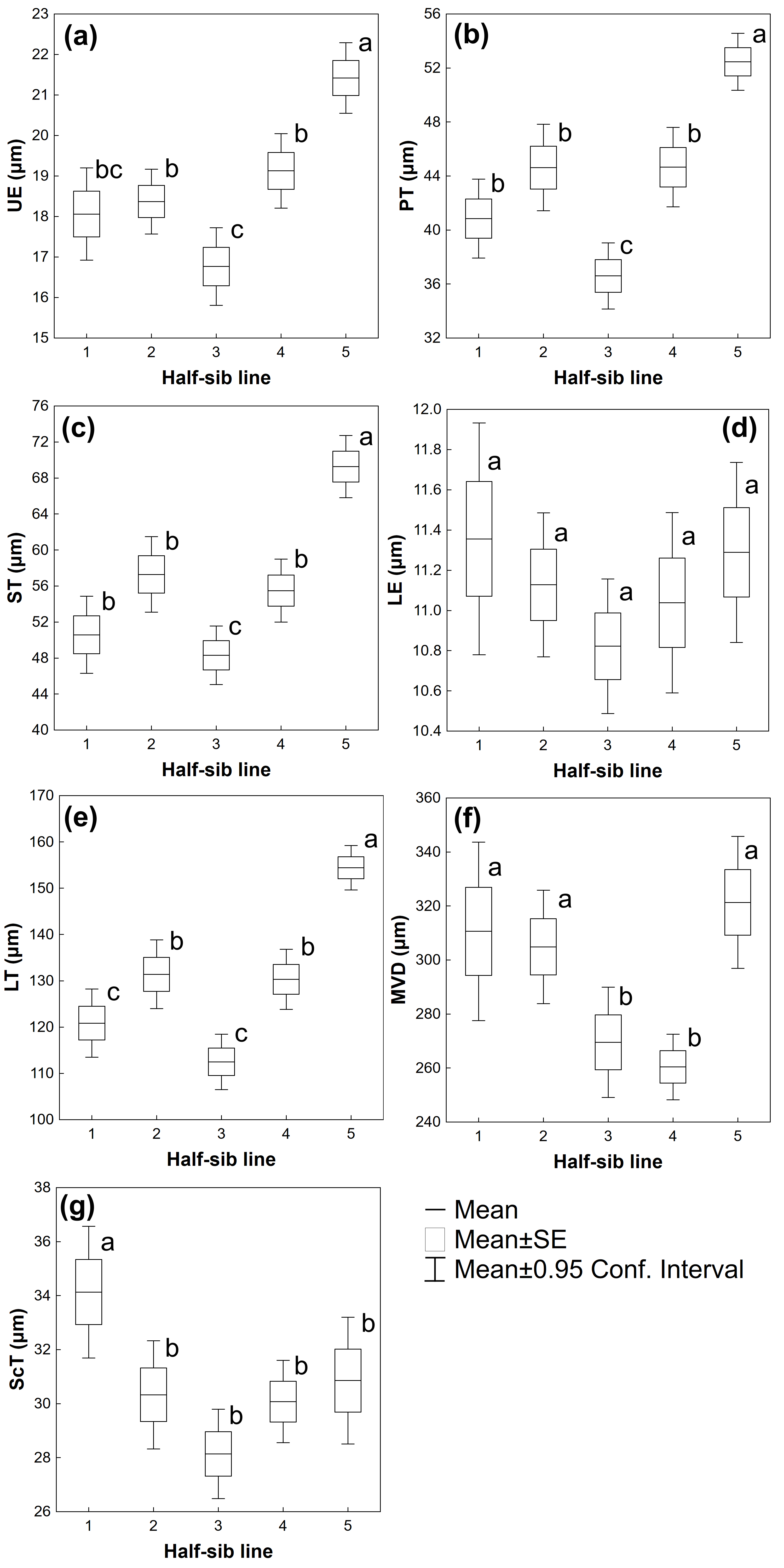
3.2. Leaf Traits
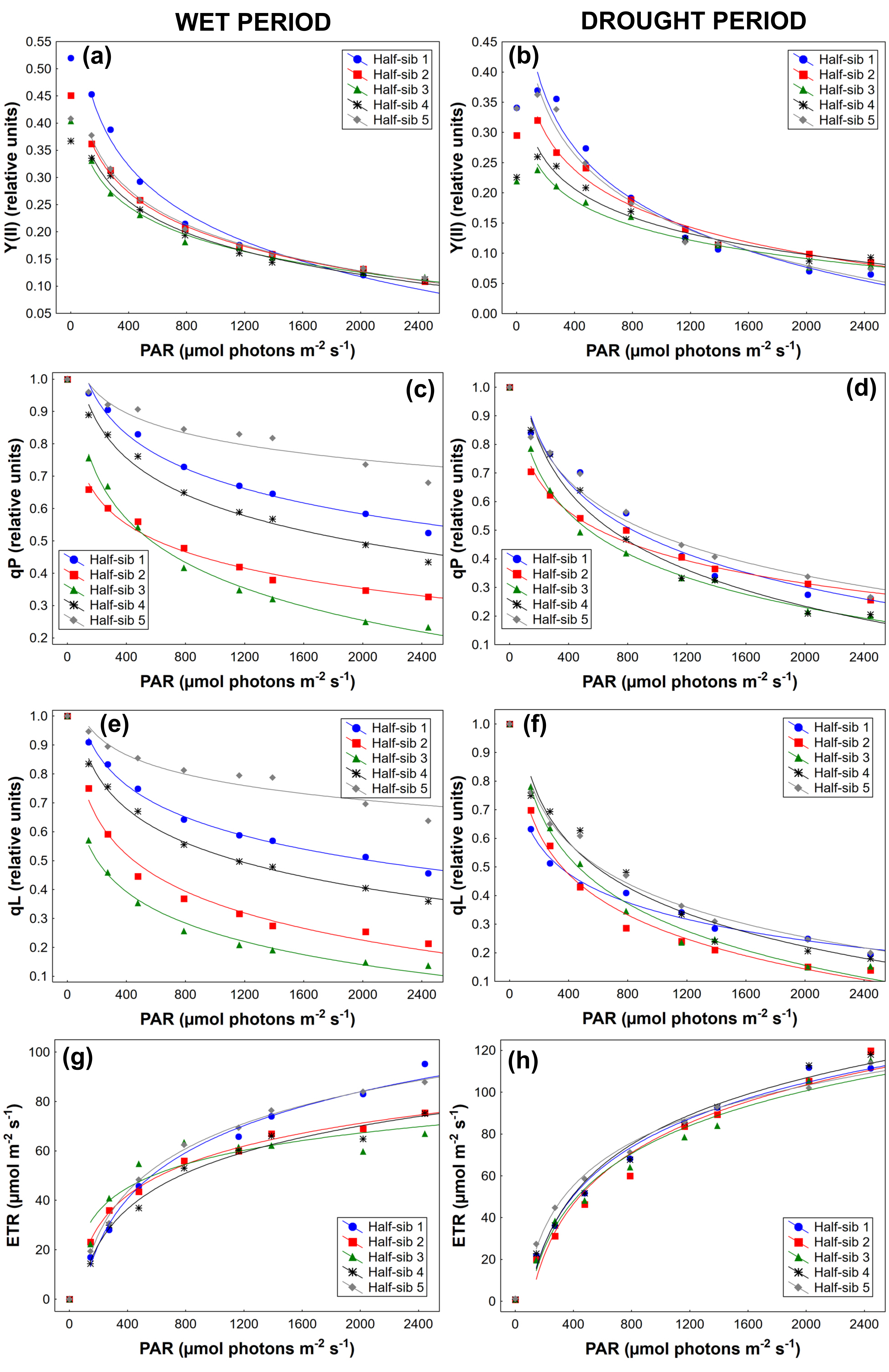
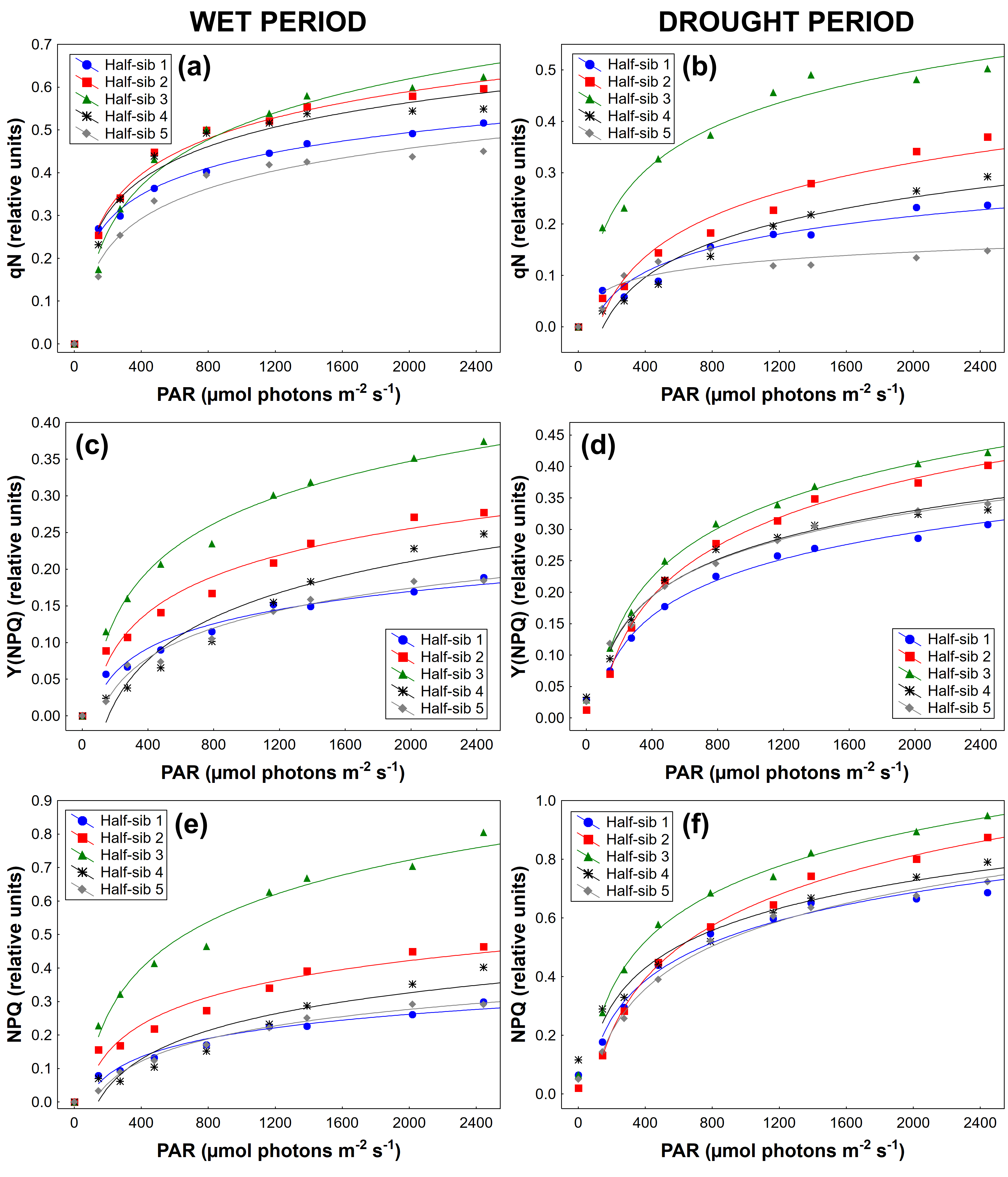
3.3. Chlorophyll a Fluorescence
4. Discussion
4.1. Plant Biometry
4.2. Leaf Traits
4.3. Chlorophyll a Fluorescence
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front Plant Sci. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Lehner, F.; Deser, C.; Sanderson, B.M. Future risk of record-breaking summer temperatures and its mitigation. Clim. Chang. 2018, 146, 363–375. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.J.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Lloret, F.; Breshears, D.D. Drought-induced forest decline: Causes, scope and implications. Biol. Lett. 2011, 8, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Ivetić, V.; Aleksić, J.M. Response of rare and endangered species Picea omorika to climate change: The need for speed. Reforesta 2016, 1, 81–89. [Google Scholar] [CrossRef]
- Živanović, S. Impact of drought in Serbia on fire vulnerability of forests. Int. J. Bioautomat. 2017, 21, 217–226. [Google Scholar]
- Clark, J.S.; Iverson, L.; Woodall, C.W.; Allen, C.D.; Bell, D.M.; Bragg, D.C.; D’Amato, A.; Davis, F.W.; Hersh, M.H.; Ibanez, I.; et al. The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Global Chang. Biol. 2016, 22, 2329–2352. [Google Scholar] [CrossRef]
- Čehulić, I.; Sever, K.; Katičić Bogdan, I.; Jazbec, A.; Škvorc, Ž.; Bogdan, S. Drought impact on leaf phenology and spring frost susceptibility in a Quercus robur L. provenance trial. Forests 2019, 10, 50. [Google Scholar] [CrossRef]
- Annighöfer, P.; Beckschäfer, P.; Vor, T.; Ammer, C. Regeneration patterns of European oak species (Quercus petraea (Matt.) Liebl., Quercus robur L.) in dependence of environment and neighborhood. PLoS ONE 2015, 10, e0134935. [Google Scholar]
- Thomas, F.M.; Gausling, T. Morphological and physiological responses of oak seedlings (Quercus petraea and Q. robur) to moderate drought. Ann. For. Sci. 2000, 57, 325–333. [Google Scholar] [CrossRef]
- Thomas, F.M. Recent advances in cause-effect research on oak decline in Europe. CAB Rev. Perspect. Agricul. Vet. Sci. Nutr. Nat. Res. 2008, 3, 1–12. [Google Scholar] [CrossRef]
- Drekić, M.; Poljaković-Pajnik, L.; Orlović, S.; Kovačević, B.; Vasić, V.; Pilipović, A. Results of multiannual monitoring of tree crown condition. Poplar 2014, 193/194, 23–35. [Google Scholar]
- Mikac, S.; Žmegač, A.; Trlin, D.; Paulić, V.; Oršanić, M.; Anić, I. Drought-induced shift in tree response to climate in floodplain forests of Southeastern Europe. Sci. Rep. 2018, 8, 16495. [Google Scholar] [CrossRef]
- Fotelli, M.N.; Radoglou, K.M.; Constantinidou, H.I.A. Water stress responses of seedlings of four Mediterranean oak species. Tree Physiol. 2000, 20, 1065–1075. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, H.; Kong, D.; Yan, F.; Liu, D.; Zhang, Y. Effects of gradual soil drought stress on the growth, biomass partitioning, and chlorophyll fluorescence of Prunus mongolica seedlings. Turk. J. Biol. 2015, 39, 532–539. [Google Scholar] [CrossRef]
- Li, J.; Cang, Z.; Jiao, F.; Bai, X.; Zhang, D.; Zhai, R. Influence of drought stress on photosynthetic characteristics and protective enzymes of potato at seedling stage. J. Saudi. Soc. Agric. Sci. 2017, 16, 82–88. [Google Scholar] [CrossRef]
- Kramer, D.M.; Evans, J.R. The importance of energy balance in improving photosynthetic productivity. Plant Physiol. 2011, 155, 70–78. [Google Scholar] [CrossRef]
- Stojnić, S.; Trudić, B.; Galović, V.; Šimunovački, Đ.; Đorđević, B.; Rađević, V.; Orlović, S. Conservation of pedunculate oak (Quercus robur L.): Genetic resources at the territory of public enterprise ’Vojvodinašume’. Poplar 2014, 193/194, 47–71. [Google Scholar]
- Gentilesca, T.; Camarero, J.J.; Colangelo, M.; Nole, A.; Ripullone, F. Drought-induced oak decline in the western Mediterranean region: An overview on current evidences, mechanisms and management options to improve forest resilience. iForest 2017, 10, 796–806. [Google Scholar] [CrossRef]
- Arend, M.; Kuster, T.; Günthardt-Goerg, M.S.; Dobbertin, M. Provenance-specific growth responses to drought and air warming in three European oak species (Quercus robur, Q. petraea and Q. pubescens). Tree Physiol. 2011, 31, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Bojović, M.; Nikolić, N.; Borišev, M.; Pajević, S.; Horák, R.; Pavlović, L.; Vaštag, E. The effect of drought stress and recovery on pedunculate oak populations grown in semi-controlled conditions. Poplar 2017, 199, 194–207. [Google Scholar]
- Vander Mijnsbrugge, K.; Turcsán, A.; Maes, J.; Duchêne, N.; Meeus, S.; Van der Aa, B.; Steppe, K.; Steenackers, M. Taxon-Independent and Taxon-Dependent Responses to Drought in Seedlings from Quercus robur L., Q. petraea (Matt.) Liebl. and Their Morphological Intermediates. Forests 2017, 8, 407. [Google Scholar] [CrossRef]
- Deligöz, A.; Bayar, E. Drought stress responses of seedlings of two oak species (Quercus cerris and Quercus robur). Turk. J. Agric. 2018, 42, 114–123. [Google Scholar] [CrossRef]
- Aydinşakir, K.; Büyüktaş, D.; Nazmi, D.İ.N.Ç.; Karaca, C. Impact of salinity stress on growing, seedling development and water consumption of peanut (Arachis hypogaea cv. NC-7). Akdeniz Üniversitesi Ziraat Fakültesi Dergisi 2015, 28. [Google Scholar]
- Fenner, M.K.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005; p. 260. [Google Scholar]
- DeEll, J.R.; Toivonen, P.M.A. Practical Applications of Chlorophyll Fluorescence in Plant Biology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; p. 132. [Google Scholar]
- Krstić, J.; Orlović, S.; Galić, Z.; Pilipović, A.; Stojnić, S. Seasonal changes in leaf gas exchange parameters in Platanus acerifolia Willd. and Acer pseudoplatanus L. seedlings on undeveloped alluvial soil (fluvisol). Forestry 2014, 1–2, 163–178. [Google Scholar]
- Pšidová, E.; Živčák, M.; Stojnić, S.; Orlović, S.; Gömöry, D.; Kučerová, J.; Ditmarováa, L.; Střelcovád, K.; Brestič, M.; Kalaji, H.M. Altitude of origin influences the responses of PSII photochemistry to heat waves in European beech (Fagus sylvatica L.). Environ. Exp. Bot. 2018, 152, 97–106. [Google Scholar] [CrossRef]
- Bai, T.; Li, C.; Li, C.; Liang, D.; Ma, F. Contrasting hypoxia tolerance and adaptation in Malus species is linked to differences in stomatal behavior and photosynthesis. Physiol. Plant. 2013, 147, 514–523. [Google Scholar] [CrossRef]
- Batos, B.; Vilotić, D.; Orlović, S.; Miljković, D. Inter and intra-population variation of leaf stomatal traits of Quercus robur L. in northern Serbia. Arch. Biol. Sci. 2010, 62, 1125–1136. [Google Scholar] [CrossRef]
- Stojnić, S.; Orlović, S.; Miljković, D.; Galić, Z.; Kebert, M.; von Wuehlisch, G. Provenance plasticity of European beech leaf traits under differing environmental conditions at two Serbian common garden sites. Eur. J. For. Res. 2015, 134, 1109–1125. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, K.; Fan, D.; Li, G.; Zheng, Y.; Yu, L.; Yang, R. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ. Exp. Bot. 2011, 71, 174–183. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Genty, B.; Harbinson, J.; Cailly, A.L.; Rizza, F. Fate of Excitation at PS II in Leaves: The Non-Photochemical Side. In Proceedings of the Third BBSRC Robert Hill Symposium on Photosynthesis, Sheffield, UK, 31 March–3 April 1996. [Google Scholar]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- van Kooten, O.; Snel, J. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New flux parameters for the determination of QA redox state and excitation fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Xu, F.; Guo, W.; Wang, R.; Xu, W.; Du, N.; Wang, Y. Leaf movement and photosynthetic plasticity of black locust (Robinia pseudoacacia) alleviate stress under different light and water conditions. Acta Physiol. Plant. 2009, 31, 553–563. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13; TIBCO Software Inc.: Palo Alto, CA, USA, 2017. [Google Scholar]
- Sánchez-Gómez, D.; Robson, T.M.; Gascó, A.; Gil-Pelegrín, E.; Aranda, I. Differences in the leaf functional traits of six beech (Fagus sylvatica L.) populations are reflected in their response to water limitation. Environ. Exp. Bot. 2013, 87, 110–119. [Google Scholar] [CrossRef]
- Stojanović, D.B.; Levanič, T.; Matović, B.; Orlović, S. Growth decrease and mortality of oak floodplain forests as a response to change of water regime and climate. Eur. J. For. Res. 2015, 134, 555–567. [Google Scholar] [CrossRef]
- Čater, M.; Batič, F. Groundwater and light conditions as factors in the survival of pedunculate oak (Quercus robur L.) seedlings. Eur. J. For. Res. 2006, 125, 419–426. [Google Scholar] [CrossRef]
- Stojanović, D.B.; Matović, B.; Orlović, S.; Kržič, A.; Trudić, B.; Galić, Z.; Stojnić, S.; Pekeč, S. Future of the main important forest tree species in Serbia from the climate change perspective. SEEFOR 2014, 5, 117–124. [Google Scholar] [CrossRef]
- Gratani, L. Plant phenotypic plasticity in response to environmental factors. Adv. Botany 2014. [Google Scholar] [CrossRef]
- Weinig, C. Plasticity versus canalization: Population differences in the timing of shade-avoidance responses. Evolution 2000, 54, 441–451. [Google Scholar] [CrossRef]
- Steinger, T.; Roy, B.A.; Stanton, M.L. Evolution in stressful environments II: Adaptive value and costs of plasticity in response to low light in Sinapis arvensis. J. Evol. Biol. 2003, 16, 313–323. [Google Scholar] [CrossRef]
- Zida, D.; Tigabu, M.; Sawadogo, L.; Odén, P.C. Initial seedling morphological characteristics and field performance of two Sudanian savanna species in relation to nursery production period and watering regimes. For. Ecol. Manag. 2008, 255, 2151–2162. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Scippa, G.S.; Chiatante, D. Fine-root morphological and growth traits in a Turkey oak stand in relation to seasonal changes in soil moisture in the southern Apennines, Italy. Ecol. Res. 2012, 27, 725–733. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, W.H.; Ma, C.; Zhou, J.Y. Changes in morphological, physiological, and biochemical responses to different levels of drought stress in Chinese cork oak (Quercus variabilis Bl.) seedlings. Russ. J. Plant Physiol. 2013, 60, 681–692. [Google Scholar] [CrossRef]
- Zhang, X.; Zang, R.; Li, C. Population differences in physiological and morphological adaptations of Populus davidiana seedlings in response to progressive drought stress. Plant Sci. 2004, 166, 791–797. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Baesso, B.; Santamaria, R.; Scippa, S.G.; Chiatante, D. Drought and fire stress influence seedling competition in oak forests: Fine-root dynamics as indicator of adaptation strategies to climate change. Reforesta 2016, 1, 86–105. [Google Scholar] [CrossRef]
- Riaz, A.; Younis, A.; Taj, A.R.; Karim, A.; Tariq, U.; Munir, S.; Riaz, S. Effect of drought stress on growth and flowering of marigold (Tagetes erecta L.). Pak. J. Bot. 2013, 45, 123–131. [Google Scholar]
- Poulos, H.M.; Goodale, U.M.; Berlyn, G.P. Drought response of two Mexican oak species, Quercus laceyi and Q. sideroxyla (Fagaceae), in relation to elevational position. Am. J. Bot. 2007, 94, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Vasques, A.; Chirino, E.; Vilagrosa, A.; Vallejo, V.R.; Keizer, J.J. The role of seed provenance in the early development of Arbutus unedo seedlings under contrasting watering conditions. Environ. Exp. Bot. 2013, 96, 11–19. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Samarah, N.; Mullen, R.; Cianzio, S. Size distribution and mineral nutrients of soybean seeds in response to drought stress. J. Plant Nutr. 2007, 27, 815–835. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.H.; Xu, A.H.; Nie, L.X.; Huang, J.L.; Peng, S.B. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Li, S.; Lü, X.; Wang, X.; Han, X. Changes in specific leaf area of dominant plants in temperate grasslands along a 2500-km transect in northern. China Sci. Rep. 2017, 7, 10780. [Google Scholar]
- Kurz Besson, C.; Lobo-do-Vale, R.; Rodrigues, M.L.; Almeida, P.; Herd, A.; Grant, P.M.; Soares, D.T.; Schmidt, M.; Otieno, D.; Keenan, T.F.; et al. Cork oak physiological responses to manipulated water availability in a Mediterranean woodland. Agric. For. Meteorol. 2014, 184, 230–242. [Google Scholar] [CrossRef]
- Bruschi, P. Geographical variation in morphology of Quercus petraea (Matt.) Liebl. as related to drought stress. Plant Biosyst. 2010, 144, 298–307. [Google Scholar] [CrossRef]
- Liu, J.F.; Arend, M.; Yang, W.J.; Schaub, M.; Ni, Y.Y.; Gessler, A.; Jiang, Z.-P.; Rigling, A.; Li, M.H. Effects of drought on leaf carbon source and growth of European beech are modulated by soil type. Sci. Rep. 2017, 7, 42462. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.; Richardson, A.D. Stomatal length correlates with elevation of growth in four temperate species. J. Sustain. For. 2009, 28, 63–73. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate–stomata action under the control of phytohormones in drought stress conditions. Front Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef]
- Kim, T.H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant. Cell Environ. 2005, 28, 834–849. [Google Scholar]
- Gallé, A.; Haldimann, P.; Feller, U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol. 2007, 174, 799–810. [Google Scholar] [CrossRef]
- Varone, L.; Ribas-Carbo, M.; Cardona, C.; Gallé, A.; Medrano, H.; Gratani, L.; Flexas, J. Stomatal and non-stomatal limitations to photosynthesis in seedlings and saplings of Mediterranean species pre-conditioned and aged in nurseries: Different response to water stress. Environ. Exp. Bot. 2012, 75, 235–247. [Google Scholar] [CrossRef]
- Ennajeh, M.; Vadel, A.M.; Cochard, H.; Khemira, H. Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J. Hortic. Sci. Biotech. 2010, 85, 289–294. [Google Scholar] [CrossRef]
- Sankar, B.; Karthishwaran, K.; Somasundaram, R. Leaf anatomical changes in peanut plants in relation to drought stress with or without paclobutrazol and ABA. J. Phytol. 2013, 5, 25–29. [Google Scholar]
- Alves-Silva, E.; Santos, J.C.; Cornelissen, T.G. How many leaves are enough? The influence of sample size on estimates of plant developmental instability and leaf asymmetry. Ecol. Indic. 2018, 89, 912–924. [Google Scholar] [CrossRef]
- Olsen, J.T.; Caudle, K.L.; Johnson, L.C.; Baer, S.G.; Maricle, B.R. Environmental and genetic variationin leaf anatomy among populations of Andropogon gerardii (Poaceae) along a precipitation gradient. Am. J. Bot. 2013, 100, 1957–1968. [Google Scholar] [CrossRef]
- Otto, M.S.G.; Francisco, J.G.; Gonsalez, B.T.; de Almeida Calvo, L.; de Mattos, E.M.; de Almeida, M.; de Andrade Moral, R.; Demétrio, C.G.B.; Stape, J.L.; de Oliveira, R.F. Changes in γ-aminobutyric acid concentration, gas exchange, and leaf anatomy in Eucalyptus clones under drought stress and rewatering. Acta Physiol. Plant. 2017, 39, 208. [Google Scholar] [CrossRef]
- Pan, X.; Qiu, Q.; Li, J.; Wang, J.; He, Q.; Su, Y.; Ma, J. Drought resistance evaluation based on leaf anatomical structures of 25 shrubs on the Tibetan Plateau. J. South China Agric. Univ. 2015, 36, 61–68. [Google Scholar]
- Valladares, F.; Chico, J.; Aranda, I.; Balaguer, L.; Dizengremel, P.; Manrique, E.; Dreyer, E. The greater seedling high-light tolerance of Quercus robur over Fagus sylvatica is linked to a greater physiological plasticity. Trees 2002, 16, 395–403. [Google Scholar] [CrossRef]
- Wang, X.; Arora, R.; Horner, H.T.; Krebs, S.L. Structural adaptations in overwintering leaves of thermonastic and non-thermonastic Rhododendron species. J. Am. Soc. Hortic. Sci. 2008, 133, 768–776. [Google Scholar] [CrossRef]
- Kulyaa, C.; Siangliwb, J.L.; Toojindab, T.; Lontoma, W.; Pattanagula, W.; Sriyota, N.; Sanitchon, J.; Theerakulpisuta, P. Variation in leaf anatomical characteristics in chromosomal segment substitution lines of KDML105 carrying drought tolerant QTL segments. ScienceAsia 2018, 44, 197–211. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osorio, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Silva, E.C.; Nogueira, R.J.; Vale, F.H.; Araújo, F.P.D.; Pimenta, M.A. Stomatal changes induced by intermittent drought in four umbu tree genotypes. Braz. J. Plant Physiol. 2009, 21, 33–42. [Google Scholar] [CrossRef]
- de Sousa, C.A.F.; de Paiva, D.S.; Casari, R.A.D.C.N.; de Oliveira, N.G.; Molinari, H.B.C.; Kobayashi, A.K.; Magalhães, P.C.; Gomide, R.L.; Souza, M.T. A procedure for maize genotypes discrimination to drought by chlorophyll fluorescence imaging rapid light curves. Plant Methods 2017, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.; Jajoo, A.; Poudyal, R.S.; Timilsina, R.; Park, Y.S.; Aro, E.M.; Nam, H.G.; Lee, C.H. Towards a critical understanding of the photosystem II repair mechanism and its regulation during stress conditions. FEBS Lett. 2013, 587, 3372–3381. [Google Scholar] [CrossRef] [PubMed]
- Cendrero-Mateo, M.P.; Moran, M.S.; Papuga, S.A.; Thorp, K.R.; Alonso, L.; Moreno, J.; Ponce-Campos, G.; Wang, G. Plant chlorophyll fluorescence: Active and passive measurements at canopy and leaf scales with different nitrogen treatments. J. Exp. Bot. 2015, 67, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Naumann, J.C.; Young, D.R.; Anderson, J.E. Linking leaf chlorophyll fluorescence properties to physiological responses for detection of salt and drought stress in coastal plant species. Physiol. Plant. 2007, 131, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Isoda, A. Effects of water stress on leaf temperature and chlorophyll fluorescence parameters in cotton and peanut. Plant Prod. Sci. 2010, 13, 269–278. [Google Scholar]
- Jamnická, G.; Ditmarová, Ľ.; Kurjak, D.; Kmeť, J.; Pšidová, E.; Macková, M.; Gömöry, D.; Střelcová, K. The soil hydrogel improved photosynthetic performance of beech seedlings treated under drought. Plant Soil Environ. 2013, 10, 446–451. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Koller, S.; Holland, V.; Brüggemann, W. Effects of drought stress on the evergreen Quercus ilex L., the deciduous Q. robur L. and their hybrid Q.× turneri Willd. Photosynthica 2013, 51, 574–582. [Google Scholar] [CrossRef]
- Cocozza, C.; De Miguel, M.; Pšidová, E.; Marino, S.; Maiuro, L.; Alvino, A.; Tognetti, R. Variation in ecophysiological traits and drought tolerance of beech (Fagus sylvatica L.) seedlings from different populations. Front Plant Sci. 2016, 7, 886. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H.; Yang, J. Photosynthetic properties and potentials for improvement of photosynthesis in pale green leaf rice under high light conditions. Front Plant Sci. 2017, 8, 1082. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Struik, P.C. Constraints to the potential efficiency of converting solar radiation into phytoenergy in annual crops: From leaf biochemistry to canopy physiology and crop ecology. J. Exp. Bot. 2015, 66, 6535–6549. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, M.H.; Pietkiewicz, S. Some physiological indices to be exploited as a crucial tool in plant breeding. Plant Breed. Seeds Sci. 2004, 49, 19–39. [Google Scholar]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2014, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Zlatev, Z. Drought-induced changes in chlorophyll fluorescence of young wheat plants. Biotechnol. Biotechnol. Equip. 2009, 23, 438–441. [Google Scholar] [CrossRef]
| Traits | Acronym | Unit |
|---|---|---|
| Biometrical | ||
| Seedling height | SH | cm |
| Root length | RL | cm |
| Root collar diameter | RCD | mm |
| Root to shoot ratio of dry mass | R:S | / |
| Specific leaf area | SLA | mm2 mg−1 |
| Leaf anatomical | ||
| Stomatal density | SD | number per mm2 |
| Stomatal guard cell length | LA | μm |
| Stomatal guard cell width | WB | μm |
| Stomatal aperture length | La | μm |
| Stomatal aperture width | Wb | μm |
| Adaxial epidermis thickness | UE | μm |
| Palisade parenchyma thickness | PT | μm |
| Spongy parenchyma thickness | ST | μm |
| Abaxial epidermis thickness | LE | μm |
| Lamina thickness | LT | μm |
| Main vein diameter | MVD | μm |
| Sclerenchyma thickness | ScT | μm |
| Physiological | ||
| Effective quantum yield (efficiency) of PS II photochemistry | Y(II) | relative units |
| Coefficient of photochemical quenching | qP | relative units |
| Coefficient of photochemical fluorescence quenching assuming interconnected PS II antennae | qL | relative units |
| Electron transport rate | ETR | μmol m−2 s−1 |
| Coefficient of non-photochemical quenching | qN | relative units |
| Quantum yield of regulated energy dissipation | Y(NPQ) | relative units |
| Stern-Volmer type non-photochemical fluorescence quenching | NPQ | relative units |
| Source of Variation | SH | RL | RCD | R:S | SLA |
|---|---|---|---|---|---|
| Half-sib lines | F(4,40) = 4.7145 p ≤ 0.01 | F(4,40) = 2.9197 p ≤ 0.05 | F(4,40) = 1.7240 p = 0.1644 | F(4,40) = 5.4992 p ≤ 0.01 | F(4,130) = 4.1857 p ≤ 0.01 |
| Source of Variation | SD | LA | WB | La | Wb |
|---|---|---|---|---|---|
| Half-sib lines | F(4,220) = 24.905 p ≤ 0.001 | F(4,1120) = 34.836 p ≤ 0.001 | F(4,1120) = 9.1665 p ≤ 0.01 | F(4,1120) = 63.292 p ≤ 0.001 | F(4,1120) = 16.463 p ≤ 0.001 |
| Source of Variation | UE | PT | ST | LE | LT | MVD | ScT |
|---|---|---|---|---|---|---|---|
| Half-sib lines | F(4,215) = 13.988 p ≤ 0.001 | F(4,215) = 18.681 p ≤ 0.001 | F(4,215) = 19.542 p ≤ 0.001 | F(4,215) = 0.95489 p = 0.433 | F(4,215) = 24.500 p ≤ 0.001 | F(4,213) = 5.7836 p ≤ 0.001 | F(4,212) = 4.4786 p ≤ 0.01 |
| Source of Variation | qN | Y(II) | qP | Y(NPQ) | qL | NPQ | ETR |
|---|---|---|---|---|---|---|---|
| Period (P) | F(1,44) = 54.558 p < 0.01 | F(1,44) = 92.453 p < 0.01 | F(1,44) = 50.512 p < 0.01 | F(1,44) = 21.862 p < 0.01 | F(1,44) = 28.264 p < 0.01 | F(1,44) = 43.319 p < 0.01 | F(1,44) = 65.399 p < 0.01 |
| Half-sib lines (H) | F(3,122)=12.497 p < 0.01 | F(3,120) = 6.666 p < 0.01 | F(3,124) = 26.197 p < 0.01 | F(2,104) = 36.358 p < 0.01 | F(3,129) = 34.354 p < 0.01 | F(3,115) = 25.838 p < 0.01 | F(2,103) = 2.081 p > 0.05 |
| P × H | F(3,140) = 3.277 p < 0.05 | F(2,101) = 6.257 p < 0.05 | F(4,156) = 15.049 p < 0.01 | F(2,104) = 12.647 p < 0.01 | F(3,134) = 17.650 p < 0.01 | F(2,96) = 6.715 p < 0.05 | F(3,133) = 1.799 p < 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vastag, E.; Cocozza, C.; Orlović, S.; Kesić, L.; Kresoja, M.; Stojnić, S. Half-Sib Lines of Pedunculate Oak (Quercus robur L.) Respond Differently to Drought Through Biometrical, Anatomical and Physiological Traits. Forests 2020, 11, 153. https://doi.org/10.3390/f11020153
Vastag E, Cocozza C, Orlović S, Kesić L, Kresoja M, Stojnić S. Half-Sib Lines of Pedunculate Oak (Quercus robur L.) Respond Differently to Drought Through Biometrical, Anatomical and Physiological Traits. Forests. 2020; 11(2):153. https://doi.org/10.3390/f11020153
Chicago/Turabian StyleVastag, Erna, Claudia Cocozza, Saša Orlović, Lazar Kesić, Milena Kresoja, and Srdjan Stojnić. 2020. "Half-Sib Lines of Pedunculate Oak (Quercus robur L.) Respond Differently to Drought Through Biometrical, Anatomical and Physiological Traits" Forests 11, no. 2: 153. https://doi.org/10.3390/f11020153
APA StyleVastag, E., Cocozza, C., Orlović, S., Kesić, L., Kresoja, M., & Stojnić, S. (2020). Half-Sib Lines of Pedunculate Oak (Quercus robur L.) Respond Differently to Drought Through Biometrical, Anatomical and Physiological Traits. Forests, 11(2), 153. https://doi.org/10.3390/f11020153








