Abstract
Acacia spp. are widespread all over the Portuguese territory, representing a threat to local biodiversity and to the productivity of the forest sector. The measures adopted in some countries for their eradication or to control their propagation are expensive, have been considered unfeasible from practical and economical perspectives, and have generated large amounts of residue that must be valorized in a sustainable way. This review brings together information on the valorization of bark, wood, leaves, flowers, pods, seeds, roots, and exudates from Acacia spp., through the production of high-value bioactive extracts (e.g., antioxidant, antimicrobial, anti-inflammatory, antidiabetic, antiviral, anthelmintic, or pesticidal agents, suitable to be explored by pharmaceutical, nutraceutical, cosmetics, and food and feed industries), its incorporation in innovative materials (e.g., polymers and composites, nanomaterials, low-cost adsorbents), as well as through the application of advanced thermochemical processes (e.g., flash pyrolysis) and pre-treatments to decompose biomass in its structural components, regarding the production of biofuels along with valuable chemicals derived from cellulose, hemicellulose, and lignin. The knowledge of this research is important to encourage an efficient and sustainable valorization of Acacia spp. within a biorefinery concept, which can bring a significant economic return from the valorization of these residues, simultaneously contributing to forest cleaning and management, to reduce the risk of fires, and to improve the social-economic development of rural areas.
1. Introduction
The genus Acacia comprises a large group of more than 1350 species, widely distributed throughout tropical and warm temperate areas of the world [1]. Most of the species are native to Australia but are spread all over the world because of their wide variety of uses and economic importance such as for ornamental purposes, for sand and dune stabilization, as a fuel through the production of woodfuel and charcoal, as an important source of fodder, tannins for the leather industry, gums, and essences for perfumes [2,3,4]. In the Portuguese territory its introduction was mainly for stabilization of dunes and soil [5], as well as for ornamental purposes [6].
However, its worldwide cultivation has led to its establishment and invasion in many regions of the world, with negative impacts on biodiversity and on ecosystem properties and functions [7]. Some key features contributing to the success of Acacia spp. as invaders include their phenotypic plasticity, which confers them with the ability to adapt to changing environments; their large seed production and accumulation of massive seed banks for long periods; their high capacity for vegetative reproduction from rhizomes after disturbances, such as fires and cuttings; and their allelopathic properties, inhibiting the growth of neighboring native species [8,9,10].
Acacia spp. are considered invasive in the Portuguese territory [11], where the occupied area almost doubled from 1995 to 2010, passing from 2701 ha to 5351 ha, according to the last national forest inventory [12]. Amongst invasive Acacia spp., A. cyclops A. Cunn. ex G. Don fil., A. dealbata Link, A. longifolia (Andrews) Willd., A. melanoxylon R. Br., A. saligna (Labill.) H. L. Wendl., A. retinodes Schlecht., A. karroo Hayne, A. mearnsii De Wild. and A. pycnantha Bentham are the most representative. Figure 1 shows examples of invaded areas by Acacia spp. in the Portuguese territory.
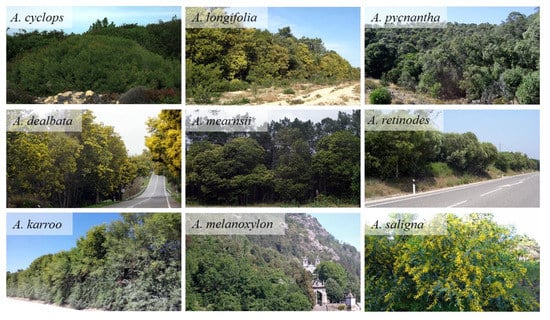
Figure 1.
Examples of invaded areas by Acacia spp. in the Portuguese territory (elaborated from images taken from Plantas Invasoras em Portugal (http://invasoras.pt), accessed on 1 November 2020).
The threat to protected areas, and the negative impacts on the ecosystems and on the productivity of the forest sector have motivated expensive removal initiatives by local authorities in order to minimize its proliferation [13], generating high amounts of biomass which is usually burned for energy production or landfilled. The valorization of some Acacia spp. for energy may have a positive contribution to the economic sustainability of the involved operations [14], but this option is often limited due to collection and transportation costs. However, finding high-value applications for these species can contribute to overcome these costs and make the process economically sustainable. Furthermore, given the magnitude of the invasion, the radicular system of these species, the dispersion of seeds in the natural environments, and the years necessary before soil nutrients and processes return to similar pre-invasion levels, their elimination by removal has been considered unfeasible from practical and economical perspectives [15], and has the potential of by itself to cause significant environmental impacts, namely the destabilization of large volumes of soil.
Acacia spp. fractions, namely bark, wood, leaves, flowers, pods, seeds or roots, are rich sources of bioactive secondary metabolites (e.g., amines and alkaloids, cyanogenic glycosides, cyclitols, fatty acids and seed oils, gums, non-protein amino acids, terpenes, tannins and other flavonoids and simple phenolics) [4,16,17] that have been used in traditional medicine for a wide range of ailments, such as diabetes, worm infection, dysmenorrhea, eczema, malaria, gout, jaundice, abdominal pain, kidney problems, constipation, leprosy, piles, pneumonia, rheumatism, fever and cancer [18]. These secondary metabolites can play an important role in reducing oxidative stress, by acting as antioxidants, and possess antimicrobial properties, which are important in the development of alternatives to antibiotics due to the increasing resistance to the conventional antimicrobial agents [18]. Besides, other important biological activities, such as anti-inflammatory, antiviral, anticancer, antidiabetic, immunomodulatory, hepatoprotective, cardioprotective and anthelmintic, were already reported for extracts obtained from Acacia spp. [18] In this way, the production of bioactive preparations of Acacia spp., either from well-established areas or from the biomass generated in the control operations, can be an option to bring an important economic return, overcoming expenses with collection and transport, while simultaneously contributing to motivating forest cleaning and management actions, reducing the risk of fires and improving the social-economic development of rural areas. Moreover, the valorization of its flowers, as an example, will have a positive contribution to the control of the proliferation by preventing the formation of seeds, thus minimizing the spread through the seed dispersal route [19]. Other well-studied applications of Acacia spp. biomass fractions with the potential to contribute to the industrial exploitation of these species, and therefore to the control of their unregulated widespread, include its incorporation in polymers and composites, micro and nanomaterials or as adsorbents.
This review presents various high-value applications of different biomass fractions from Portuguese invasive Acacia spp. (e.g., bark, wood, leaves, flowers, pods, seeds and roots) Besides the potential applications in the lucrative nutraceutical, pharmaceutical, cosmetic and food and feed industries, the production of innovative materials and biofuels will be also discussed. Under the current transition towards a biobased economy, the cost-efficient use of biomass for the production of biobased products and energy is facilitated by biorefining systems [20]. Therefore, the knowledge of these options is essential to enable the valorization of this resource using a biorefinery approach, focused on the sustainable processing of biomass to yield a spectrum of marketable products and energy [21]. It has been strongly recommended by specialists the search for these novel integrative and cost-effective solutions, involving the collaboration of society, politicians and stakeholders, as necessary to achieve sustainable control of acacias [15].
2. Bioactive Extracts from Acacia spp. Plant Components
2.1. Bark
Overall, the bark is a by-product and is highly available from the adopted proliferation control operations or from the logging industries, whose disposal may cause environmental problems. For example, the bark of A. dealbata is frequently removed without felling the tree to reduce or prevent sprouting, and the bark of A. melanoxylon can come from industries that valorize the high-quality wood of this species [22]. Research has been conducted regarding the production of high-value products extracted from this biomass fraction, where it can be seen that Acacia spp. are one of a few 24 species that currently have commercialized products retrieved from their bark, especially for the leather and adhesive industries [23,24]. Recent research has shown that the bark of Acacia spp. contains important chemicals with the potential to be applied in recently growing and profitable industries, such as nutraceutical, cosmetic, pharmaceutical or food industries (see Table 1).

Table 1.
Reported biological activities for extracts of Acacia spp. bark.
2.1.1. Commercial Tannin-Rich Extracts From Bark
The most known class of compounds present in the bark of Acacia spp. are the flavonoids, where tannins, and more specifically the proanthocyanidins, are included. After the discovery of the incredible superoxide scavenging activity of an aqueous extract obtained from black wattle (A. mearnsii), approximately 10 times higher than that of the pine bark supplements Pycnogenol® and Enzogenol®, clinical studies were made on the human safety use of this extract, which has been commercialized in Japan since 2007 with the label Acapolia® [38]. The biological activities demonstrated by the proanthocyanidins derived from A. mearnsii bark, including antioxidant, antimicrobial, anti-obesity, antidiabetic, anti-inflammatory, among others, are extensively reviewed in the literature, the role of proanthocyanidin oligomers having 5-deoxyflavan-3-ol units and the presence of distinctive flavan-3-ols like such as robinetinidol and fisetinidol being highlighted [33,34,38]. Another field of application of tannin bark extract from Acacia spp. is their inclusion in animal feed. Several studies used commercial tannin-rich extract obtained from A. mearnsii bark and highlighted its effect as a methane-mitigating agent for dairy cows [35]; in decreasing the urinary excretion of urea in sheep [39]; in reducing the urinary nitrogene excretion and improving the amino acids supply in Holstein steers without significantly affecting the total organic matter digestibility [36]; or as anthelmintic agents in sheep artificially infected with parasites Trichostrongylus colubriformis and Haemonchus contortus [37]. However, precautions must be taken and further research must be developed, since reducing effects on in vivo nutrient digestibility and/or negative impacts on energy intake were observed in sheep and wethers [40,41].
2.1.2. Antioxidants, Anti-Inflammatory and Anti-Viral Agents from Bark
Sowndhararajan et al. [25] compared acetone and methanol as extraction solvents of previously petroleum ether defatted bark of Indian acacias, including A. dealbata, aiming to obtain antioxidants. The authors observed better outcomes using acetone, with remarkable results from free radical scavenging assays against DPPH, ABTS and OH free radicals, and from ferric-reducing antioxidant power, metal chelation, phosphomolybdenum reduction and peroxidation inhibition assays, which were comparable or in some cases higher than those obtained with the widely used antioxidants BHT (butylated hydroxytoluene) and α-tocopherol. The authors found strong positive correlations between these antioxidant activities and the phenolic and flavonoid contents of the extracts. This acetone extract also demonstrated effective inhibition of H2O2-mediated oxidative stress in human hepatoma (HepG2) cells, in a concentration-dependent manner (25, 50 and 75 mg/mL), which gives it the potential to be explored as a therapeutic agent in preventing oxidative stress-mediated diseases [26].
Treating inflammation has become the focus of global scientific research because of its implication in virtually all human and animal diseases, and because conventional drugs used to ameliorate this phenomenon are too expensive, toxic, and are not commonly available to the entire population of the world [30]. Therefore, research has been done to find anti-inflammatory agents of natural origin, with Acacia spp. contributing in a significant manner to this purpose. Sowndhararajan et al. [28] demonstrated that an acetone extract of A. dealbata bark effectively inhibited the production of nitric oxide and suppressed the expressions of cyclooxygenase (COX-2), inducible nitric oxide synthase (iNOS) and tumor necrosis factor (TNF-α) in lipopolysaccharide (LPS) -stimulated RAW 264.7 macrophage cells, in a concentration-dependent manner (25, 50 and 75 mg/mL). These results suggest its potential use as a source of anti-inflammatory agents by suppressing the expression of pro-inflammatory mediators. Similarly, hydroalcoholic extracts from the bark of A. longifolia, A. farnesiana and A. tortilis showed anti-inflammatory activity by inhibition of COX-1 and COX-2 enzymes [32]. An in vivo study demonstrated the anti-inflammatory and also the analgesic activity of an aqueous extract from the stem bark of A. karroo, even when used by the oral route [30]. Anti-inflammatory activity was accessed using the carrageenan-induced and histamine-induced rat paw oedema models, a strong reduction of the formation of oedema at 100 and 200 mg/kg doses for both models being observed. Analgesic activity was evaluated in mice using acetic acid-induced writhing response and the tail immersion test, with both 100 and 200 mg/kg doses showing a significant reduction in the number of writhes and increasing the reaction time to pain after 30 min of oral administration of the extract [30]. The effects of the A. karroo extract were comparable to the observed with the reference drug indomethacin, administrated at a 10 mg/kg dose [30].
Extracts of the bark of A. karroo showed promise for the results as anti-HIV agents of natural origin. Mulaudzi et al. [31] demonstrated the potent HIV-1 reverse transcriptase inhibitory activity by hydromethanolic and aqueous extracts (over 70% of inhibition at 1 mg/mL based on COX assay), exhibiting IC50 (i.e., the necessary concentration to attain 50% of inhibition) values of 30 μg/mL and 100 μg/mL, respectively [31]. Since medicines used to fight HIV are expensive and not available for the entire population, the use of drugs of natural origin can contribute to ameliorating this situation, and the extracts of A. karroo are good candidates to be further studied for that purpose [31].
2.1.3. Antimicrobial Agents from Bark
An important feature of extracts from plant origin is their antimicrobial activity, contributing to finding alternatives to synthetic antibiotics, due to the increasing pathogenic resistance to conventional antimicrobial agents [42]. Microbial strains already studied with bark extracts of Acacia spp. are summarized in Table 2, where it can be seen that polar and apolar extracts from the bark of A. dealbata, A. melanoxylon and A. karroo, have shown antimicrobial activity against a variety of Gram-positive and Gram-negative bacteria and fungi. The reported values are comparable to the ones obtained with antibiotics ampicillin, gentamicin, tetracycline, cefotaxime and amphotericin B, which showed MIC values in the range 10–1250 μg/mL [29]. Comparing them with other species, Neiva et al. [22] found better antimicrobial activity against bacteria and fungi when using ethanolic and aqueous A. dealbata extract than when using the corresponding Eucalyptus globulus or Picea abies bark extracts. The extract of A. karroo exhibited a activity similar to those found with other species traditionally used to treat venereal diseases, such as Aloe chabaudii, Adansonia digitata, Bolusanthus speciosus, Elephantorrhiza burkei, Ekebergia capensis, Grewia occidentalis, Osyris lanceolata, Peltophorum africanum, Pterocarpus angolensis, Pappea capensis or Ximenia caffra [31].

Table 2.
Microbial strains inhibited by Acacia spp. bark extracts.
2.1.4. Properties of the Lipophilic Fraction from Bark
The exploitation of lipophilic compounds from Acacia spp. bark has also been tried in the last few years. Freire et al. investigated bark of A. dealbata, A. melanoxylon, A. longifolia and A. retinodes for their sterol content using dichloromethane as an extraction solvent, having found free sterol contents between 213 mg/kg (A.melanoxylon) and 321 mg/kg (A. longifolia) relative to dried bark, with particularly high amounts of spinasterol (96–192 mg/kg of dry bark) and dihydrospinasterol (92–126 mg/kg of dry bark) [43]. The corresponding unusual steryl glucosides, spinasteryl glucodside (88–186 mg/kg) and dihydrospinasteryl glucoside (58–166 mg/kg of dry bark) were also identified in significant amounts in the barks of A. dealbata, A. longifolia and A. melanoxylon [43]. The biological activities of sterols are extensively reviewed in the literature [44,45], including important roles in reducing cholesterol levels in the blood, or as antidiabetic and anticancer agents. Specific bioactivity for spinasterol, dihydrospinasterol and respective glucosides were also referred, such as antitumorigenic potential and therapeutic potential in modulating the diabetic neuropathy evidenced by spinasterol, potent inhibitory effect on the Epstein-Barr virus early antigen attributed to dihydrospinasterol, and anticarcinogenic and cytotoxic potential exhibited by dihydrospinasterol glucoside [43]. Recently, a broader characterization of the lipophylic fraction was performed for A. dealbata bark [27]. Terpenic compounds (lupenone and lupenyl acetate) represent the major lipophilic family in A. dealbata bark (3451 mg/kg of dry bark), followed by long-chain aliphatic alcohols (1083 mg/kg of dry bark), fatty acids (1060 mg/kg of dry bark) (with more than 10% of these being unsaturated fatty acids), monoglycerides (692 mg/kg of dry bark), sterols (484 mg/kg of dry bark), aromatic compounds (29 mg/kg of dry bark) and others (744 mg/kg of dry bark), among which was α-tocopherol (a type of vitamin E) (46 mg/kg of dry bark) [27]. Terpenic compounds, and more specifically lupenone, were reported to have good therapeutic potential in inflammation, virus infection, diabetes, cancer, and treatment of Chagas disease [46], and the positive effects of unsaturated fatty acids on the cardiovascular, respiratory, gastrointestinal, renal and immune systems are well known [47]. A cytotoxicity screening of the above-described lipophilic A. dealbata bark extract in several mammalian cell lines representing brain, immune system, skin, lung and liver cells, revealed encouraging results regarding its future incorporation in oral or topic pharmaceutical or nutraceutical formulations since there is a lack of toxicity in liver hepatocytes (HepG2), epidermis keratinocytes (HaCaT) and dermis fibroblasts (NIH/3T3) [27].
2.2. Wood
Wood from Acacia spp. can be an abundantly available resource from removal operations or as a residue from logging industries that use Acacia spp. as a raw material. As a rule, wood has a lower organic solvent and water-soluble extractives, including polyphenols, when compared to bark [27,43,48,49]. However, these extractives may contain bioactive secondary metabolites that can be exploited for incorporation in formulations of nutraceuticals, cosmetics or other high-value applications. Extractive content for some Acacia spp. has been found in the range of 4%–10% relative to dried wood [13,50,51]. Table 3 resumes the reported biological activities of wood extracts from some species.

Table 3.
Reported biological activities for extracts of Acacia spp. wood.
2.2.1. Antimicrobial Agents from Wood
Yildiz et al. [29] observed that methanolic extracts from sapwood and heartwood of A. dealbata were revealed to be effective against L. monocytogenes, S. aureus and K. pneumoniae. MIC values ranged between 39 and 625 μg/mL [29]. The extract was effective as the antibiotic ampicillin against S. aureus. A methanolic extract of A. karroo stem demonstrated efficiency as antimicrobial agent against several antibiotic-resistant bacteria (Methicillin-resistant S.aureus (MRSA), β-lactamase positive (βL+) E. coli (βL+ EC), Ampicillin-resistant K. pneumoniae (ARKP), Carbenicillin-resistant P. aeruginosa (CRPA) and Chloramphenicol-resistant Citrobacter freundii (CRCF)), fungi (C. albicans and Microsporum audouinii) and against the highly pathogenic Mycobacterium tuberculosis and non-pathogenic Mycobacterium smegmatis mycobacteria [52]. MIC values varied in the range 78–313 μg/mL for bacteria (against 10–20 μg/mL showed by the antibiotic gentamicin), between 78 and 156 μg/mL for fungi (against 20 μg/mL for the antibiotic nystatin) and 1250–2500 μg/mL for mycobacteria (against values lower than 1 μg/mL for the antibiotics ciprofloxacin and isoniazid) [52]. This antimicrobial activity of A. karroo wood extract was of the same magnitude or higher than that observed with wood extracts of other medicinal plants, like Acokanthera oppositifolia, Erythrophleum lasianthum and Ptaeroxylon obliquum [52]. These results encourage the possible use of wood extracts from Acacia spp. in the treatment of microbial infections, especially against bacteria.
2.2.2. Properties of the Lipophilic Fraction from Wood
The lipophilic extractives from Acacia spp. wood were also characterized, and the corresponding biological activities and potential applications were addressed in the literature. The main classes of compounds found in dichloromethane extracts of wood of A. mearnsii with ages between 4 and 13 years old, were fatty acids (27–95 mg/kg of dry wood), sterols (21–56 mg/kg of dry wood) and aromatic compounds (1–13 mg/kg of dry wood), followed by smaller amounts of long chain aliphatic alcohol (0–3 mg/kg of dry wood) and hydrocarbons (0–3 mg/kg of dry wood) [53]. Sterols from the wood of A. dealbata, A. melanoxylon, A. longifolia and A. retinodes were found in amounts between 335 mg/kg (A.longifolia) and 652 mg/kg (A. retinodes) relative to dried wood, with particularly high amounts of spinasterol (128–359 mg/kg of dry bark), dihydrospinasterol (109–286 mg/kg of dry wood) and stigmasterol (only found in A. longifolia–61 mg/kg of dry wood) [43]. The steryl glucosides spinasteryl glucodside (12–80 mg/kg of dry bark) and dihydrospinasteryl glucoside (20–79 mg/kg of dry bark) were also identified in significant amounts in the wood of all studied species, and campesteryl glucoside was identified only in A. longifolia wood (36 mg/kg of dry wood) [43]. A more extensive characterization of the lipophilic fraction of A. dealbata wood revealed that sterols (spinasterol, sitostanol and dihydrospinasterol) represent the major lipophilic family in A. dealbata wood (total of 590 mg/kg of dry wood), followed by fatty acids (290 mg/kg of dry wood, with almost 41% of them being unsaturated fatty acids), long-chain aliphatic alcohols (44 mg/kg of dry wood), aromatic compounds (27 mg/kg of dry wood) and monoglycerides (18 mg/kg of dry wood) [27]. This lipophilic fraction exhibited none or low cytotoxicity at the tested doses in different mammalian cell lines representing brain, immune system, skin, lung and liver cells, highlighting the potential to be further exploited for oral or topic nutraceutical or pharmaceutical applications, given the lack of toxicity observed in liver hepatocytes (HepG2), epidermis keratinocytes (HaCaT) and dermis fibroblasts (NIH/3T3) [27]. The benefic biological activities of sterols (e.g., anti-cholesterol, antidiabetic, anticancer) are well-known [44,45], as well as the positive effects of unsaturated fatty acids in some human systems (e.g., cardiovascular, respiratory, gastrointestinal, renal and immune systems) [47].
2.3. Leaves
Leaves are a highly available component of Acacia spp., and is mainly responsible for the liberation of allelopathic compounds, which significantly contribute to their success as invaders. These compounds may be volatile or non-volatile compounds liberated by lixiviation, and the research into their identification and confirmation of allelopathic effects has contributed to interesting revelations about their potential applications as bioherbicides or even in pharmaceutical formulations. Besides the benefits that can be obtained from the allelopathic chemicals, the extraction of other bioactive compounds from Acacia spp. leaves has been atempted in the last few years using polar or non-polar solvents to recover a wide range of hydrophilic and lipophilic components, and interesting biological activities have been demonstrated (summarized in Table 4).

Table 4.
Reported biological activities for extracts of Acacia spp. leaves.
2.3.1. Potential Biopesticide Agents from Leaves
The continuous use of synthetic herbicides has resulted in the evolution of herbicide-resistant weeds and environmental pollution due to the toxicity associated with these products. The search for alternatives in the less toxic allelochemicals released by some plants has been a trend in the last few years, which will be a more sustainable and environmentally friendly approach to support the change to more sustainable agricultural practices [73]. In this view, invasive plants like Acacia spp. naturally came as candidates for this purpose, taking advantage of the allelochemicals these plants release to inhibit the growth of native species and their establishment. Souza-Alonso et al. [74] demonstrated the allelopathic effect of volatile compounds from A. dealbata leaves on Trifolium subterraneum, Lolium multiflorum and Medicago sativa species, having identified phytol as the most abundant, representing almost half of the entire composition (44%) [74]. Phytol is a very interesting compound that has been pointed at as a candidate for a broad range of applications in the pharmaceutical and biotechnological industry, with recent investigations demonstrating its anxiolytic, metabolism-modulating, cytotoxic, antioxidant, autophagy and apoptosis-inducing, antinociceptive, anti-inflammatory, immune-modulating and antimicrobial effects [75]. A. longifolia leaves proved their phytotoxicity against Portuguese native L. multiflorum, T. subterraneum and Plantago lanceolata species, having identified 2-heptanol (28%), nerol (19%) and geraniol (7%) as the most abundant [76]. Nerol and geraniol are particularly important compounds due to their biological activities. Nerol evidenced significant inhibition of L. monocytogenes bacteria biofilms [77] and exhibited better herbicidal effects than the commercial herbicide 2,4-D against the important weeds Amaranthus retroflexus, Chenopodium album and Rumex crispus [78]. Geraniol is a commercially important terpene alcohol in the flavour and fragrance industries, and presents several relevant biological activities, such as an insecticidal and insect repellent, a chemoprotective agent for cancer, antimicrobial, antioxidant and anti-inflammatory effects, among others reviewed by Chen and Viljoen [79].
Non-volatile compounds of A. dealbata showed allelopathic capacity against lettuce (Lactuta sativa), being identified resorcinol, benzophenone and the non-protein amino acid maculosin as the most representative chemicals in leaves [56]. Resorcinol and resorcinol derivatives, as well as benzophenone, are referred to as possessing unique characteristics for developing drugs suitable for treating various human diseases due to their antioxidant, anti-inflammatory, antimicrobial, anticancer, antiviral, antidiabetic and hepato and cardiovascular protection, among other important biological actions reviewed by Durairaj [80] and Wu et al. [81]. In turn, maculosin was considred an ideal prototype for creating a safe and environmentally friendly antiknapweed herbicide [82].
Several polar leaf extracts of Acacia spp. exhibited promising properties for their application in biopesticides to control weeds and plant pathogens. An ethanolic extract from A. longifolia leaves showed allelopathic capacity against L. sativa by causing a small reduction on the germination percentage (4% reduction) and speed (5% reduction), but significantly decreasing the growth of hypocotyls by 41% [61]. From A. melanoxylon leaves, an hydromethanolic extract and a water extract showed promising results having in view the development of bioherbicides, given the inhibitory effect on the growth of black gram (Phaseolus mungo) and Cassia accidentalis and Cyperus rotundus weeds [63], and on the growth of Galician native species Dactylis glomerata, Lolium perenne and Rumex acetosa [64], respectively. Aqueous and organic extracts of leaves of A. saligna were also tested on the seeds of wheat (Triticum aestivum) and L. sativa crops, and on two weeds (Peganum harmala and Silybum marianum) by El Ayeb et al. [68] Water extracts reduced seed germination of the crops to a small extent (5%–10% for L. sativa, and 7%–11% for T. aestivum), but inhibition of weeds did not even reach 40% (28–35% against P. harmala, and 17%–38% for S. marianum). Organic extracts inhibited seed germination of crops by higher extents (9%–32% for L. sativa, and 5%–37% for T. aestivum), but were very efficient against weeds (maximum of 80% of inhibition for P. harmala, and 82% for S. marianum) [68]. These findings, together with the abovementioned allelopathic effects of volatile and non-volatile compounds identified in Acacia spp., make leaves an important raw material to be considered for the development of pesticides of natural origin.
2.3.2. Antimicrobial and Antioxidant Agents from Leaves
A. dealbata, A. farnesiana, A. karroo, A. pycnantha and A. saligna are some of the most studied Acacia spp. for the development of antibiotic agents, with several studies showing the antimicrobial effects of leaf extracts against pathogenic bacteria and fungi. A summary of the microorganism strains for which leaf extracts showed inhibition activity is presented in Table 5.

Table 5.
Microbial strains inhibited by Acacia spp. leaf extracts.
A broad range of Gram-positive and Gram-negative bacteria, and fungi are sensitive to leaf extracts. The ethanolic extract of A. dealbata showed a higher inhibition against B. cereus than the antibiotic gentamicin (inhibition diameter between one and twofold the diameter exhibited by the antibiotic), possibly due to the presence of phenolic acids such as syringic, p-coumaric, ferulic and ellagic acids [54]. Extracts of A. dealbata showed antibacterial activity of the same magnitude as that observed with Eucalyptus nicholii [54] and Olea europaea [55]. Quercetin-galloylglucoside, rutin, quercetin-pentoside, diosmetin diglycoside and especially quercetin-deoxyhexoside, were found in the A. farnesiana extract and are suspected to be involved in the observed bioactivity [57]. Besides antimicrobial properties, the A. farnesiana extract was proved to have low toxicity and good antioxidant properties, accessed by the ability to chelate iron and reduce the power and scavenging capacity against nitric oxide and DPPH free radicals [57]. In A. karroo, multiple classes of compounds were already identified in their leaves that may be involved in its antimicrobial activity, such as flavonoids, phenols, phytosterols, proanthocyanidin, tannins or terpenes [83]. The antimicrobial effects of A. karroo leaf extracts are probably due to the presence of epicatechin, β-sitosterol and epigallocatechin, some of the most abundant compounds present in the active extracts [58]. The antimicrobial activity against the microorganisms studied by Nielsen et al. [52] and Nyila et al. [58] was lower than that observed with the drugs gentamicin, nystatin, ciprofloxacin, isoniazid and erythromycin, but were of the same magnitude as that observed with ampicillin, chloramphenicol and nyastin for the usually more resistant Gram-negative bacteria studied by Cock and van Vuuren [60] (studied microorganisms showed in Table 5). The extracts of A. karroo exhibited better antimicrobial activity than many African medicinal plants already studied, such as Eucomis autumnalis, Senecio inonartus, Drimia altissima, Aloe arborescens, Acokanthera oppositifolia, Bulbine latifolia, Curtisia dentata, Erythrophleum lasianthum, Ptaeroxylon obliquum, Searsia burchellii and Sansevieria hyacinthoides, among others [52,58,60]. A. pycnantha extract was found to contain alkaloids, sterols and triterpenes, tannins and phenols, saponins and flavonoids [66], having been already identified as individual compounds of n-hexadecanoic acid, octadecanoic acid and phytol, which have recognized antimicrobial activity [67]. The activity of A. pycnantha extracts were comparable to the observed with tetracyclin and ciprofloxacin (16–22 mm of inhibition diameter) [66] and cefoxitin (20–28 mm of inhibition diameter) [67]. The antimicrobial activity evidenced by A. saligna extracts may be attributed to phenolic compounds, including complex flavonoids that were identified in the extracts, such as gallic, protocatioic, chlorogenic, p-hydroxybenzoic, p-coumaric, syringic, vanillic and salicylic acids [70], and also catechin, 7-O-galloylcatechin, myricetin-3-O-α-l-arabinopyranoside, quercetin-3-O-β-d-glucopyranoside, quercetin-3-O-α-l-arabinopyranoside, apigenin-7-O-β-d-glucopyranoside and luteolin-7-O-β-d-glucopyranoside [71]. The aqueous and organic extracts of A. saligna evaluated by Noreen et al. [69] revealed inhibition diameters approximately between 20 to 75% of the ones showed by azithromycin and ciprofloxacin, while the hydroethanolic extracts evaluated by Gumgumjee and Hajar [70] were of the same magnitude of the obtained with streptomycin and ciprofloxacin with bacteria (19–27 mm, at 10 mg/mL)), and with amphoteracin and nyastin with fungi (25–32 mm, at 10 mg/mL).
These findings highlight that extracts from leaves of Acacia spp. and can be considered as a potential source of natural antimicrobials, which is of importance, since health hazards are frequently associated with the use of chemical antimicrobial agents or food preservatives.
2.3.3. Anthelmintic Agents from Leaves
In addition to antimicrobial effects, anthelmintic action was also observed with aqueous extracts of A. melanoxylon and A. karroo. Payne et al. [65] observed anthelmintic activity by a water extract from a mix of leaves and flowers of A. melanoxylon against cyathostomins parasites, which is very common in horses. The extract completely inhibited larval growth at a concentration of 1400 μg/mL, encouraging its further study to possibly be included in parasite management programs for horses [65]. In turn, Mølgaard et al. [84] evaluated the anthelmintic effects of a water extract of A. karroo leaves against schistosomules of the trematode Schistosoma mansoni and cysticercoids of the cestode Hymenolepis diminuta. The extract killed the newly excysted cysticercoids of tapeworms within an hour when incubated in a culture medium, showing a lethal concentration of 3100 μg/mL. This lethal concentration decreased to 800 μg/mL when considering a period of 24 h. However, the activity against schistosomules was considered week, with the extract showing a lethal concentration of 103 mg/mL [84]. Anthelmintic effects on goats infected with Haemonchus contortus by the inclusion of A. karroo leaves in their diet was also reported [85,86].
2.3.4. Anti-Inflammatory, and Antitumoral Agents from Leaves
Extracts from leaves also have been proven to be good candidates for the production of plant-based anti-inflammatory agents, showing strong potential as a natural source of functional components related to human health. Xiong et al. [62] found that crude A. mearnsii extract, but especially the purified proanthocyanidin-rich fraction, depressed reactive oxygen species in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells, and inhibited the release of nitric oxide, and significantly inhibited the mRNA expression levels of the anti-inflammatory interleukine IL-1β, cyclooxygenase COX-2, inducible nitric oxide synthase (iNOS) and interleukin-6 (IL-6) in a dose-dependent manner (1–100 μg/mL). In turn, Gabr et al. [32] showed the anti-inflammatory activity of aqueous ethanolic extracts of A. longifolia leaves by inhibition of COX-1 and COX-2 enzymes. A. longifolia leaves were shown to contain relevant bioactive compounds, such as catechin, myricetin 3-O-rhamnoside, kaempferol 3-O-glucoside, quercitrin (quercetin 3-O-rhamnoside) and luteolin [32].
Other relevant effects of a leaf extract were observed with A. saligna leaves by Gedara and Galala [72]. Authors isolated from a crude 70% aqueous methanol extract the spirostane saponine (25S)-5β-spirostan-3β-yl-3-O-β-d-xylopyranosyl (1→3)-O-β-d-xylopyranosyl (1→4)-β-d-galactopyranoside, that showed potent cytotoxic activity against HEPG2 (liver cancer) cell line (IC50 of 2.8 μg/mL), the biflavonoid glycoside myricetin-3-O-rhamnoside (C7-O-C7) myricetin-3-O-rhamnoside, that showed potent free radical scavenging activity against ABTS free radicals, besides erythrodiol, 3β-O-trans-p-coumaroyl-erythrodiol, quercetin-3-O-α-l-rhamnoside and myricetin-3-O-α-l-rhamnoside, that showed cytotoxicity and antioxidant activities, but to a lower extent.
2.3.5. Properties of the Lipophilic Fraction from Leaves
An extensive characterization of the lipophilic fraction of A. dealbata leaves revealed that terpenic compounds (14,635 mg/kg of dry leaf) represent the major lipophilic class (with lupenone representing almost half of the terpenics), followed by long-chain aliphatic alcohols (1892 mg/kg of dry leaf), fatty acids (1747 mg/kg of dry leaf, with almost 19% of them being unsaturated fatty acids), sterols (636 mg/kg of dry leaf), aromatic compounds (112 mg/kg of dry leaf), monoglycerides (49 mg/kg of dry leaf), and other compounds, including a remarkable quantity of a-tocopherol of 1936 mg/kg of dry leaf [27]. This lipophilic fraction showed good potential for the development of oral or topic nutraceutical or pharmaceutical applications, since it was revealed to be non-toxic to different mammalian cell lines representing brain, immune system, skin, lung and liver cells [27].
In turn, Borges et al. [55] found interesting free radical scavenging activities against DPPH (45–226 μM of trolox equivalents per gram of fresh leaf) and ABTS (393–539 μM of trolox equivalents per gram of fresh leaf) free radicals.
2.4. Flowers
Flowers of Portuguese invasive Acacia spp. can be of great economic importance, since they are important sources of essences for the cosmetic and perfume industries and are of valuable anthochlor pigments (chalcones), in addition to relevant biological activities that have been found in their extracts. Moreover, its exploitation can be of crucial importance to the control of the propagation of Acacia spp., once its collection during the flowering period prevents the formation of seeds, so hindering plant proliferation through the seed dispersal route [19]. The biological activities and uses of Acacia spp. flowers are summarized in Table 6.

Table 6.
Reported biological activities for extracts of Acacia spp. flowers.
2.4.1. Essences from Flowers
The intense and pleasant aroma of the flowers of A. dealbata is pronounced. The absolute oil, obtained from its flowers by hexane or petroleum ether extraction followed by fractionation with ethyl alcohol, is used in many perfumes, with a worldwide production estimated at five tons per year [87]. The chemical composition of the absolute oil was reported by Perriot et al. [87] to know its constituents and to better understand its possible impact on both human health and the environment. Straight-chain compounds from C6 to C26 with different functional groups (hydro-carbons, esters, aldehydes, diethyl acetals, alcohols and ketones) were identified, with near 20% of volatiles (mainly mainly alkanes, fatty acids (palmitic), and (Z)-heptadec-8-ene (6%)), 50% of semi-volatiles (triterpenic ketones (lupenone, 20%) and alcohols (lupeol, 8%)) and 30% of non-volatiles (aromatic and fatty acid triterpenic esters). Previous studies of A. dealbata absolute oil showed that it is neither an irritant, nor is it toxic by ingestion, and it does not show toxicity or phitotoxicity for microorganisms [87].
2.4.2. Pigments and Dyes from Flowers
Currently, industries are returning to the use natural pigments or dyes to reduce the dangers caused by the synthetic materials (e.g., disruption of aquatic life through industrial discharges and adverse effects on human health, such as in the central nervous system, cancer of the thyroid and tumors of the adrenal glands as well as allergenic reactions), since natural molecules are mostly known to be eco-friendly, biodegradable, low toxic, less allergenic and non-carcinogenic [94]. Moreover, some natural pigment molecules, such as chalcones, are generally known for their antibacterial and antifungal activities [93]. Chalcones are well-known anthochlor pigments and have been used for coloring textile materials for decades, and the flowers of some Acacia spp. can be an important source of this valuable material, which is responsible for its yellow colour. Moreover, [92] isolated two chalcone glucosides from the flowers of A. saligna, isosalipurposide (4,2′,4′,6′-tetrahydroxychalcone 2′-glucoside) and 4,2′,4′,6′-tetrahydroxychalcone 4-glucoside, using preparative chromatographic techniques on a crude 95% ethanol extract. With the same procedure, the same author isolated the anthochlor chalcononaringenin 2′-[O-rhamnosyl-(I→4)-xyloside] (4,2′,4′,6′-tetrahydroxychalcone 2′-[O-rhamnosyl-(I→4)-xyloside]) [88], and later two more anthochlors were obtained: the chalcone glucoside 4,2′,4′,6′-tetrahydroxy-3-methoxychalcone 2′-O-β-D-glucoside and the aurone cernuoside (4, 6,3′,4′-tetrahydroxyaurone 4-O-fl-D-glucoside) [89]. Amongst these chalcones, isosalipurposide was studied for its application as a dye on wool [93], and on the coloration of polylactic acid byopolymer, commonly used in food packaging [94]. Ghouila et al. [93] observed that isosalipurposide is more effective if used at a lower pH (pH 2) and lower temperatures (best result at 60 °C) to avoid its degradation. It provided bright hues with good colour fastness properties, which is improved if using zinc sulphate as a mordant [93]. In turn, Fajraoui et al. [94] studied several processing parameters to obtain a well finished and yellow coloured polylactic acid film, obtainig the best performance when using a dye concentration of 0.5% and blending at 50 rpm during 5 min at 90 °C, suggesting that these conditions allow the pigment to be able to disperse in the intermolecular chains of the amorphous byoplymer.
2.4.3. Potential Bioherbicide Agents from Flowers
As described for leaves, the search for natural herbicides is fundamental in the change for more sustainable agricultural practices. Along with leaves and leaf litter, flowers play an important role in releasing allelopathic compounds, and the allelopathic effects of Acacia spp. on native flora mostly occurs during the flowering period [97,98]. Investigation of the allelopathic properties of flowers and the identification of individual volatile and non-volatile chemicals have been made and can bring important information for the development of bioherbicides. The allelopathic effects of A. dealbata and A. longifolia were observed in several Portuguese and Spanish native species, such as Medicago sativa, Plantago lanceolate, Trifolium subterraneum and especially on Lolium multiflorum [74,76]. Amongst the most abundant compounds identified in the volatiles, and probably responsible for the allelopathic effects, there are heptadecadiene (23% in A. dealbata and 7% in A. longifolia), n-nonadecane (19% in A. dealbata and 14% in A. longifolia), n-tricosane (11% in A. dealbata and 12% in A. longifolia), octadecene (9% in A. dealbata), nonanal (8% in A. longifolia) and kaurene (8% in A. longifolia) [74,76].
The allelopathic effect of the non-volatile fraction of flowers was accessed for A. dealbata, which showed the allelopathic capacity against L. sativa [56]. The most abundant compounds found in the non-volatile fraction were anisal, methyl p-hydroxycinnamate and p-anysil alcohol [56]. Anisal is widely used in flavor and fragrance indutries, methyl p-hydroxycinnamate previously showed anti-inflammatory activity [99], and nematicidal effects were associated with p-anysil alcohol [100]. Lorenzo et al. [19] explored the potential herbicidal effect of two compounds present in flowers of A. dealbata, methyl cinnamate and methyl anisate, against well-established plants, namely lettuce (L. sativa), the widely consumed wheat crop (Triticum aestivum), and against rigid ryegrass (Lolium rigidum Gaudin), a common weed in winter cereal and wheat crops. The authors found methyl cinnamate as the most active compound, which showed selective phytotoxicity by inhibiting early stem and radicle growth of dicotyledonous lettuce (60% and 89%, respectively) and monocotyledonous L. rigidum (76% and 87%, respectively), but not affecting wheat. These results encouraged the exploitation of A. dealbata flowers as a source of potential bioherbicides, namely to be applied in wheat crops infested by rigid ryegrass [19]. A water extract from A. melanoxylon leaves evidenced promising results as a potential bioherbicide agent, given the inhibitory effect on the growth of Galician native species [64]. The extract showed mean LC50 values (i.e., the dose for 50% inhibition of seedling growth) of 43% against Lolium perenne, 40% against Rumex acetosa and 53% against L. sativa (doses referred to dilutions of the whole liquid crude extract) [64]. Water and organic extracts of flowers of A. saligna also exhibited potential for their application as bioherbicides [68]. These were tested on the seeds of wheat (T. aestivum) and lettuce (L. sativa) crops, and on two weeds (Peganum harmala and S. marianum). Water extracts reduced seed germination of the crops to a low extent (0%–2% for lettuce and 3%–13% for wheat), but were not remarkably efficient against weeds (maximum of 13% of inhibition for S. marianum) [68]. Organic extracts inhibited the seed germination of crops to higher extents (11%–40% for lettuce, and 0%–23% for wheat), but were very much efficient against weeds (maximum of 77% of inhibition for P. harmala, and 80% for S. marianum) [68].
2.4.4. Antimicrobial Agents from Flowers
Flower extracts have demonstrated antimocrobial effects as well. Edrah et al. [66] observed antibacterial activity of ethanol and water extracts of A. pycnatha leaves against S. aureus, S. epidermis, K. pneumoniae and E. coli, with inhibition diameters in the range 6–10 mm, against 16–22 mm showed by tetracyclin and ciprofloxacin. In turn, Al-Huqail et al. [96] evaluated the antibacterial activity of water extract of A. saligna flowers against phytopathogenic bacteria Agrobacterium tumefaciens, Enterobacter cloacae, Erwinia amylovora and Pectobacterium carotovorum subsp. carotovorum, causal agents of different infectious plant symptoms, such as blackleg, brown or soft rot on potato tuber and stems, and tumors on olive and other ornamental plants. The author tested the extract also against fungi Fusarium culmorum, Rhizoctonia solani and Penicillium chrysogenum, which cause root rot, cankers and green fruit rot, respectively. These fungi can colonize and cause pigmentation and discoloration of different wood and wood-based products in humid conditions. All bacteria were sensitive to the extract, with MIC values ranging within 100–300 µg/mL, against 16–35 µg/mL obtained with the antibiotic tobramycin [96]. For the fungi, the best performance was attained at a concentration of 3%, with inhibition percentages between 29% and 66% [96]. The authors found benzoic acid, caffeine, o-coumaric acid, p-hydroxy benzoic acid and ellagic acid to be the most abundant phenolics (present in the extract in concentrations between 12 to 162 mg/100 g), and naringenin, quercetin and kaempferol as the most abundant flavonoids (44 to 145 mg/100 g) [96].
2.4.5. Other Applications of Flower Extracts
Other important characteristics were found for flower extracts that gave them potential to be applied in pharmaceutics, cosmetics or in therapeutics against Alzheimer’s disease.
Casas et al. [90] found a good free radical scavenging activity of crude ethanol extract against ABTS free radicals (850 mg of Trolox equivalents per gram of extract), which is probably attributed to the presence of phenolic compounds (270 mg of gallic acid equivalents per gram of extract). The authors found butanol, ethyl acetate, hexane and the crude ethanol extracts to be protective against neutrophils oxidative bursts, accessed in venous blood collected from healthy human volunteers, at concentrations in the range 63 to 500 μg/mL, and showed moderate cytotoxic activity against colon carcinoma HCT-116 and lung adenocarcinoma A549 cells [90]. Moreover, encouraging results were obtained with the application of extracts as solvents in the preparation of hydrogels. Hydrogels with attractive mechanic properties were produced, thus opening a new possibility for the valorization of A. dealbata flowers through their inclusion in the preparation of these materials for personal care products, cosmetics or pharmaceuticals, taking advantage of their antiradical and anti-proliferative potential [90].
Soto et al. [91] evaluated the potential of the use of some vegetal raw materials, including A. dealbata flowers, in personal-care products (hand cream, body oil, shampoo, clay mask, body exfoliating cream and skin cleanser), with the evaluation being made by participants attributing a classification for some sensory attributes and overall acceptance. Between the four assayed vegetal extracts (grape pomace, Pinus pinaster wood chips, A. dealbata flowers and Lentinus edodes), the floral aroma and the yellow color provided by the A. dealbata flower extract to all produced cosmetic products, make the acacia-based products preferred by all consumers, independent of gender or age [91]. Besides, the A. dealbata extract was the one with the highest antioxidant activity, given by the ferric-reducing antioxidant power assay (170 mg ascorbic acid equivalents per gram of extract) and the ABTS free radical scavenging assay (700 mg of trolox equivalents per gram of extract) [91].
Presently, amongst the treatments used against Alzheimer’s disease are included the use of acetylcholinesterase inhibitors and antioxidants [101,102]. Ghribia et al. [95] evaluated the antioxidant and anti-acetylcholinesterase activities of extracts (dichloromethane, ethyl acetate, butanol and aqueous) and of the compounds isolated from flowers of A. saligna (isosalipurposide, quercetin and naringenin). In terms of IC50 values (i.e., the extract concentration providing 50% inhibition), quercetin (IC50 = 5 μg/mL) and ethyl acetate (IC50 = 67 μg/mL) showed the best antiradicalar activity against DPPH free radicals; dichloromethane extract displayed the best antiradicalar activity against ABTS free radical (IC50 = 78 μg/mL); and ethyl acetate extract was the one with the best reducing power. All extracts showed a dose-dependent acetylcholinesterase inhibition (IC50 between 16.03 and 52.04 µg/mL), with the butanolic extract being the most potent sample [95]. The antioxidant and anti-acetylcholinesterase activity exhibited by these extracts encourage its further study for the development of alternative therapeutics to fight neurological disorders.
2.5. Pods and Seeds
Collection of pods and seeds to obtain valuable products can be another way to promote the control of the proliferation of Acacia spp., by decreasing the amount of seeds in soils. The most reported biological activities are summarized in Table 7.

Table 7.
Reported biological activities for extracts of Acacia spp. pods and seeds.
2.5.1. Bioactive Properties of Pods
Pods are formed after the flowering period. Studies about bioactive properties of pods from Portuguese invasive Acacia spp. are scarce. To the best of our knowledge, the focus has been mainly on the allelopathic efects of pods. Aguilera et al. [56] demonstrated the allelopathic capacity of a water extract from pods of A. dealbata against lettuce (L. sativa), identifying stigmasterol and d-alpha-tocopherol quinone as the most abundant chemicals in pods [56]. Jelassi et al. [103] evaluated the allelopathic potential of A. cyclops, A. mollissima and A. saligna pods and seeds also on the germination of lettuce. The authors observed effects on the growth of roots and shoots, but found that the most pronounced effect was observed with an ethyl acetate extract from A. saligna seeds, that completely inhibited seed germination. The saponic triterpene glycoside 3-O-β-d-xylopyranosyl-(1→4)-α-l-rhampyranosyl-(1→6)-β-d-glucopyranoside, named mollisside B, isolated from the butanolic extract of A. cyclops pods, strongly inhibited lettuce root (88.21%) and shoot (63.68%) growth, being proposed as a lead compound for the development of new herbicides [103]. In turn, A. farnesiana forms pods with good antioxidant properties, having been observed to protect against oxidative-induced damages in pig kidney cells LLC-PK1 and in in vivo studies with gerbils [104].
Considering the remarkable activities already demonstrated by pods of other acacia species, more research must be conducted into the extraction of bioactive compounds from Portuguese invasive Acacia spp. Extracts from pods of A. nilotica showed good antimicrobial activity [108,109], antidiabetic and hypolipidemic effects [110], antihyperglycemic properties [111], antihypertensive and antispasmodic actions [112] and enhanced wound healing by alleviating oxidative stress and suppressing pro-inflammatory cytokines [113]. Tannins, saponins and flavonoids are known classes of compounds present in the pods of other species [109], some of these having already been identified, such as catechin, catechin-7-O-gallate, catechin-4′-O-gallate, catechin-3′-O-gallate, gallic acid, quercetin and quercetin 3-O-glucoside [111].
2.5.2. Nutritional Potential of Seeds
The continuous increase in population has led to an increased demand for high-quality seed oils, and along with this, the necessity to diversify the sources of vegetable oil has emerged to meet that demand. Between the Portuguese invasive Acacia spp., the most studied for this purpose is A. saligna, whose oil has already been widely characterized and very good properties have been reported [114,115]. Youzbachi et al. [115] evaluated the composition of 12 A. saligna ecotypes, finding an oil content between 87 and 119 g/kg of dry seeds, with saturated fatty acid content in the range 12-13%, monounsaturated fatty acid content of 22%–25% and a high polyunsaturated fatty acid content between 62 and 66%. Smaller proportions of stearic (1%–2%), vaccenic (1%–2%) and palmitoleic (0%–1%) acids were also quantified. The most representative saturated fatty acid was palmitic acid (9%–10%) and oleic acid was the most abundant monounsaturated fatty acid (20%–23%) and a remarkable content of linoleic acid (ω6) was found (61%–65%) [115]. The benefits of the inclusion of unsaturated fatty acids in the human diet are well known, such as reduction of serum cholesterol, atherosclerosis and on the modulation and/or prevention of cardiovascular deseases, type 2 diabetes, and several types of cancer [114,115,116]. Along with such notable content of polyunsaturated fatty acids on its oil, A. saligna seeds also contain phenolics (1860–1970 mg of gallic acid equivalents per kg of dry seeds) and flavonoids (310–490 mg of rutin equivalents per kg of dry seeds), which confer to seeds important antioxidant properties, demonstrated by DPPH and ABTS free radical scavenging assays on methanolic extracts (430–660 μM and 140–580 μM of trolox equivalents, respectively [115]. A similar composition for seed oils was observed by Jelassi et al. [114] with A. cyclops, A. saligna and A. mollissima, that found oil contents between 9 and 11%, saturated fatty acid contents ranging from 16%–17%, monounsaturated fatty acids in the range 21%–26% and polyunsaturated fatty acids between 57 and 62%. Besides, the authors determined also the phenolic composition of the seed oils, founding syringic (35%–39%) and ferulic (31%–34%) acids as the dominant phenolic compounds, and minor amounts of other phenolic acids, such as o–coumaric (1%–10%), p-coumaric (3%–7%) and protocatechuic (ca. 7%) [114]. These studies confirm that A. saligna could be considered as a potential food source of ω6 fats and antioxidants, showing that its seeds have the potential to replace conventional oil types such as soybean, olive, corn, coconut, peanut, cottonseed, palm, sunflower and rapeseed oils, which are more expensive and are not affordable to a considerable population in developing countries. The nutritional value of A. retinodes seeds was also investigated, having being found with a different composition profile when compared with A. saligna seeds. Shelat et al. [117] observed A. retinode seeds to contain a high protein (28%) and dietary fibre (34%) content and 16% of oil. The oil is characterized by 30% of saturated fatty acids, 53% of monounsaturated fatty acids and 18% of polyunsaturated fatty acids, with palmitic acid (18%), oleic acid (50%) and linoleic acid (16%) the most representative fatty acids [117].
Acacia seeds are characterized also by a mineral composition enriched in potassium (237–907 mg/100 g of dry seed), calcium and magnesium (61–509 mg/100 g of dry seed), phosphorous (20–85 mg/100 g of dry seed) [115,118] and proteins (10–14 g/100 g of dry seed) [118]. Hannachi et al. [118] investigated the chemical composition of seven Acacia species, including A.saligna, having found for this species 14% of seed storage protein, comprising 3% of albumin, 6% of globulin, 3% of prolamin and 2% of glutelin; potassium, phosphorous and calcium and magnesium contents of 493, 85 and 73 mg/100 g of dry seed, respectively; a phenolic content of 191 mg of gallic acid equivalents per 100 g of dry seed; flavonoids content of 29 mg of rutin equivalents per 100 g of dry seed; and a carotenoid content of 6 mg/kg of dry seed. In addition, the authors also found seeds to exhibit antioxidant activity, accessed by the DPPH (880 μM of trolox equivalents) and ABTS (1290 μM of trolox equivalents) free radical scavenging assays [118]. These results makes seeds of Acacia spp., including those of the Portuguese invasive A. saligna, an interesting natural source of protein, minerals and antioxidants, with potential to be explored in the development of functional foods and dietary supplements.
2.5.3. Bioactive Properties of Seeds
In addition to the abovementioned properties, seeds extracts already demonstrated immunomodulatory, antidiabete, antimicrobial and antinematode effects. El Abbouyi et al. [105] investigated the effects of a water extract of A. saligna seeds on superoxide anions generation in rat pleural polymorphonuclear leukocytes, activated by opsonized zymosan, having observed a significant stimulatory effect in a dose-dependent manner. Moreover, the extract enhanced superoxide anions generation by polymorphonuclear leukocytes pre-treated with the anti-inflammatory diclofenac. The authors suggest that this stimulatory effect is probably due to the presence of polyphenols, such as tannins and/or lignins in the extract [105]. Kumar [106] studied the antidiabetic activity of a 70% ethanol extract of seed of A. melanoxylon in streptozotocin-induced diabetic rats. The extract, administered orally at 100, 200 and 400 mg/kg of body weight doses, exhibited significant hypoglycemic activities, significantly reducing blood glucose levels even at the lowest dosage (from 302 to 225 mg/dL) as compared with a diabetic control. Moreover, the administration of the extract avoided the decrease in body weight verified in the diabetic rats without treatment (reduced from 228 to 212 g), and showed instead a continuous improvement in body weight from 208 to 211 g, when using the 100 mg/kg dosage. Lectin-like protein extracted from A. farnesiana seeds showed bacteriostatic effects against Xanthomonas axonopodis pv. passiflorae and, especially, against Clavibacter michiganensis subsp. michiganensis, inhibiting the growth of these phytopathogenic bacteria by 78% and 92%, respectively [107]. Moreover, anti-nematode properties were also found against the root-knot nematode Meloidogyne incognita, involving both egg hatching and motility and reduction in larval mobility, suggesting its potential use as a component of integrated pest management programs [107].
2.6. Root
Within the Portuguese invasive Acacia spp., the most studied is what concerns the valorization of the roots is A. karroo (Table 8). The roots of A. karroo have been used in traditional medicine in some African regions for decades, as an aphrodisiac or in remedies for colic, convulsions, dizziness, general body pains, gonorrhea, malaria, sexually transmitted diseases, syphilis, urinary schistosomiasis, venereal diseases, diarrhea, etc. [83]

Table 8.
Reported biological activities for extracts of A. karroo roots.
2.6.1. Antimicrobial and Anthelmintic Agents from Roots
Extracts from A. karroo have demonstrated good antimicrobial activities. Priyanka et al. [59] verified good antibacterial activity of chloroform, ethanol, ethyl acetate and methanol extracts against S. aureus, B. subtillis, E. coli, S. typhi, P. aeruginosa, K. pneumoniae and P. vulgaris bacteria. The ethyl acetate extract caused the maximum zone of inhibition against S. aureus (33 mm) and the lowest against E. coli (9 mm). Tshikalange et al. [119] and Mamba et al. [120] investigated the antimicrobial activities of several plants traditionally used in the treatment of sexually transmitted diseases, including A. karroo. Tshikalange et al. [119] found the antimicrobial effect of an ethanolic extract against S. aureus, E. coli, K. oxytoca, K. pneumoniae subsp. Pneumoniae, and N. gonorrhoeae bacteria and C. albicans fungi, with MIC values in the range 400–1600 μg/mL [119]. Mamba et al. [120] accessed the efficacy of an hydroethanolic extract against Gardnerella vaginalis, Neisseria gonorrhoeae and Oligella ureolytica bacteria, and against C. albicans fungi, having observed the lowest MIC values against N. gonorrhoeae and C. albicans (800 μg/mL, against 10 μg/mL exhibited by the antibiotic ciprofloxacin), with G. vaginalis showing the highest resistance (6300 μg/mL). The A. karroo root extracts were among the most effective against most of the studied microorganisms, compared with other plants traditionally used to treat sexually transmitted deseases, such as Abrus precatorius, Diospyros mespiliformis, Ipomoea crassipes, Senna petersiana, Ximenia caffra, Elaeodendron croceum, Elaeodendron transvaalense, Hilliardiella nudicaulis, Jasminum fluminense, Peltophorum africanum, Schotia capitata, among others [119,120].
Extracts of A. karroo root also exhibited anthelmintic effects against tapeworms, evaluated by Mølgaard et al. [84] using newly excysted tapeworms (cestodes) of Hymenolepis diminuta. The water extract killed the newly excysted cysticercoids within an hour when incubated in a culture medium, showing a letal concentration of 17 mg/mL. This letal concentration decreased to 2 mg/mL when considering a period of 24 h. Besides verifying anthelmintic activity by the root extract, the authors observed that the extract obtained from the leaves proved to be even more effective [84].
2.6.2. Antioxidants and Anti-Inflammatory Agents from Roots
In addition to the antimicrobial properties against microorganisms commonly associated with causing many sexually transmitted diseases and urinary tract infections, the ethanolic extracts showed other important characteristics in this context, namely its safety, anti-inflammatory activity, and efficacy against HIV-1 virus [119,120]. The ethanol extract of the A. karroo root showed remarkable antiradicalar activity given by the DPPH free radical scavenging assay, exhibiting an IC50 value ((i.e., extract concentration providing 50% inhibition) of 830 μg/mL, lower than that of ascorbic acid used as control (1440 μg/mL) [119]. Moreover, the authors demonstrated the safety of its use by confirming its low citoxicity, accessed against Vero African monkey cells lines, having found an IC50 value of 115 μg/mL [119]. In turn, Mamba et al. [120] demonstrated the anti-inflammatory and anti-HIV-1 activity by a hydroethanolic extract of A. karroo. The anti-inflammatory activity was studied by measuring the inhibition of pro-inflammatory enzyme 15-lipoxygenase (15-LOX), with the extract showing an IC50 value of 62 μg/mL (i.e., concentrations that resulted in 50% 15-LOX inhibition), comparable with that obtained with the positive control quercetin (IC50 = 49 μg/mL). The antiviral activity against the HIV-1 virus was investigated measuring the inhibition of the HIV-1 reverse transcriptase enzyme, which is vital in the lifecycle of the HIV virus. The authors found that the A. karroo extract is amongst the ones with better inhibitory activity, showing an inhibitory percentage of 67%, demonstrating that it could be a good source of potent compounds for a therapeutic strategy against the HIV-1 reverse transcriptase enzyme [120].
2.7. Exhudates and Gum
Exudates from Acacia spp. are of great economic importance, due to their numerous biological activities and wide range of applications, and are one of the most traded products derived from these species. Gum from A. senegal or A. seyal, commercially known as gum arabic, is a global commodity generally harvested from stems and branches of these species in Africa and Western Asia [121]. It is a complex, branched heteropolysaccharide, either neutral or slightly acidic, and is composed of 1, 3-linked β-D-galactopyranosyl units, currently used in foods, pharmaceuticals and many other industries, taking advantage of its antioxidant properties, positive effects on renal function, reduction of blood glucose concentration, improvement of intestinal absorption, improvement of the lipid metabolism, improvement of the cardiovascular system, protection against dental erosion and hepatic protection, among others [121,122]. In the pharmaceutical industry, it is also used as a carrier of drugs; in the food industry it is used in confectionery, bakery, dairy, beverages, as a microencapsulating agent, as a stabilizer in frozen products like ice and ice cream, absorbing water and producing a finer texture and in the cosmetic industry its is used as smoothener in lotions and protective creams and as an adhesive in facial masks or face powders [122]. In food products, it received the number E414, being classified as an emulsifier, stabilizer, thickener or glazing agent.
Nevertheless, the search for alternatives to A. senegal or A. seyal gum is important to decrease the dependence on gum arabic, or to provide more affordable solutions to developing countries by using endogenous plants to achieve similar results. This has been the case for A. karroo and A. mearnsii. Gum of A. karroo has been used as an alternative to gum arabic in some African countries. It can be used in the commercial production of sweets and other confectioneries, and has been traditionally used in the treatment of several ailments, such as abscesses, diarrhoea, dysentery, haemorrhage, inflammation of eyes, mouth ulcers, oral thrush and osteomyelitis [83]. Structural and physical chemical properties of A. mearnsii gum have been investigated and compared with commercial gum arabic, to better understand its behaviour and possible applications as an alternative to gum arabic [123,124]. Grein et al. [123] observed that A. mernsii gum is more efficient in lowering the surface tension of water and saline solutions and is more efficient in emulsifying castor oil droplets, suggesting that polysaccharides from A. mearnsii are candidates as substitutes of currently commercialized arabic gums, even exhibiting improved properties, depending on their application.
An A. pycnantha exudate was characterized for its water-soluble prebiotic compounds [125]. It was found that the exudate contains 79% of water-soluble carbohydrates and 3% of protein. The carbohydrates are mainly constituted by galactose (78%) and arabinose (22%), while the dominant aminoacids are hydroxyproline (23%), aspartic acid (12%), leucine (11%) and proline (7%). Amongst the studied species by Vidanarachchi et al. [125], the A. pycnantha exudate was the one with the highest percentages of hydroxyproline and proline. Proline plays an important role in protein synthesis and structure, metabolism (particularly the synthesis of arginine, polyamines and glutamate via pyrroline-5-carboxylate) and nutrition, as well as in wound healing, antioxidative reactions and immune responses, while hydroxyproline can participate in the synthesis of glycine (essential for chickens), pyruvate and glucose (important for ruminants), and may also scavenge oxidants and regulate the redox state of cells [126]. These represent interesting compounds to be included in the development of dietary supplementation to benefit the health, growth and development of animals and humans [126]. Vidanarachchi et al. [127] studied the effect of some plant extracts, including an exudate of A. pycnantha, on growth performance, intestinal morphology, microflora composition and activity in broiler chickens. The authors observed that the prebiotic-rich plant extracts had no negative effect on the performance of broilers, beneficially modulating the composition of the microflora in the ileum and caeca by increasing the number of lactobacilli, and reducing harmful bacteria, such as potential pathogenic coliforms and Clostridium perfringens bacteria [127].
3. Material Applications of Acacia spp. Biomass
In addition to bioactive extracts and aromatic oil, with potential uses in the nutraceutical, pharmaceutical, cosmetic, perfume, food or feed industries, a diversity of other interesting materials can be obtained from Acacia spp. Some of these materials are already available on a commercial scale and will not be further discussed. These include the use of A. mearnsii for the production of tannins for the leather industry and for the manufacture of adhesives, which has been implemented since the sixties, as well the manufacture of coagulants used in the wastewater treatment, which were also commercialized about forty years ago [34,38,128]. Besides these known applications, recent studies have shown that the potential of the Acacia spp. biomass or extracts obtained from it can have new applications with high added value, as in the polymer and composite industries, in the production of nanomaterials, in the manufacture of new adsorbents for the removal of pollutants as alternatives to conventional activated carbon, or wood and steel protection (Table 9).

Table 9.
Material applications from Acacia spp. components.
3.1. Polymers and Composites from Bark
The growing awareness of sustainability in the polymer industry has led to the development not only of biopolymers but also of bio-based additives, such as stabilisers, that are often required to extend the service life of the material. Bridson et al. [129] investigated the use of a commercial tannin extract of A. mearnsii as a stabilizer of linear low density polyethylene films. The authors successfully increased the thermo-oxidative and UV stability of the films when using tannin extract compounded with co-polymers (ethylene vinyl alcohol, maleic anhydride modified polyethylene or ethylene acrylic ester maleic anhydride terpolymer) as additives. The tannin extract improved the strength and elongation retention of the polymer after the accelerated weathering, performed with UV light, elevated temperature and humidity to simulate in-service environmental conditions, probably due to radical scavenging reactions that reduces oxidation and limits the damage to the polymer [129]. The A. mearnsii extract is already produced at a commercial scale, so it can represent a cost-competitive additive for this purpose for industrial applications.
After the extraction of tannins from the bark of A. mearnsii, hundreds of tons of bark residue are produced, which represents an environmental problem if a proper strategy is not adopted. This residue is usually burned, but the alternative use of exhausted bark as a filler in polypropylene composites was investigated by Taflick et al. [135] The authors studied the impact of the addition of bark in the mechanical and thermal properties of the produced materials, using maleic anhydride as a compatibilizer to improve the adhesion between the matrix and the bark particles. The incorporation of 10% of bark particles with sizes ranging from 106 to 425 nm produced composites with higher impact properties, higher crystallization and degradation temperatures and comparable tensile strength, compared with the polymer matrix without the addition of bark [135]. These composites were considered suitable for use in non-structural applications, such as in decoration, sound isolation and household goods, bringing the advantages of reducing the environmental impact of using synthetic polymers and adding value to an abundant waste formed after tannin production from A. mearnsii bark.
3.2. Micro and Nanomaterials from Leaves and Bark
Shashanka and Kumara Swamy [139] successfully synthesized colloidal silver nanoparticles using a water extract of leaves of A. melanoxylon, and studied their application as a dopamine and hydrogen peroxide sensor. Dopamine and hydrogen peroxide are important intermediates in environmental and biological systems, whose determination has become important and has been given tremendous attention by neuroscientists and chemists in biomedical and bioanalytical research. The colloidal silver nanoparticles were prepared by treating the water A. melanoxylon extract with silver nitrate and were then used in the preparation of a carbon paste electrode, by mixing it with graphite powder and silicon oil [139]. The addition of silver nanoparticles prepared this way enhanced the sensitivity of the fabricated electrode towards the oxidation of both dopamine and hydrogen peroxide, which occurred at a physiological pH, by a diffusion-controlled process, and with a linear increase in peak current with different concentrations of both products [139].
Superparamagnetic nanoparticles based on a core consisting of iron oxides can have relevant applications, such as biomedical uses as drug delivery systems or as adsorbents to remove pollutants from wastewaters [142]. Research for the preparation of these materials in an eco-friendly way is important for the sustainability of the process, and the use of green processes and biobased nanoparticles were investigated by Khan et al. [130], using a tannin extract of A. mearnsii. The authors prepared superparamagnetic nanoparticles containing Fe3O4 cores in biochar, by carbonizing the precipitate formed at pH 10 from the mixture of tannin and ferric chloride and ferrous chloride solutions. The tannin molecules contain a large number of phenolic-OH groups capable of taking part in redox reactions that result in the reduction of the iron salt and formation of stabilized iron oxide nanoparticles at pH 10 [130]. The authors produced superparamagnetic nanoparticles with average sizes in the range 18–35 nm, with surface areas suitable for use in the adsorption of heavy metals from wastewater [130].
The concept of “active packaging” for food or pharmaceutical products arose a few years ago, with the intention of creating a packaging material to extend the shelf-life or the expiration date of products, which can be achieved by exerting antioxidant effects to protect against oxidation, UV radiation or moisture [131]. The current trend is moving towards the use of green and sustainable resources, where nanocellulose and bio-based additives with antioxidant properties can play an important role. Missio et al. [131] prepared a nanocellulose film containing 190 mg/g of proanthocyanidin-rich tannin extract of A. mearnsii, which was incorporated into the matrix by mechanical fibrillation of cellulose pulp and tannin mixture. The addition of the tannin extract improved the surface hydrophobicity, which resulted in a 6-fold enhancement in their air-barrier properties, while the mechanic features of the cellulose matrix were not significantly affected [131]. Moreover, good resistance to several organic solvents was verified, as well as a slow release of antioxidant components upon soaking (55% of the original tannins in one weak), thus showing the potential use of tannin-enhanced biofilms as a valid green and non-toxic material for food, pharmaceutical or cosmetic products [131].
Nanofiber membranes are used for diverse objectives, from the removal of pollutants from wastewater to biomedical, tissue engineering and regenerative medicine applications [143]. Poly (e-caprolactone) is one of the most important synthetic polymers used for developing nanofiber scaffolds, for repairing soft and hard tissues, due to its cytocompatibility, biodegradability and mechanical resistance, but it has also some limitations, such as high hydrophobicity and low water absorptivity [132]. To overcome these shortcomings, Martins et al. [132] produced a novel poly (ε-caprolactone) electrospun membrane, by blending poly (ε-caprolactone) with a commercial amino-functionalized tannin extracted from A. mearnsii, to be used as a scaffold for wound healing and tissue repair. The authors used different percentages of tannin incorporation (from 0% to 22%), which could be tuned to impart anchorage, adhesion and proliferation of human adipose-derived stem cells. Moreover, the incorporation of the tannin extract conferred bactericidal properties to the produced membrane against Pseudomonas aeruginosa, particularly with 22% of incorporation [132].
Dos Santos et al. [133] produced nanoparticles containing A. mearnsii tannin extract, encapsulated using sol-gel methods, and evaluated its application as antimicrobial agents. Amongst the sol-gel methods used, the acidic and alkaline routes affected the structure of the tannins during the synthesis and decreased the antibacterial activity of the material, while a non-hydrolytic route affected the final texture of the encapsulated materials, hindering the release of the extract. Only the silicate sol-gel option, performed by mixing a sodium silicate solution with dissolved extract in acidic medium, at 50 °C during 48–72 h, produced a material with a very small particle size (<1 nm), promoting a gradual release of tannins in aqueous medium and an improved interaction with microorganisms (moderate activity against Staphylococcus aureus, Aspergillus niger and Candida sp.).
Grasel et al. [134] successfully incorporated A. mearnsii tannin extract on lactic-co-glycolic acid microparticles, having obtained 73% of encapsulation efficiency and a particle size distribution between 0.6 and 2.4 μm. The authors demonstrated in vitro the biocompatibility of the produced material with normal cell lines (Vero), with no effects on cell proliferation being observed after 24 h, using microparticle concentrations up to 200 μg/mL.
These findings for the utilization of A. mearnsii bark extract deserve further attention and research for the implementation at larger scales, not only due to the improved properties given by the tannin extract to silver nanoparticles, superparamagnetic nanoparticles, nanocellulose films and nanofiber membranes, but mainly because this extract can be delivered from well-established procedures at a commercial scale, and can be provided at a relatively low cost.
Another way to valorize the exhausted A. mearnsii bark produced after the extraction of tannins, avoiding an environmental problem, is its use in the production of cellulose nanocrystals, which are used usually for polymer reinforcement, as well as in transparent films for several applications [136]. The common procedure for isolating cellulose involves costly and time-consuming extraction steps to remove extractives, but Taflick et al. [136] demonstrated, with exhausted A. mearnsii bark, that this step can be avoided if the purpose is producing cellulose nanocrystals. Only the common steps of delignification, bleaching and hydrolysis were applied, but without using chlorinated chemicals in the bleaching step. It was found that differences in the isolated cellulose, with the process without solvent extraction showing a higher crystallinity index (86% versus 82%), larger and more agglomerated particles, and higher residual mass after thermal degradation; however, no significant difference was observed in the morphology and thermal stability of obtained nanocrystals. Such a result represents lower costs with solvents and a more eco-friendly process to produce cellulose nanocrystals, using a bark residue.
3.3. Alternative Adsorbents from Bark, Wood and Leaves
The interest in the development of low-cost adsorbents for the removal of pollutants from wastewaters, such as dyes, heavy metals or organic compounds, has increased with the increased awareness to finding environmentally friendly and sustainable processes for that purpose, and preferentially at an affordable cost to make them usable in developing countries [144,145]. Conventional activated carbon is still the most-used adsorbent, but its widespread use is frequently restricted due to its high cost, besides other issues related to its efficiency and the cost of its regeneration, and its disposal at the end of its life cycle [145]. In this context, biomass from invasive species, such as Acacia spp., are good candidates due to their high abundance and availability, either in nature or as an industrial by-product from wood industries, charcoal production or tannin industries.
Silva et al. [137] prepared an alternative adsorbent using A. mearnsii bark waste from a tannin industry. The bark was pretreated by steam explosion, the adsorbent was prepared by an acetosolv method (boiling for 24 h a mixture of steam-exploded bark, acetone and a small percentage of sulfuric acid) and is then used for crystal violet dye removal. Besides the small surface area determined for the adsorbent (near 5 m2/g), this procedure promotes a chemical modification of lignin and polysaccharides by the solvent and the acid, generating new functional groups like ketones, ethers, open aromatic rings and carbonyl groups, that can act as new sites for adsorption [137]. The authors observed removal percentages of 95% from an initial solution containing a 50 mg/L dye concentration, after two hours of contact, using an adsorbent dosage of 750 mg/L, at pH of 10. The maximum adsorption capacity was estimated at 280 mg of dye per gram of adsorbent [137]. These results illustrate a way to aggregate value to bark waste, by producing an effective dye adsorbent, and using a simple and low-cost method, without further activation steps.
Singh et al. [146] tried the removal of heavy metals Ni(II) and Zn(II) from aqueous solutions using an industrially produced A. karroo charcoal. The authors performed a single activation process by boiling the charcoal in acidic medium (hydrochloric acid solution, 1 M) for 5 h and found removal percentages of Ni(II) and Zn(II) metals 90 and 83% at a dose of 15 g/L. The maximum adsorption capacities for Ni(II) and Zn(II) metals were estimated in 9 and 8 mg of metal per gram of adsorbent, at an optimum pH of 6 and 4, respectively. These findings show that this material holds good potential for removing heavy metals from aqueous solutions and could be used for desalinating metal ions from industrial wastewater [146]. Moreover, its production can be made using a discarded fraction from the charcoal production, which can be obtained at a regular rate and at a low cost.
Beckinghausen et al. [138] tried the removal of ammonium from artificial wastewater (1000 mg/L of ammonium solution) in a fixed-bed column, using activated biochar made from A. mearnsii woodchips. The woodchips were carbonized at 400 °C for two hours and then two activation processes were compared-steam activation at 850 °C and hydrogen peroxide activation by shaking the biochar in a 10% hydrogen peroxide solution. The steam activation proved to be the most suitable activation method, producing a material with a surface area of 340 m2/g, with an adsorption capacity of 1651 μg of ammonium per gram of adsorbent. Moreover, the steam activation decreased the desorption capacity of the ammonium-loaded adsorbent, making it suitable to use as a solid fertilizer, improving soil nitrogen content and plant growth [138]. The process needs to be improved to increase the adsorption capacity for ammonium, but this approach has the advantage of producing a material that can remove ammonium from wastewater, and then be used as a soil fertilizer, returning a considerable amount of carbon into the earth [138].
Another alternative approach was performed by Taşkıran et al. [140], through the use of silver nanoparticles produced with A. saligna leaves extract in a dye adsorption. The silver nanoparticles were produced by mixing the liquid water extract with silver nitrate, with the extract promoting the reduction of silver cations to its metallic form, and then used in the adsorption of the Basic Red 46 dye. The optimal conditions were found as pH 7, a temperature of 35 °C and an adsorbent dosage of 1 g/L, being estimated to have a maximum adsorption capacity of 126 mg of dye per gram of adsorbent. Moreover, the authors demonstrated that the reduction of dye concentration to a level below that required by legislation can be attained using four adsorption stages (i.e., four batches of adsorption), with the effluent of the first batch entering into the second batch, and so on [140].
3.4. Wood and Steel Protection Using Bark and Leaf Extracts
The use of natural extracts to protect materials from degradation or corrosion, instead of using synthetic formulations, is of great importance when a change to more sustainable and environmentally friendly industrial practices is required.
Taking advantage of the antioxidant and antimicrobial properties of extracts of A. dealbata, Yildiz et al. [29] tried to use methanol extracts from this species as wood (Scots pine) protecting agents. The decay resistance of Scots pine wood against the brown-rot fungus (Coniophora puteana) was accessed comparing the impregnation of wood samples with water or methanol extracts from A. dealbata sapwood, heartwood and bark, using concentrations of 3% and 5% by weight. The weight loss caused by the fungus varied in the range of 7% to 24% for treated samples, against almost 40% of weight loss verified with untreated samples. Bark extracts were overall more effective than wood extracts, and methanol was the most appropriate solvent, being the highest protection observed when using a concentration of 5% of methanol bark extract [29]. Although these experiments did not yet provide adequate protection to meet the requirements of European standards, these are encouraging results and deserve a further investigation to try higher concentrations or the mixing with other non-toxic antifungal materials [29].
The efficiency of A. saligna leaf extract as an environmentally friendly inhibitor for mild steel was investigated by Avci and Keleş [141], using a sulphuric acid solution (1 M) as the corrosive agent. The authors concluded that water leaves extract adsorbs onto the mild steel surface and form a protective film against metal corrosion. The corrosion inhibition efficiencies determined by potentiodynamic polarization measurements and by electrochemical impedance spectroscopy techniques were in agreement, with the highest values observed when using an extract concentration of 20 g/L (93 and 95% for 1 h of immersion, respectively). The presence of the extract increased the activation energy for the corrosion process and slowed the corrosion rate down, even in long immersion experiments (up to 120 h of immersion time).
4. Energy from Acacia spp. and Biorefinery Approaches
The high availability of residues of Acacia spp. makes them an interesting resource to be studied, having in mind the production of solid, liquid or gaseous biofuels. This gains even more significance with a view to meeting targets for the incorporation of renewable energies according to the new directive on the promotion of the use of energy from renewable sources [147]. The solid biomass fuels, such as wood chips or pellets are now in the scope of the new directive. Its production to obtain heat or power by thermochemical processes can be an easy way to retrieve some value from Acacia spp. residues and mitigate eradication costs [14], instead of being landfilled or left in place, but more investigation must be made to access combustion, gasification or pyrolysis properties when using the Portuguese acacias.
4.1. Combustion and Pyrolysis
The overall chemical composition of the wood of some Portuguese Acacia spp. reported in the literature is summarized in Table 10. It is essentially a carbon-based material and its chemical composition does not significantly differ from other biomass sources [148]. The reported lower heating values lay between 18100–18400 kJ/kg [149,150].
Studies on combustion performance have been made using domestic boilers, where it was observed that CO and NOx emissions are much higher than those observed with the combustion of pine, especially in the case of NOx emissions, that are more than three times higher in the case of A. dealbata pellets [151]. This can be improved if combustion takes place in a fluidized bed combustor, but CO and NOx emissions from acacia pellets are still considerably higher than those which occur with pine pellets [150]. Concerning the NOx emissions, the higher values observed with acacia are due to the high nitrogen content found in these species (ca. 1% in A. dealbata, five times higher than in pine) [150]. Furthermore, the particulate emissions are higher (two times higher) when combusting acacia than when using pine, either in domestic or in fluidized bed boilers, according to experiments made with A. longifolia (whole tree, including bark, treetops and branches) [149,152].

Table 10.
Chemical composition of acacia wood (values expressed as weight percentage, on a dry basis).
Table 10.
Chemical composition of acacia wood (values expressed as weight percentage, on a dry basis).
| Chemical Composition | Value | Reference |
|---|---|---|
| Structural composition | ||
| Cellulose | 36%–45% | [50,153,154,155,156] |
| Hemicelluloses | 14%–28% | |
| Lignin | 19%–47% | |
| Extractives | 5%–9% | |
| Proximate composition | ||
| Volatile matter | 75%–88% | [50,149,150,152,157] |
| Fixed carbon | 8%–19% | |
| Ashes | 1%–3% | |
| Elemental composition | ||
| C | 47%–50% | [149,150,152,154,157] |
| H | 5%–6% | |
| O | 41%–47% | |
| N | ca. 1% | |
| S | <1% |
Further investigations on the impact of operational parameters or biomass pre-treatments in the performance of the selected thermochemical process are needed to better access the suitability of Portuguese invasive Acacia spp. for energy recovery purposes. For instance, torrefaction [158] and hydrothermal carbonization [159,160] of wood of other species of Acacia has been tried and successfully improved its calorific value, carbon content and mechanical properties. Even its co-gasification with plastic (polyethylene terephthalate) was already considered a possibility [161].
The feasibility of the valorization by flash pyrolysis of forest shrub wastes, including of A. dealbata, was accessed by Amutio et al. [154], in a conical spouted bed reactor operating at 500 °C, with a continuous biomass feed and char removal. A high bio-oil yield of 72 wt.% was obtained, with a char yield of 23 wt.% and a low gas yield of 5 wt.%. The produced bio-oils are composed mainly of water (concentration of 40 wt.%), phenols (13 wt.%), ketones (9 wt.%), catechols (8%), acids (7 wt.%), and furans (5 wt.%), with lower contents of alkyl phenols (3 wt.%), aldehydes (2 wt.%), guaiacols (2 wt.%), saccharides (2 wt.%) and alcohols (1 wt.%). This process originated high bio-oil yields with a suitable composition for extracting individual components and further energetic or catalytic upgrading to produce hydrocarbons or syngas, thus allowing the production of chemicals and energy [154]. The produced char also evidenced a much higher calorific value of 27,500 kJ/kg when compared with the original biomass (17100 kJ/kg), making it more suitable for energy recovery, besides other possible material applications such as in soil amendment or as adsorbents [154]. Pyrolysis kinetics of these wastes were later studied in detail by the same authors [162].
Furtado et al. [155] studied the composition of the pyrolysis liquor of A. mearnsii wood, in pyrolysis trials carried out in similar conditions to those used in the charcoal production. This liquor is usually released to the atmosphere in the traditional brick ovens, but its condensation can be easily performed, minimizing the amount of gas liberated and making possible the valorization of the condensables, along with the charcoal. This condensate contains important valuable chemicals derived from the thermal degradation of the lignocellulosic constituents, such as carboxylic acids, phenols, aldehydes, low molecular mass lignin fragments (e.g., guayacol, syringol and eugenol), substituted aromatic compounds (e.g., vanillin, ethyl vanilli and 2-methoxy-4-propenyl-phenol), as well as polysaccharide derivatives from cellulose and hemicellulose depolymerization that occurs at higher temperatures (e.g., furan, 2-acetylfuran, methyl-2-furoate and furfural) [155]. Amongst these chemicals, eugenol is worth hightlighting and is used in dentistry, perfumes and food flavoring and protection; guaiacol, used in the pharmaceutical industry; syringol, used as a fuel additive and as a surfactant; 5-methylfurfural, which is an important flavor of balsamic vinegar; and 4-hydroxy-3-methoxyethylbenzaldehyde, which has a high commercial value for nutraceutical, pharmaceutical, cosmetics, agrochemical and dyeing purposes [155].
4.2. Decomposition of Biomass in the Main Constituents
More efficient use of biomass can be achieved by opting for a biorefinery approach, seeking for obtaining high-value chemical products and/or other materials and energy. For this purpose, the decomposition of biomass in its main constituents (cellulose, hemicelluloses and lignin) and the further valorization of each, has been suggested [163,164,165]. This can be achieved with the help of physical pretreatments (milling, microwaves, sonication, gamma radiation, pyrolysis), chemical pretreatments (acid hydrolysis, alkaline hydrolysis, ozonation, treatment with ionic liquids, deep-eutectic solvents or organic solvents (organosolv)), physico-chemical pretreatments (steam explosion, carbon dioxide explosion, ammonia fiber explosion (AFEX), hydrothermal treatment (autohydrolysis)), or even biological pretreatments (white rot, brown rot, soft rot, bacterial or enzymatic treatments, pickling), which has already been reviewed in the literature [163,164,165].
A common approach is the application of one or a combination of pretreatments to obtain a cellulose-enriched solid, by removing lignin or hemicelluloses, making it more susceptible to enzymatic hydrolysis and fermentation processes to produce liquid biofuels, while simultaneously obtaining valuable monosaccharides, sugar oligomers and other degradation products from hemicelluloses and lignin (such as furfural, 5-hydroxymethylfurfural, organic acids and phenolic compounds). A summary of the reported practices for invasive Acacia spp. is provided in Table 11.

Table 11.
Pretreatment approaches for the decomposition of Acacia biomass.
Delignification of acacia wood was investigated by Domínguez et al. [50], who fractionated milled A. dealbata wood (250 μm to 1 mm) using an organosolv process with mixtures of glycerol-water (40%–80% glycerol) in a 600 mL stainless steel Parr reactor, with temperatures ranging from 200–230 °C, for 30 to 120 min, at a liquid to solid ratio of 6 and 10 g/g. This process promotes the delignification of the wood using a cheap industrial by-product, preserving cellulose in the solid phase, which can be later used as a substrate for enzymatic hydrolysis. The most favorable condition was using 80% glycerol at 230 °C for one hour, with a liquid to solid ratio of 6 g/g and introducing sodium hydroxide 1% in the washing steps, that led to the removal of 80% of the initially present lignin, allowing a solid yield of 51% (relative to the initial raw material) that preserved 93% and 23% of the initial cellulose and xylan contents, respectively [50]. The enzymatic saccharification yielded glucose concentrations up to 85 g/L, with almost complete cellulose conversion into glucose, thus confirming the potential of glycerol as an environmentally friendly fractionation agent for biorefineries [50]. Another way to promote delignification of biomass is by using pretreatment with acetate-based ionic liquids, which also promotes some loss of crystallinity of cellulose [167]. Yáñez et al. [156] treated milled A. dealbata wood (1–250 μm) with the ionic liquid 1-ethyl-3-methylimidazolium acetate in flasks at temperatures between 90 and 150 °C, for 30 to 1440 min, using a solid loading of 8 wt%, and testing water, methanol, ethanol and acetone-water (1:1 V/V) as anti-solvents to precipitate cellulose. The optimal condition (150 °C for 30 min, and water as anti-solvent) led to the removal of almost 20% of lignin and the preservation of 88% of cellulose and 66% of xylan in the solid fraction (82% of the initial raw material). Comparing with the untreated material, occurred an increase in cellulose and xylan conversion to fermentable sugars after enzymatic hydrolysis by 13-fold and 20-fold, respectively [156].
Solubilization of hemicelluloses was accessed by Yáñez et al. [166] by promoting the autohydrolysis of A. dealbata wood in a stainless steel Parr reactor at temperatures ranging within 170–240 °C and using a liquid to solid ratio of 8 kg of water per kg of oven-dried wood. In the selected condition (215 °C), this procedure led to 70% of xylan conversion into xylooligosaccharides, whereas cellulose and lignin remained in the solid phase with little alteration. This method reduced the hemecelluloses content in the remaining solid phase from 16 to near 8%, and increased the cellulose and lignin contents from 42 to 65% and from 19 to 27%, respectively [166]. The valorization of the solid phase (approximately 70% of the initial material) can be completed by subsequent processing, such as delignification and/or enzymatic hydrolysis of cellulose, enabling, for example, the production of bioethanol. The liquid phase contains saccharides (including monosaccharides and sugar oligomers suitable as ingredients of functional foods or as carbon sources for chemical or biotechnological processes), sugar-degradation compounds (furfural) and acetic acid [166]. Acuña et al. [153] investigated the potential of wood from A. melanoxylon and several Eucalyptus spp. for the production of bioethanol, considering also an autohydrolysis pretreatment (liquid to solid ratio of 6:1 (v/w), a heating rate of 3 °C/min and cooking at 194 °C for 1 min), followed by simultaneous saccharification and fermentation of the remaining cellulose-and lignin-enriched solid. A. melanoxylon outstands with a high average theoretical ethanol yield, after 48 months of plant growth, that reached 396 L of ethanol per ton of wood [153]. This estimation must be interpreted in the context of the referred work, that considered a plantation in marginal lands, with the sites presenting serious nutritional and hydric deficits, leading to low yields of wood production [153].
Acid hydrolysis also favors the solubilization of the hemicelluloses fraction, as demonstrated by Ferreira et al. [13], studying the potential of stalks and leaves of A. dealbata for bioethanol production. The authors pretreated the biomass with diluted sulfuric acid in various experimental conditions (up to approximately 4% of sulfuric acid (relative to dry biomass), 100–201 °C, 5–55 min and a liquid to solid ratio of 5:1), and then tried different methods for the production of ethanol from the obtained slurry, namely: the fermentation of the water-soluble fraction (72 h, 30 °C); the simultaneous saccharification and fermentation of the whole slurry (72 h, 30 °C); the simultaneous saccharification and fermentation (72 h, 30 °C), and the separate hydrolysis and fermentation (enzymatic saccharification for 72 h at 50 °C and fermentation for 24 hrs at 30 °C), in the case of the water-insoluble fraction (solid phase). Pretreatment at 180 °C with 0.8% of sulfuric acid for 15 min led to an increase of cellulose and acid-insoluble lignin in the solid phase from 43 to 62% and from 21 to 44%., respectively, while completely removing xylan. This condition led to the production of the highest ethanol concentration (10 g ethanol/L) by using separate hydrolysis and fermentation of the water-insoluble fraction [13]. With simultaneous saccharification and fermentation, results obtained for the water-insoluble fraction were better than those obtained with the whole slurry (6.8–7.5 g ethanol/L against ca. 1 g ethanol/L), which is due to the higher concentration of the fermentation inhibitors furfural and 5-hydroxymethylfurfural in the slurry, while the fermentation of the water-soluble fraction gave 1.9 to 2.6 g ethanol/L [13]. The separate hydrolysis and fermentation led to the lowest presence of inhibitors due to the washing operations involved until the fermentation step. It is worth noting that the global ethanol concentration can be increased by running a parallel fermentation of the water-soluble fraction together with the separate hydrolysis and fermentation processes [13].
4.3. Integrated Scheme for the Valorization of Invasive Acacia spp.
Taking into consideration the valuable extracts that can be obtained from the various fractions of the invasive Acacia spp., and the abovementioned decomposition techniques to obtain valuable chemicals and biofuels, an integrated approach can be suggested for more efficient use of these biomass sources. Since the majority of the reported extraction processes involves mild conditions for obtaining extractives from biomass, there will be negligible impacts on its lignocellulosic structure, thus allowing the application of physical, chemical, physico-chemical or biological pre-treatments to the spent biomass to obtain more products, materials and solid or liquid biofuels.
The scheme in Figure 2 represents the possible options for the valorization of Portuguese invasive Acacia spp., based on what was already studied with these species. Instead of being directly burned for energy recovery, directly submitted to thermochemical processes or decomposed in its main constituents, an extraction step may be applied to recover high-value extracts suitable to use in pharmaceutical, food and cosmetic industries, or in the growing nutraceutical industry. The spent biomass can then be submitted to thermochemical processes (route A in the diagram), such as combustion, gasification or pyrolysis. These options allow the production of heat and power, as well as important intermediates that can be further explored to obtain chemicals and fuels, such as the syngas obtained by gasification or the pyrolysis bio-oil, and also chars that can be the basis for the development of low-cost adsorbents or used in soil amendment [168,169]. In another way, spent biomass can be submitted to the abovementioned decomposition pre-treatments to obtain biofuels (especially bioethanol) from the hydrolysis and fermentation of the cellulose and hemicelluloses fraction, and important chemicals from the decomposition of lignin and hemicelluloses [163,164,165]. Ethanol, xylitol and other sugar alcohols, 2,3–butanediol, methyl ethyl ketone, 2-butanol, acetic acid, lactic acid, levulinic acid, fumaric acid, succinic acid, furfural and hydroxymethylfurfural are a few examples of industrially useful chemicals that can be derived from hemicelluloses [170,171]. From lignin, a broad range of chemicals can also be obtained, such as guaiacol, vanillin and a large variaty of other phenols and phenolic acids, acetylene, ethylene and acetic acid [164,171].
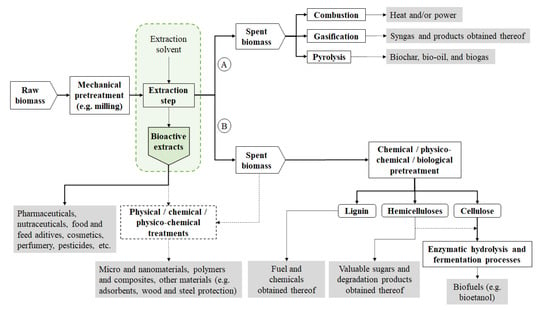
Figure 2.
Scheme for the valorization of biomass from Portuguese invasive Acacia spp., integrating an extraction step before application of advanced thermochemical processes (A) or biomass decomposition pre-treatments (B).
5. Conclusions
Acacia spp. are widespread all over the Portuguese territory, as well as across other territories around the world where they are considered invasive. Large amounts of residue has been generated from the proliferation control operations, whose valorization in an economic and environmentally sustainable manner is urgent. This review unified the results of the valorization of the various fractions of Acacia spp. present in the Portuguese territory, with a view to obtaining high-value extracts, chemicals, innovative materials and biofuels for energy recovery. The knowledge of these outputs is important regarding its sustainable valorization from a biorefinery perspective.
The general conclusion of researchers is that bark, wood, leaves, flowers, pods, seeds, roots and exudates can be exploited for their important biological activities, namely antioxidant, antimicrobial, anti-inflammatory, antitumoral, antidiabetic, antiviral, anthelmintic or pesticidal activities, and thus have the potential to be used by several profitable industries like pharmaceutical, nutraceutical, cosmetic or food and feed industries. Moreover, the incorporation of some fractions of the tree, or their extracts, in polymers and composites, nanomaterials and in the production of low-cost adsorbents as substitutes of commercial activated carbon, have been researched and highlighted by scientists.
Advanced decomposition processes to promote the fractionation of the biomass in its structural constituents (cellulose, hemicelluloses and lignin) have been studied in the last few years and are considered the basis for the valorization of these species in a biorefinery approach, taking advantage of the production of biofuels from the hydrolysis and fermentation of cellulose and hemicelluloses, as well as of the valuable chemicals that can be derived from the decomposition of hemicelluloses and lignin. Nevertheless, given the high-value products that can be extracted from Acacia spp., a scheme for the application of these decomposition procedures or of advanced thermochemical techniques (e.g., gasification or pyrolysis) to the spent biomass obtained from extraction processes is suggested, allowing us to obtain a broader range of products from the biomass.
Author Contributions
All the authors of this manuscript have contributed substantially to the work here reported. Conceptualization, R.C., M.G., J.C.Q., M.P.D.; Supervision, M.G., J.C.Q.; Writing—original draft preparation, R.C.; Writing-review and editing, R.C., M.G., J.C.Q., M.P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Portuguese public agency FCT-Fundação para a Ciência e Tecnologia through the PhD grant SFRH/BD/133300/2017. This work was also supported by FCT-Fundação para a Ciência e Tecnologia within the R&D Units Project Scope: UIDB/04077/2020.
Acknowledgments
The authors would like to acknowledge FCT–Fundação para a Ciência e Tecnologia for the funding provided for this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maslin, B.R. Synoptic overview of Acacia sensu lato (Leguminosae: Mimosoideae) in East and Southeast Asia. Gard. Bull. Singap. 2015, 67, 231. [Google Scholar] [CrossRef]
- Breton, C.; Guerin, J.; Ducatillion, C.; Médail, F.; Kull, C.A.; Bervillé, A. Taming the wild and “wilding” the tame: Tree breeding and dispersal in Australia and the Mediterranean. Plant Sci. 2008, 175, 197–205. [Google Scholar] [CrossRef]
- Kull, C.A.; Shackleton, C.M.; Cunningham, P.J.; Ducatillon, C.; Dufour-Dror, J.-M.; Esler, K.J.; Friday, J.B.; Gouveia, A.C.; Griffin, A.R.; Marchante, E.; et al. Adoption, use and perception of Australian acacias around the world. Divers. Distrib. 2011, 17, 822–836. [Google Scholar] [CrossRef]
- Seigler, D.S. Phytochemistry of Acacia-Sensu lato. Biochem. Syst. Ecol. 2003, 31, 845–873. [Google Scholar] [CrossRef]
- Marchante, H.; Marchante, E.; Freitas, H. Invasion of the Portuguese dune ecosystems by the exotic species Acacia longifolia (Andrews) Willd.: Effects at the community level. In Plant Invasions: Ecological Threats and Management Solutions; Child, L.E., Brock, J.H., Brundu, G., Prach, K., Pyšek, P., Wade, P.M., Williamson, M., Eds.; Backhuys Publishers: Kerkwerve, The Netherlands, 2003; pp. 75–85. [Google Scholar]
- Nunes, L.J.R.; Meireles, C.I.R.; Gomes, C.J.P.; Ribeiro, N.M.C.A. Historical development of the Portuguese forest: The introduction of invasive species. Forests 2019, 10, 974. [Google Scholar] [CrossRef]
- Miller, J.T.; Murphy, D.J.; Brown, G.K.; Richardson, D.M.; González-Orozco, C.E. The evolution and phylogenetic placement of invasive Australian Acacia species. Divers. Distrib. 2011, 17, 848–860. [Google Scholar] [CrossRef]
- Lorenzo, P.; González, L.; Reigosa, M.J. The genus Acacia as invader: The characteristic case of Acacia dealbata Link in Europe. Ann. For. Sci. 2010, 67, 1–11. [Google Scholar] [CrossRef]
- Le Maitre, D.C.; Gaertner, M.; Marchante, E.; Ens, E.-J.; Holmes, P.M.; Pauchard, A.; O’Farrell, P.J.; Rogers, A.M.; Blanchard, R.; Blignaut, J.; et al. Impacts of invasive Australian acacias: Implications for management and restoration. Divers. Distrib. 2011, 17, 1015–1029. [Google Scholar] [CrossRef]
- Fuentes-Ramírez, A.; Pauchard, A.; Cavieres, L.A.; García, R.A. Survival and growth of Acacia dealbata vs. native trees across an invasion front in. For. Ecol. Manag. 2011, 261, 1003–1009. [Google Scholar] [CrossRef]
- Presidência do Conselho de Ministros Decreto-Lei n.o 92/2019 de 10 de Julho. Diário da República, 10 July 2019; 3428–3442.
- ICNF IFN6. Áreas dos Usos do solo e das Espécies Florestais de Portugal Continental, Resultados Preliminares; Instituto da Conservação da Natureza e das Florestas: Lisboa, Portugal, 2013. [Google Scholar]
- Ferreira, S.; Gil, N.; Queiroz, J.A.; Duarte, A.P.; Domingues, F.C. An evaluation of the potential of Acacia dealbata as raw material for bioethanol production. Bioresour. Technol. 2011, 102, 4766–4773. [Google Scholar] [CrossRef]
- Carneiro, M.; Moreira, R.; Gominho, J.; Fabião, A. Could control of invasive acacias be a source of biomass for energy under mediterranean conditions? Chem. Eng. Trans. 2014, 37, 187–192. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; Rodríguez, J.; González, L.; Lorenzo, P. Here to stay. Recent advances and perspectives about Acacia invasion in Mediterranean areas. Ann. For. Sci. 2017, 74. [Google Scholar] [CrossRef]
- Jæger, D.; O’Leary, M.C.; Weinstein, P.; Møller, B.L.; Semple, S.J. Phytochemistry and bioactivity of Acacia sensu stricto (Fabaceae: Mimosoideae). Phytochem. Rev. 2019, 18, 129–172. [Google Scholar] [CrossRef]
- Pereira, F.B.M.; Domingues, F.M.J.; Silva, A.M.S. Triterpenes from Acacia dealbata. Nat. Prod. Lett. 1996, 8, 97–103. [Google Scholar] [CrossRef]
- Subhan, N.; Burrows, G.E.; Kerr, P.G.; Obied, H.K. Phytochemistry, Ethnomedicine, and Pharmacology of Acacia. In Studies in Natural Products Chemistry; Elsevier BV: Amsterdam, The Netherlands, 2018; Volume 57, pp. 247–326. ISBN 9780444640574. [Google Scholar]
- Lorenzo, P.; Reboredo-Durán, J.; Muñoz, L.; Freitas, H.; González, L. Herbicidal properties of the commercial formulation of methyl cinnamate, a natural compound in the invasive silver wattle (Acacia dealbata). Weed Sci. 2019, 68, 69–78. [Google Scholar] [CrossRef]
- De Jung, E.; Jungmeier, G. Chapter 1—Biorefinery Concepts in Comparison to Petrochemical Refineries. In Industrial Biorefineries and White Biotechnology; Elsevier B.V: Amsterdam, The Netherlands, 2015; pp. 3–33. ISBN 9780444634535. [Google Scholar]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Neiva, D.M.; Luís, Â.; Gominho, J.; Domingues, F.; Duarte, A.P.; Pereira, H. Bark residues valorization potential regarding antioxidant and antimicrobial extracts. Wood Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, S.; Yuan, Z.; Leitch, M.; Charles, C. Valorization of bark for chemicals and materials: A review. Renew. Sustain. Energy Rev. 2013, 26, 560–578. [Google Scholar] [CrossRef]
- Pásztory, Z.; Mohácsiné, I.R.; Gorbacheva, G.; Börcsök, Z. The utilization of tree bark. BioResources 2016, 11, 7859–7888. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Joseph, J.M.; Manian, S. Antioxidant and free radical scavenging activities of Indian acacias: Acacia leucophloea (Roxb.) willd., Acacia ferruginea dc., Acacia dealbata link. and Acacia pennata (l.) willd. Int. J. Food Prop. 2013, 16, 1717–1729. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Hong, S.; Jhoo, J.W.; Kim, S.; Chin, N.L. Effect of acetone extract from stem bark of Acacia species (A. dealbata, A. ferruginea and A. leucophloea) on antioxidant enzymes status in hydrogen peroxide-induced HepG2 cells. Saudi J. Biol. Sci. 2015, 22, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.D.; Moreira, P.; Resende, J.; Cruz, M.T.; Pereira, C.M.F.; Silva, A.M.S.; Santos, S.A.O.; Silvestre, A.J.D. Characterization and cytotoxicity assessment of the lipophilic fractions of different morphological parts of acacia dealbata. Int. J. Mol. Sci. 2020, 21, 1814. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Santhanam, R.; Hong, S.; Jhoo, J.W.; Kim, S. Suppressive effects of acetone extract from the stem bark of three Acacia species on nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Asian Pac. J. Trop. Biomed. 2016, 6, 658–664. [Google Scholar] [CrossRef]
- Yildiz, S.; Gürgen, A.; Can, Z.; Tabbouche, S.A.; Kiliç, A.O. Some bioactive properties of Acacia dealbata extracts and their potential utilization in wood protection. Drewno 2018, 61, 81–97. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Sofidiya, M.O.; Masika, P.J.; Afolayan, A.J. Anti-inflammatory and analgesic activities of the aqueous extract of Acacia karroo stem bark in experimental animals. Basic Clin. Pharmacol. Toxicol. 2008, 103, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Mulaudzi, R.B.; Ndhlala, A.R.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Antimicrobial properties and phenolic contents of medicinal plants used by the Venda people for conditions related to venereal diseases. J. Ethnopharmacol. 2011, 135, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Gabr, S.; Nikles, S.; Pferschy Wenzig, E.M.; Ardjomand-Woelkart, K.; Hathout, R.M.; El-Ahmady, S.; Motaal, A.A.; Singab, A.; Bauer, R. Characterization and optimization of phenolics extracts from Acacia species in relevance to their anti-inflammatory activity. Biochem. Syst. Ecol. 2018, 78, 21–30. [Google Scholar] [CrossRef]
- Ogawa, S.; Matsuo, Y.; Tanaka, T.; Yazaki, Y. Utilization of flavonoid compounds from bark and wood. III. Application in health foods. Molecules 2018, 23, 1860. [Google Scholar] [CrossRef]
- Ogawa, S.; Yazaki, Y. Tannins from Acacia mearnsii De Wild. Bark: Tannin determination and biological activities. Molecules 2018, 23, 837. [Google Scholar] [CrossRef]
- Denninger, T.M.; Schwarm, A.; Birkinshaw, A.; Terranova, M.; Dohme-Meier, F.; Münger, A.; Eggerschwiler, L.; Bapst, B.; Wegmann, S.; Clauss, M.; et al. Immediate effect of Acacia mearnsii tannins on methane emissions and milk fatty acid profiles of dairy cows. Anim. Feed Sci. Technol. 2020, 261. [Google Scholar] [CrossRef]
- Orlandi, T.; Kozloski, G.V.; Alves, T.P.; Mesquita, F.R.; Ávila, S.C. Digestibility, ruminal fermentation and duodenal flux of amino acids in steers fed grass forage plus concentrate containing increasing levels of Acacia mearnsii tannin extract. Anim. Feed Sci. Technol. 2015, 210, 37–45. [Google Scholar] [CrossRef]
- Tavares Lima, P.D.M.; Crouzoulon, P.; Sanches, T.P.; Zabre, G.; Kabore, A.; Niderkorn, V.; Hoste, H.; Talamini do Amarante, A.F.; Costa-Junior, L.M.; Abdalla, A.L.; et al. Effects of Acacia mearnsii supplementation on nutrition, parasitological, blood parameters and methane emissions in Santa Inês sheep infected with Trichostrongylus colubriformis and Haemonchus contortus. Exp. Parasitol. 2019, 207. [Google Scholar] [CrossRef]
- Yazaki, Y. Utilization of flavonoid compounds from bark and wood: A review. Nat. Prod. Commun. 2015, 10, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, T.; Stefanello, S.; Mezzomo, M.P.; Pozo, C.A.; Kozloski, G.V. Impact of a tannin extract on digestibility and net flux of metabolites across splanchnic tissues of sheep. Anim. Feed Sci. Technol. 2020, 261, 114384. [Google Scholar] [CrossRef]
- Gerlach, K.; Pries, M.; Südekum, K.H. Effect of condensed tannin supplementation on in vivo nutrient digestibilities and energy values of concentrates in sheep. Small Rumin. Res. 2018, 161, 57–62. [Google Scholar] [CrossRef]
- Kozloski, G.V.; Härter, C.J.; Hentz, F.; de Ávila, S.C.; Orlandi, T.; Stefanello, C.M. Intake, digestibility and nutrients supply to wethers fed ryegrass and intraruminally infused with levels of Acacia mearnsii tannin extract. Small Rumin. Res. 2012, 106, 125–130. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These Mechanisms with Bacterial Resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Coelho, D.S.C.; Santos, N.M.; Silvestre, A.J.D.; Neto, C.P. Identification of Δ7 phytosterols and phytosteryl glucosides in the wood and bark of several Acacia species. Lipids 2005, 40, 317–322. [Google Scholar] [CrossRef]
- Miras-Moreno, B.; Sabater-Jara, A.B.; Pedreno, M.A.; Almagro, L. Bioactivity of Phytosterols and Their Production in Plant in Vitro Cultures. J. Agric. Food Chem. 2016, 64, 7049–7058. [Google Scholar] [CrossRef]
- Kaur, N.; Chaudhary, J.; Jain, A.; Kishore, L. Stigmasterol: A compreensive review. Int. J. Pharm. Sci. Res. 2011, 2, 2259–2265. [Google Scholar] [CrossRef]
- Xu, F.; Huang, X.; Wu, H.; Wang, X. Beneficial health effects of lupenone triterpene: A review. Biomed. Pharmacother. 2018, 103, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Gill, I.; Valivety, R. Polyunsaturated fatty acids, part 1: Occurrence, biological activities and applications. Trends Biotechnol. 1997, 15, 401–409. [Google Scholar] [CrossRef]
- Fradinho, D.M.; Neto, C.P.; Evtuguin, D.; Jorge, F.C.; Irle, M.A.; Gil, M.H.; Pedrosa de Jesus, J. Chemical characterisation of bark and of alkaline bark extracts from maritime pine grown in Portugal. Ind. Crops Prod. 2002. [Google Scholar] [CrossRef]
- Miranda, I.; Gominho, J.; Mirra, I.; Pereira, H. Chemical characterization of barks from Picea abies and Pinus sylvestris after fractioning into different particle sizes. Ind. Crops Prod. 2012, 36, 395–400. [Google Scholar] [CrossRef]
- Domínguez, E.; Romaní, A.; Alonso, J.L.; Parajó, J.C.; Yáñez, R. A biorefinery approach based on fractionation with a cheap industrial by-product for getting value from an invasive woody species. Bioresour. Technol. 2014, 173, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.; Baptista, I.; Gominho, J.; Pereira, H. The influence of heartwood on the pulping properties of Acacia melanoxylon wood. J. Wood Sci. 2008, 54, 464–469. [Google Scholar] [CrossRef]
- Nielsen, T.R.H.; Kuete, V.; Jäger, A.K.; Meyer, J.J.M.; Lall, N. Antimicrobial activity of selected South African medicinal plants. BMC Complement. Altern. Med. 2012, 12, 1. [Google Scholar] [CrossRef]
- Oliveira, G.R.A.; dos Grasel, F.S.; de Pinho, G.P.; Silvério, F.O. Comparison of chemical composition of lipophilic extracts from Acacia mearnsii De Wild. wood of different ages. Ind. Crops Prod. 2020, 147, 112200. [Google Scholar] [CrossRef]
- Silva, E.; Fernandes, S.; Bacelar, E.; Sampaio, A. Antimicrobial activity of aqueous, ethanolic and methanolic leaf extracts from Acacia spp. and Eucalyptus nicholii. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 130–134. [Google Scholar] [CrossRef][Green Version]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of techniques and solvents on the antimicrobial and antioxidant potential of extracts from acacia dealbata and olea europaea. Antibiotics 2020, 9, 48. [Google Scholar] [CrossRef]
- Aguilera, N.; Becerra, J.; Villaseñor-Parada, C.; Lorenzo, P.; González, L.; Hernández, V. Effects and identification of chemical compounds released from the invasive Acacia dealbata Link. Chem. Ecol. 2015, 31, 479–493. [Google Scholar] [CrossRef]
- Ramli, S.; Harada, K.I.; Ruangrungsi, N. Antioxidant, antimicrobial and cytotoxicity activities of Acacia farnesiana (L.) Willd. Leaves ethanolic extract. Pharmacogn. J. 2011, 3, 50–58. [Google Scholar] [CrossRef]
- Nyila, M.A.; Leonard, C.M.; Hussein, A.A.; Lall, N. Activity of South African medicinal plants against Listeria monocytogenes biofilms, and isolation of active compounds from Acacia karroo. S. Afr. J. Bot. 2012, 78, 220–227. [Google Scholar] [CrossRef]
- Priyanka, C.; Kumar, P.; Bankar, S.P.; Karthik, L. In vitro antibacterial activity and gas chromatography–mass spectroscopy analysis of Acacia karoo and Ziziphus mauritiana extracts. J. Taibah Univ. Sci. 2015, 9, 13–19. [Google Scholar] [CrossRef]
- Cock, I.E.; van Vuuren, S.F. South African food and medicinal plant extracts as potential antimicrobial food agents. J. Food Sci. Technol. 2015, 52, 6879–6899. [Google Scholar] [CrossRef]
- Lima, C.P.; Cunico, M.M.; Auer, C.G.; Miguel, O.G.; Miguel, M.D.; da Silva, C.B.; Andrade, C.A.; Kerber, V.A. Potencial alelopático e antifúngico do extrato das folhas de Acacia longifolia (Andr.) Willd. Visão Acad. 2013, 14, 16–25. [Google Scholar] [CrossRef]
- Xiong, J.; Grace, M.H.; Esposito, D.; Wang, F.; Lila, M.A. Phytochemical characterization and anti-inflammatory properties of Acacia mearnsii leaves. Nat. Prod. Commun. 2016, 11, 649–653. [Google Scholar] [CrossRef]
- Manikandan, M.; Jayakumar, M.; Eyini, M.; Bong-Seop, K. The Bioefficacy of Methanol Crude Leaf Extract of Acacia melanoxylon. Korean J. Ecol. 1998, 21, 805–808. [Google Scholar]
- Hussain, M.I.; González, L.; Reigosa, M.J. Allelopathic potential of Acacia melanoxylon on the germination and root growth of native species. Weed Biol. Manag. 2011, 11, 18–28. [Google Scholar] [CrossRef]
- Payne, S.E.; Kotze, A.C.; Durmic, Z.; Vercoe, P.E. Australian plants show anthelmintic activity toward equine cyathostomins in vitro. Vet. Parasitol. 2013, 196, 153–160. [Google Scholar] [CrossRef]
- Edrah, S.M.; Alafid, F.; Shmeala, H.; Abobaker, D.M. Phytochemical analysis and antibacterial activity of Acacia pycnantha from Alkhums Libya. In Proceedings of the 2nd Annual Conference on Theories and Applications of Basic and Biosciences, University of Misurata, Misurata, Lybia, 1 September 2018; pp. 704–712. [Google Scholar]
- Mahmoud, M.F.; Alrumman, S.A.; Hesham, A.E.L. Biological activities of some Acacia spp. (Fabaceae) against new clinical isolates identified by ribosomal RNA gene-based phylogenetic analysis. Pak. J. Pharm. Sci. 2016, 29, 221–229. [Google Scholar] [PubMed]
- El Ayeb, A.; Ben Jannet, H.; Harzallah-Skhiri, F. Effects of Acacia cyanophylla Lindl. extracts on seed germination and seedling growth of four crop and weed plants. Turkish J. Biol. 2013, 37, 305–314. [Google Scholar] [CrossRef]
- Noreen, I.; Iqbal, A.; Rabbi, F.-; Muhammad, A.; Shah, Z.; Rahman, Z.U. Antimicrobial activity of different solvents extracts of Acacia cyanophylla. Pakistan J. Weed Sci. Res. 2017, 23, 79–90. [Google Scholar]
- Gumgumjee, N.M.; Hajar, A.S. Antimicrobial efficacy of Acacia saligna (Labill.) HL Wendl. and Cordia sinensis Lam. leaves extracts against some pathogenic microorganisms. Int. J. Microbiol. Immunol. Res. 2015, 3, 51–57. [Google Scholar]
- El-Toumy, S.A.; Salib, J.Y.; Mohamed, W.M.; Morsy, F.A. Phytochemical and antimicrobial studies on Acacia saligna leaves. Egypt. J. Chem. 2010, 53, 705–717. [Google Scholar] [CrossRef]
- Gedara, S.R.; Galala, A.A. New cytotoxic spirostane saponin and biflavonoid glycoside from the leaves of Acacia saligna (Labill.) H.L. Wendl. Nat. Prod. Res. 2014, 28, 324–329. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; González, L.; Cavaleiro, C. Ambient has become strained. Identification of acacia dealbata link volatiles interfering with germination and early growth of native species. J. Chem. Ecol. 2014, 40, 1051–1061. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; González, L.; López-Nogueira, A.; Cavaleiro, C.; Pedrol, N. Volatile organic compounds of Acacia longifolia and their effects on germination and early growth of species from invaded habitats. Chem. Ecol. 2018, 34, 126–145. [Google Scholar] [CrossRef]
- Leonard, C.M.; Virijevic, S.; Regnier, T.; Combrinck, S. Bioactivity of selected essential oils and some components on Listeria monocytogenes biofilms. S. Afr. J. Bot. 2010, 76, 676–680. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Sutay, S. Inhibitory effects of monoterpenes on seed germination and seedling growth. Z. Naturforsch. Sect. C J. Biosci. 2007, 62, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Viljoen, A.M. Geraniol-A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Durairaj, R.B. Resorcinol Chemistry in Pharmaceuticals Applications. In Resorcinol: Chemistry, Technology and Applications; Springer: Berlin/Heidelberg, Germany, 2005; pp. 633–715. [Google Scholar]
- Wu, S.B.; Long, C.; Kennelly, E.J. Structural diversity and bioactivities of natural benzophenones. Nat. Prod. Rep. 2014, 31, 1158–1174. [Google Scholar] [CrossRef]
- Bobylev, M.M.; Bobyleva, L.I.; Strobel, G.A. Synthesis and bioactivity of analogs of maculosin, a host-specific phytotoxin produced by Alternaria alternata on spotted knapweed (Centaurea maculosa). J. Agric. Food Chem. 1996, 44, 3960–3964. [Google Scholar] [CrossRef]
- Maroyi, A. Acacia karroo Hayne: Ethnomedicinal uses, phytochemistry and pharmacology of an important medicinal plant in southern Africa. Asian Pac. J. Trop. Med. 2017, 10, 351–360. [Google Scholar] [CrossRef]
- Mølgaard, P.; Nielsen, S.B.; Rasmussen, D.E.; Drummond, R.B.; Makaza, N.; Andreassen, J. Anthelmintic screening of Zimbabwean plants traditionally used against schistosomiasis. J. Ethnopharmacol. 2001, 74, 257–264. [Google Scholar] [CrossRef]
- Kahiya, C.; Mukaratirwa, S.; Thamsborg, S.M. Effects of Acacia nilotica and Acacia karoo diets on Haemonchus contortus infection in goats. Vet. Parasitol. 2003, 115, 265–274. [Google Scholar] [CrossRef]
- Marume, U.; Chimonyo, M.; Dzama, K. Influence of dietary supplementation with Acacia karroo on experimental haemonchosis in indigenous Xhosa lop-eared goats of South Africa. Livest. Sci. 2012, 144, 132–139. [Google Scholar] [CrossRef]
- Perriot, R.; Breme, K.; Meierhenrich, U.J.; Carenini, E.; Ferrando, G.; Baldovini, N. Chemical Composition of French Mimosa Absolute Oil. Agric. Food Chem. 2010, 58, 1844–1849. [Google Scholar] [CrossRef]
- Imperato, F. A chalcone glycoside from Acacia dealbata. Phytochemistry 1982, 21, 480–481. [Google Scholar] [CrossRef]
- Imperato, F. A new chalcone glucoside and cernuoside from the flowers of Acacia dealbata. Experientia 1982, 38, 67–68. [Google Scholar] [CrossRef]
- Casas, M.P.; Conde, E.; Ribeiro, D.; Fernandes, E.; Domínguez, H.; Torres, M.D. Bioactive properties of Acacia dealbata flowers extracts. Waste Biomass Valorization 2019. [Google Scholar] [CrossRef]
- Soto, M.L.; Parada, M.; Falqué, E.; Domínguez, H. Personal-care products formulated with natural antioxidant extracts. Cosmetics 2018, 5, 13. [Google Scholar] [CrossRef]
- Imperato, F. A new chalcone glucoside and Isosalipurposide from Acacia cyanophylla. Phytochemistry 1977, 17, 822–823. [Google Scholar] [CrossRef]
- Ghouila, H.; Meksi, N.; Haddar, W.; Mhenni, M.F.; Jannet, H.B. Extraction, identification and dyeing studies of Isosalipurposide, a natural chalcone dye from Acacia cyanophylla flowers on wool. Ind. Crops Prod. 2012, 35, 31–36. [Google Scholar] [CrossRef]
- Fajraoui, A.; Ben Nasr, J.; Lacoste, C.; Amar, M.B.; Dony, P.; Odof, S.; El Halouani, F. Coloration of the polylactic acid with the natural dye extracted from acacia cyanophylla flowers. Polym. Test. 2019, 78, 105988. [Google Scholar] [CrossRef]
- Ghribia, L.; Ghouilaa, H.; Omrib, A.; Besbesb, M.; Hichem, H. Ben Antioxidant and anti-acetylcholinesterase activities of extracts and secondary metabolites from Acacia cyanophylla. Asian Pac. J. Trop. Biomed. 2014, 4, S417–S423. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Behiry, S.I.; Salem, M.Z.M.; Ali, H.M.; Siddiqui, M.H.; Salem, A.Z.M. Antifungal, Antibacterial, and Antioxidant Activities of Acacia Saligna (Labill.) H. L. Wendl. Flower Extract: HPLC Analysis of Phenolic and Flavonoid Compounds. Molecules 2019, 24, 700. [Google Scholar] [CrossRef]
- Lorenzo, P.; Palomera-Pérez, A.; Reigosa, M.J.; González, L. Allelopathic interference of invasive Acacia dealbata Link on the physiological parameters of native understory species. Plant Ecol. 2011, 212, 403–412. [Google Scholar] [CrossRef]
- Lorenzo, P.; Pazos-Malvido, E.; Reigosa, M.J.; González, L. Differential responses to allelopathic compounds released by the invasive Acacia dealbata Link (Mimosaceae) indicate stimulation of its own seed. Aust. J. Bot. 2010, 58, 546–553. [Google Scholar] [CrossRef]
- Vo, V.A.; Lee, J.; Shin, S.; Kwon, J.; Lee, H.J.; Kim, S.; Kwon, Y.; Chun, W. Methyl p-Hydroxycinnamate Suppresses Lipopolysaccharide- Induced Inflammatory Responses through Akt Phosphorylation. Biomol. Ther. 2014, 22, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Mayer, A.; Anke, H.; Sterner, O. Fatty acids and other compounds with nematicidal activity from cultures of basidiomycetes. Planta Med. 1994, 60, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Srikrishna, S.; Castellani, R.J.; Perry, G. Antioxidants in the Prevention and Treatment of Alzheimer’s Disease. In Nutritional Antioxidant Therapies: Treatments and Perspectives; Springer International Publishing: Cham, Switzerland, 2018; pp. 523–553. ISBN 9783319676258. [Google Scholar]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Jelassi, A.; El Ayeb-Zakhama, A.; Nejma, A.B.; Chaari, A.; Harzallah-Skhiri, F.; Jannet, H.B. Ben Phytochemical composition and allelopathic potential of three Tunisian Acacia species. Ind. Crops Prod. 2016, 83, 339–345. [Google Scholar] [CrossRef]
- Puga, C.D.; Hilario, M.C.; Mendoza, J.G.E.; Campos, O.M.; Jijón, E.M.; Martínez, M.D.; Izazaga, M.A.Á.; Solano, J.Á.L.; Chaverri, J.P. Antioxidant activity and protection against oxidative-induced damage of Acacia shaffneri and Acacia farnesiana pods extracts: In vitro and in vivo assays. BMC Complement. Altern. Med. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- El Abbouyi, A.; Toumi, M.; El Hachimi, Y.; Jossang, A. In vitro effects of aqueous seeds extract of Acacia cyanophylla on the opsonized zymosan-induced superoxide anions production by rat polymorphonuclear leukocytes. J. Ethnopharmacol. 2004, 91, 159–165. [Google Scholar] [CrossRef]
- Kumar, S. Anti-Diabetic Activity of Hydroalcoholic Extract of Acacia melanoxylon Linn. Seeds in Streptozotocin Induced Diabetic Rats. J. Diabetes Res. Ther. 2016, 2. [Google Scholar] [CrossRef]
- Santi-Gadelha, T.; Rocha, B.A.M.; Gadelha, C.A.A.; Silva, H.C.; Castellon, R.E.R.; Gonçalves, F.J.T.; Toyama, D.O.; Toyama, M.H.; de Souza, A.J.F.; Beriam, L.O.S.; et al. Effects of a lectin-like protein isolated from Acacia farnesiana seeds on phytopathogenic bacterial strains and root-knot nematode. Pestic. Biochem. Physiol. 2012, 103, 15–22. [Google Scholar] [CrossRef]
- Abdallah, E. Antibacterial Efficacy of Acacia nilotica (L.) Pods Growing in Sudan against Some Bacterial Pathogens. Int. J. Curr. Res. Biosci. Plant. Biol. 2016, 3, 6–11. [Google Scholar] [CrossRef]
- Auwal, M.S.; Shuaibu, A.; Ibrahim, A.; Mustapha, M. Antibacterial properties of crude pod extract of Acacia nilotica (Fabaceae). Haryana Vet. 2015, 54, 29–32. [Google Scholar]
- Ahmad, M.; Zaman, F.; Sharif, T.; Zabta Ch., M. Antidiabetic and hypolipidemic effects of Aqueous Methanolic extract of acacia nilotica pods in alloxan-induced diabetic rabbits. Scand. J. Lab. Anim. Sci. 2008, 35, 29–30. [Google Scholar]
- Omara, E.A.; Nada, S.A.; Razik, A.; Farrag, H.; Sharaf, W.M.; El-toumy, S.A. Phytomedicine Therapeutic effect of Acacia nilotica pods extract on streptozotocin induced diabetic nephropathy in rat. Eur. J. Integr. Med. 2012, 19, 1059–1067. [Google Scholar] [CrossRef]
- Gilani, A.H.; Shaheen, F.; Zaman, M.; Janbaz, K.H.; Shah, B.H.; Akhtar, M.S. Studies on antihypertensive and antispasmodic activities of methanol extract of Acacia nilotica pods. Phyther. Res. 1999, 13, 665–669. [Google Scholar] [CrossRef]
- Kankara, S. Acacia Nilotica Pods ’ Water Extract Enhances Wound Healing in Sprague-Dawley Rats By Alleviating Oxidative Stress and Suppressing Pro-Inflammatory Cytokines. Nig. J. Sc. Res. 2018, 16, 202–210. [Google Scholar]
- Jelassi, A.; Cheraief, I.; Hamza, M.A.; Jannet, H. Ben Chemical composition and characteristic profiles of seed oils from three Tunisian Acacia species. J. Food Compos. Anal. 2014, 33, 49–54. [Google Scholar] [CrossRef]
- Youzbachi, N.; Elfalleh, W.; Tlili, N.; Gregoire, S.; Berdeaux, O.; Salles, C.; Triki, S.; Khouja, M.L.; Khaldi, A.; Nasri, N. Unexploited Acacia cyanophylla seeds: Potential food sources of ω6 fatty acids and antioxidants? J. Sci. Food Agric. 2012, 92, 1526–1532. [Google Scholar] [CrossRef]
- Kumar, N.G.; Contaifer, D.; Madurantakam, P.; Carbone, S.; Price, E.T.; Van Tassell, B.; Brophy, D.F.; Wijesinghe, D.S. Dietary bioactive fatty acids as modulators of immune function: Implications on human health. Nutrients 2019, 11, 2974. [Google Scholar] [CrossRef]
- Shelat, K.J.; Adiamo, O.Q.; Mantilla, S.M.O.; Smyth, H.E.; Tinggi, U.; Hickey, S.; Rühmann, B.; Sieber, V.; Sultanbawa, Y. Overall Nutritional and Sensory Profile of Di ff erent Species of Australian Wattle Seeds (Acacia spp.): Potential Food Sources in the Arid and Semi-Arid Regions. Foods 2019, 8, 482. [Google Scholar] [CrossRef]
- Hannachi, H.; Elfalleh, W.; Ennajeh, I.; Laajel, M.; Khouja, M.L.; Ferchichi, A.; Nasri, N. Chemicals profiling and antioxidants activities of acacia seeds. J. Med. Plant. Res. 2011, 5, 6869–6875. [Google Scholar] [CrossRef]
- Tshikalange, T.E.; Mamba, P.; Adebayo, S.A. Antimicrobial, antioxidant and cytotoxicity studies of medicinal plants used in the treatment of sexually transmitted diseases. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1891–1895. [Google Scholar]
- Mamba, P.; Adebayo, S.A.; Tshikalange, T.E. Anti-microbial, anti-inflammatory and HIV-1 reverse transcriptase activity of selected South African plants used to treat sexually transmitted diseases. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1870–1876. [Google Scholar]
- Patel, S.; Goyal, A. Applications of natural polymer gum Arabic: A review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Musa, H.H.; Ahmed, A.A.; Musa, T.H. Chemistry, Biological, and Pharmacological Properties of Gum Arabic. In Bioactive Molecules in Food; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 1–18. ISBN 9783319545288. [Google Scholar]
- Grein, A.; Da Silva, B.C.; Wendel, C.F.; Tischer, C.A.; Sierakowski, M.R.; Moura, A.B.D.; Iacomini, M.; Gorin, P.A.J.; Simas-Tosin, F.F.; Riegel-Vidotti, I.C. Structural characterization and emulsifying properties of polysaccharides of Acacia mearnsii de Wild gum. Carbohydr. Polym. 2013, 92, 312–320. [Google Scholar] [CrossRef]
- Grein-Iankovski, A.; Ferreira, J.G.L.; Orth, E.S.; Sierakowski, M.R.; Cardoso, M.B.; Simas, F.F.; Riegel-Vidotti, I.C. A comprehensive study of the relation between structural and physical chemical properties of acacia gums. Food Hydrocoll. 2018, 85, 167–175. [Google Scholar] [CrossRef]
- Vidanarachchi, J.K.; Iji, P.A.; Mikkelsen, L.L.; Sims, I.; Choct, M. Isolation and characterization of water-soluble prebiotic compounds from Australian and New Zealand plants. Carbohydr. Polym. 2009, 77, 670–676. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef]
- Vidanarachchi, J.K.; Elangovan, A.V.; Mikkelsen, L.L.; Choct, M.; Iji, P.A. Effect of some plant extracts on growth performance, intestinal morphology, microflora composition and activity in broiler chickens. Anim. Prod. Sci. 2010, 50, 880–889. [Google Scholar] [CrossRef]
- Chan, J.M.; Day, P.; Feely, J.; Thompson, R.; Little, K.M.; Norris, C.H. Acacia mearnsii industry overview: Current status, key research and development issues. South. For. 2015, 77, 19–30. [Google Scholar] [CrossRef]
- Bridson, J.H.; Kaur, J.; Zhang, Z.; Donaldson, L.; Fernyhough, A. Polymeric flavonoids processed with co-polymers as UV and thermal stabilisers for polyethylene films. Polym. Degrad. Stab. 2015, 122, 18–24. [Google Scholar] [CrossRef]
- Khan, M.Y.; Mangrich, A.S.; Schultz, J.; Grasel, F.S.; Mattoso, N.; Mosca, D.H. Green chemistry preparation of superparamagnetic nanoparticles containing Fe3O4 cores in biochar. J. Anal. Appl. Pyrolysis 2015, 116, 42–48. [Google Scholar] [CrossRef]
- Missio, A.L.; Mattos, B.D.; de Ferreira, D.F.; Magalhães, W.L.E.; Bertuol, D.A.; Gatto, D.A.; Petutschnigg, A.; Tondi, G. Nanocellulose-tannin films: From trees to sustainable active packaging. J. Clean. Prod. 2018, 184, 143–151. [Google Scholar] [CrossRef]
- Martins, A.F.; Facchi, S.P.; da Câmara, P.C.F.; Camargo, S.E.A.; Camargo, C.H.R.; Popat, K.C.; Kipper, M.J. Novel poly(ε-caprolactone)/amino-functionalized tannin electrospun membranes as scaffolds for tissue engineering. J. Colloid Interface Sci. 2018, 525, 21–30. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.; Vargas, Á.; Fronza, N.; dos Santos, J.H.Z. Structural, textural and morphological characteristics of tannins from Acacia mearnsii encapsulated using sol-gel methods: Applications as antimicrobial agents. Colloids Surf. B Biointerfaces 2017, 151, 26–33. [Google Scholar] [CrossRef]
- Dos Grasel, F.S.; Behrens, M.C.; Strassburger, D.; Einloft, S.; Diz, F.M.; Morrone, F.B.; Wolf, C.R.; Ligabue, R.A. Synthesis, characterization and in vitro cytotoxicity of acacia mearnsii proanthocyanidin-loaded plga microparticles. Braz. J. Chem. Eng. 2019, 36, 239–250. [Google Scholar] [CrossRef]
- Taflick, T.; Maich, É.G.; Dias Ferreira, L.; Bica, C.I.D.; Rodrigues, S.R.S.; Nachtigall, S.M.B. Acacia bark residues as filler in polypropylene composites. Polimeros 2015, 25, 289–295. [Google Scholar] [CrossRef]
- Taflick, T.; Schwendler, L.A.; Rosa, S.M.L.; Bica, C.I.D.; Nachtigall, S.M.B. Cellulose nanocrystals from acacia bark–Influence of solvent extraction. Int. J. Biol. Macromol. 2017, 101, 553–561. [Google Scholar] [CrossRef]
- Da Silva, J.S.; da Rosa, M.P.; Beck, P.H.; Peres, E.C.; Dotto, G.L.; Kessler, F.; Grasel, F.S. Preparation of an alternative adsorbent from Acacia Mearnsii wastes through acetosolv method and its application for dye removal. J. Clean. Prod. 2018, 180, 386–394. [Google Scholar] [CrossRef]
- Beckinghausen, A.; Reynders, J.; Merckel, R.; Wen, Y.; Marais, H.; Schwede, S. Post-pyrolysis treatments of biochars from sewage sludge and A. mearnsii for ammonia (NH 4 -n) recovery. Appl. Energy 2020, 271, 115212. [Google Scholar] [CrossRef]
- Shashanka, R.; Kumara Swamy, B.E. Biosynthesis of silver nanoparticles using leaves of Acacia melanoxylon and their application as dopamine and hydrogen peroxide sensors. Phys. Chem. Res. 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Taşkıran, F.; Uzunoğlu, D.; Özer, A. Biosynthesis, characterisation and determination of adsorbent properties of silver nanoparticles with cyprus acacia (acacia cyanophylla) leaf extract. Anadolu Univ. J. Sci. Technol. A Appl. Sci. Eng. 2017, 18, 733–745. [Google Scholar] [CrossRef]
- Avci, G.; Keleş, Y. Aqueous extract of Acacia cyanophylla leaves as environmentally friendly inhibitor for mild steel corrosion in 1 M H2SO4 solution. Surf. Interface Anal. 2011, 43, 1311–1317. [Google Scholar] [CrossRef]
- Devi, S.M.; Nivetha, A.; Prabha, I. Superparamagnetic Properties and Significant Applications of Iron Oxide Nanoparticles for Astonishing Efficacy—a Review. J. Supercond. Nov. Magn. 2019, 32, 127–144. [Google Scholar] [CrossRef]
- Ismail, A.; Hilal, N.; Jaafar, J.; Wright, C. Nanofiber Membranes for Medical, Environmental, and Energy Applications; Ismail, A., Hilal, N., Jaafar, J., Wright, C., Eds.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781351174046. [Google Scholar]
- Sulyman, M.; Namiesnik, J.; Gierak, A. Low-cost adsorbents derived from agricultural by-products/wastes for enhancing contaminant uptakes from wastewater: A review. Pol. J. Environ. Stud. 2017, 26, 479–510. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, R.; Ali, A. Application of Acacia karroo charcoal for desalinating Ni(II) and Zn(II) from aqueous solutions through batch mode. J. Water Reuse Desalin. 2013, 3, 268–276. [Google Scholar] [CrossRef]
- Directive (EU) 2018/2001 of the European Parliament and of the Council Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the promotion of the use of energy from renewable sources. Off. J. Eur. Union 2018, L 328, 82–209.
- McKendry, P. Energy production from biomass (Part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Vicente, E.D.; Vicente, A.M.; Evtyugina, M.; Carvalho, R.; Tarelho, L.A.C.; Paniagua, S.; Nunes, T.; Otero, M.; Calvo, L.F.; Alves, C. Emissions from residential pellet combustion of an invasive acacia species. Renew. Energy 2019, 140, 319–329. [Google Scholar] [CrossRef]
- Ferreira, T.; Marques, E.; Almeida, D.; Pereira, C.; Paiva, J.M.; Pinho, C. Monitoring Fluidization Quality and Combustion Efficiency of Invasive species pellets. In Proceedings of the 15th Brazilian Congress of Thermal Sciences and Engineering, Belém, Brazil, 10–13 November 2014. [Google Scholar]
- Ferreira, T.; Paiva, J.M.; Pinho, C. Performance assessment of invasive Acacia dealbata as a fuel for a domestic pellet boiler. In Proceedings of the 8th Conference on Sustainable Development of Energy, Water and Environmental Systems, Dubrovnik, Croatia, 22–27 September 2013; pp. 1–10. [Google Scholar]
- Ribeiro, J.P.; Vicente, E.D.; Alves, C.; Querol, X.; Amato, F.; Tarelho, L.A.C. Characteristics of ash and particle emissions during bubbling fluidised bed combustion of three types of residual forest biomass. Environ. Sci. Pollut. Res. 2017, 24, 10018–10029. [Google Scholar] [CrossRef]
- Acuña, E.; Cancino, J.; Rubilar, R.; Parra, C. Bioethanol potential from high density short rotation woody crops on marginal lands in central Chile. Cerne 2017, 23, 133–145. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Alvarez, J.; Moreira, R.; Duarte, G.; Nunes, J.; Olazar, M.; Bilbao, J. Flash pyrolysis of forestry residues from the Portuguese Central Inland Region within the framework of the BioREFINA-Ter project. Bioresour. Technol. 2013, 129, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Furtado, C.M.; Dos Santos Stolz, A.; Pinto, F.L.; Moura, A.B.D.; Pont Morisso, F.D.; Pitarelo, A.P.; Ramos, L.P.; Von Mühlen, C.; Riegel-Vidotti, I.C. Pyroligneous liquor produced from Acacia Mearnsii de wild wood under controlled conditions as a renewable source of chemicals. Quim. Nova 2015, 38, 1068–1074. [Google Scholar] [CrossRef]
- Yáñez, R.; Gómez, B.; Martínez, M.; Gullón, B.; Alonso, J.L. Valorization of an invasive woody species, Acacia dealbata, by means of Ionic liquid pretreatment and enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2014, 89, 1337–1343. [Google Scholar] [CrossRef]
- Saldarriaga, J.F.; Aguado, R.; Pablos, A.; Amutio, M.; Olazar, M.; Bilbao, J. Fast characterization of biomass fuels by thermogravimetric analysis (TGA). Fuel 2015, 140, 744–751. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Gajek, M.; Zajemska, M.; Jayaraman, K.; Gokalp, I. Combustion and kinetic parameters estimation of torrefied pine, acacia and Miscanthus giganteus using experimental and modelling techniques. Bioresour. Technol. 2017, 243, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.; Magdziarz, A.; Jayaraman, K.; Szymańska-Chargot, M.; Gökalp, I. Hydrothermal carbonization characteristics of sewage sludge and lignocellulosic biomass. A comparative study. Biomass Bioenergy 2019, 120, 166–175. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Kalemba-Rec, I.; Szymańska-Chargot, M. Upgrading of green waste into carbon-rich solid biofuel by hydrothermal carbonization: The effect of process parameters on hydrochar derived from acacia. Energy 2020, 202. [Google Scholar] [CrossRef]
- Radenahmad, N.; Rahman, I.S.A.; Morni, N.A.H.; Azad, A.K. Acacia-polyethylene terephthalate co- gasification as renewable energy resource. Int. J. Renew. Energy Res. 2018, 8, 1612–1620. [Google Scholar]
- Amutio, M.; Lopez, G.; Alvarez, J.; Moreira, R.; Duarte, G.; Nunes, J.; Olazar, M.; Bilbao, J. Pyrolysis kinetics of forestry residues from the Portuguese Central Inland Region. Chem. Eng. Res. Des. 2013, 91, 2682–2690. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Narron, R.H.; Kim, H.; Chang, H.M.; Jameel, H.; Park, S. Biomass pretreatments capable of enabling lignin valorization in a biorefinery process. Curr. Opin. Biotechnol. 2016, 38, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, R.; Romaní, A.; Garrote, G.; Alonso, J.L.; Parajó, J.C. Processing of Acacia dealbata in Aqueous Media: First Step of a Wood Biorefinery. Ind. Eng. Chem. Res. 2009, 48, 6618–6626. [Google Scholar] [CrossRef]
- Doherty, T.V.; Mora-Pale, M.; Foley, S.E.; Linhardt, R.J.; Dordick, J.S. Ionic liquid solvent properties as predictors of lignocellulose pretreatment efficacy. Green Chem. 2010, 12, 1967–1975. [Google Scholar] [CrossRef]
- Benedetti, V.; Patuzzi, F.; Baratieri, M. Gasification Char as a Potential Substitute of Activated Carbon in Adsorption Applications. Energy Procedia 2017, 105, 712–717. [Google Scholar] [CrossRef]
- Akhtar, A.; Krepl, V.; Ivanova, T. A Combined Overview of Combustion, Pyrolysis, and Gasification of Biomass. Energy Fuels 2018, 32, 7294–7318. [Google Scholar] [CrossRef]
- Ji, X.J.; Huang, H.; Nie, Z.K.; Qu, L.; Xu, Q.; Tsao, G.T. Fuels and chemicals from hemicellulose sugars. Adv. Biochem. Eng. Biotechnol. 2012, 128, 199–224. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-based chemicals from renewable biomass for integrated biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).