Spatial Patterns and Interspecific Associations During Natural Regeneration in Three Types of Secondary Forest in the Central Part of the Greater Khingan Mountains, Heilongjiang Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Plot Design and Survey
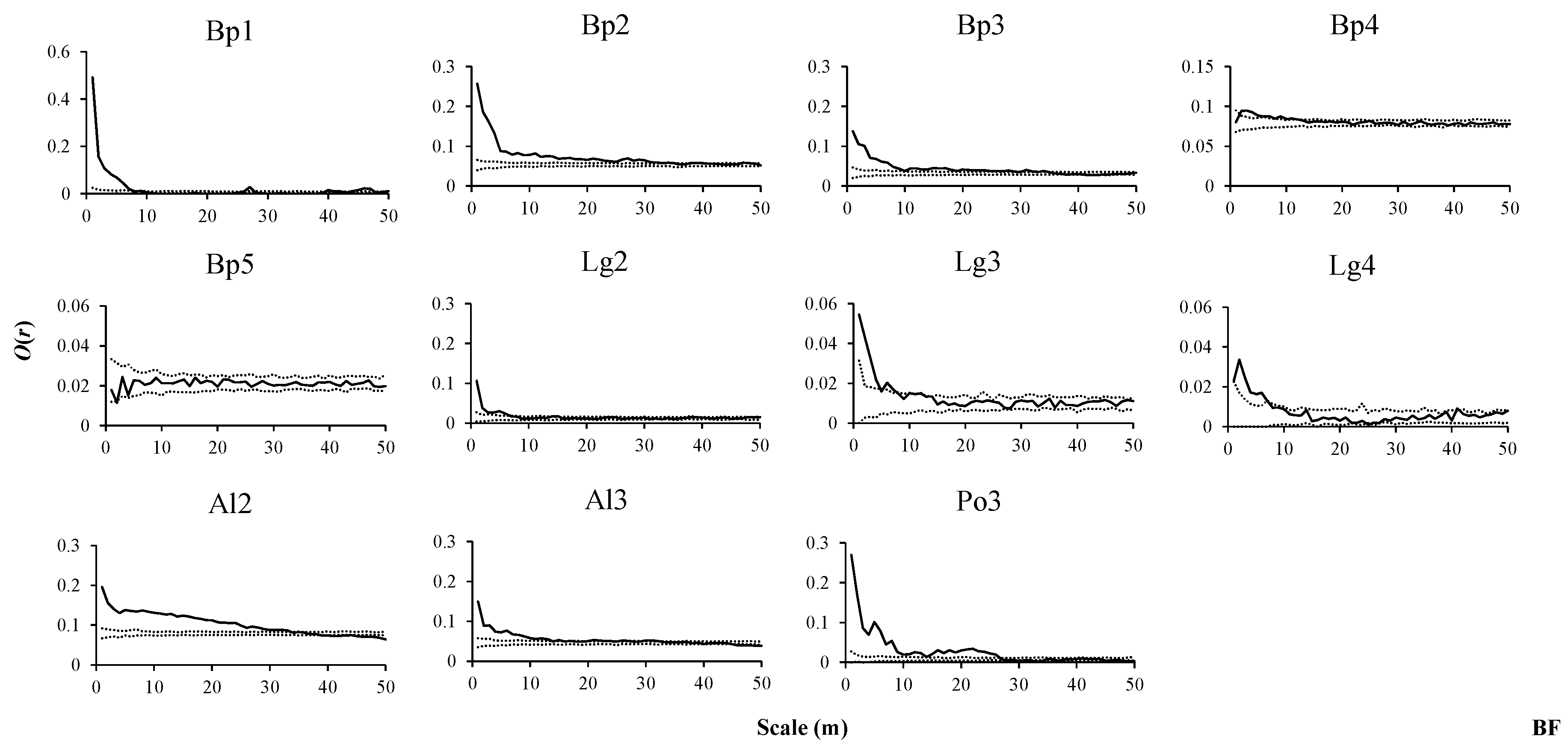
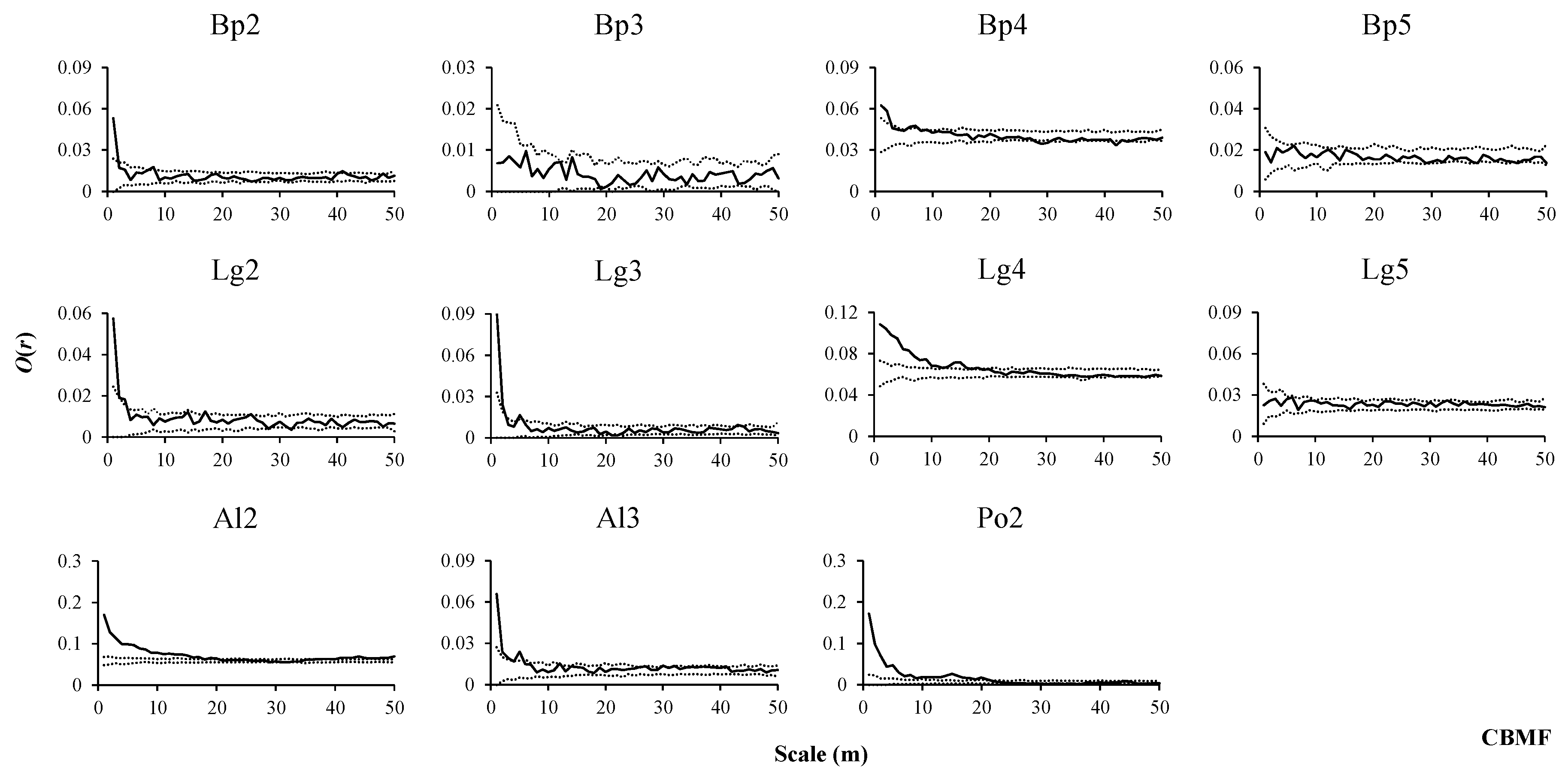
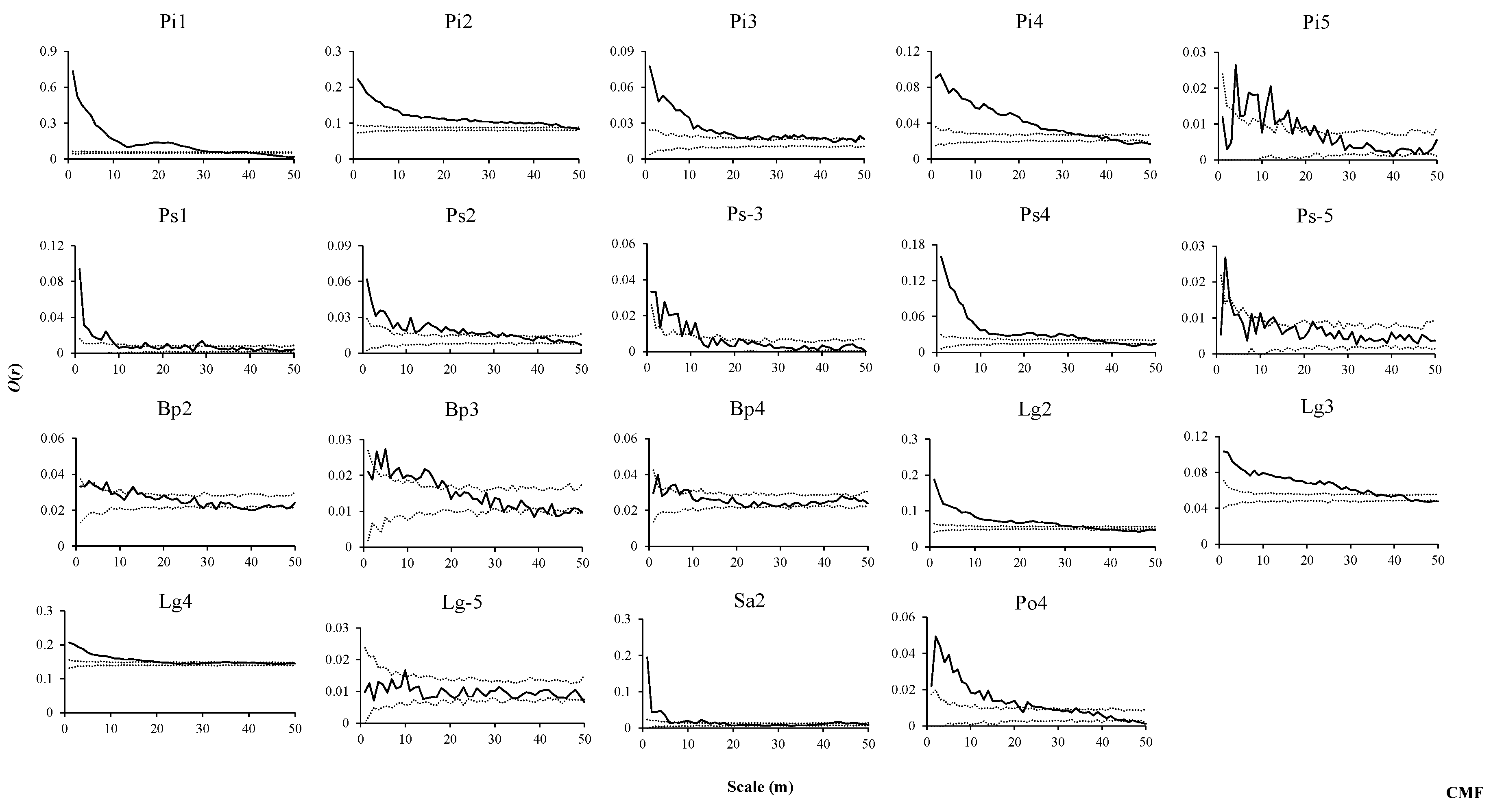
2.3. Spatial Pattern Analysis
3. Results
3.1. Population Structure
3.2. Spatial Distribution Patterns of Species in Three Forest Types
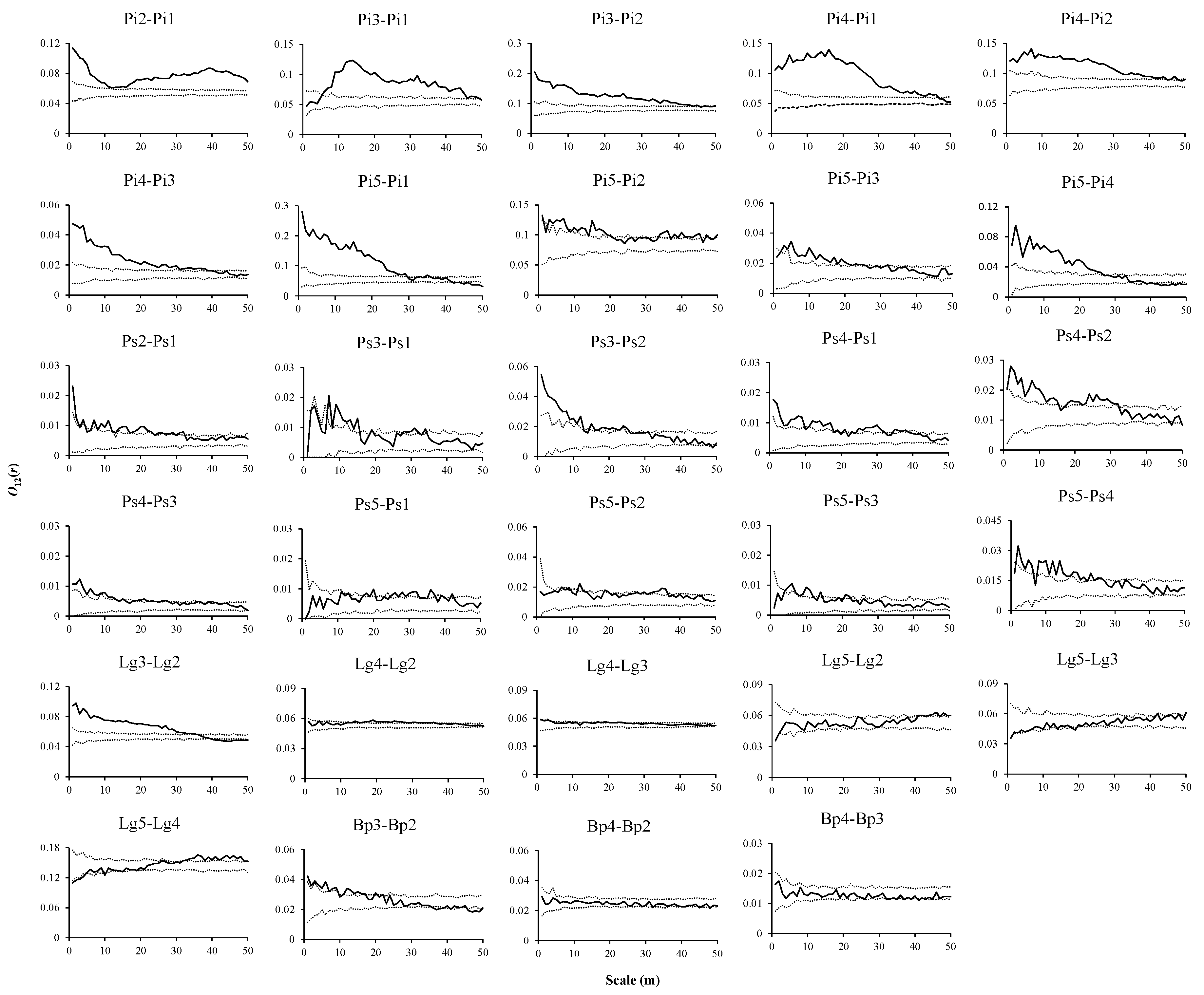
3.3. Intraspecific Spatial Associations in Three Forest Types
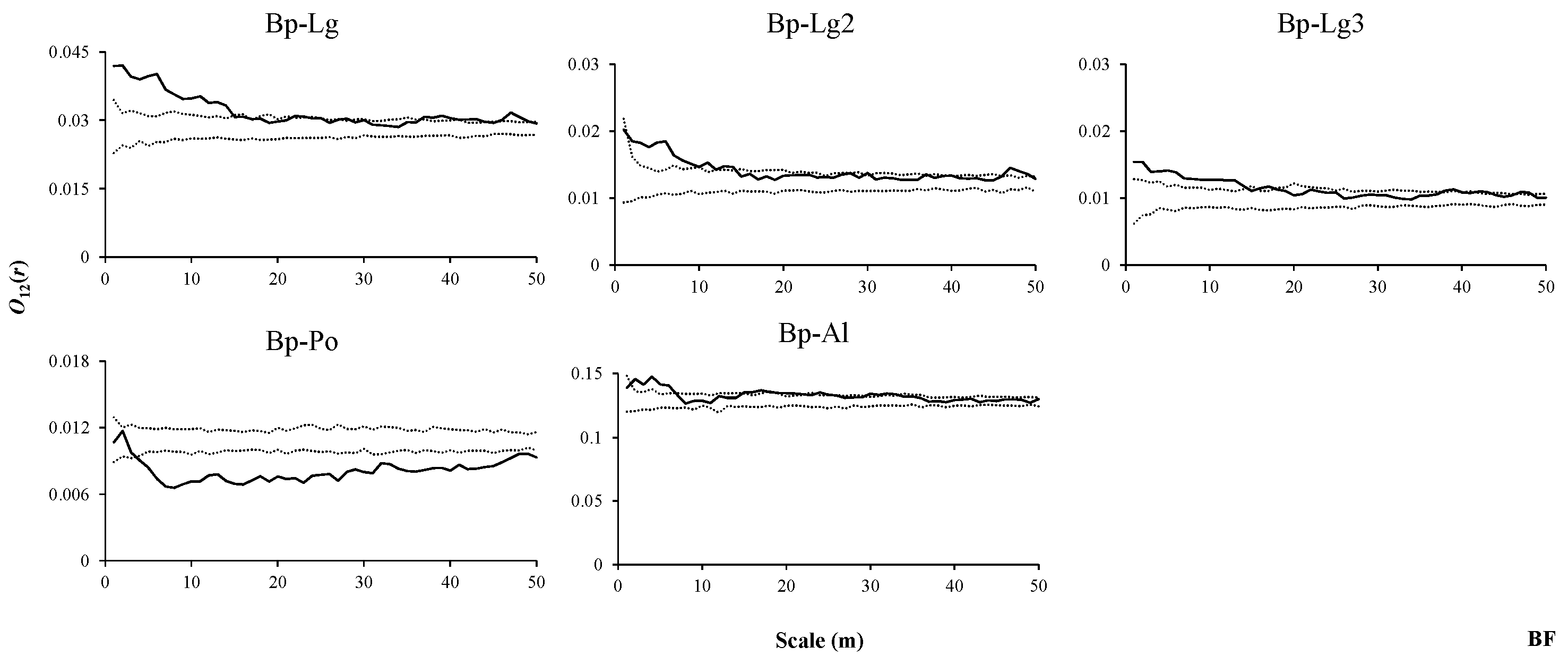
3.4. Interspecific Associations of Dominant Tree Species with Other Trees at Different Stages in Three Forest Types
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, D.; Zhang, G.; Zhu, J.; Wang, G.; Zhu, C.; Yan, Q.; Zhang, J. Early natural regeneration patterns of woody species within gaps in a temperate secondary forest. Eur. J. Forest Res. 2019, 138, 991–1003. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, D.; Zhang, W. Effects of gaps on regeneration of woody plants: A meta-analysis. J. Forestry. Res. 2014, 25, 501–510. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, J.; He, H.; Dai, L. Effects of natural and human-assisted regeneration on landscape dynamics in a Korean pine forest in Northeast China. PLoS ONE 2013, 8, e82414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, D.; Zhou, L.; Zhou, W.; Ding, H.; Wang, Q.; Wang, Y.; Wu, X.; Dai, L. Forest management in Northeast China: History, problems, and challenges. Environ. Manage. 2011, 48, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Z.; Li, F.R.; Jia, W.W.; Wang, S.F. Water conservation of litterfall in different mixed forest types of white birch and larch in Daxing’an Mountain. J. Northeast For. Univ. 2014, 42, 43–46+52. (In Chinese) [Google Scholar]
- Feng, Y.Q.; Chen, C.F.; Qin, L.; He, Y.T.; Wang, P.; Duan, Y.X.; Wang, Y.F.; He, Y.J. Effects of different management models on stand structure and plant diversity of natural secondary forests of Quercus Mongolica. Sci. Silv. Sin. 2018, 54, 12–21. [Google Scholar]
- State Forestry Administration of the People’s Republic of China. Eighth National Forest Resource Inventory Report (2009–2013), February 25, 2014; State Forestry Administration of the People’s Republic of China: Beijing, China, 2014. [Google Scholar]
- Nagashima, H. The processes of height-rank determination among individuals and neighbourhood effects in Chenopodium album L. Stands. Ann. Bot. 1999, 83, 501–507. [Google Scholar] [CrossRef][Green Version]
- Cranston, B.H.; Hermanutz, L. Seed-seedling conflict in conifers as a result of plant-plant interactions at the forest-tundra ecotone. Plant Ecol. Divers. 2013, 6, 319–327. [Google Scholar] [CrossRef]
- Frelich, L.E.; Sugita, S.; Reich, P.B.; Davis, M.B.; Friedman, S.K. Neighbourhood effects in forests: Implications for within stand patch structure. J. Ecol. 1998, 86, 149–161. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Liu, S.; Zang, R.; He, F.; Spence, J.R. Partial recovery of a tropical rain forest a half-century after clear-cut and selective logging. J. Appl. Ecol. 2015, 52, 1044–1052. [Google Scholar] [CrossRef]
- Nanami, S.; Kawaguchi, H.; Tateno, R.; Li, C.; Katagiri, S. Sprouting traits and population structure of co-occurring Castanopsis species in an evergreen broad-leaved forest in southern China. Ecol. Res. 2004, 19, 341–348. [Google Scholar] [CrossRef]
- Szwagrzyk, J.; Szewczyk, J.; Bodziarczyk, J. Dynamics of seedling banks in beech forest: Results of a 10-year study on germination, growth and survival. For. Ecol. Manag. 2001, 141, 237–250. [Google Scholar] [CrossRef]
- Gupta, B.; Gupta, T.K. Analysis of tree diversity and factors affecting natural regeneration in fragmented dry deciduous forests of lateritic West Bengal. Trop. Ecol. 2019, 60, 405–414. [Google Scholar] [CrossRef]
- Baruch, Z. Ordination and classification of vegetation along an altitudinal gradient in the Venezuelan páramos. Vegetatio 1984, 55, 115–126. [Google Scholar]
- Vergarechea, M.; del Río, M.; Gordo, J.; Martín, R.; Cubero, D.; Calama, R. Spatio-temporal variation of natural regeneration in Pinus pinea and Pinus pinaster Mediterranean forests in Spain. Eur. J. Forest Res. 2019, 138, 313–326. [Google Scholar] [CrossRef]
- Cole, R.J.; Holl, K.D.; Keene, C.L.; Zahawi, R.A. Direct seeding of late-successional trees to restore tropical montane forest. For. Ecol. Manag. 2011, 261, 1590–1597. [Google Scholar] [CrossRef]
- Wiegand, T.; Gunatilleke, S.; Gunatilleke, N. Species associations in a heterogeneous Sri Lankan dipterocarp forest. Am. Nat. 2007, 170, E77–E95. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Pinard, M.A. Ecological knowledge of regeneration from seed in neotropical forest trees: Implications for natural forest management. For. Ecol. Manag. 1998, 112, 87–99. [Google Scholar] [CrossRef]
- Luo, Z.R.; Yu, M.J.; Chen, D.L.; Wu, Y.G.; Ding, B.Y. Spatial associations of tree species in a subtropical evergreen broad-leaved forest. J. Plant. Ecol. 2012, 5, 346–355. [Google Scholar] [CrossRef]
- Marimon, B.S.; Felfili, J.M.; Lima, E.S.; Duarte, W.M.G.; Marimon-Ju’nior, B.H. Environmental determinants for natural regeneration of gallery forest at the Cerrado/Amazonia boundaries in Brazil. Acta Amazon. 2010, 40, 107–118. [Google Scholar] [CrossRef]
- Devaney, J.L.; Jansen, M.A.K.; Whelan, P.M. Spatial patterns of natural regeneration in stands of English yew (Taxus baccata L.); Negative neighbourhood effects. For. Ecol. Manag. 2014, 321, 52–60. [Google Scholar] [CrossRef]
- Guo, Y.X.; Hu, Y.N.; Li, G.; Wang, D.X.; Yang, J.J.; Yang, G.H. Spatial pattern and spatial Association of Betula albosinensis at different developmental stages at Taibai Mountain. Sci. Silv. Sin. 2014, 50, 9–14. (In Chinese) [Google Scholar]
- Wright, S.J.; Muller-landau, H.C.; Calderón, O.; Hernandéz, A. Annual and spatial variation in seedfall and seedling recruitment in a neotropical forest. Ecology 2005, 86, 848–860. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Z. Spatial heterogeneity and forest regeneration. Chin. J. Appl. Ecol. 2002, 13, 615–619. [Google Scholar]
- D’Amato, A.W.; Orwig, D.A.; Foster, D.R. Understory vegetation in old-growth and second-growth Tsuga canadensis forests in western Massachusetts. For. Ecol. Manag. 2009, 257, 1043–1052. [Google Scholar] [CrossRef]
- Muller-Landau, H.C.; Wright, S.J.; Calderón, O.; Condit, R.; Hubbell, S.P. Interspecific variation in primary seed dispersal in a tropical forest. J. Ecol. 2008, 96, 653–667. [Google Scholar] [CrossRef]
- Christie, D.A.; Armesto, J.J. Regeneration microsites and tree species coexistence in temperate rain forests of Chiloé Island, Chile. J. Ecol. 2003, 91, 776–784. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, X.; Zhang, C.; Zhao, X.; Gadow, K.V. Effects of density dependence in a temperate forest in northeastern China. Sci. Rep. 2016, 6, 32844. [Google Scholar] [CrossRef]
- Miao, N.; Liu, S.; Yu, H.; Shi, Z.; Moermond, T.; Liu, Y. Spatial analysis of remnant tree effects in a secondary Abies-Betula forest on the eastern edge of the Qinghai–Tibetan Plateau, China. For. Ecol. Manag. 2014, 313, 104–111. [Google Scholar] [CrossRef]
- Nathan, R. Long-Distance Dispersal of Plants. Science 2006, 313, 786–788. [Google Scholar] [CrossRef]
- Fang, J.; Wang, X.; Liu, Y.; Tang, Z.; White, P.S.; Sanders, N.J. Multi-scale patterns of forest structure and species composition in relation to climate in northeast China. Ecography 2012, 35, 1072–1082. [Google Scholar] [CrossRef]
- Xing, H.; Li, F.; Jia, W.; Zhuang, C. Spatial structure of natural mixed forests in Daxing’an Mountain. J. Northeast. For. Univ. 2014, 42, 6–10. [Google Scholar]
- Lei, N.; Liu, Y.; Sa, R.; Tie, N. Structure characteristics of Larix gmelinii natural forest in Daxing’an Mountains. J. Northeast. For. Univ. 2017, 45, 8–12. (In Chinese) [Google Scholar]
- Bao, Y.; Zhang, Q.L.; Wang, L.M. Comparative study on characteristics of stand structures in Larix gmelinii forests of different origin. J. Northeast. For. Univ. 2013, 41, 18–21. (In Chinese) [Google Scholar]
- Jia, W.; Xie, X.; Jiang, W.; Li, F. Spatial distribution pattern of seedlings and saplings of three forest types by natural regeneration in Daxin’an Mountains Xinlin Forestry Bureau, China. Chin. J. Appl. Ecol. 2017, 28, 2813–2822. (In Chinese) [Google Scholar]
- Gong, Z.T. Chinese Soil Taxonomy; Science Press: Beijing, China, 1999; p. 615. (In Chinese) [Google Scholar]
- Wiegand, T.; Moloney, K.A. Rings, circles, and null-models for point pattern analysis in ecology. Oikos. 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Wiegand, T.; Kissling, W.D.; Cipriotti, P.A.; Aguiar, M.R. Extending point pattern analysis for objects of finite size and irregular shape. J. Ecol. 2006, 94, 825–837. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Baker, P.; Bunyavejchewin, S.; Gunatilleke, S.; Gunatilleke, N.; Hubbell, S.P.; Foster, R.B.; Itoh, A.; LaFrankie, J.V.; et al. Spatial patterns in the distribution of tropical tree species. Science 2000, 288, 1414–1418. [Google Scholar] [CrossRef]
- Erfanifard, Y.; Stereńczak, K. Intra- and interspecific interactions of Scots pine and European beech in mixed secondary forests. Acta Oecol. 2017, 78, 15–25. [Google Scholar] [CrossRef]
- Stoyan, D.; Penttinen, A. Recent applications of point process methods in forestry statistics. Stat. Sci. 2000, 15, 61–78. [Google Scholar]
- Law, R.; Illian, J.; Burslem, D.F.R.P.; Gratzer, G.; Gunatilleke, C.V.S.; Gunatilleke, I.A.U.N. Ecological information from spatial patterns of plants: Insights from point process theory. J. Ecol. 2009, 97, 616–628. [Google Scholar] [CrossRef]
- Wang, X.; Wiegand, T.; Hao, Z.; Li, B.; Ye, J.; Lin, F. Species associations in an old-growth temperate forest in north-eastern China. J. Ecol. 2010, 98, 674–686. [Google Scholar] [CrossRef]
- Wiegand, T.; Moloney, K.A. Handbook of Spatial Point-Pattern Analysis in Ecology; CRC Press: New York, NY, USA, 2014. [Google Scholar]
- Perry, G.L.W.; Miller, B.P.; Enright, N.J. A comparison of methods for the statistical analysis of spatial point patterns in plant ecology. Plant. Ecol. 2006, 187, 59–82. [Google Scholar] [CrossRef]
- Schiffers, K.; Schurr, F.M.; Tielbörger, K.; Urbach, C.; Moloney, K.; Jeltsch, F. Dealing with virtual aggregation–a new index for analysing heterogeneous point patterns. Ecography 2008, 31, 545–555. [Google Scholar] [CrossRef]
- Gavrikov, V.; Stoyan, D. The use of marked point processes in ecological and environmental forest studies. Environ. Ecol. Stat. 1995, 2, 331–344. [Google Scholar] [CrossRef]
- Clark, J.S.; Silman, M.; Kern, R.; Macklin, E.; Hillerislambers, J. Seed dispersal near and far: Patterns across temperate and tropical forests. Ecology 1999, 80, 1475–1494. [Google Scholar] [CrossRef]
- Cain, M.L.; Milligan, B.G.; Strand, A.E. Long-distance seed dispersal in plant populations. Am. J. Bot. 2000, 87, 1217–1227. [Google Scholar] [CrossRef]
- Das, A.; Battles, J.; Stephenson, N.L.; Van Mantgem, P.J. The contribution of competition to tree mortality in old-growth coniferous forests. For. Ecol. Manag. 2011, 261, 1203–1213. [Google Scholar] [CrossRef]
- Wiegand, T.; Gunatilleke, C.V.S.; Gunatilleke, I.A.U.N.; Huth, A. How individual species structure diversity in tropical forests. Proc. Natl. Acad. Sci. USA 2007, 104, 19029–19033. [Google Scholar] [CrossRef]
- Wiegand, T.; Gunatilleke, S.; Gunatilleke, N.; Okuda, T. Analyzing the spatial structure of a Sri Lankan tree species with multiple scales of clustering. Ecology. 2007, 88, 3088–3102. [Google Scholar] [CrossRef]
- Janzen, D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Connell, J.H. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In Dynamics of Populations; Center for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1971. [Google Scholar]
- Law, R.; Purves, D.W.; Murrell, D.J.; Dieckmann, U. Causes and effects of small-scale spatial structure in plant populations. In Integrating Ecology and Evolution in a Spatial Context; Silvertown, J., Antonovics, J., Eds.; Blackwell Science: Oxford, UK, 2001; pp. 21–44. [Google Scholar]
- Liu, Z.; Li, Z. Perspectives on small-scale spatial structure of plant species in plant communities. Acta Phytoecol. Sin. 2005, 29, 1020–1028. (In Chinese) [Google Scholar]
- Laungani, R.; Knops, J.M.H. Species-driven changes in nitrogen cycling can provide a mechanism for plant invasions. Proc. Natl. Acad. Sci. USA 2009, 106, 12400–12405. [Google Scholar] [CrossRef] [PubMed]
- Fibich, P.; Leps, J.; Novotny, V.; Klimes, P.; Tesitel, J.; Molem, K.; Damas, K.; Weiblen, G.D. Spatial patterns of tree species distribution in New Guinea primary and secondary lowland rain forest. J. Veg. Sci. 2016, 27, 328–339. [Google Scholar] [CrossRef]
- Deng, X.; Liu, Y.; Wu, Y. Interconnection among dominant plant populations of Castanopsis community in Jinggang Mountain Nature Reserve. Acta Phytoecol. Sin. 2003, 27, 531–536. (In Chinese) [Google Scholar]
- Read, Q.D.; Henning, J.A.; Sanders, N.J. Intraspecific variation in traits reduces ability of trait-based models to predict community structure. J. Veg. Sci. 2017, 28, 1070–1081. [Google Scholar] [CrossRef]
- García-Cervigón, A.I.; Gazol, A.; Sanz, V.; Camarero, J.J.; Olano, J.M. Intraspecific competition replaces interspecific facilitation as abiotic stress decreases: The shifting nature of plant–plant interactions. Perspect. Plant. Ecol. Evol. Syst. 2013, 15, 226–236. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Hendriks, M.; Ravenek, J.M.; Smit-Tiekstra, A.E.; Van Der Paauw, J.W.; De Caluwe, H.; Van Der Putten, W.H.; De Kroon, H.; Mommer, L.; Smit-Tiekstra, A.E. Spatial heterogeneity of plant-soil feedback affects root interactions and interspecific competition. New Phytol. 2015, 207, 830–840. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Kang, B.; Zhang, G.; Liu, P.; Du, Y.; Yu, F. Interspecific associations of dominant plant populations in secondary forest of Pinus armandii in Qinling Mountains. Sci. Silv. Sin. 2015, 51, 12–21. (In Chinese) [Google Scholar]
- Haugaasen, T.; Peres, C.A. Interspecific primate associations in Amazonian flooded and unflooded forests. Primates 2009, 50, 239–251. [Google Scholar] [CrossRef]
- Roxburgh, S.H.; Chesson, P. A new method for detecting species associations with spatially autocorrelated data. Ecology 1998, 79, 2180–2192. [Google Scholar] [CrossRef]
- Virágh, K.; Bartha, S. Interspecific associations in different successional stages of Brachypodium pinnatum grassland after deforestation in Hungary. Tiscia 1998, 31, 3–12. [Google Scholar]
- Hou, J.H.; Mi, X.C.; Liu, C.R.; Ma, K.P. Spatial patterns and associations in a Quercus-Betula forest in northern China. J. Veg. Sci. 2004, 15, 407–414. [Google Scholar]
- Leathwick, J.R.; Mitchell, N.D. Forest pattern, climate and vulcanism in central North Island, New Zealand. J. Veg. Sci. 1992, 3, 603–616. [Google Scholar] [CrossRef]
- Barot, S.; Gignoux, J.; Menaut, J.-C. Demography of a savanna palm tree: Predictions from comprehensive spatial pattern analysis. Ecology 1999, 80, 1987–2005. [Google Scholar] [CrossRef]
- Getzin, S.; Dean, C.; He, F.; Trofymow, J.A.; Wiegand, K.; Wiegand, T. Spatial patterns and competition of tree species in a Douglas-fir chronosequence on Vancouver Island. Ecography 2006, 29, 671–682. [Google Scholar] [CrossRef]


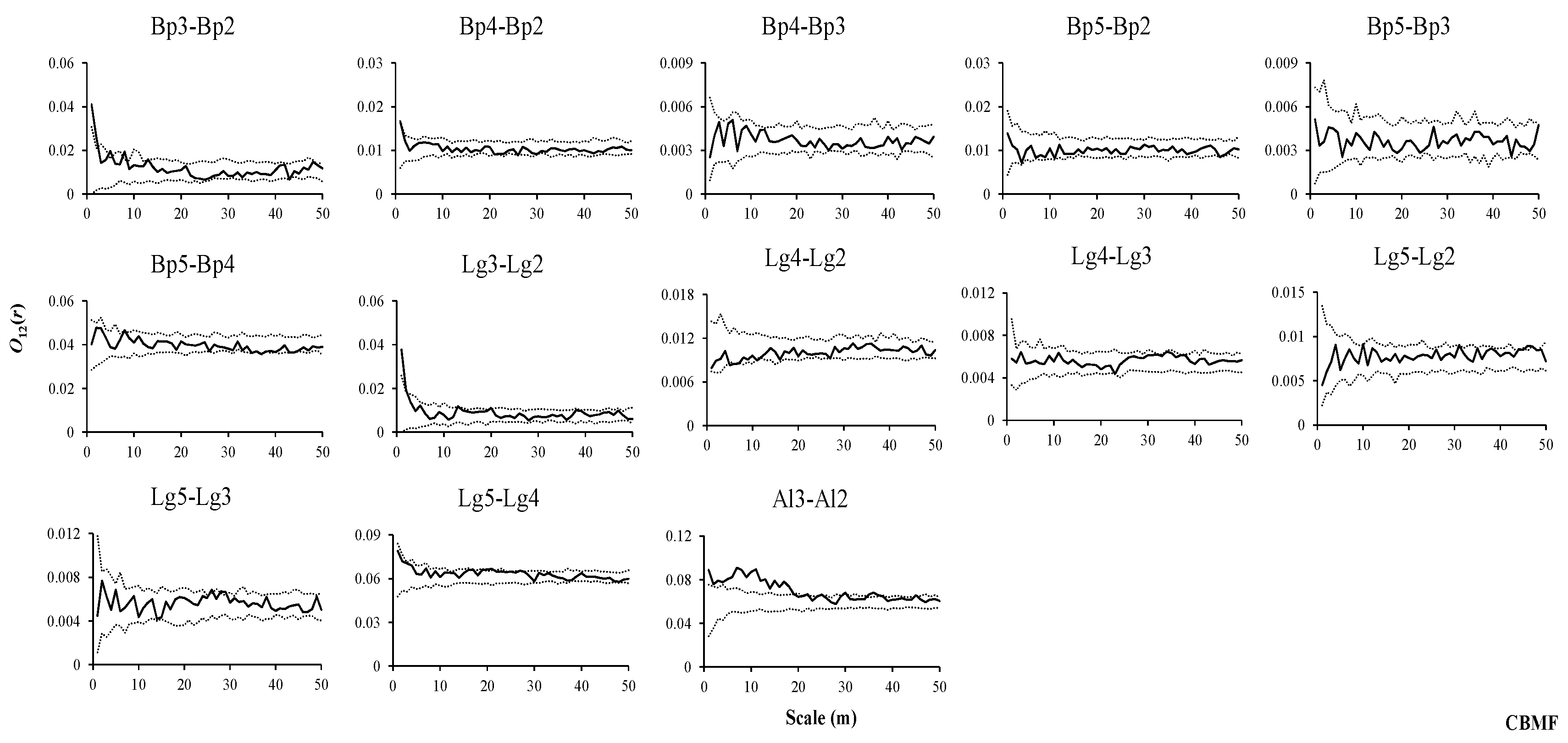


| Types | Species | Total Stems | A | B | C | D | E | BA (m2/ha) | BA (%) |
|---|---|---|---|---|---|---|---|---|---|
| Lg | 282 | 1 | 123 | 98 | 46 | 14 | 0.935 | 6.8 | |
| Bp | 1926 | 62 | 538 | 322 | 791 | 213 | 12.552 | 90.9 | |
| BF | Po | 108 | 0 | 10 | 83 | 14 | 1 | 0.117 | 0.8 |
| Al | 1285 | 0 | 795 | 464 | 26 | 0 | 0.182 | 1.3 | |
| Sa | 8 | 0 | 1 | 3 | 4 | 0 | 0.022 | 0.2 | |
| Total | 3609 | 63 | 1467 | 970 | 881 | 228 | 13.808 | 100.0 | |
| Lg | 970 | 4 | 74 | 54 | 612 | 226 | 11.434 | 58.5 | |
| Bp | 728 | 9 | 105 | 37 | 404 | 173 | 7.762 | 39.7 | |
| Ps | 8 | 0 | 0 | 0 | 4 | 4 | 0.247 | 1.3 | |
| CBMF | Pi | 2 | 0 | 0 | 0 | 1 | 1 | 0.042 | 0.2 |
| Po | 75 | 0 | 64 | 5 | 4 | 2 | 0.067 | 0.3 | |
| Al | 695 | 2 | 590 | 103 | 0 | 0 | 0 | 0 | |
| Sa | 4 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | |
| Total | 2482 | 15 | 836 | 200 | 1025 | 406 | 19.6 | 100.0 | |
| Lg | 2720 | 12 | 531 | 527 | 1537 | 113 | 13.005 | 62.0 | |
| Bp | 644 | 6 | 251 | 132 | 243 | 12 | 1.577 | 7.5 | |
| CMF | Ps | 351 | 48 | 115 | 30 | 111 | 47 | 2.424 | 11.6 |
| Pi | 1789 | 546 | 841 | 136 | 223 | 43 | 2.728 | 13.0 | |
| Po | 133 | 1 | 27 | 23 | 56 | 26 | 1.226 | 5.9 | |
| Al | 11 | 0 | 6 | 5 | 0 | 0 | 0 | 0 | |
| Sa | 125 | 2 | 105 | 18 | 0 | 0 | 0 | 0 | |
| Qm | 26 | 22 | 4 | 0 | 0 | 0 | 0 | 0 | |
| Total | 5797 | 637 | 1880 | 871 | 2170 | 241 | 20.960 | 100.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Dong, L.; Liu, Q.; Liu, Z. Spatial Patterns and Interspecific Associations During Natural Regeneration in Three Types of Secondary Forest in the Central Part of the Greater Khingan Mountains, Heilongjiang Province, China. Forests 2020, 11, 152. https://doi.org/10.3390/f11020152
Zhang L, Dong L, Liu Q, Liu Z. Spatial Patterns and Interspecific Associations During Natural Regeneration in Three Types of Secondary Forest in the Central Part of the Greater Khingan Mountains, Heilongjiang Province, China. Forests. 2020; 11(2):152. https://doi.org/10.3390/f11020152
Chicago/Turabian StyleZhang, Lingyu, Lingbo Dong, Qiang Liu, and Zhaogang Liu. 2020. "Spatial Patterns and Interspecific Associations During Natural Regeneration in Three Types of Secondary Forest in the Central Part of the Greater Khingan Mountains, Heilongjiang Province, China" Forests 11, no. 2: 152. https://doi.org/10.3390/f11020152
APA StyleZhang, L., Dong, L., Liu, Q., & Liu, Z. (2020). Spatial Patterns and Interspecific Associations During Natural Regeneration in Three Types of Secondary Forest in the Central Part of the Greater Khingan Mountains, Heilongjiang Province, China. Forests, 11(2), 152. https://doi.org/10.3390/f11020152





