Abstract
Research Highlights: Data indicated that fire severity modulates natural regeneration of Cytisus scoparius and Salix atrocinerea communities and drives much stronger effects on the germination of the dominant species. Background and Objectives: Previous studies demonstrated that fire severity induces different behaviours in plant species. Mother plant age is an important feature that must also be considered in plans of forest restoration. The objectives were to determine, in field studies, the effect of fire severity on the natural regeneration of C. scoparius and S. atrocinerea communities, to know the role of mother plant age on the germination of seeds of C. scoparius and S. atrocinerea, and to quantify their germination response at different levels of fire severity, in laboratory settings. Material and Methods: We have analysed the role of fire severity on the natural regeneration of C. scoparius and S. atrocinerea communities considering cover and height. Forty 30 × 30 m plots were randomly located in C. scoparius and S. atrocinerea communities. Fire severity on the germination of dominant species was tested through different levels of smoke, charcoal, ash, and heat. Results: High severity reduced the vertical cover and growth in height of the two communities and favoured the increase of cover of woody species in the C. scoparius community and herbaceous species in the S. atrocinerea community. Mother plant age determined germination percentages of C. scoparius seeds. Germination of C. scoparius was increased by moderate heat, and heat and smoke; and fire severity greatly reduced germination of S. atrocinerea. Conclusions: The regeneration responses after fire were largely controlled by interactions between the fire severity and the individual species regeneration strategies. For restoration purposes, C. scoparius seeds should be treated with 80 °C and smoke for 10 min, in order to increase germination; however, Salix seeds should be used without treatment and immediately after dispersion.
1. Introduction
Fire regimes are expected to change in many areas of the world due to changes in climate and land use, causing larger, more frequent, and more severe fires [1,2,3]. Southern Europe is one of the most vulnerable regions to climate change [4]. One of the most important attributes that modulates the structure of plant communities is the severity of fire (loss or change in ecosystem biomass) [5,6,7]. The severity of fire can have a strong impact on early succession after fire, which will ultimately determine the structure and functioning of forest ecosystems [8]. Understanding the relation between fire severity and the natural regeneration of communities is essential for mitigating the adverse effects of fires and for maintaining beneficial ecosystem functions and services [9].
The post-fire restoration of burned areas is a major task for international administrations and forest services. The relevance of post-fire restoration is likely to increase as fire regimes worldwide are expected to be altered, with an increase in fire recurrence, extent, and/or severity of wildfires due to the ongoing climate change [10,11].
Among other factors, natural regeneration is dependent on the regenerative strategies of the species [12]. Fire severity influences the post-fire regeneration of plants [13,14], which is also strongly dependent on the woody or herbaceous plant habits and the regenerative strategies of species [15,16]. Species from fire-prone environments have developed several strategies that allow them to survive recurrent fires: According to their post-fire regeneration, plants are divided into seeders and resprouters [17,18]. Resprouters tend to survive fire primarily by sprouting new shoots from epicormic or lignotuberous buds but can also reproduce by occasional seed germination in periods between fires [19,20].
Obligate seeders do not resprout and are often killed by fire; however, some of them depend on fire to trigger germination from a canopy or soil seed bank to provide population replacement, and thus can be short-lived with a high population turnover [20]. In the Atlantic areas of Europe, there are two species widely distributed and dominant in the mixed shrub and herbaceous communities where they occur: Cytisus scoparius L. (Link) and Salix atrocinerea Brot. These two species have very different reproductive strategies: C. scoparius is an obligate seeder shrub and S. atrocinerea is a facultative resprouter tree. Their communities are composed of woody and herbaceous species [21,22].
A major indicator of how a forest responds to fire is the reproductive strategy of the species [12,23,24]. Germination and dormancy can vary depending on the age of the mother plant. The mother plant age has remarkable effects on the progeny’s response to disturbance [25,26,27,28]. In some species with hard seeds and a persistent seed bank, mother plant age plays an important role on the germinative response to fire [29]. Thus, the age of the mother plant is an important feature that must be taken into account in studies of reproductive behaviour after fire. The severity of a fire is usually simulated through the main factors of fire: Heat and smoke in the majority and also charcoal and ash in the most complete studies [30,31].
These factors usually produce very different responses depending on the species and also on the level or quantity of the factor studied [23,32,33,34]. Although there are numerous studies on the germination response of different species in relation to fire, there are very few studies conducted on S. atrocinerea [21] and C. scoparius [35,36], and none that combine the effects of the severity of the fire and the age of the mother plant.
On the other hand, the characteristics of S. atrocinerea and C. scoparius as pioneer species, with great capacity to colonise open spaces, great seed production, and rapid growth, make them suitable species to restore burned areas [37] and minimise the invasion of degraded lands by invasive species [38]. Also, Salix is widely used in restoring damaged ecosystems. It provides a low cost-effective material for stabilisation and restoration of disturbed landscapes, phytoremediation, both riparian and upland erosion control, and biomass production [39].
We hypothesize that fire severity determines the natural regeneration of S. atrocinerea and C. scoparius communities, influences the germination behaviour of these two dominant species, and drives different regeneration patterns on communities with contrasting reproductive strategies of dominant species. For these reasons, the following objectives were proposed:
(1) To determine the effect fire severity on the natural regeneration of woody and herbaceous species of C. scoparius and S. atrocinerea communities considering cover and height.
(2) To know the role of mother plant age on the germination of seeds of C. scoparius and S. atrocinerea, to quantify their germination response at different levels of fire severity through the main factors of fire (smoke, charcoal, ash, and temperature) and, in species with fire stimulating effects, to evaluate combined fire factors on germination of seeds of mother plants with different ages.
2. Materials and Methods
2.1. Biological Material
Scotch broom, C. scoparius (Leguminosae), is a long-lived shrub native to Western and Central Europe [40]. Pioneer and ubiquitous, it grows in moors, coppices, and understories, from plains to 1700 m in altitude. It has been widely introduced as an ornamental plant and it can now be found naturalised in temperate areas of eastern and western USA and Canada, Hawaii, Chile and Argentina, eastern New Zealand, south-eastern Australia, India, Iran, Japan, and Western Cape, South Africa [22,41,42,43,44,45]. Sexual maturity has been observed after 3 years [45]. Mature populations with more than 7 years of age reached 3500 seeds/m2 [46] and buried seeds can remain dormant in the soil for periods of up to 60–80 years [47,48]. C. scoparius seeds have a hard cover and are dispersed by spontaneous opening of fruit or dehiscence [49]. Seeds with impermeable coatings, like Cytisus seeds, usually exhibit an important physical dormancy [50]. C. scoparius seeds are dispersed by pod over a short distance. This species shows seed dormancy and develops a persistent seed-bank [45,51].
Willow, S. atrocinerea (Salicaceae) is a fast growing tree found in southwest, central and northern Europe, and northern Africa. It is found in permanently moist soils, nitrified or not, that are preferably poor in bases. The maximum altitude that it can reach is 2000 m [52]. It is a species of riparian environments or flooded soils. A mature population of Salix sp. can produce 7650 seeds m−2·year−1 [53]. Seed dispersal is favoured by wind and water [54]. Salix seeds are very lightweight (<0.005 g) could produce about 5·106 seeds kg−1 [55] and they lose their viability very quickly in natural conditions [56], (personal observation), with 50% reduction in just one or two weeks [56]. Therefore, water availability at this stage is essential. The direct sun requirement for development (heliophilic) and its rooting capability from plant segments enable this species to colonise open environments [57,58,59,60].
2.2. Study Areas
The study was developed in an Atlantic coastal region, located in the southwest of Europe (Figure 1A). The two study areas were Monte Pindo (Pindo, A Coruña, Spain, UTM 29T 490710 4748473, ETRS89), for the field study and for C. scoparius seed collection, and Brañas of Reveirugas (Baio, A Coruña, Spain, UTM 29T 503600 4779811, ETRS89) for S. atrocinerea seed collection. The seeds were picked in 2015; C. scoparius seeds in July and S. atrocinerea in May. C. scoparius is a seeder species and the community of old C. scoparius was established after a fire occurred 10 years before seed collection. S. atrocinerea old plants had been established in the area 13 years before and had not experienced fire or cutting. The young mother plants were established in an area that suffers periodic disturbances, the last disturbance took place 3 years ago (personal observation).
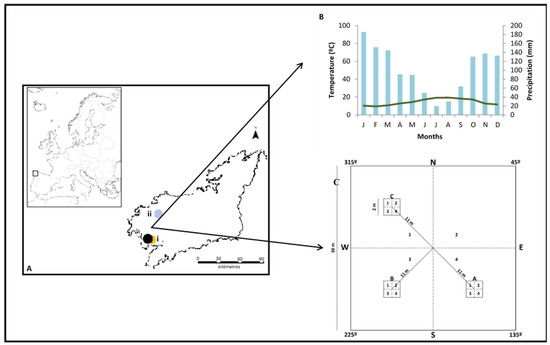
Figure 1.
(A) Map of Europe with the situation of the province of A Coruña where the two sampling areas stand in: (i) Monte Pindo, where the black circle is the area affected by the fire and the orange circle the control zone and (ii) Brañas of Reveirugas. (B) Ombrothermic diagram of the studied area. (C) Scheme of the plot of 30 m × 30 m, the 2 m × 2 m subplots, and the 1 m × 1 m units.
These areas have a granitic substrate [61] and acidic soils. Average annual rainfall and temperature are 1470 mm and 12.5 °C respectively [62]. Precipitation is distributed throughout the year and there is no summer drought (Figure 1B). Monte Pindo experienced a big fire in September 2013 that burned 1.900 ha of forested lands and left burned areas with varying degrees of severity. Before the fire, the dominant vegetation in Monte Pindo were Pinus pinaster Aiton pine forests with undergrowth of Ulex europaeus L., C. scoparius, Rubus ulmifolius Schott, Halimium lasianthum (Lam.) and wet areas were dominated by S. atrocinerea. In Brañas of Reveirugas the dominant vegetation was S. atrocinerea tree stand with Betula celtiberica Rothm. and Vasc. and Alnus glutinosa (L.) Gaertn intermixed and of U. europaeus and Genista berberidea Lange undergrowth.
2.3. Post-Fire Regeneration
To reach objective 1, areas of different severity (high severity (HS), and low severity (LS)) were selected in the Pindo Mount study area. In order to allocate field plots in all burn severity situations we followed a stratified random design. Specifically, Calvo et al. performed the burn severity map [63] of the last fire using the differenced normalized burn ratio (dNBR) [64], which is the standard burn severity spectral index [6]. The data source used to calculate this index were Landsat 8 scenes from August 30th 2013 and September 15th 2013. Landsat images, which have a spatial resolution of 30 m × 30 m, were atmospherically corrected using the FLAASH (The Fast Line-of-sight Atmospheric Analysis of Spectral Hypercubes) algorithm and topographically corrected with the C-correction, obtaining the land surface reflectance for each band (see Fernández-García et al. for further details [6,65]). Then the dNBR was calculated as follows:
- NBR = (ρ5 − ρ7)/(ρ5 + ρ7)
- Dnbr = (NBRpre − NBRpost) − offset.
NBR being the normalized burn ratio index; ρ5, the land surface reflectance of Landsat 8 band 5 (Near Infrared); ρ7, the land surface reflectance of Landsat 8 band 7 (Short Wave Infrared 2); dNBR, the differenced normalized burn ratio with values ranging from −2 to 2; and offset, the average dNBR value from pixels in homogeneous unburned areas.
The burned area was classified into low and high severity categories using the dNBR value of 0.55 as the threshold [13,65]. We distribute field plots proportionally to each severity category, establishing a minimum of five plots.
After severity determination, stratified sampling was carried out. We considered an ecosystem of C. scoparius or S. atrocinerea provided that the dominant species was present in the 12 sampling units of 1 × 1 m. Forty 30 × 30 m plots were randomly located in ecosystems of C. scoparious and S. atrocinerea. Ten were in high severity and ten were in low severity in each community. In addition, 10 control plots (not burned) (at least 15 years without burning) were sampled, five with the presence of C. scoparius and five with the presence of S. atrocinerea. Burned and unburned plots were separated between 0.9 and 6.5 km. In each plot, three subplots of 2 × 2 m were delimited. The subplots were divided in four 1 × 1 m sampling units (Figure 1C). Inside every sampling unit, the following measures were taken by visual estimates: the vertical cover, that is the percentage of vegetation cover present in different layers located vertically from the ground up, in three vertical stratum (0–1 m, 1–4 m, >4 m); the percentage of horizontal cover of herbaceous species and woody species; and the average height of the community. To determine the height of the community, one measurement was taken in each sampling unit of 1 × 1 m, obtaining 12 height datapoints by plot. The horizontal cover and the height of the community are good indicators of the speed of recovery of the vegetation. The vertical cover serves to identify if the architecture of the vegetation has been recovered. For these reasons, we collected all three measures. Plant cover and height were measured during the summers of 2015, 2016, and 2017.
2.4. Viability and germination
Since, in preliminary essays C. scoparius presented low germination, the seeds of this species were subjected to a viability test to check the health of the seed. Scarified seeds of C. scoparius were subjected to a solution of 2,3,3-triphenyl tetrazolium chloride salt at 1%. Five replicates, of 25 seeds each, were made [29,38]. The seeds were kept in the solution for 48 h in the dark and then all tincted seeds were recorded as live seeds. Also, in preliminary essays, S. atrocinerea presented a very high germination, which indicated a high viability as well.
To achieve objective 2, C. scoparius seeds from mother plants of different ages: 3 and 10 years old (Young and Old plants respectively, henceforth YP and OP) were picked in Monte Pindo. On the other hand, seeds of S. atrocinerea from a population with two different ages were selected in Brañas of Reveirugas. Mother plants were 3 and 13 years old (Young and Old plants respectively).
The treatments were carried out for both species following the methodology of Cruz et al. [29]. From each treatment and mother plant age, five replications of 25 seeds were made per Petri dish, following ISTA (International Seed Testing Association) standards [66].
The fire-related factors tested were smoke, charcoal, ash, and heat. Smoke treatments were obtained by exposing the seeds for 5, 10, and 15 min to a smoke-saturated atmosphere following the methodology of other works [23,67,68]. Two charcoal treatments were applied, one with charcoal from the studied species (C. scoparius or S. atrocinerea) and the other one with charcoal from U. europaeus, the most abundant shrub species in the understory of forest ecosystems in northwestern Spain. The seeds were incubated in Petri dishes on 0.26 g (411 kg/ha) of charcoal from the corresponding species. This is the equivalent amount of charcoal measured by Ohlson and Tryterud [69] after experimental fires in the boreal forests of Scandinavia and is the dose used in previous works [70].
The applied treatments of ash were Ash1 (0.027 g/Petri dish, 43.5 kg/ha), Ash2 (0.055 g/Petri dish, 87 kg/ha), Ash3 (0.11 g/Petri dish, 174 kg/ha), Ash4 (0.275 g/Petri dish, 435 kg/ha), and Ash5 (0.55 g/Petri dish, 870 kg/ha). These quantities correspond to multiples of the quantity collected by Soto el al. [71] following a controlled burning conducted in an Atlantic shrubland of southwestern Europe. The ash was obtained from the total combustion and the charcoal from the partial combustion (~20 min) of dried material (principally branches and leaves) of C. scoparius and S. atrocinerea. Ash was separated from charcoal and other fractions with a 0.4 mm mesh size sieve and the charcoal from the rest of the charred material with a 2.1 mm mesh size sieve. Ash and charcoal were then placed on the respective dishes and the seeds were sown on top.
A forced air oven was used to apply the thermal shocks to the seeds. The tested temperatures were 80 °C, 110 °C, 150 °C, and 200 °C and the exposure times were 5 and 10 min. Temperatures and exposure times correspond to those registered at different soil depths during forest fires and experimental burns by Auld and O’Connell [72] and Bradstock and Auld [73] in Mediterranean ecosystems of the south-east of Australia, by Dunn and DeBano [74] in chaparrals of California, by Trabaud [75] in French garrigues and Díaz-Fierros et al. [76] in shrublands of northwestern Spain.
Lastly, in species stimulated by fire the combined effects of the fire factors were tested. We selected the treatments that increased germination the most and we performed the following combined treatments:
- SCAH: Smoke 10 min + C. scoparius Charcoal + Ash 1 + 80 °C, 10 min
- SH: Smoke 10 min + 80 °C, 10 min
- CA: C. scoparius Charcoal + Ash 1
Immediately after the application of each treatment, every Petri dish was watered with 4 mL of distilled water and thereafter with a suitable amount of water needed to allow germination of the seeds. The seeds were incubated in a germination chamber. Following other authors [36,68], the incubation photoperiod was 16 h of light at 24 °C and 8 h of darkness at 16 °C; to simulate the thermophotoperiod which coincides with that registered in the northwest of the Iberian Peninsula during summer. Germination was monitored three times every week until the germination ended (several days without germinated seeds in both treatments).
With the viability data of the seeds, the percentage of viability was calculated, and with the germination data, the percentage of germination, and the germination rate through T50 (average of the time it takes to produce 50% of the total germination) were calculated.
2.5. Statistical Procedures
C. scoparius and S. atrocinerea fire responses were statistically analysed through both the data obtained in post-fire regeneration and in the germination tests.
Linear mixed-effects models were used to evaluate the effects of Severity and Years after fire on post-fire regeneration with a 0.05 level of significance. Independent analyses for C. scoparius and S. atrocinerea were carried out in a fully factorial analysis with the fixed factors Severity and Year. The random effects of Sampling Unit and Subplot, subsequently nested within the random variable Plot, were added to account for the spatial relationships in the field data. Control plots were also included in the analysis as unburnt. The LSD (Least Significant Difference) method was used as a post-hoc test.
A fully factorial General linear model (GLM) with a 0.05 level of significance was used to test the effects of fire, scarification treatments, and mother plant age on the germination percentage and T50 of C. scoparius and S. atrocinerea seeds. In order to meet the assumptions of the analysis, the germination percentage and the T50 data were square-root transformed. Treatments with less than three replicates over 0% germination were removed from the T50 analysis. Tukey tests were used for a posteriori comparisons.
To perform these statistics, IBM SPSS Statistics 24 was used.
3. Results
3.1. Natural Regeneration
The vertical structure of both communities reflects important differences between them; the community of C. scoparius is a high shrubland community, in which the development of the layer over 4 m high is scarce and, in contrast, the community of S. atrocinerea is a forest community that tends to increase the cover of the upper stratum over time (Table 1). After the fire, the community of C. scoparius quickly recovered the 0–1 m stratum, exceeding the values of the unburnt community (49.5%) both in LS (81.4% after 2 years, 84.6% after 3 years, and 86% after 4 years) and in HS (92.9% after 2 years, 94.8% after 3 years, and 95.9% after 4 years). There are highly significant differences between unburnt and post-fire cover at both levels of severity (p < 0.001).

Table 1.
The stratum cover (x±sd) reached in unburnt areas and burned areas 2, 3, and 4 years after fire with different fire severity levels in the C. scoparius and S. atrocinerea communities.
The second stratum, 1–4 m high, reaches 63.3% of cover in the unburned community and after fire began a slow recovery that was stronger in LS areas (11.6% after 2 years, 19.2% after 3 years, and 22.8% after 4 years) than in HS areas (5.5% after 2 years, 5.3% after 3 years, and 10.4 after 4 years). Also in this second layer, the differences recorded between the unburnt area and burnt area with high and low severity were statically significant (p < 0.001). The third stratum, more than 4 m high, shows an average value of 38.7% in unburned areas, after fire only in areas of low severity. Some cover was detected and it was lower below 1% in the 4 years post-fire.
Highly significant differences were detected between the unburnt areas and burnt areas, LS and HS burnt areas (p < 0.001). In the unburnt S. atrocinerea community the 0–1 m stratum presented high percentage of cover (80.8%), after LS of fire plants cover recovered quickly and in the second year (74.1%) it is near to the unburnt value. There were no significant differences between the unburnt cover and LS burnt cover in 0–1 m stratum (Table 1). However, after HS fire, the regeneration of vegetation was so fast that 3 and 4 years after, the post-fire cover (85.1% and 89.3% respectively) surpassed the pre-fire cover which led to significant differences between them (p < 0.001, Table 2).

Table 2.
T50 (x ± sd) reached by C. scoparius and S. atrocinerea seeds of Old and Young mother plants under fire treatments.
The unburnt 1–4 m layer is little dense (32.5%) and after fire recovered more slowly in HS areas than in LS areas, but even after 4 years the cover percentages were lower than those of unburnt areas (p < 0.001, Table 2). The >4 m stratum presented 77.1% of cover pre-fire and it was significantly different to all cover values after fire. Natural regeneration of this stratum was very slow. Two, 3, and 4 years after fire in LS areas had percentages under 1%. In HS areas the >4 m layer totally disappeared.
In terms of horizontal cover, we found that the woody cover of the C. scoparius community and S. atrocinerea community tended to increase over time after the Monte Pindo 2013 wildfire (Figure 2a,b, p < 0.001). In the unburnt area, the C. scoparius community covered 91.4% of land and, 4 years after fire, reached 60.7% in LS and 75.8% in HS. In this community, the woody cover reached during the third and fourth year after fire was significantly greater than that obtained during the second year (Figure 2a). Fire severity affected woody species cover so that the C. scoparius community reached greater cover in HS areas (Figure 2a, p < 0.001). In the S. atrocinerea community, woody cover of the unburned areas (97.7%) was significantly greater than that achieved in the post-fire years studied (67.3% in LS and 66% in HS after 4 years of post-fire regeneration). In this community, each year the woody cover increased significantly compared to the previous one (Figure 2b, p < 0.001), but the severity did not significantly affect the regeneration of the woody cover (Figure 2b).
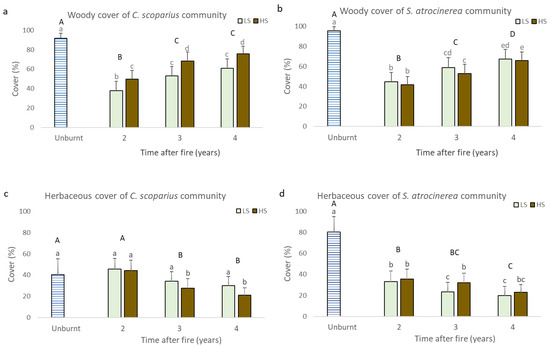
Figure 2.
(a–d): The horizontal cover of woody and herbaceous species reached in unburnt areas and burned areas 2, 3, and 4 years after fire with different fire severity levels in C. scoparius and S. atrocinerea communities. a: woody cover of C. scoparius community, b: woody cover of S. atrocinerea community, c: herbaceous cover of C. scoparius community, d: herbaceous cover of S. atrocinerea community, HS: high severity, LS: low severity. Different lowercase letters indicate differences between severities and different uppercase letters indicate differences between unburnt and post-fire years.
The herbaceous cover of the C. scoparius community was significantly lower in the unburnt areas (40.6%) than in the burnt areas (62.9% in LS and 72.6% in HS after 4 years post-fire regeneration), with both LS and HS (p < 0.001, Figure 2c,d). In the S. atrocinerea community, the herbaceous cover of unburnt areas (80.2%) was significantly higher than that of burnt areas (45.5% in LS and 66.8% in HS areas after 4 years post-fire regeneration) (p < 0.001, Figure 2d). After fire, herbaceous cover did not change over time either in the C. scoparius community (Figure 2c, p = 0.980) or in the S. atrocinerea community (Figure 2d, p = 0.197), but the severity did significantly affect regeneration in each year of study (p < 0.001 in both communities). The cover obtained in HS areas was greater than LS areas.
The average height of the C. scoparius community, in 4 years of vegetation development (102.5 cm in LS and 74.3 cm in HS), did not reach the height of the unburned community (223.0 cm). Yet, we detected a significant increase in the height over time (Figure 3a, p < 0.001) and LS favoured the growth in height of this community during the third and fourth year after fire (p < 0.001). In the S. atrocinerea community, differences between the average height of the unburned community (800.2 cm) and the regenerated community (99.5 cm in LS and 62.8 cm in HS) were even more noticeable (Figure 3b). Significant differences were detected between the unburned community and the regenerated community at 2, 3, and 4 years after fire (p < 0.001), and also between the two levels of severity within each post-fire year studied (p < 0.001).
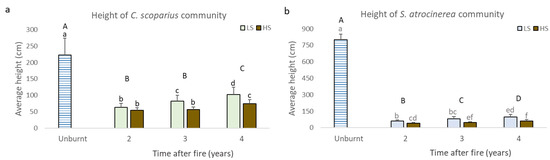
Figure 3.
(a,b): The average height reached in unburnt areas and burned areas 2, 3, and 4 years after fire with different fire severity level in the C. scoparius and S. atrocinerea communities. a: height of C. scoparius community, b: height of S. atrocinerea community, HS: high severity, LS: low severity. Different lowercase letters indicate differences between severities and different uppercase letters indicate differences between unburnt and post-fire years.
3.2. Viability and Germination Response
The viability of C. scoparius seeds was 100% in seeds from both mother plant ages. With respect to S. atrocinerea, some replicates of different treatments of both plant mother age reached 100% germination, and the viability of S. atrocinerea was high as well.
C. scoparius seeds of both ages presented important differences in the percentage of germination, then the seeds from Young Plants (YP) obtained an average value of 9.9% and the seeds from Old Plants (OP) obtained a value of 12.3%. Major differences were found among the other treatments and the applied GLM detected highly significant differences between the seeds from different mother plant ages (p < 0.001).
Within each age, the different treatments provided very different germination, and the GLM detected significant differences (p < 0.001) between the control and the fire treatments. The control germination percentages of C. scoparius were low; 4% for seeds from YP and 3.2% for seeds from OP (Figure 4a). Smoke treatments and charcoal treatments did not significantly modify the germination of the seeds from the two age classes of mother plants. The treatments of Ash 1, Ash 2, and Ash 3 in seeds from both mother plant ages, and Ash 4 in seeds from OP did not modify the germination of C. scoparius. However, Ash 4, in seeds of YP, and Ash 5, in all the seeds, completely annulled their germination.
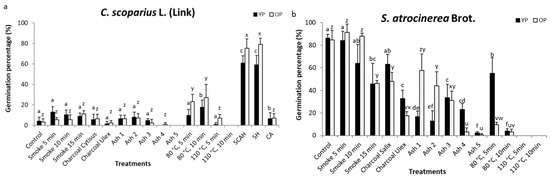
Figure 4.
The germination percentages (x ± sd) reached by C. scoparius (a) and S. atrocinerea (b) seeds of young and old plants after fire treatments. Different letters on the bars indicate significant differences between the treatments and the control of the same mother plant ages.
The heat treatments produced different germinative responses depending on the intensity of the treatment, 80 °C, 10 min, in seeds from YP; and 80 °C, 5 min, and 80 °C, 10 min, in seeds from OP, increased germination. The increase was eight times the control value with the last treatment. On the other hand, 80 °C, 5 min and 110 °C, 5 min, in seeds from YP, and 110 °C, 5 min, in seeds from OP, did not modify the control germination while 110 °C, 10 min or more severe heat treatments completely inhibited the germination of seeds from both mother plant ages.
Since the seeds of this species act positively upon stimulation, combined treatments were performed. We found that the combination of smoke, charcoal, ash, and heat (SCAH), on the one hand, and smoke and heat (SH), on the other, produced a very sharp increase in germination reaching levels of 60% for seeds from YP and 80% in seeds from OP (Figure 4a). This behaviour multiplied germination control of the seeds by 15 from YP and by 26 those of OP. Conversely, the combined treatment of charcoal and ash (CA) did not significantly modify the germination.
Among the treatments, significant differences were also found in S. atrocinerea (p < 0.001). The S. atrocinerea percentage of germination in the Control treatment reached 86.4% in seeds from YP and 84.8% in seeds from OP (Figure 4b). The smoke treatments Smoke 5 min and Smoke 10 min provided very high germination percentages and similar to the control, being higher in the seeds from OP than in seeds from YP. Within 15 min of exposure to smoke there was a significant reduction of germination in the seeds of both age classes, reaching 45.6% and 46.4% of germination in seeds from YP and OP respectively. Salix charcoal produced germination similar to the Control in seeds from YP (63.2%) but significantly lower in the seeds from OP (48%). Ulex charcoal significantly reduced germination at half the Control level in the seeds from YP (32.8%) and a quarter in the seeds from OP (17.6%).
All the ash treatments tested reduce the germination of the seeds of mother plants of both ages, but in the seeds from YP, Ash1, Ash2 and Ash 5 produced very strong inhibitions giving percentages of 16.8%, 12.8%, and 2.4% respectively, while with Ash 3 and Ash 4 the percentages of germination are slightly higher (33.6% and 23.2% respectively). In the seeds from OP, the reduction in germination is greater with a higher ash concentration, reaching values of between 57.6% with Ash 1 and 0.8% with Ash 5. The heat treatments also strongly inhibit germination and with 110 °C, 5 min the inhibition is total. It should be noted that seeds from YP are more resistant to the negative effects of heat and germinate more (55.2%) than seeds from OP (13.6%) when subjected to 80 °C for 5 min.
3.3. Germination Timing (T50)
On average, half of the C. scoparius germination occurs in 45.8 days in seeds from YP and in 46.9 days in seeds from OP (Table 2). The T50 Control of seeds from YP was 23 days, while the T50 Control of seeds from OP was 66 days. A tendency can be highlighted in both ages: As the applied treatments (smoke, ash, or thermal shock) increased in intensity level, the T50 value increased; however, these values were never significantly larger than for the control treatments. The combined treatments carried out reached T50 between 40 and 70 days, depending on the treatment and the age analysed. Despite these differences, there were no significant differences between the T50 values of seeds from the two mother plant ages, nor between fire treatments (p = 0.930).
The germination of S. atrocinerea seeds (6.1 ± 0.5 days) were very fast compared with the germination of C. scoparius seeds (46.4 ± 15 days, Table 2). The germination of S. atrocinerea occurred between 4 and 10 days in almost all the treatments carried out on this species, both in YP seeds and in OP seeds and there were no significant differences between them (6.2 days in YP seeds and 6 days in OP seeds). Nevertheless, there were significant differences between some fire treatments and the Control (p < 0.001). The T50 value of S. atrocinerea in the Control was around 5 days for both ages. There were high significant differences between the Control and Charcoal Ulex, Ash3, Ash4, Ash5, 80 °C, 5 min, and 80 °C, 10 min in YP seeds and only 80 °C, 10 min in OP seeds. Likely, Ash4 and Ash5 would also be significantly different if there were enough replicates with germination. The treatments of smoke, Salix charcoal, and low doses of ash did not modify the speed of germination.
4. Discussion
Fire severity modulates the natural regeneration of the communities of C. scoparius and S. atrocinerea and performs much stronger effects on the germination of these species.
4.1. Fire Effects on Natural Regeneration
Fire severity affected the recovery of the C. scoparius community and S. atrocinerea community upper stratum, so that the more the severity the lower the recovery. The physiognomy of both communities before the fire was distinct and reflected in their vertical structure. The most abundant stratum in the C. scoparius community was intermediate stratum, while in the S. atrocinerea community the low and high stratum were dominant. The fire simplified the vertical structure of both communities turning them into communities dominated by the low stratum and with near elimination of the high stratum. Álvarez et al. [77] found this same effect in forests of Quercus pyrenaica; Willd and Muñoz et al. [21] in the heathlands of Erica ciliaris Loefl. ex L. and Erica tetralix L.
C. scoparius and S. atrocinerea are the taller and more abundant species of their respective communities, likely, they will need more than four years to regenerate the upper stratum, even in a favourable microclimate for plant growth, such as southwestern Europe [78]. Dzwonko et al. [79] also found different effects of fire severity in the different vertical stratum of Salix cinerea L. in forests of Poland and, in their study, the upper stratum did not begin to recover until the seventh year after fire.
In the C. scoparius community, woody cover remained below the unburnt community cover, with increases year after year. The larger increase being in HS areas over LS areas. The studied C. scoparius community was composed of several species with stimulated germination by fire as C. scoparius [36], (this study), U. europaeus [80], or Halimiun alyssoides (Lam.) Greuter [36] had a very abundant soil seed bank [81,82,83]. Probably high temperatures and smoke of HS areas strongly stimulated the germination of the seeds of soil bank [36] this study, resulting in a large number of seedlings in the first year after fire that increased in cover over time.
Fernández-García et al. [13] found that HS favoured development of cover of shrub species in P. pinaster ecosystems with very similar species composition of underwood, and Bowman et al. [84] found the same in Eucalyptus delegatensis F.Muell. ex R.T.Baker ecosystems (an ecosystem dominated by a seeder species and with a very different specific composition). The herbaceous species cover reached the cover of the unburnt community in the second year post-fire and after that it decreased, particularly in HS areas. Likely, the greatest development of woody cover reduced light intake into the lower strata [85] and that led to a lower cover over time and less in HS than in LS.
In the S. atrocinerea community, four years of regeneration was not enough to match the woody and herbaceous cover of the unburnt community. S. atrocinerea disperses its seeds in early spring and does not form persistent seed banks. Rather, its seeds germinate quickly or die within a few weeks [56], personal observation. The Monte Pindo 2013 large fire occurred in the summer and at that time there were no seeds available to germinate, both in the burned area and outside of it. The post-fire regeneration of S. atrocinerea initially occurred only after regrowth. In later years, seeds from unburnt areas could have arrived but competition for resources was already high in the second post-fire year. As in the C. scoparius community, the decline of the herbaceous cover of S. atrocinerea community over time could be related to woody cover increases [86].
Severity reduced growth in the height of the two communities. The average height of both communities began to recover slowly after the fire and fire severity marked differences in regeneration, with recovery being faster in LS areas more than in HS areas. The lower height growth in the HS areas may be due to lower soil fertility. In acidic soils, like those in this study, Fernández-García et al. [87] found inverse relationships between various soil properties (microbial biomass, phosphatase, β-glucosidase, and organic C) and fire severity. Also, for a month and a half after the Monte Pindo fire, there was very heavy rainfall (305 L/m−2) with 1 episode of rain of 61.8 L/m−2 in 24 h and three episodes of rain with higher amounts up to 30 L/m−2 in 24 h [62]. Due to the abundant rainfall and steep slope (approximately 25%), massive amounts of soil and fertility were dragged. The damage was probably greater in the HS than in the LS areas as the stability of the soil aggregates and the soil organic carbon decreases with severity [88].
4.2. Mother Plant Effect
The mother plant effect on the germination of the two studied species were different. The mother plant age determined germination percentages of C. scoparius seeds, but not of S. atrocinerea, and seeds of OP germinated more in response to fire cues (heat and smoke). Germination speed was independent of the mother plant age in both species. This also occurs in other species such as P. pinaster and Acacia melanoxylon R.Br. [29,89].
4.3. Fire factors Effects
Fire severity, through combustion or lethal heating during burning, directly determines the availability of post-fire in situ propagules generated [90]; however, it can also lead to greater recruitment of seedlings and resprouts [12,38] and, likely, this affects the natural regeneration of communities. Our results showed that the germination behaviour of C. scoparius and S. atrocinerea were strongly influenced by certain fire factors. They did not modify the germination rate of C. scoparius but exerted large effects on the germination percentage. Smoke did not modify the control germination of C. scoparius but did on S. atrocinerea; it was reduced after 15 min of exposure to smoke.
In other studies with leguminous species such as C. scoparius and Cytisus striatus (Hill) Rothm. [36] or Acacia melanoxylon [67] no significant effects of smoke on germination were found. In contrast, Pérez-Fernández and Rodríguez-Echevarria [91] detected smoke stimulating effects in Trifolium angustifolium L. and Retama sphaerocarpa (L.) Boiss with exposure to smoke for 10 min but not for 20 min. Reyes and Trabaud [92] detected germination inhibition of Cistus albidus L., Fumana ericoides (Cav.) Gand., Bituminaria bituminosa (L.) C.H.Stirt. and Emerus major Mill. It is possible that each species has a different sensitivity to smoke and that short exposure times stimulate or maintain it around control values. An extended exposure to smoke would likely lead to germination reduction.
Charcoal did not modify C. scoparius germination and reduced S. atrocinerea germination, with Ulex charcoal having a stronger inhibition effect than Salix charcoal. In other leguminous species, charcoal inhibition of germination was not found either, such as Acacia dealbata Link. and A. melanoxylon [70] or T. angustifolium and R. sphaerocarpa [91]. The charcoal effect seem to depend on the nature of the charcoal [70].
Thermal shocks of 80 °C for 5 and 10 min stimulated the germination of the seeds of C. scoparius OP. The seeds from C. scoparius YP only were stimulated to germinate with exposures to 80 °C for a period of 10 min. In addition, the combined treatments that included heat and smoke (SCAH and SH) greatly stimulated the germination of C. scoparius by multiplying the control germination of the seeds of young plants by 15 and that of mature plants by 26 [93]. It also found stimulation of germination by smoke or heat in 77% of 30 Mediterranean species [93].
More severe heat treatments inhibited the germination of C. scoparius seeds of both mother plant ages. Cocks and Stock [94] found optimal germination of the fynbos legume species to be between 80 °C and 100 °C; whereas the highest mortality occurred at longer durations of 100 °C exposure and 120 °C. Similar trends were found by Auld and O’Connell [72], whereas Riveiro et al. [31] determined that exposure to 150 °C is lethal to seeds of Daucus carota L., Helichrysum foetidum (L.) Moench., and Oenothera glazioviana Micheli.
The seeds of S. atrocinerea showed a high control germination in a very short time (85% in 7 days), a characteristic shared with other Salix species such as Salix caroliniana Michx. [95], and a large reduction in their percentage and Germination speed with virtually all fire factors. Only the smoke for 5 and 10 min did not change its control percentages. The seeds of YP resist well at 80 °C for 5 min and the seeds of OP for the low doses of ash. Therefore, after a fire, the recovery of S. atrocinerea populations, through germination, would be limited to seeds from unburned areas and for the short period of time during which the seeds are dispersed and viable. However, this resprouting strategy allows Salix species a very good recovery [96,97] (this study).
5. Conclusions
The regeneration response after fire in Monte Pindo was largely controlled by interactions between fire severity and species regeneration strategies, as found in many other studies of mediterranean ecosystems [63,98,99]. The C. scoparius community seemed to be better adapted to fire than the S. atrocinerea community, as its woody horizontal cover 4 years after fire was very close to the unburnt plots cover. The woody cover was greater in high-severity areas that in those of low severity, and the germination of C. scoparius seeds was greatly stimulated by heat and particularly, by the combination of heat and smoke. In addition, C. scoparius seeds are viable for a long time and form abundant soil seed banks, from which they can restore their populations after fire.
In contrast, the horizontal woody cover of the S. atrocinerea community did not approach that of the unburnt community. Recovery was similar in low-severity and high-severity areas, and fire factors tended not to inhibit or increase S atrocinerea germination. Also, the seed viability of S. atrocinerea is lost quickly, preventing the formation of a seed bank. The germination results provide an important reference for post-fire management. When using C. scoparius seeds for revegetation, for example, they should be treated previously with a combined heat treatment of 80 °C and smoke for 10 min, as it is cheap and very effective. However, Salix seeds should be used without treatment and immediately after dispersion.
Author Contributions
Conceptualization: Ó.C.; Methodology: Ó.C. and O.R.; Formal Analysis: Ó.C. and J.G.-D.; Investigation: C.G.-G. and Ó.C.; Resources: Ó.C., O.R. and J.G.-D.; Writing—original draft: Ó.C. and O.R.; Writing—review and editing: Ó.C., S.F.R., M.C. and O.R.; Visualization: Ó.C.; Project Administration: O.R.; Funding acquisition: M.C. and O.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Spanish Ministry of Economy, Industry and Competitiveness, the Spanish Ministry of Science, Innovation and Universities, the Castilla y León Regional Government, the Galicia Regional Government and the European Regional Development Fund (ERDF) in the framework of the GESFIRE (AGL2013-48189-C2-2-R), FIRESEVES (AGL2017-86075-C2-2-R) and SEFIRECYL (LE001PE17) projects and the Competitive Reference Group BIOAPLIC (ED431C2019/07) and the Strategic Researcher Cluster BioReDeS (ED431E 2018/09).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brondízio, E.S.; Gatzweiler, F.W.; Zografos, C.; Kumar, M.; Kadekodi, G.K.; McNeely, J.A.; Xu, J.; Martinez-Alier, J. The Socio-Cultural Context of Ecosystem and Biodiversity Valuation. In The Economics of Ecosystems and Biodiversity: Ecological and Economic Foundations; Routledge: London, UK, 2012; pp. 149–182. [Google Scholar] [CrossRef]
- Aragão, L.E.O.C.; Anderson, L.O.; Fonseca, M.G.; Rosan, T.M.; Vedovato, L.B.; Wagner, F.H.; Silva, C.V.J.; Silva Junior, C.H.L.; Arai, E.; Aguiar, A.P.; et al. 21st Century Drought-Related Fires Counteract the Decline of Amazon Deforestation Carbon Emissions. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; Laudicina, V.A.; Parra, A.; Albert-Belda, E.; Moreno, J.M. Drought and Its Legacy Modulate the Post-Fire Recovery of Soil Functionality and Microbial Community Structure in a Mediterranean Shrubland. Glob. Chang. Biol. 2019, 25, 1409–1427. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate Change Projections for the Mediterranean Region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Keeley, J.E.; Pausas, J.G.; Rundel, P.W.; Bond, W.J.; Bradstock, R.A. Fire as an Evolutionary Pressure Shaping Plant Traits. Trends Plant Sci. 2011, 16, 406–411. [Google Scholar] [CrossRef]
- Fernández-García, V.; Santamarta, M.; Fernández-Manso, A.; Quintano, C.; Marcos, E.; Calvo, L. Burn Severity Metrics in Fire-Prone Pine Ecosystems along a Climatic Gradient Using Landsat Imagery. Remote Sens. Environ. 2018, 206, 205–217. [Google Scholar] [CrossRef]
- Andrus, R.A.; Veblen, T.T.; Harvey, B.J.; Hart, S.J. Fire Severity Unaffected by Spruce Beetle Outbreak in Spruce-Fir Forests in Southwestern Colorado. Ecol. Appl. 2016, 26, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, J.F.; Chapin, F.S.; Hollingsworth, T.N.; Mack, M.C.; Romanovsky, V.; Turetsky, M. Fire, Climate Change, and Forest Resilience in Interior Alaska1. Can. J. For. Res. 2010, 40, 1302–1312. [Google Scholar] [CrossRef]
- Fang, L.; Yang, J.; White, M.; Liu, Z. Predicting Potential Fire Severity Using Vegetation, Topography and Surface Moisture Availability in a Eurasian Boreal Forest Landscape. Forests 2018, 9, 130. [Google Scholar] [CrossRef]
- Westerling, A.L.; Hidalgo, H.G.; Cayan, D.R.; Swetnam, T.W. Warming and Earlier Spring Increase Western U.S. Forest Wildfire Activity. Science 2006, 313, 940–943. [Google Scholar] [CrossRef]
- Keane, R.E.; Cary, G.J.; Parsons, R. Using Simulation to Map Fire Regimes: An Evaluation of Approaches, Strategies, and Limitations. Int. J. Wildl. Fire 2003, 12, 309–322. [Google Scholar] [CrossRef]
- Van Leeuwen, M.; Nieuwenhuis, M. Retrieval of Forest Structural Parameters Using LiDAR Remote Sensing. Eur. J. For. Res. 2010, 129, 749–770. [Google Scholar] [CrossRef]
- Fernández-García, V.; Fulé, P.Z.; Marcos, E.; Calvo, L. The Role of Fire Frequency and Severity on the Regeneration of Mediterranean Serotinous Pines under Different Environmental Conditions. For. Ecol. Manag. 2019, 444, 59–68. [Google Scholar] [CrossRef]
- Brais, S.; David, P.; Ouimet, R. Impacts of Wild Fire Severity and Salvage Harvesting on the Nutrient Balance of Jack Pine and Black Spruce Boreal Stands. For. Ecol. Manag. 2000, 137, 231–243. [Google Scholar] [CrossRef]
- Pausas, J.G. Response of Plant Functional Types to Changes in the Fire Regime in Mediterranean Ecosystems: A Simulation Approach. J. Veg. Sci. 1999, 10, 717–722. [Google Scholar] [CrossRef]
- Broncano, M.J.; Retana, J. Topography and Forest Composition Affecting the Variability in Fire Severity and Post-Fire Regeneration Occurring after a Large Fire in the Mediterranean Basin. Int. J. Wildl. Fire 2004, 13, 209–216. [Google Scholar] [CrossRef]
- Keeley, J.E. Resilience of mediterranean shrub communities to fires. In Resilience in Mediterranean-Type Ecosystems. Tasks for Vegetation Science; Dell, B., Hopkins, A.J.M., Lamont, B.B., Eds.; Springer: Dordrecht, The Netherlands, 1986; Volume 16. [Google Scholar]
- Agee, J.K. The Landscape Ecology of Western Forest Fire Regimes. Northwest Sci. 1998, 72, 24–34. [Google Scholar]
- Lamont, B.B.; Wiens, D. Are Seed Set and Speciation Rates Always Low among Species That Resprout after Fire, and Why? Evol. Ecol. 2003, 17, 277–292. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Evolutionary Ecology of Resprouting and Seeding in Fire-Prone Ecosystems. New Phytol. 2014, 204, 55–65. [Google Scholar] [CrossRef]
- Muñoz, A.; Alvarez, R.; Pesqueira, X.; García Duro, J.; Reyes, O.; Casal, M. Burning in the Management of Heathlands of Erica Ciliaris and Erica Tetralix: Effects on Structure and Diversity. NACC Nova Acta Científica Compostelana 2010, 19, 69–81. [Google Scholar]
- Peterson, D.J.; Prasad, R. The Biology of Canadian Weeds. 109. Cytisus scoparius (L.) Link. Can. J. Plant Sci. 1998, 78, 497–504. [Google Scholar] [CrossRef]
- Reyes, O.; Casal, M. Regeneration Models and Plant Regenerative Types Related to the Intensity of Fire in Atlantic Shrubland and Woodland Species. J. Veg. Sci. 2008, 19, 575–583. [Google Scholar] [CrossRef]
- Denham, A.J.; Whelan, R.J.; Auld, T.D.; Denham, R.J. The Coupling of Recruitment and Disturbance by Fire in Two Resprouting Proteaceae Species. Plant Ecol. 2011, 212, 471–481. [Google Scholar] [CrossRef]
- Andersson, L.; Milberg, P. Variation in Seed Dormancy among Mother Plants, Populations and Years of Seed Collection. Seed Sci. Res. 1998, 8, 29–38. [Google Scholar] [CrossRef]
- Aly, M.M.; El-Sayed, H.E.A.; Jastaniah, S.D. Synergistic Effect between Azotobacter vinelandii and Streptomyces sp. Isolated From Saline Soil on Seed Germination and Growth of Wheat Plant. J. Am. Sci. 2012, 88, 667–676. [Google Scholar]
- Latzel, V.; Dospělová, L.; Klimešová, J. Annuals Sprouting Adventitiously from the Hypocotyl: Their Compensatory Growth and Implications for Weed Management. Biologia 2009, 64, 923–929. [Google Scholar] [CrossRef]
- Latzel, V.; Klimešová, J. Transgenerational Plasticity in Clonal Plants. Evol. Ecol. 2010, 24, 1537–1543. [Google Scholar] [CrossRef]
- Cruz, O.; García-Duro, J.; Casal, M.; Reyes, O. Can the Mother Plant Age of Acacia melanoxylon (Leguminosae) Modulate the Germinative Response to Fire? Aust. J. Bot. 2017, 65, 593–600. [Google Scholar] [CrossRef]
- García-Duro, J.; Cruz, O.; Casal, M.; Reyes, O. Fire as Driver of the Expansion of Paraserianthes lophantha (Willd.) I. C. Nielsen in SW Europe. Biol. Invasions 2019, 21, 1427–1438. [Google Scholar] [CrossRef]
- Riveiro, S.F.; García-Duro, J.; Cruz, Ó.; Casal, M.; Reyes, O. Fire Effects on Germination Response of the Native Species Daucus carota and the Invasive Alien Species Helichrysum foetidum and Oenothera glazioviana. Glob. Ecol. Conserv. 2019, 20, e00730. [Google Scholar] [CrossRef]
- Calvo, L.; Hernández, V.; Valbuena, L.; Taboada, A. Provenance and Seed Mass Determine Seed Tolerance to High Temperatures Associated to Forest Fires in Pinus pinaster. Ann. For. Sci. 2016, 73, 381–391. [Google Scholar] [CrossRef]
- Herranz, J.M.; Ferrandis, P.; Martínez-Sánchez, J.J. Influence of Heat on Seed Germination of Nine Woody Cistaceae Species. Int. J. Wildl. Fire 2000, 9, 173–182. [Google Scholar] [CrossRef]
- Zupo, T.; Jaime Baeza, M.; Fidelis, A. The Effect of Simulated Heat-Shock and Daily Temperature Fluctuations on Seed Germination of Four Species from Fire-Prone Ecosystems. Acta Bot. Bras. 2016, 30, 514–519. [Google Scholar] [CrossRef]
- Tarrega, R.; Calvo, L.; Trabaud, L. Effect of High Temperatures on Seed Germination of Two Woody Leguminosae. Vegetatio 1992, 102, 139–147. [Google Scholar] [CrossRef]
- Rivas, M.; Reyes, O.; Casal, M. Influence of Heat and Smoke Treatments on the Germination of Six Leguminous Shrubby Species. Int. J. Wildl. Fire 2006, 15, 73–80. [Google Scholar] [CrossRef]
- Fernández-Abascal, I.; Tárrega, R.; Luis-Calabuig, E. Ten Years of Recovery after Experimental Fire in a Heathland: Effects of Sowing Native Species. For. Ecol. Manag. 2004, 203, 147–156. [Google Scholar] [CrossRef]
- Arán, D.; García-Duro, J.; Cruz, O.; Casal, M.; Reyes, O. Understanding Biological Characteristics of Acacia melanoxylon in Relation to Fire to Implement Control Measurements. Ann. For. Sci. 2017, 74. [Google Scholar] [CrossRef]
- Kuzovkina, Y.A.; Knee, M.; Quigley, M.F. Cadmium and Copper Uptake and Translocation in Five Willow (Salix L.) Species. Int. J. Phytoremediat. 2004, 6, 269–287. [Google Scholar] [CrossRef]
- González-André, F.; Ortiz, J.M. Leaf and Stem Anatomy of Species of Cytisophyllum, Cytisus, Chamaecytisus, Genista. Sect. Teline (Fabaceae: Genisteae) as an Add for Taxonomy. Isr. J. Plant Sci. 1994, 42, 213–225. [Google Scholar] [CrossRef]
- Barkley, T.M.; Holm, L.; Pancho, J.V.; Herberger, J.P.; Plucknett, D.L. A Geographical Atlas of World Weeds. Brittonia 1980, 32, 127. [Google Scholar] [CrossRef]
- Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia, 2nd ed.; Cuthbertson, E.G., Ed.; Inkata Press: Melbourne, Australia, 2001. [Google Scholar]
- Cullen, J.; Julien, M.; McFadyen, R. Biological Control of Weeds in Australia; CSIRO Publishing: Melbourne, Australia, 2019. [Google Scholar] [CrossRef]
- Impacts of Broom (Cytisus scoparius) in Western North America. Available online: https://www.cabdirect.org/cabdirect/abstract/20003030302 (accessed on 22 October 2019).
- Memmott, J.; Fowler, S.V.; Syrett, P.; Hosking, J.R. What Makes Broom a Major Problem. In Proceedings of the Brighton Crop Protection Conference, Weeds, Brighton, UK, 22–25 November 1993; British Crop Protection Council (BCPC): Thornton Heath, Surrey, 1993; pp. 753–758. [Google Scholar]
- Hoshovsky, M. Element Stewardship Abstract for Cytisus scoparius and Genista monspessulanus; The Nature Conservancy: Arlington County, VA, USA, 1986. [Google Scholar]
- Schmid, R.; Bossard, C.C.; Randall, J.M.; Hoshovsky, M.C. Invasive Plants of California’s Wildlands. Taxon 2001, 50, 627. [Google Scholar] [CrossRef]
- Oneto, S.R.; Kyser, G.B.; DiTomaso, J.M. Efficacy of Mechanical and Herbicide Control Methods for Scotch Broom (Cytisus scoparius) and Cost Analysis of Chemical Control Options. Invasive Plant Sci. Manag. 2010, 3, 421–428. [Google Scholar] [CrossRef]
- Magda, D.; Chambon-Dubreuil, E.; Agreil, C.; Gleizes, B.; Jarry, M. Demographic Analysis of a Dominant Shrub (Cytisus scoparius): Prospects for Encroachment Control. Basic Appl. Ecol. 2009, 10, 631–639. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Paynter, Q.; Fowler, S.V.; Memmott, J.; Shaw, R.H.; Sheppard, A.W. Determinants of Broom (Cytisus scoparius (L.) Link) Abundance in Europe. Plant Prot. Q. 2000, 15, 149–155. [Google Scholar]
- USDA; ARS; National Genetic Resources Program. Germplasm Resources Information Network—(GRIN) [Base de Datos en Línea]; National Germplasm Resources Laboratory: Beltsville, Maryland, 2019; 20. Available online: http://www.ars-grin.gov/cgi-bin/npgs/html/index (accessed on 20 December 2019).
- Gage, E.A.; Cooper, D.J. Patterns of Willow Seed Dispersal, Seed Entrapment, and Seedling Establishment in a Heavily Browsed Montane Riparian Ecosystem. Can. J. Bot. 2005, 83, 678–687. [Google Scholar] [CrossRef]
- Rey, F.; Labonne, S. Resprout and Survival of Willow (Salix) Cuttings on Bioengineering Structures in Actively Eroding Gullies in Marls in a Mountainous Mediterranean Climate: A Large-Scale Experiment in the Francon Catchment (Southern Alps, France). Environ. Manag. 2015, 56, 971–983. [Google Scholar] [CrossRef]
- Catalán, G. Semillas de árboles y Arbustos Forestales; Instituto Nacional para la Conservación de la Naturaleza. Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1991; pp. 145–147. [Google Scholar]
- Karrenberg, S.; Suter, M. Phenotypic Trade-Offs in the Sexual Reproduction of Salicaceae from Flood Plains. Am. J. Bot. 2003, 90, 749–754. [Google Scholar] [CrossRef]
- Zsuffa, L. Genetic Improvement of Willows for Energy Plantations. Biomass 1990, 22, 35–47. [Google Scholar] [CrossRef]
- Lindegaard, K.N.; Barker, J.H.A. Breeding Willows for Biomass. Asp. Appl. Biol. Biomass Energy Crop. 1996, 49, 155–162. [Google Scholar]
- Larsson, S. Genetic Improvement of Willow for Short-Rotation Coppice. Biomass Bioenergy 1998, 15, 23–26. [Google Scholar] [CrossRef]
- Smart, L.B.; Volk, T.A.; Lin, J.; Kopp, R.F.; Phillips, I.S.; Cameron, K.D.; White, E.H.; Abrahamson, L.P. Genetic Improvement of Shrub Willow (Salix sp.) Crops for Bioenergy and Environmental Applications in the United States. Unasylva 2005, 56, 51–55. [Google Scholar]
- Ferrero, A.; Fernández, Á.; Pérez, J.; Rubio, F.; Baltuille, J. Mapa de Rocas y Minerales Industriales de Galicia a Escala 1:250.000; Xunta de Galicia: Santiago, España, 2009. [Google Scholar]
- MeteoGalicia. Available online: https://www.meteogalicia.gal/web/inicio.action (accessed on 22 October 2019).
- Calvo, L.; Tárrega, R.; Valbuena, L.; Marcos, E.; Taboada, A.; Fernández-García, V.; Fernández-Guisuraga, J.M.; Fernández Manso, A.; Quintano, C.; de Luis, E.; et al. Climatic Conditions and Fire Regime Affect Vegetation Recovery after Large Wildfires in Pinus Forest Ecosystems. In Advances in Forest Fire Research 2018; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2018; pp. 209–217. [Google Scholar] [CrossRef][Green Version]
- Key, C.H. Ecological and Sampling Constraints on Defining Landscape Fire Severity. Fire Ecol. 2009, 2, 34–59. [Google Scholar] [CrossRef]
- Fernández-García, V.; Quintano, C.; Taboada, A.; Marcos, E.; Calvo, L.; Fernández-Manso, A. Remote sensing applied to the study of fire regime attributes and their influence on post-fire greenness recovery in pine ecosystems. Remote Sens. 2018, 10, 733. [Google Scholar] [CrossRef]
- ISTA. International Seed Testing Association. Available online: https://www.seedtest.org/en/home.html (accessed on 22 October 2019).
- Arán, D.; García-Duro, J.; Reyes, O.; Casal, M. Fire and Invasive Species: Modifications in the Germination Potential of Acacia melanoxylon, Conyza canadensis and Eucalyptus globulus. For. Ecol. Manag. 2013, 302, 7–13. [Google Scholar] [CrossRef]
- Reyes, O.; García-Duro, J.; Salgado, J. Fire Affects Soil Organic Matter and the Emergence of Pinus radiata Seedlings. Ann. For. Sci. 2015, 72, 267–275. [Google Scholar] [CrossRef]
- Ohlson, M.; Tryterud, E. Interpretation of the Charcoal Record in Forest Soils: Forest Fires and Their Production and Deposition of Macroscopic Charcoal. Holocene 2000, 10, 519–525. [Google Scholar] [CrossRef]
- Reyes, O.; Kaal, J.; Arán, D.; Gago, R.; Bernal, J.; García-Duro, J.; Basanta, M. The Effects of Ash and Black Carbon (Biochar) on Germination of Different Tree Species. Fire Ecol. 2015, 11, 119–133. [Google Scholar] [CrossRef]
- Soto, B.; Basanta, R.; Diaz-Fierros, F. Effects of Burning on Nutrient Balance in an Area of Gorse (Ulex europaeus L.) Scrub. Sci. Total Environ. 1997, 204, 271–281. [Google Scholar] [CrossRef]
- Auld, T.D.; O’Connell, M.A. Predicting Patterns of Post-fire Germination in 35 Eastern Australian Fabaceae. Aust. J. Ecol. 1991, 16, 53–70. [Google Scholar] [CrossRef]
- Bradstock, R.A.; Auld, T.D. Soil Temperatures During Experimental Bushfires in Relation to Fire Intensity: Consequences for Legume Germination and Fire Management in South-Eastern Australia. J. Appl. Ecol. 1995, 32, 76. [Google Scholar] [CrossRef]
- Dunn, P.H.; DeBano, L.F. Fire’s Effect on Biological and Chemical Properties of Chaparral Soils. In The Proceedings of Symposium on Environmental Conservation: Fire and Fuel Management in Mediterranean Ecosystems; USDA Forest Service, Washington Office: Washington, DC, USA, 1977; pp. 75–84. [Google Scholar]
- Trabaud, L. Etude Du Comportement Du Feu Dans La Garrigue de Chêne Kermès à Partir Des Températures et Des Vitesses de Propagation. Ann. Sci. For. 1979, 36, 13–38. [Google Scholar] [CrossRef]
- Díaz-Fierros, F.; Benito, E.; Vega, J.A.; Castelao, A.; Soto, B.; Pérez, R.; Taboada, T. Solute Loss and Soil Erosion in Burnt Soils from Galicia (NW Spain). In Fire and Ecosystem Dynamics; SPB Academy Publishing: The Hague, The Netherlands, 1990; pp. 103–116. [Google Scholar]
- Álvarez, R.; Muñoz, A.; Pesqueira, X.M.; García-Duro, J.; Reyes, O.; Casal, M. Spatial and Temporal Patterns in Structure and Diversity of Mediterranean Forest of Quercus pyrenaica in Relation to Fire. For. Ecol. Manag. 2009, 257, 1596–1602. [Google Scholar] [CrossRef]
- Núñez-Regueira, L.; Rodríguez-Añón, J.; Proupín, J.; Romero-García, A. Energy Evaluation of Forest Residues Originated from Pine in Galicia. Bioresour. Technol. 2003, 88, 121–130. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Loster, S.; Gawroński, S. Impact of Fire Severity on Soil Properties and the Development of Tree and Shrub Species in a Scots Pine Moist Forest Site in Southern Poland. For. Ecol. Manag. 2015, 342, 56–63. [Google Scholar] [CrossRef]
- González-Rabanal, F.; Casal, M. Effect of High Temperatures and Ash on Germination of Ten Species from Gorse Shrubland. Vegetatio 1995, 116, 123–131. [Google Scholar] [CrossRef]
- Reyes, O.; Quintero, A. Influencia Del Fuego Sobre El Banco de Semillas Del Suelo de Leguminosas Arbustivas de Cinco Comunidades Vegetales. In Montes para la Sociedad del Nuevo Milenio, Proceedings of the III Congreso Forestal Español, Graficas Coria, Sevilla, Spain, 26 September 2001; de Andalucía, J., Ed.; SECF: Sevilla, España, 2001; Volume Tomo VI, pp. 456–462. [Google Scholar]
- Valbuena, L.; Trabaud, L. Contribution of the Soil Seed Bank to Post-Fire Recovery of a Heathland. Plant Ecol. 2001, 152, 175–183. [Google Scholar] [CrossRef]
- Trabaud, L. Life and Environment in the Mediterranean; WIT Press: Southampton, UK, 2000. [Google Scholar]
- Bowman, D.M.J.S.; Murphy, B.P.; Neyland, D.L.J.; Williamson, G.J.; Prior, L.D. Abrupt Fire Regime Change May Cause Landscape-Wide Loss of Mature Obligate Seeder Forests. Glob. Chang. Biol. 2014, 20, 1008–1015. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-Regulated Plant Growth and Development. In Current Topics in Developmental Biology; Academic Press Inc.: Cambridge, MA, USA, 2010; Volume 91, pp. 29–66. [Google Scholar] [CrossRef]
- Thrippleton, T.; Bugmann, H.; Kramer-Priewasser, K.; Snell, R.S. Herbaceous Understorey: An Overlooked Player in Forest Landscape Dynamics? Ecosystems 2016, 19, 1240–1254. [Google Scholar] [CrossRef]
- Fernández-García, V.; Marcos, E.; Fernández-Guisuraga, J.M.; Taboada, A.; Suárez-Seoane, S.; Calvo, L. Impact of Burn Severity on Soil Properties in a Pinus pinaster Ecosystem Immediately after Fire. Int. J. Wildl. Fire 2019, 28, 354–364. [Google Scholar] [CrossRef]
- Francos, M.; Pereira, P.; Mataix-Solera, J.; Arcenegui, V.; Alcañiz, M.; Úbeda, X. How Clear-Cutting Affects Fire Severity and Soil Properties in a Mediterranean Ecosystem. J. Environ. Manag. 2018, 206, 625–632. [Google Scholar] [CrossRef]
- Cruz, O.; García-Duro, J.; Casal, M.; Reyes, O. Role of Serotiny on Pinus pinaster Aiton Germination and Its Relation to Mother Plant Age and Fire Severity. iForest 2019, 12, 491–497. [Google Scholar] [CrossRef]
- Wang, G.G.; Kemball, K.J. Effects of Fire Severity on Early Development of Understory Vegetation. Can. J. For. Res. 2005, 35, 254–262. [Google Scholar] [CrossRef]
- Pérez-Fernández, M.A.; Rodríguez-Echeverría, S. Effect of Smoke, Charred Wood, and Nitrogenous Compounds on Seed Germination of Ten Species from Woodland in Central-Western Spain. J. Chem. Ecol. 2003, 29, 237–251. [Google Scholar] [CrossRef]
- Reyes, O.; Trabaud, L. Germination Behaviour of 14 Mediterranean Species in Relation to Fire Factors: Smoke and Heat. Plant Ecol. 2009, 202, 113–121. [Google Scholar] [CrossRef]
- Moreira, B.; Tormo, J.; Estrelles, E.; Pausas, J.G. Disentangling the Role of Heat and Smoke as Germination Cues in Mediterranean Basin Flora. Ann. Bot. 2010, 105, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Cocks, M.P.; Stock, W.D. Heat Stimulated Germination in Relation to Seed Characteristics in Fynbos Legumes of the Western Cape Province, South Africa. S. Afr. J. Bot. 1997, 63, 129–132. [Google Scholar] [CrossRef]
- Castro-Morales, L.M.; Quintana-Ascencio, P.F.; Fauth, J.E.; Ponzio, K.J.; Hall, D.L. Environmental Factors Affecting Germination and Seedling Survival of Carolina Willow (Salix caroliniana). Wetlands 2014, 34, 469–478. [Google Scholar] [CrossRef]
- Stein, S.J.; Price, P.W.; Abrahamson, W.G.; Sacchi, C.F. The Effect of Fire on Stimulating Willow Regrowth and Subsequent Attack by Grasshoppers and Elk. Oikos 1992, 65, 190. [Google Scholar] [CrossRef]
- Lee, M.A.B.; Snyder, K.L.; Valentine-Darby, P.; Miller, S.J.; Ponzio, K.J. Dormant Season Prescribed Fire as a Management Tool for the Control of Salix caroliniana Michx. in a Floodplain Marsh. Wetl. Ecol. Manag. 2005, 13, 479–487. [Google Scholar] [CrossRef]
- Paula, S.; Arianoutsou, M.; Kazanis, D.; Tavsanoglu, Ç.; Lloret, F.; Buhk, C.; Ojeda, F.; Luna, B.; Moreno, J.M.; Rodrigo, A.; et al. Fire-Related Traits for Plant Species of the Mediterranean Basin. Ecology 2009, 90, 1420. [Google Scholar] [CrossRef]
- Díaz-Delgado, R.; Lloret, F.; Pons, X. Influence of Fire Severity on Plant Regeneration by Means of Remote Sensing Imagery. Int. J. Remote Sens. 2003, 24, 1751–1763. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).