Estimating the Potential for Forest Degradation in the Eastern United States Woodlands from an Introduction of Sudden Oak Death
Abstract
1. Introduction
1.1. Sudden Oak Death Characteristics
1.2. Sudden Oak Death Range Prediction
1.3. Present Aims
2. Materials and Methods
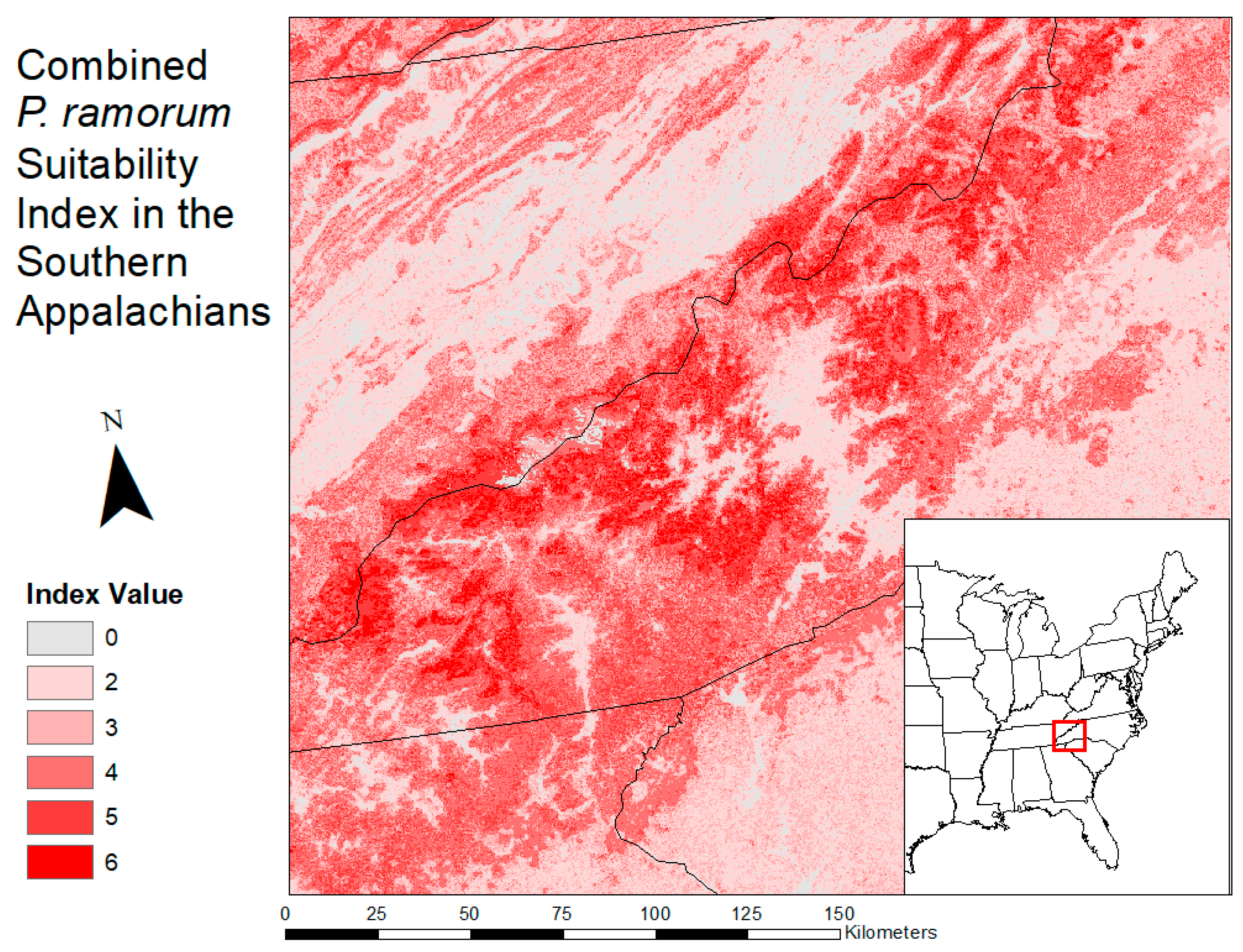
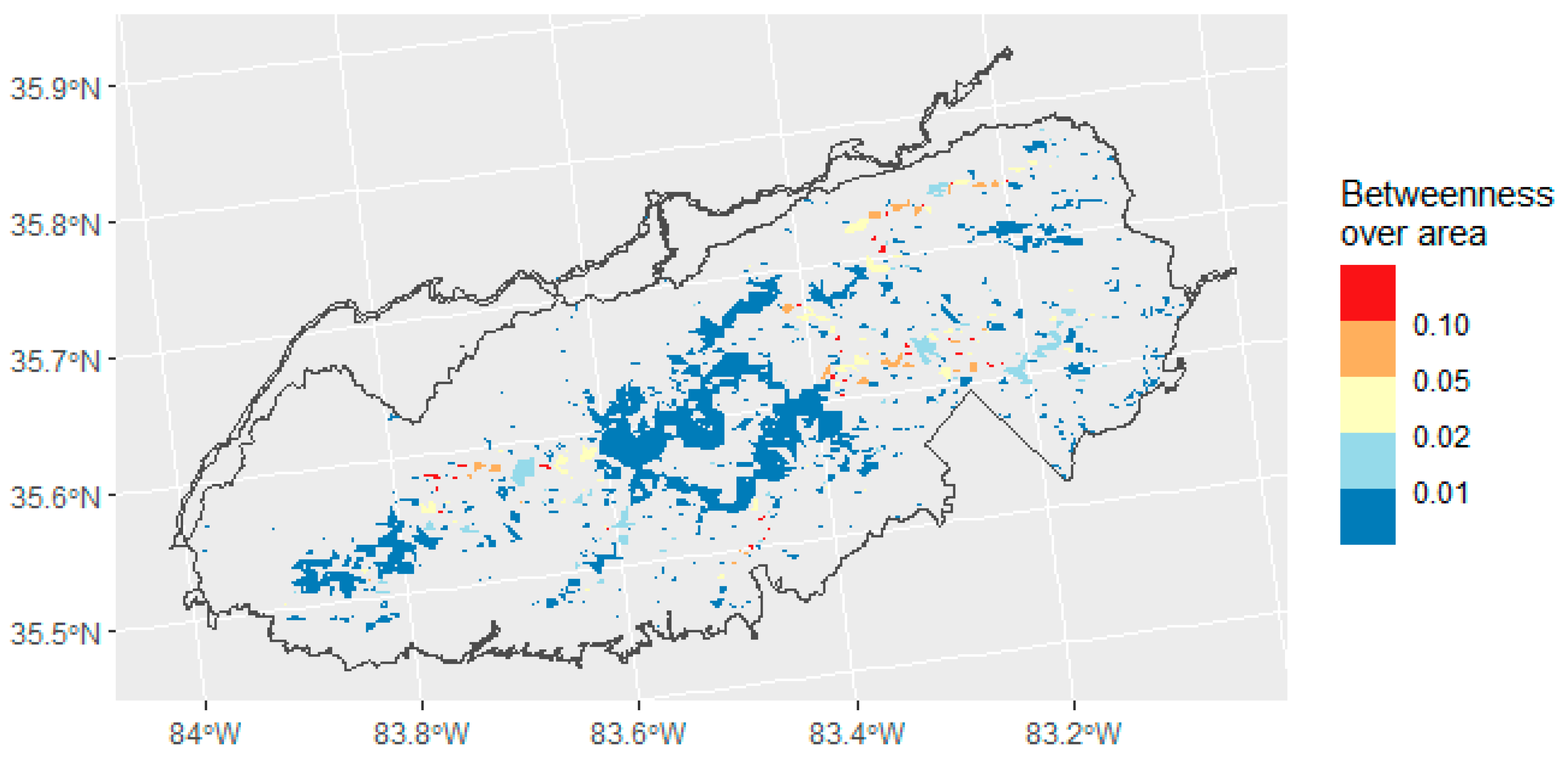
2.1. Study Area
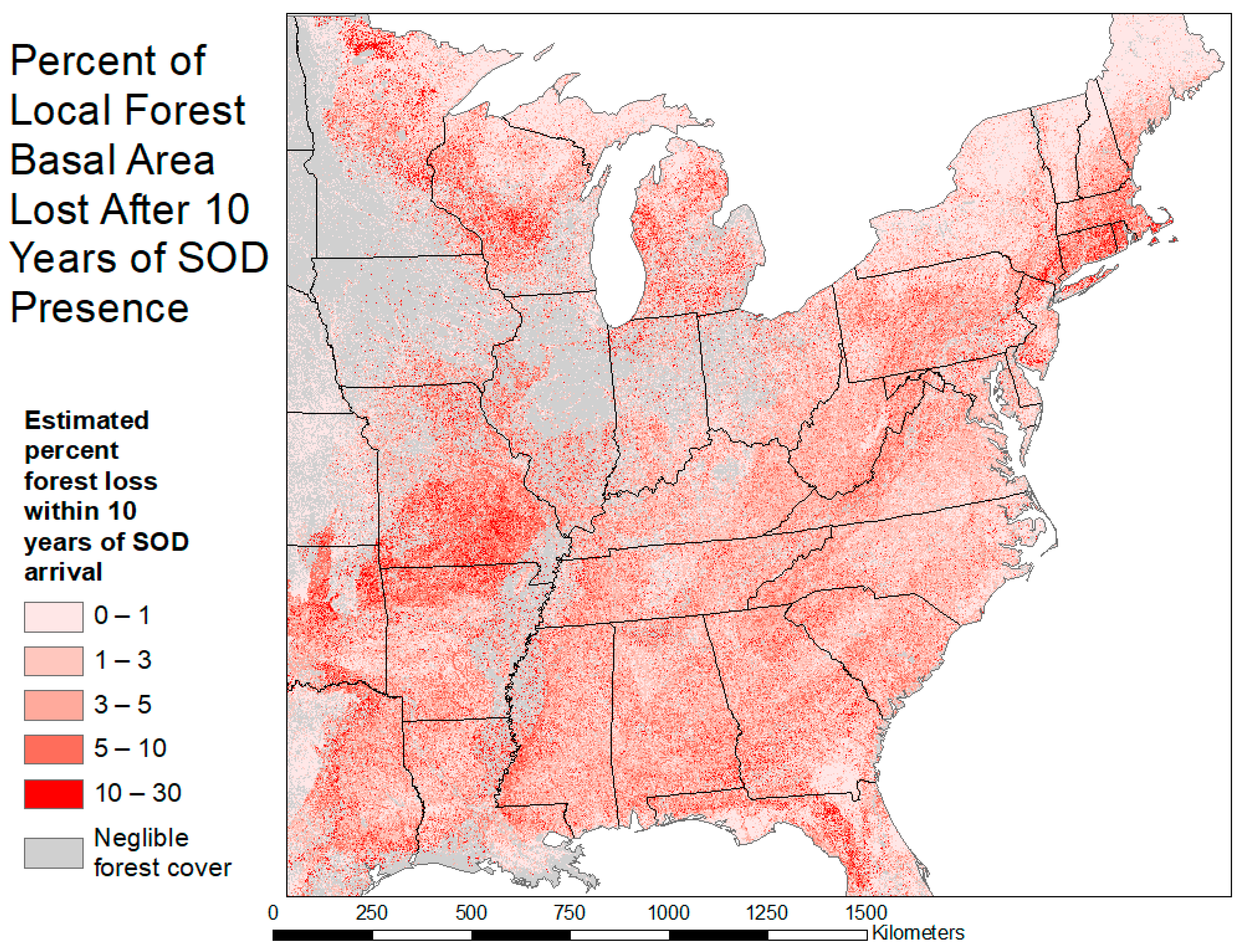
2.2. Severity Map
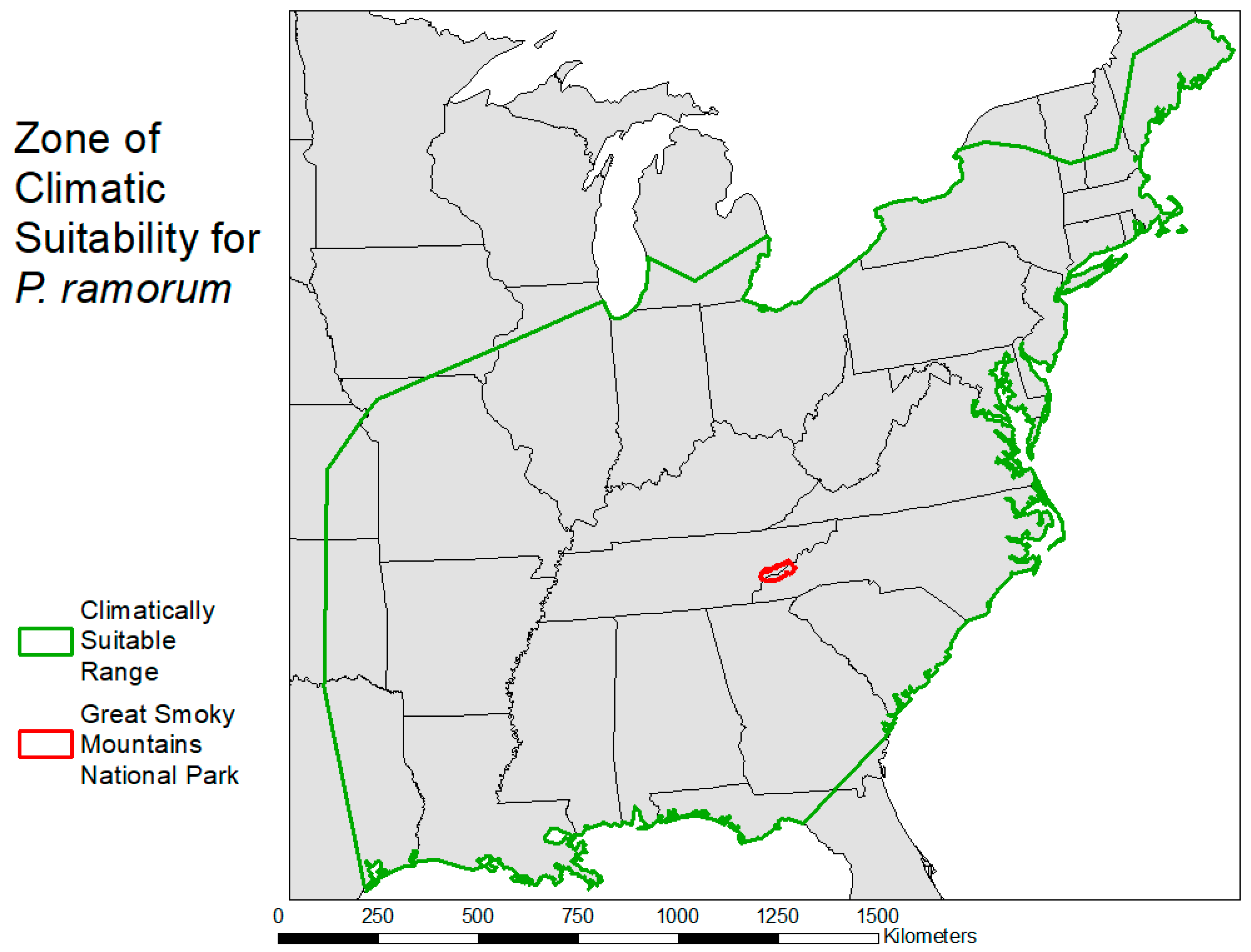
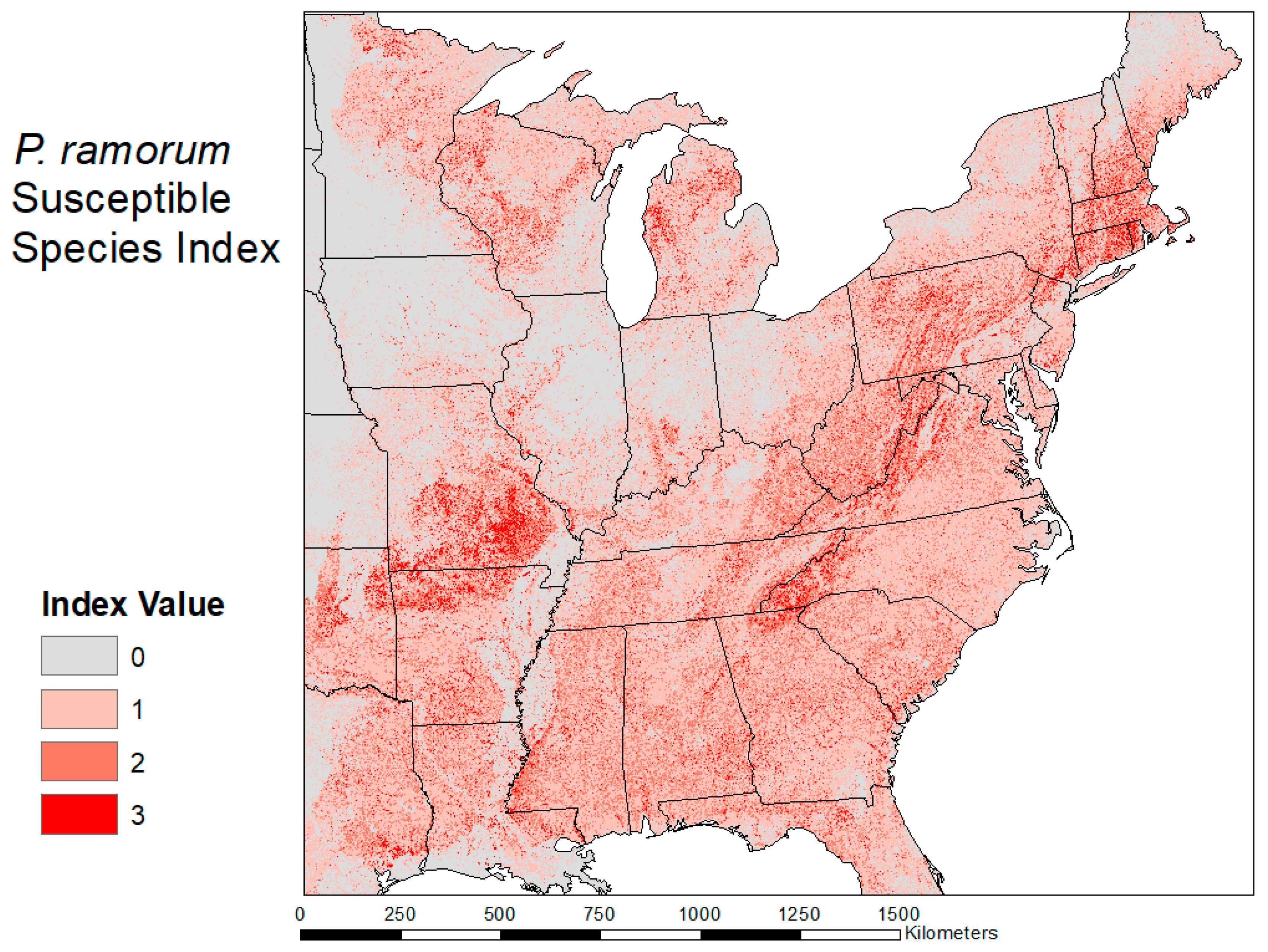
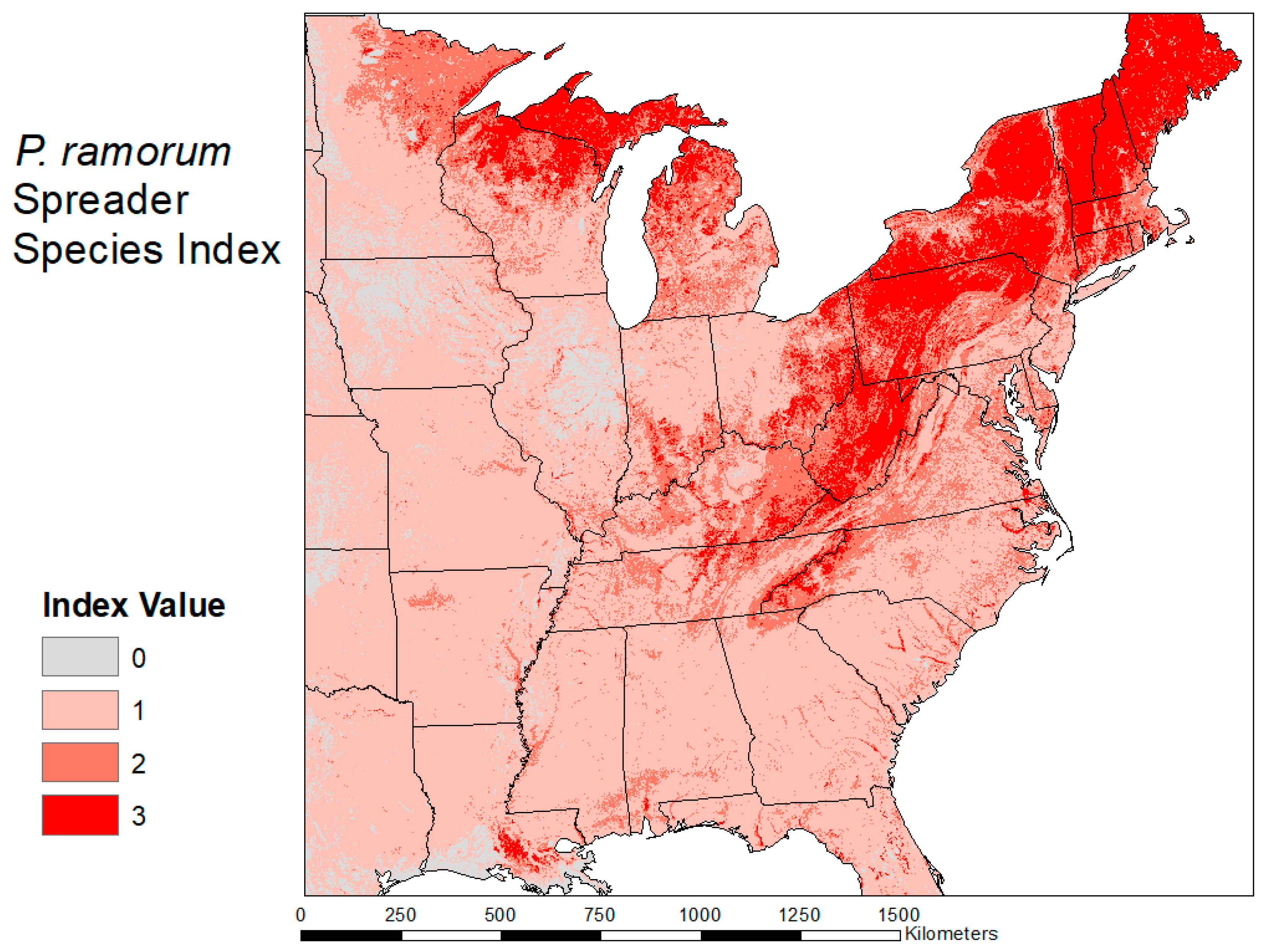
2.3. Habitat Suitability Map
2.4. Connectivity Analysis
3. Results
3.1. Severity Map
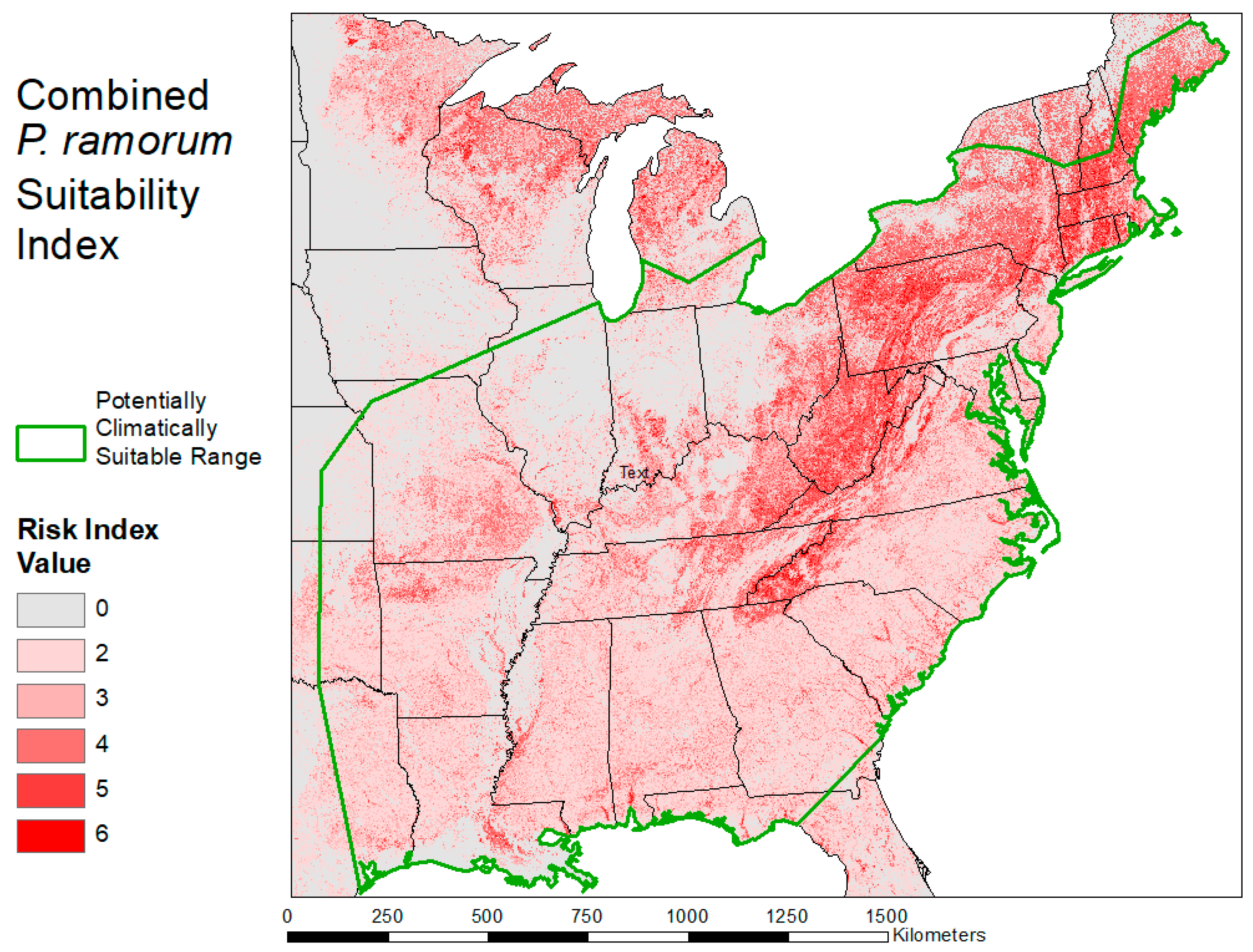
3.2. Habitat Suitability Map
3.3. Connectivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davidson, J.M.; Werres, S.; Garbelotto, M.; Hansen, E.M.; Rizzo, D.M. Sudden oak death and associated diseases caused by Phytophthora ramorum. Plant Health Prog. 2003, 4. [Google Scholar] [CrossRef]
- Rizzo, D.; Garbelotto, M. Sudden oak death: Endangering California and Oregon forest ecosystems. Front. Ecol. Environ. 2003, 1, 197–204. [Google Scholar] [CrossRef]
- McPherson, B.A.; Mori, S.R.; Wood, D.L.; Kelly, M.; Storer, A.J.; Svihra, P.; Standiford, R.B. Responses of oaks and tanoaks to the sudden oak death pathogen after 8 y of monitoring in two coastal California forests. For. Ecol. Manag. 2010, 259, 2248–2255. [Google Scholar] [CrossRef]
- Grunwald, N.J.; LeBoldus, J.M.; Hamelin, R.C. Ecology and evolution of the sudden oak death pathogen Phytophthora ramorum. Annu. Rev. Phytopathol. 2019, 57, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Bussell, E.H.; Cunniffe, N.J. Ongoing surveillance protects tanoak whilst conserving biodiversity: Applying optimal control theory to a spatial simulation model of sudden oak death. J. R. Soc. Interface 2019, 17. [Google Scholar] [CrossRef]
- Garbelotto, M.; Rizzo, D.M.; Meentemeyer, R.K.; Swiecki, T.; Owen, D.; Marshall, J.; Blomquist, C.; Bell, L.; Valachovic, Y. SODMap [Google Earth KMZ File]. 2019. Available online: https://nature.berkeley.edu/matteolab/?page_id=755 (accessed on 6 November 2019).
- Jung, T.; Pérez-Sierra, A.; Rees, H.; Scanu, B.; Bakonyi, J.; Seress, D.; Maia, C.; Harris, A.; Webber, J.; Brasier, C.; et al. Diversity of Phytophthora species in natural forests and streams and in rubber plantations in Vietnam. In Proceedings of the 8th Meeting of the International Union of Forestry Research Organizations, Phytophthora in Forests and Natural Ecosystems, Hanoi, Vietnam, 18–25 March 2017; IUFRO: Vienna, Austria, 2017; p. 56. Available online: https://www.iufro.org/fileadmin/material/publications/proceedings-archive/70209-vietnam17-abstracts.pdf (accessed on 13 December 2020).
- Ireland, K.B.; Hardy, G.E.S.J.; Kriticos, D.J. Combining inferential and deductive approaches to estimate the potential geographical range of the invasive plant pathogen, Phytophthora ramorum. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Kluza, D.A.; Vieglais, D.A.; Andreasen, J.K.; Peterson, A.T. Sudden oak death: Geographic risk estimates and predictions of origins. Plant Pathol. 2007, 56, 580–587. [Google Scholar] [CrossRef]
- Mascheretti, S.; Croucher, P.J.; Kozanitas, M.; Baker, L.; Garbelotto, M. Genetic epidemiology of the sudden oak death pathogen Phytophthora ramorum in California. Mol. Ecol. 2009, 18, 4577–4590. [Google Scholar] [CrossRef]
- Meentemeyer, R.K.; Cunniffe, N.J.; Cook, A.R.; Filipe, J.A.N.; Hunter, R.D.; Rizzo, D.M.; Gilligan, C.A. Epidemiological modeling of invasion in heterogeneous landscapes: Spread of sudden oak death in California (1990–2030). Ecosphere 2011, 2, art17. [Google Scholar] [CrossRef]
- Venette, R.C.; Cohen, S.D. Potential climatic suitability for establishment of Phytophthora ramorum within the contiguous United States. For. Ecol. Manag. 2006, 231, 18–26. [Google Scholar] [CrossRef]
- Tooley, P.W.; Browning, M.; Berner, D. Recovery of Phytophthora ramorum following exposure to temperature extremes. Plant Dis. 2008, 92, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Venette, R.C. Implication of global climate change on the distribution and activity of Phytophthora ramorum. In Proceedings of the 20th US Department of Agriculture interagency research forum on invasive species, Annapolis, MD, USA, 13–16 January 2009; McManus, K.A., Gottschalk, K.W., Eds.; USDA Forest Service: Newtown Square, PA, USA, 2009; pp. 58–59. Available online: https://www.nrs.fs.fed.us/pubs/gtr/gtr_nrs-p-51.pdf (accessed on 13 December 2020).
- Venette, R.C. Incorporating climate change into pest risk models for forest pathogens: A role for cold stress in an era of global warming? NeoBiota 2013, 18, 131–150. [Google Scholar] [CrossRef][Green Version]
- Wilson, B.T.; Lister, A.J.; Riemann, R.I.; Griffith, D.M. Live Tree Species Basal Area of the Contiguous United States (2000–2009); USDA Forest Service: Newtown Square, PA, USA, 2013. [CrossRef]
- United States Forest Service. Forest Inventory and Analysis National Program. 2019. Available online: https://www.fia.fs.fed.us/ (accessed on 6 November 2019).
- Bechtold, W.A.; Patterson, P.L. (Eds.) The enhanced forest inventory and analysis program—National sampling design and estimation procedures. In General Technical Reports; US Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2005. [Google Scholar] [CrossRef]
- Wilson, B.T.; Lister, A.J.; Riemann, R.I. A nearest-neighbor imputation approach to mapping tree species over large areas using forest inventory plots and moderate resolution raster data. For. Ecol. Manag. 2012, 271, 182–198. [Google Scholar] [CrossRef]
- USDA-APHIS. APHIS List of Hosts and Plants Associated with Phytophthora ramorum. 2020. Available online: http://www.aphis.usda.gov/plant_health/plant_pest_info/pram/downloads/pdf_files/usdaprlist.pdf (accessed on 6 November 2019).
- California Oak Mortality Task Force. Phytophthora ramorum Hosts Reported since 2012/2013 and Missing from the APHIS P. ramorum Host or Associated Host List. 2018. Available online: http://www.suddenoakdeath.org/wp-content/uploads/2018/10/P-ramorum-hosts-detected-since-2012.pdf (accessed on 17 July 2020).
- Brasier, C.M.; Webber, J. Sudden larch death. Nature 2010, 466, 824–825. [Google Scholar] [CrossRef]
- Hansen, E.M.; Kanaskie, A.; Prospero, S.; McWilliams, M.; Goheen, E.M.; Osterbauer, N.; Reeser, P.; Sutton, W. Epidemiology of Phytophthora ramorum in Oregon tan oak forests. Can. J. For. Res. 2008, 38, 1133–1143. [Google Scholar] [CrossRef]
- McRae, B.H.; Kavanagh, D.M. Linkage Mapper Connectivity Analysis Software. The Nature Conservancy, Seattle United States. 2011. Available online: http://www.circuitscape.org/linkagemapper (accessed on 10 March 2020).
- Wimberly, M.C.; Narem, D.; Bauman, P.; Carlson, B.; Ahlering, M. Grassland connectivity in fragmented agricultural landscapes of the north-central United States. Biol. Conserv. 2018, 217, 121–130. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The Igraph Software Package for Complex Network Research. 2006. Available online: http://igraph.org (accessed on 17 April 2020).
- R Core Team. R: A Language and Environment for Statistical Computing (3.6.3). 2020. Available online: https://www.R-project.org/ (accessed on 19 March 2020).
- Inman, R.M.; Pelton, M.R. Energetic production by soft and hard mast foods of American black bears in the Smoky Mountains. Ursus 2002, 13, 57–68. Available online: https://www.bearbiology.org/publications/ursus-archive/energetic-production-by-soft-and-hard-mast-foods-of-american-black-bears-in-the-smoky-mountains/ (accessed on 13 December 2020).
- Howard, J.L.; Liang, S.U.S. timber production, trade, consumption, and price statistics. 1965–2017. In Res. Pap. FPL-RP-701; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2019; pp. 1–106. Available online: https://www.fs.usda.gov/treesearch/pubs/58506 (accessed on 8 November 2020).
- Fridley, J.D. Downscaling climate over complex terrain: High finescale (<1000 m) spatial variation of near-ground temperatures in a montane forested landscape (Great Smoky Mountains). J. App. Meteorol. Climatol. 2009, 48, 1033–1049. [Google Scholar] [CrossRef]
- Brasier, C.M.; Scott, J.K. European oak declines and global warming: A theoretical assessment with special reference to the activity of Phytopthora cinnamomi. EPPO Bull. 1994, 241, 221–232. [Google Scholar] [CrossRef]
- Anagnostakis, S. Chestnut blight: The classical problem of an introduced pathogen. Mycologia 1987, 79, 23–37. [Google Scholar] [CrossRef]
- Ellis, A.M.; Vaclavik, T.; Meentemeyer, R.K. When is connectivity important? A case study of the spatial pattern of sudden oak death. Oikos 2010, 119, 485–493. [Google Scholar] [CrossRef]

| Susceptible | Spreader |
|---|---|
| Quercus s. Lobatae—Red Oaks Notholithocarpus—Tanoaks Larix—Larch | Abies—Fir Pseudotsuga—Douglas Fir Sequoia—Redwood Acer—Maple Aesculus—Buckeye Arbutus—Madrone Castanea—Chestnut Cercis—Redbud Cornus—Dogwood Eucalyptus—Eucalyptus Fagus—Beech Fraxinus—Ash Magnolia—Magnolia Prunus—Cherry/Plum/Peach Cinnamomum—Camphor tree Salix—Willow Umbellularia—California Laurel Notholithocarpus—Tanoaks Larix—Larch |
| BA Range | Associated Index Score |
|---|---|
| BA ≤ 0.1 ft2/acre (0.022 m2/ha) | 0 |
| 0.1 ft2/acre (0.022 m2/ha) < BA ≤ 10 ft2/acre (2.2 m2/ha) | 1 |
| 10 ft2/acre (2.2 m2/ha) < BA ≤ 25 ft2/acre (5.7 m2/ha) | 2 |
| BA > 25 ft2/acre (5.7 m2/ha) | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haller, D.J.; Wimberly, M.C. Estimating the Potential for Forest Degradation in the Eastern United States Woodlands from an Introduction of Sudden Oak Death. Forests 2020, 11, 1334. https://doi.org/10.3390/f11121334
Haller DJ, Wimberly MC. Estimating the Potential for Forest Degradation in the Eastern United States Woodlands from an Introduction of Sudden Oak Death. Forests. 2020; 11(12):1334. https://doi.org/10.3390/f11121334
Chicago/Turabian StyleHaller, Dillon J., and Michael C. Wimberly. 2020. "Estimating the Potential for Forest Degradation in the Eastern United States Woodlands from an Introduction of Sudden Oak Death" Forests 11, no. 12: 1334. https://doi.org/10.3390/f11121334
APA StyleHaller, D. J., & Wimberly, M. C. (2020). Estimating the Potential for Forest Degradation in the Eastern United States Woodlands from an Introduction of Sudden Oak Death. Forests, 11(12), 1334. https://doi.org/10.3390/f11121334




