Avian Response to Wildfire Severity in a Northern Boreal Region
Abstract
1. Introduction
2. Materials and Methods
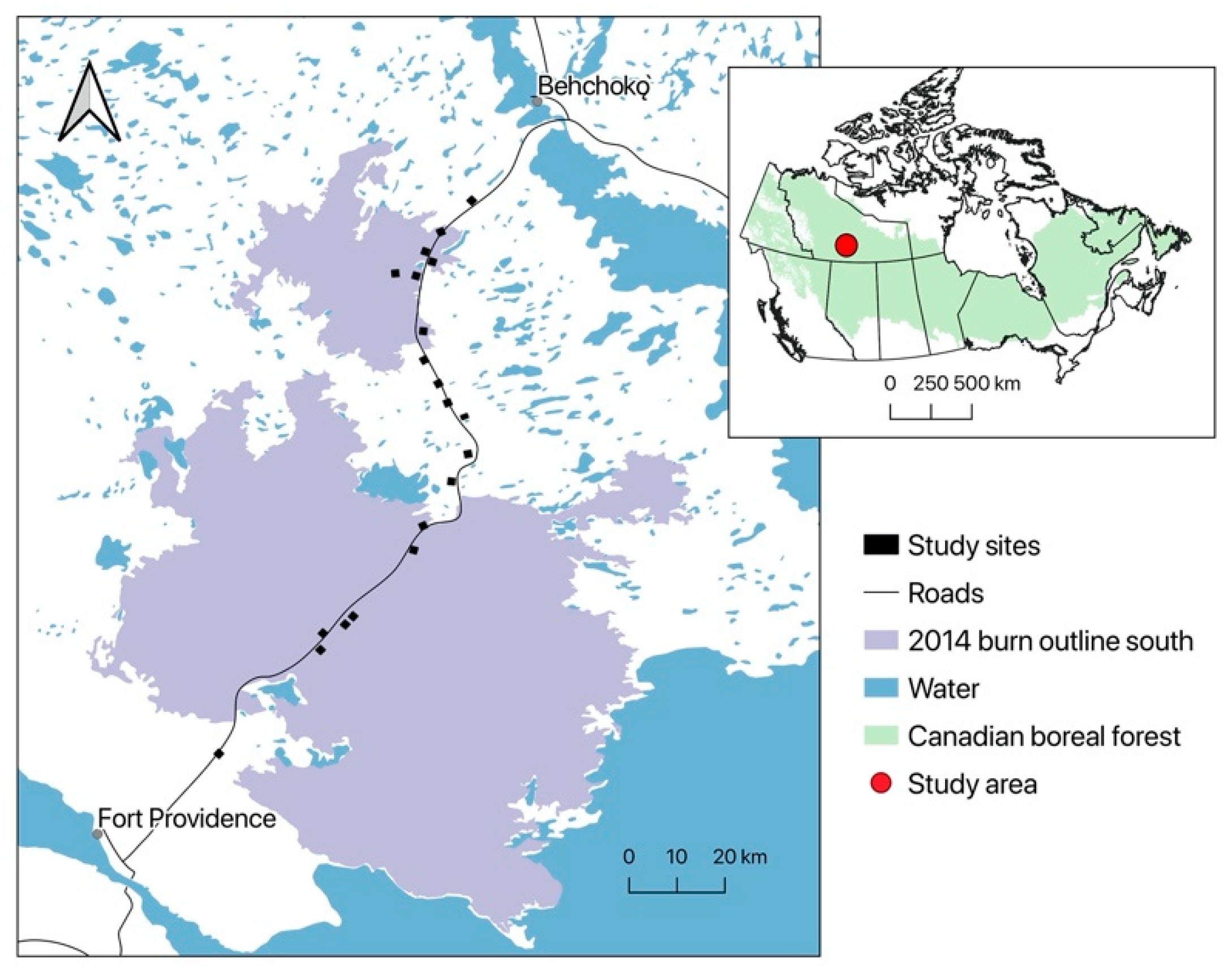
2.1. Study Area
2.2. Sampling Design and Avian Surveys
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Common Name | Scientific Name | Generalist/Specialist | Migration | Feeding | Substrate Breeding | Foraging Type | Nesting Substrate | Nesting Habitat | Total Count | Stations with Detections (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Alder Flycatcher | Empidonax alnorum | S | NM | In | Air | Sal | Sh | TSS | 59 | 12.6 |
| American Robin | Turdus migratorius | G | SDM | Om | LCS | Fo | TNs | Ur | 380 | 69.4 |
| Black-Backed Woodpecker | Picoides arcticus | S | R | In | Ba | Sca | Sn | CW | 8 | 2.0 |
| Blue-Headed Vireo | Vireo solitarius | S | SDM | In | LCS | Gl | CT | MW | 4 | 1.0 |
| Boreal Chickadee | Poecile hudsonicus | S | R | In | LCS | Gl | Sn | CW | 4 | 1.0 |
| Canada Jay | Perisoreus canadensis | G | R | Om | UC | Fo | CT | CW | 70 | 15.9 |
| Clay-Colored Sparrow | Spizella pallida | G | NM | Om | Gr | Fo | Sh | ES | 14 | 3.2 |
| Chipping Sparrow | Spizella passerina | G | SDM | Om | Gr | Fo | CT | OW | 370 | 70.6 |
| Common Nighthawk | Chordeiles minor | S | NM | In | Air | Scr | Gr | Wo | 36 | 8.6 |
| Common Yellowthroat | Geothlypis trichas | S | NM | In | LCS | Gl | Gr | Ma | 11 | 2.5 |
| Dark-Eyed Junco | Junco hyemalis | G | SDM | Om | Gr | Fo | Gr | CW | 388 | 74.8 |
| Fox Sparrow | Passerella iliaca | G | SDM | Om | Gr | Fo | Gr | Wo | 5 | 1.2 |
| Greater Yellowlegs | Tringa melanoleuca | S | SDM | Pi | Wa | Am | Gr | Bo | 5 | 1.2 |
| Hermit Thrush | Catharus guttatus | S | SDM | In | Gr | Gl | Gr | CW | 486 | 86.9 |
| Le Conte’s Sparrow | Ammodramus leconteii | G | SDM | Om | Gr | Fo | Gr | Me | 30 | 6.6 |
| Least Flycatcher | Empidonax minimus | S | NM | In | Air | Sal | DT | DW | 6 | 1.5 |
| Lesser Yellowlegs | Tringa flavipes | S | SDM | In | Wa | Gl | Gr | Bo | 101 | 23.7 |
| Lincoln’s Sparrow | Melospiza lincolnii | G | SDM | Om | Gr | Fo | Gr | Bo | 263 | 80.6 |
| Mountain Bluebird | Sialia currucoides | S | SDM | In | Gr | Gl | Sn | OW | 4 | 0.7 |
| Northern Flicker | Colaptes auratus | S | SDM | In | Gr | Gl | Sn | MW | 8 | 1.9 |
| Orange-Crowned Warbler | Vermivora celata | S | SDM | In | LCS | Gl | Gr | OW | 81 | 17.0 |
| Olive-Sided Flycatcher | Contopus cooperi | S | NM | In | Air | Sal | CT | Bo | 49 | 11.9 |
| Ovenbird | Seiurus aurocapillus | S | NM | In | Gr | Gl | Gr | DW | 6 | 1.5 |
| Palm Warbler | Dendroica palmarum | S | SDM | In | Gr | Gl | Gr | Bo | 104 | 23.0 |
| Pileated Woodpecker | Dryocopus pileatus | S | R | In | Ba | Ex | Sn | MW | 4 | 1.0 |
| Red-Winged Blackbird | Agelaius phoeniceus | S | SDM | Om | Gr | Fo | Gs | Ma | 8 | 1.5 |
| Ruby-Crowned Kinglet | Regulus calendula | S | SDM | In | UC | Gl | CT | CW | 38 | 9.1 |
| Ruffed Grouse | Bonasa umbellus | G | R | Om | Gr | Fo | Gr | MW | 33 | 7.7 |
| Savannah Sparrow | Passerculus sandwichensis | G | SDM | Om | Gr | Fo | Gr | Ag | 4 | 1.0 |
| Solitary Sandpiper | Tringa solitaria | S | NM | In | Wa | Gl | TNS | Bo | 45 | 10.1 |
| Sora | Porzana carolina | S | SDM | Om | Wa | Fo | EV | Ma | 107 | 25.4 |
| Spotted Sandpiper | Actitis macularius | S | NM | In | Sh | Gl | Gr | Be | 9 | 2.0 |
| Swamp Sparrow | Melospiza georgiana | G | SDM | Om | Gr | Fo | Gr | Ma | 56 | 12.1 |
| Swainson’s Thrush | Catharus ustulatus | G | NM | Om | Gr | Fo | CT | MW | 373 | 73.6 |
| Tennessee Warbler | Vermivora peregrina | S | NM | In | UC | Gl | Gr | Bo | 58 | 13.3 |
| Western-Wood-Pewee | Contopus sordidulus | S | NM | In | Air | Sal | CT | OW | 10 | 2.5 |
| White-Crowned Sparrow | Zonotrichia leucophrys | G | SDM | Om | Gr | Fo | Gr | Bo | 87 | 19.0 |
| White-Throated Sparrow | Zonotrichia albicollis | G | SDM | Om | Gr | Fo | Gr | ES | 299 | 55.6 |
| Yellow-Bellied Flycatcher | Empidonax flaviventris | S | NM | In | Air | Sal | Gr | Bo | 5 | 1.2 |
| Yellow-Bellied Sapsucker | Sphyrapicus varius | G | SDM | Om | Ba | Ex | DT | MW | 7 | 1.7 |
| Yellow Warbler | Setophaga petechia | S | NM | In | LCS | Gl | Sh | ES | 7 | 1.2 |
| Yellow-Rumped Warbler | Setophaga coronata | S | SDM | In | LCS | Gl | CT | CW | 193 | 41.7 |
References
- Wells, J.V.; Blancher, P. Global role for sustaining bird populations. In Boreal Birds of North America: A Hemispheric View of Their Conservation Links and Significance; Wells, J.V., Ed.; University of California Press: Berkeley, CA, USA, 2011; Chapter 2; pp. 7–22. [Google Scholar]
- Blancher, P. The Importance of Canada’s Boreal Forest to Landbirds. Available online: www.borealbirds.org/publications/importance-canadas-boreal-forest-landbirds (accessed on 2 July 2020).
- Rosenberg, K.V.; Kennedy, J.A.; Dettmers, R.; Ford, R.P.; Reynolds, D.; Alexander, J.D.; Beardmore, C.J.; Blancher, P.J.; Bogart, R.E.; Butcher, G.S.; et al. Partners in Flight Landbird Conservation Plan: 2016 Revision for Canada and Continental United States. Available online: https://partnersinflight.org/resources/the-plan/ (accessed on 2 July 2020).
- Ballard, G.; Geupel, G.R.; Nur, N.; Gardali, T. Long-term declines and decadal patterns in population trends of songbirds in western North America, 1979–1999. Condor 2003, 105, 737–755. [Google Scholar] [CrossRef]
- Environment Canada. Bird Conservation Strategy for Bird Conservation Region 6: Boreal Taiga Plains. Available online: www.canada.ca/en/environment-climate-change/services/migratory-bird-conservation/regions-strategies/description-region-6/canada.html (accessed on 2 July 2020).
- Weber, M.G.; Flannigan, M.D. Canadian boreal forest ecosystem structure and function in a changing climate: Impact on fire regimes. Environ. Rev. 1997, 5, 145–166. [Google Scholar] [CrossRef]
- ESTR Secretariat. Taiga Plains Ecozone and evidence for key findings summary. In Canadian Biodiversity: Ecosystem Status and Trends 2010, Evidence for Key Findings Summary; Report No. 13; Canadian Councils of Resource Ministers: Ottawa, ON, Canada, 2013. [Google Scholar]
- de Groot, W.J.; Cantin, A.S.; Flannigan, M.D.; Soja, A.J.; Gowman, L.M.; Newbery, A. A comparison of Canadian and Russian boreal forest fire regimes. For. Ecol. Manag. 2013, 294, 23–34. [Google Scholar] [CrossRef]
- Eberhart, K.E.; Woodard, P.M. Distribution of residual vegetation associated with large fires in Alberta. Can. J. For. Res. 1987, 17, 1207–1212. [Google Scholar] [CrossRef]
- Nielsen, S.E.; DeLancey, E.R.; Reinhardt, K.; Parisien, M.-A. Effects of lakes on wildfire activity in the boreal forests of Saskatchewan, Canada. Forests 2016, 7, 265. [Google Scholar] [CrossRef]
- Rowe, J.S.; Scotter, G.W. Fire in the boreal. Quat. Res. 1973, 3, 444–464. [Google Scholar] [CrossRef]
- Madoui, A.; Leduc, A.; Gauthier, S.; Bergeron, Y. Spatial pattern analyses of post-fire residual stands in the black spruce boreal forest of western Quebec. Int. J. Wildland Fire 2010, 19, 1110–1126. [Google Scholar] [CrossRef]
- Weber, M.G.; Stocks, B.J. Forest fires and sustainability in the boreal forests of Canada. Ambio 1998, 27, 545–550. [Google Scholar]
- Bourgeau-Chavez, L.L.; Alexander, M.E.; Stocks, B.J.; Kasischke, E.S. Distribution of Forest Ecosystems and the Role of Fire in the North American Boreal Region. In Fire, Climate Change, and Carbon Cycling in the Boreal Forest; Kasischke, E.S., Stocks, B.J., Eds.; Springer: New York, NY, USA, 2000; pp. 111–131. [Google Scholar]
- National Wetlands Working Group. The Canadian Wetland Classification System, 2nd ed.; Warner, B.G., Rubec, C.D.A., Eds.; Wetlands Research Centre, University of Waterloo: Waterloo, ON, Canada, 1998. [Google Scholar]
- Ecosystem Classification Group. Ecological Regions of the Northwest Territories—Taiga Plains; Report; Department of Environment and Natural Resources, Government of the Northwest Territories: Yellowknife, NT, Canada, 2009; 173p.
- Kettridge, N.; Turetsky, M.R.; Sherwood, J.H.; Thompson, D.K.; Miller, C.A.; Benscoter, B.W.; Flannigan, M.D.; Wotton, B.M.; Waddington, J.M. Moderate drop in water table increases peatland vulnerability to post-fire regime. Sci. Rep. 2015, 5, 8063. [Google Scholar] [CrossRef]
- Hokanson, K.J.; Lukenbach, M.C.; Devito, K.J.; Kettridge, N.; Petrone, R.M.; Waddington, J.M. Groundwater connectivity controls peat burn severity in the boreal plains. Ecohydrology 2015, 9, 574–584. [Google Scholar] [CrossRef]
- Whitman, E.; Parisien, M.-A.; Thompson, D.K.; Hall, R.J.; Skakun, R.S.; Flannigan, M.D. Variability and drivers of burn severity in the northwestern Canadian boreal forest. Ecosphere 2018, 9, e02128. [Google Scholar] [CrossRef]
- Flannigan, M.; Stocks, B.J.; Turetsky, M.; Wotton, M. Impacts of climate change on fire activity and fire management in the circumboreal forest. Glob. Chang. Biol. 2009, 15, 549–560. [Google Scholar] [CrossRef]
- Wirth, C. Fire Regime and Tree Diversity in Boreal Forests: Implications for the Carbon Cycle. In Forest Diversity and Function: Temperate and Boreal Systems; Springer: Berlin/Heidelberg, Germany, 2005; pp. 309–344. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Gaston, K.J. Spatial patterns in the species richness of birds in the new world. Ecography 1996, 19, 369–375. [Google Scholar] [CrossRef]
- Swanson, M.E.; Franklin, J.F.; Beschta, R.L.; Crisafulli, C.M.; DellaSala, D.M.; Hutto, R.L.; Lindenmayer, D.B.; Swanson, F.J. The forgotten stage of forest succession: Early-successional ecosystems on forest sites. Front. Ecol. Environ. 2011, 9, 117–125. [Google Scholar] [CrossRef]
- Hogg, E.H.; Wein, R.H. Impacts of drought on forest growth and regeneration following fire in southwestern Yukon, Canada. Can. J. For. Res. 2005, 35, 2141–2150. [Google Scholar] [CrossRef]
- Alberta Biodiveristy Monitoring Institute. Biodiversity Browser. Available online: https://abmi.ca/home/data-analytics/biobrowser-home (accessed on 17 October 2020).
- Hobson, K.; Schieck, J. Changes in bird communities in boreal mixedwood forest: Harvest and wildfire effects over 30 years. Ecol. Appl. 1999, 9, 849–863. [Google Scholar] [CrossRef]
- Imbeau, L.; Savard, J.L.; Gagnon, R. Comparing bird assemblages in successional black spruce stands originating from fire and logging. Can. J. Zool. 1999, 77, 1850–1860. [Google Scholar] [CrossRef]
- Morissette, J.L.; Cobb, T.P.; Brigham, R.M.; James, P.C. The response of boreal forest songbird communities to fire and post-fire harvesting. Can. J. For. Res. 2002, 32, 2169–2183. [Google Scholar] [CrossRef]
- Schieck, J.; Song, S.J. Changes in bird communities throughout succession following fire and harvest in boreal forests of western North America: Literature review and meta-analyses. Can. J. For. Res. 2006, 36, 1299–1318. [Google Scholar] [CrossRef]
- Azeria, E.T.; Ibarzabal, J.; Hébert, C.; Boucher, J.; Imbeau, L.; Savard, J.P. Differential response of bird functional traits to post-fire salvage logging in a boreal forest ecosystem. Acta Oecol. 2011, 37, 220–229. [Google Scholar] [CrossRef]
- Haney, A.; Apfelbaum, S.; Burris, J.M. Thirty years of post-fire succession in a southern boreal forest bird community. Am. Midl. Nat. 2008, 159, 421–433. [Google Scholar] [CrossRef]
- Hutto, R.L. Composition of bird communities composition following stand-replacement fires in northern Rocky Mountain (U.S.A) conifer forests. Conserv. Biol. 1995, 9, 1041–1058. [Google Scholar] [CrossRef]
- Van Wilgenburg, S.L.; Hobson, K.A. Landscape-scale disturbance and boreal forest birds: Can large single-pass harvest approximate fires? For. Ecol. Manag. 2008, 256, 136–146. [Google Scholar] [CrossRef]
- Nappi, A.; Drapeau, P.; Savard, J.P.L. Salvage logging after wildfire in the boreal forest: Is it becoming a hot issue for wildlife? For. Chron. 2004, 80, 67–74. [Google Scholar] [CrossRef]
- James, F.C. Ordinations of Habitat Relationships among Breeding Birds. Wilson Bull. 1971, 83, 215–236. [Google Scholar]
- Hutto, R.L.; Patterson, D.A. Positive effects of fire on birds may appear only under narrow combinations of fire severity and time-since-fire. Int. J. Wildland Fire 2016, 25, 1074–1085. [Google Scholar] [CrossRef]
- Latif, Q.S.; Sanderlin, J.S.; Saab, V.A.; Block, W.M.; Dudley, J.G. Avian relationships with wildfire at two dry forest locations with different historical fire regimes. Ecosphere 2016, 7, e01346. [Google Scholar] [CrossRef]
- Northwest Territories Department of Environment and Natural Resources. 2014 NWT Fire Season: Review Report; Government of the Northwest Territories: Yellowknife, NT, Canada, 2015.
- Northwest Territories Department of Environment and Natural Resources. Annual Area Burned and Number of Fires. Available online: www.enr.gov.nt.ca/en/state-environment/143-annual-area-burned-and-number-fires (accessed on 5 December 2020).
- Government of Canada (2018, March 1) Station Results—1981–2010 Climate Normals and Averages (Fort Simpson, Hay River, Yellowknife). Available online: https://climate.weather.gc.ca/climate_normals (accessed on 16 November 2018).
- Whitman, E. Extended Assessment Differenced Normalized Burn Ratio (dNBR) Maps for Large 2014 Wildfires in the Northwest Territories, Derived from Landsat 8—OLI and Landsat 5—TM Imagery [Raster Geotiff]; Natural Resources Canada; Canadian Forest Service & University of Alberta: Edmonton, AB, Canada, 2015. [Google Scholar]
- Key, C.H.; Benson, N.C. Landscape Assessment: Sampling and Analysis Methods: Firemon: Fire Effects Monitoring and Inventory System; General Technical Report, RMRS-GTR-164-CD; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006.
- Rose, E.T.; Simons, T.R.; Klein, R.; McKerrow, A.J. Normalized burn ratios link fire severity with patterns of avian occurrence. Landsc. Ecol. 2016, 31, 1537. [Google Scholar] [CrossRef]
- Wilkerson, R.L.; Tingley, M.W.; Ruiz-gutie, V.; Howell, C.A.; Siegel, R.B. Pyrodiversity promotes avian diversity over the decade following forest fire. Proc. R. Soc. Biol. 2016, 283, 20161703. [Google Scholar]
- Miquelajauregui, Y.; Cumming, S.G.; Gauthier, S. Modelling variable fire severity in boreal forests: Effects of fire intensity and stand structure. PLoS ONE 2016, 11, e0150073. [Google Scholar] [CrossRef]
- Northwest Territories Department of Environment and Natural Resources. Northwest Territories Forest Vegetation Inventory Standards with Softcopy Supplements; Northwest Territories Department of Environment and Natural Resources: Yellowknife, NT, Canada, 2012.
- Shonfield, J.; Bayne, E. Autonomous recording units in avian ecological research: Current use and future applications. Avian Conserv. Ecol. 2017, 12, 14. [Google Scholar] [CrossRef]
- Hutto, R.; Stutzman, R. Humans versus autonomous recording units: A comparison of point-count results. J. Field Ornithol. 2009, 80, 387–398. [Google Scholar] [CrossRef]
- Venier, L.A.; Holmes, S.B.; Holborn, G.W.; McIlwrick, K.A.; Brown, G. Evaluation of an automated recording device for monitoring forest birds. Wildl. Soc. Bull. 2012, 36, 30–39. [Google Scholar] [CrossRef]
- Pankratz, R.F.; Hache, S.; Sólymos, P.; Bayne, E.M. Potential benefits of augmenting road-based breeding bird surveys with autonomous recordings. Avian Conserv. Ecol. 2017, 12, 18. [Google Scholar] [CrossRef]
- Yip, D.A.; Knight, E.C.; Haave-Audet, E.; Wilson, S.J.; Charchuk, C.; Scott, C.D.; Sólymos, P.; Bayne, E.M. Sound level measurements from audio recordings provide objective distance estimates for distance sampling wildlife populations. Remote Sens. Ecol. Conserv. 2020, 6, 301–315. [Google Scholar] [CrossRef]
- Mackenzie, D.; Nichols, J.; Royle, J.; Pollock, K.; Bailey, L.; Hines, J. Occupancy Estimation and Modelling, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Sólymos, P.; Matsuoka, S.M.; Bayne, E.M.; Lele, S.R.; Fontaine, P.; Cumming, S.G.; Stralberg, D.; Schmiegelow, F.K.; Song, S.J. Calibrating indices of avian density from non-standardized survey data: Making the most of a messy situation. Methods Ecol. Evol. 2013, 4, 1047–1058. [Google Scholar] [CrossRef]
- Sólymos, P. QPAD Version 3 Documentation—Technical Report; Boreal Avian Modelling Project: Edmonton, AB, Canada, 2016; Available online: https://zenodo.org/record/3251111#.X81pTOlKhTY (accessed on 5 December 2020).
- Gagic, V.; Bartomeus, I.; Jonsson, T.; Taylor, A.; Winqvist, C.; Fischer, C.; Slade, E.M.; Steffan-Dewenter, I.; Emmerson, M.; Potts, S.G.; et al. Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc. R. Soc. Biol. 2015, 282, 2014–2620. [Google Scholar] [CrossRef]
- Schmiegelow, F.K.; Machtans, C.S.; Hannnon, S.J. Are boreal birds resilient to forest fragmentation? An experimental study of short-term community responses. Ecology 1997, 78, 1914–1932. [Google Scholar] [CrossRef]
- Devictor, V.; Julliard, R.; Clavel, J.; Jiguet, F.; Lee, A.; Couvet, D. Functional biotic homogenization of bird communities in disturbed landscapes. Glob. Ecol. Biogeogr. 2008, 17, 252–261. [Google Scholar] [CrossRef]
- Virkkala, R.; Lehikoinen, A. Birds on the move in the face of climate change: High species turnover in northern Europe. Ecol. Evol. 2017, 7, 8201–8209. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0-12. 2014. Available online: https://cran.r-project.org/web/packages/FD (accessed on 5 December 2020).
- Schleuter, D.S.; Aufresne, M.D.; Assol, F.M. A user’s guide to functional diversity indices. Ecol. Monogr. 2010, 80, 469–484. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.4-1. 2016. Available online: https://cran.r-project.org/web/packages/vegan (accessed on 5 December 2020).
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the spatial component of ecological variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Anderson, M.J. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef]
- Vanderhoff, N.; Pyle, P.; Pattern, M.A.; Sallabanks, R.; James, F.C. American Robin (Turdus migratorius), version 2.0. In Birds of North America; Rodewald, P.G., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2016. [Google Scholar]
- Schneider, R.R.; Devito, K.; Kettridge, N.; Bayne, E. Moving beyond bioclimatic envelope models: Integrating upland forest and peatland processes to predict ecosystem transitions under climate change in the western Canadian boreal plain. Ecohydrology 2016, 9, 899–908. [Google Scholar] [CrossRef]
- Stralberg, D.; Arseneault, D.; Baltzer, J.L.; Barber, Q.E.; Bayne, E.M.; Boulanger, Y.; Brown, C.D.; Cooke, H.A.; Devito, K.; Edwards, J.; et al. Climate-change refugia in boreal North America: What, where, and for how long? Front. Ecol. Environ. 2020, 18, 261–270. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada. North American Breeding Bird Survey—Canadian Trends Website, Data-Version 2015; Environment and Climate Change Canada: Gatineau, QC, Canada, 2017. Available online: https://wildlife-species.canada.ca/breeding-bird-survey-results (accessed on 5 December 2020).
- Fontaine, J.B.; Kennedy, P.L. Meta-analysis of avian and small-mammal response to fire severity and fire surrogate treatments in U.S. fire-prone forests. Ecol. Appl. 2012, 22, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Zlonis, E.J.; Walton, N.G.; Sturtevant, B.R.; Wolter, P.T.; Niemi, G.J. Burn severity and heterogeneity mediate avian response to wildfire in a hemiboreal forest. For. Ecol. Manag. 2019, 439, 70–80. [Google Scholar] [CrossRef]
- Taillie, P.J.; Burnett, R.D.; Robert, L.J.; Campos, B.R.; Peterson, M.N.; Moorman, C.E. Interacting and non-linear avian responses to mixed-severity wildfire and time since fire. Ecosphere 2018, 9, e02291. [Google Scholar] [CrossRef]
- Kirkpatrick, C.; Conway, C.J.; Jones, P.B. Distribution and Relative Abundance of Forest Birds in Relation to Burn Severity in Southeastern Arizona. J. Wildl. Manag. 2006, 70, 1005–1012. [Google Scholar] [CrossRef]
- Stephens, J.L.; Ausprey, I.J.; Seavy, N.E.; Alexander, J.D. Fire severity affects mixed broadleaf–conifer forest bird communities: Results for 9 years following fire. 2015. Condor Ornithol. Appl. 2015, 13, 430–446. [Google Scholar]
- Kochtubajda, B.; Flannigan, M.D.; Gyakum, J.R.; Stewart, R.E.; Logan, K.A.; Nguyen, T.V. Lightning and fires in the Northwest Territories and responses to future climate change. Arctic 2006, 59, 211–221. [Google Scholar] [CrossRef]
- Héon, J.; Arseneault, D.; Parisien, M. Resistance of the boreal forest to high burn rates. Proc. Natl. Acad. Sci. USA 2014, 111, 13888–13893. [Google Scholar] [CrossRef] [PubMed]
- Campos-Ruiz, R.; Parisien, M.; Flannigan, M.D. Temporal patterns of wildfire activity in areas of contrasting human influence in the Canadian boreal forest. Forests 2018, 9, 159. [Google Scholar] [CrossRef]
- Stralberg, D.; Carroll, C.; Pedlar, J.H.; Wilsey, C.B.; McKenney, D.W.; Nielsen, S.E. Macrorefugia for North American trees and songbirds: Climatic limiting factors and multi-scale topographic influences. Glob. Ecol. Biogeogr. 2018, 27, 690–703. [Google Scholar] [CrossRef]
- Tremblay, J.A.; Boulanger, Y.; Cyr, D.; Taylor, A.R.; Price, D.T.; St-Laurent, M.-H. Harvesting interacts with climate change to affect future habitat quality of a focal species in eastern Canada’s boreal forest. PLoS ONE 2019, 13, e0191645. [Google Scholar] [CrossRef]


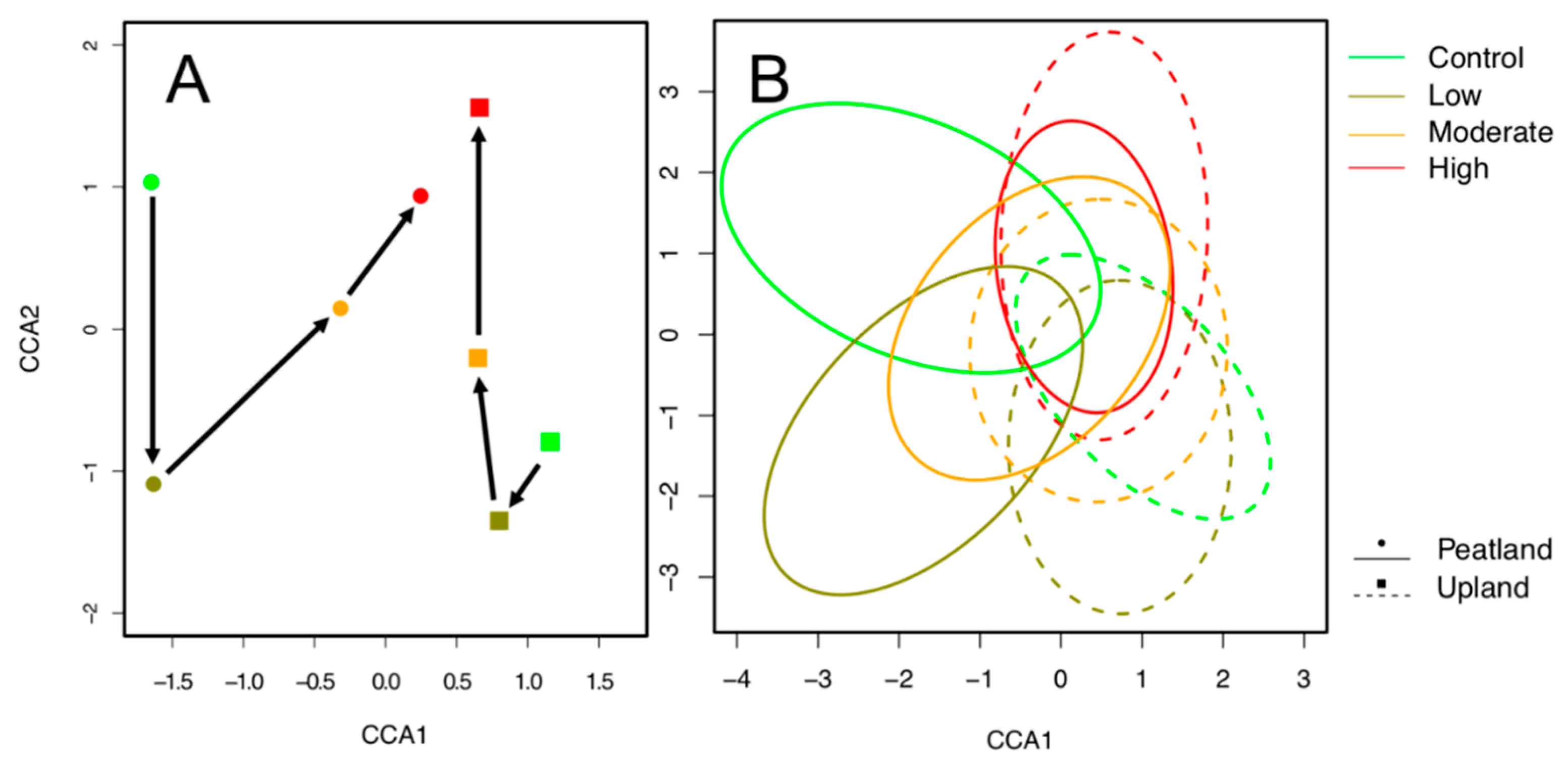
| Species | # Stations | Best Model | Vegetation Type | Control | Low | Moderate | High |
|---|---|---|---|---|---|---|---|
| Species with lower occupancy rates in burned areas | |||||||
| Alder Flycatcher | 51 | psi(vegtype + burn_binary) p(.) | Peatland | 0.33 0.22–0.47 | 0.17 0.12–0.24 | 0.17 0.12–0.24 | 0.17 0.12–0.24 |
| (r = 0.90) | Upland | 0.11 0.07–0.18 | 0.05 0.03–0.08 | 0.05 0.03–0.08 | 0.05 0.03–0.08 | ||
| dens(vegtype × burn_rank + year) − NB | Peatland | 0.73 0.11–1.35 | 0.31 0.11–0.52 | 0.13 0.03–0.23 | 0.06 0–0.11 | ||
| Upland | 0.03 0.01–0.05 | 0.03 0.02–0.04 | 0.03 0.02–0.04 | 0.03 0.01–0.04 | |||
| Common Yellowthroat | 10 | psi(vegtype + burn binary) p(doy) | Peatland | 0.18 0.0–0.41 | 0.02 0.0–0.05 | 0 | 0 |
| (r = 0.98) | Upland | 0 | 0 | 0 | 0 | ||
| dens(vegtype + burn_rank) − NB | Peatland | 0.12 0.02–0.21 | 0.02 0–0.04 | 0 | 0 | ||
| Upland | 0 | 0 | 0 | 0 | |||
| Orange-Crowned Warbler | 69 | psi(vegtype + burn_rank) p(doy) | Peatland | 0.62 0.42–0.78 | 0.42 0.28–0.58 | 0.25 0.16–0.38 | 0.14 0.07–0.24 |
| (r = 0.98) | Upland | 0.34 0.22–0.48 | 0.19 0.13–0.28 | 0.10 0.06–0.16 | 0.05 0.02–0.09 | ||
| dens(vegtype + burn_rank + year) − NB | Peatland | 1.55 0.63–2.57 | 0.71 0.37–1.04 | 0.32 0.16–0.59 | 0.15 0.05–0.25 | ||
| Upland | 0.51 0.25–0.76 | 0.23 0.14–0.33 | 0.11 0.05–0.16 | 0.05 0.02–0.08 | |||
| Ruby-Crowned Kinglet | 37 | psi(burn_binary) p(doy + year) | Both veg. types | 0.42 0.30–0.68 | 0.03 0.01–0.06 | 0.03 0.01–0.06 | 0.03 0.01–0.06 |
| (r = 0.86) | dens(vegtype + burn_cat.) − NB | Peatland | 0.13 0.03–0.23 | 0.01 0–0.02 | 0.01 0–0.02 | 0 | |
| Upland | 0.42 0.15–0.70 | 0.01 0–0.03 | 0.02 0–0.04 | 0 | |||
| Ruffed Grouse | 31 | psi(burn_binary) p(year) | Both veg. types | 0.48 0.31–0.65 | 0.01 0–0.04 | 0.01 0–0.04 | 0.01 0–0.04 |
| (r = 1) | dens(burn_rank) − P | Both veg. types | 0.10 0.0–0.21 | 0.01 0–0.02 | 0 | 0 | |
| Swainson’s Thrush | 299 | psi(burn_cat.) p(doy + year) | Both veg. types | 1 | 0.73 0.64–0.81 | 0.66 0.55–0.76 | 0.52 0.43–0.60 |
| (r = 0.99) | dens(burn_rank + year + sdoy) − P | Both veg. types | 0.88 0.78–0.98 | 0.60 0.54–0.64 | 0.39 0.35–0.43 | 0.26 0.22–0.30 | |
| Swamp Sparrow | 49 | psi(vegtype + burn_rank) p(year) | Peatland | 0.44 0.27–0.63 | 0.37 0.23–0.53 | 0.30 0.19–0.45 | 0.24 0.13–0.41 |
| Upland | 0.17 0.09–0.30 | 0.14 0.08–0.22 | 0.10 0.06–0.17 | 0.08 0.04–0.15 | |||
| dens(vegtype × burn_rank + year) − NB | Peatland | 0.63 0.05–1.20 | 0.23 0.07–0.38 | 0.08 0.02–0.15 | 0.03 0–0.06 | ||
| Upland | 0.02 0–0.04 | 0.02 0–0.04 | 0.03 0.01–0.04 | 0.03 0.01–0.06 | |||
| Yellow-Rumped Warbler | 170 | psi(vegtype + burn_rank) p(.) | Peatland | 0.58 0.46–0.70 | 0.41 0.31–0.51 | 0.25 0.18–0.33 | 0.14 0.09–0.20 |
| (r = 0.81) | Upland | 0.72 0.62–0.80 | 0.55 0.47–0.63 | 0.37 0.30–0.45 | 0.22 0.16–0.30 | ||
| dens(vegtype × burn_rank + year) − P | Peatland | 0.26 0.13–0.39 | 0.19 0.13–0.26 | 0.14 0.09–0.20 | 0.10 0.04–0.17 | ||
| Upland | 1.31 1.07–1.55 | 0.67 0.58–0.77 | 0.34 0.28–0.41 | 0.18 0.12–0.23 | |||
| Species with higher occupancy rates in burned areas | |||||||
| American Robin | 282 | psi(burn_rank) p(doy + year) | Both veg. types | 0.45 0.36–0.54 | 0.73 0.65–0.80 | 0.90 0.81–0.95 | 0.97 0.91–0.99 |
| (r = 0.98) | dens(burn_rank + year)-P | Both veg. types | 0.19 0.15–0.23 | 0.41 0.35–0.46 | 0.54 0.48–0.60 | 0.72 0.66–0.78 | |
| Canada Jay | 65 | psi (burn_binary) p(doy) | Both veg. types | 0.44 0.14–0.79 | 0.76 0.12–0.99 | 0.76 0.12–0.99 | 0.76 0.12–0.99 |
| (r = n/a) | dens(.) − P | Both veg. types | 0.15 0.11–0.18 | 0.15 0.11–0.18 | 0.15 0.11–0.18 | 0.15 0.11–0.18 | |
| Chipping Sparrow | 287 | psi (vegtype + burn_binary) p(doy + year) | Peatland | 0.69 0.56–0.80 | 0.79 0.68–0.87 | 0.86 0.76–092 | 0.91 0.81–0.96 |
| (r = 0.78) | Upland | 0.58 0.48–0.68 | 0.69 0.62–0.76 | 0.79 0.71–0.85 | 0.86 0.77–0.92 | ||
| dens(vegtype + burn_binary + year) − P | Peatland | 0.66 0.52–0.80 | 1.03 0.91–1.16 | 1.03 0.91–1.16 | 1.03 0.91–1.16 | ||
| Upland | 0.45 0.36–0.54 | 0.69 0.62–0.77 | 0.69 0.62–0.77 | 0.69 0.62–0.77 | |||
| Dark-Eyed Junco | 304 | psi(burn_binary) p(doy + year) | Both veg. types | 0.68 0.57–0.77 | 0.87 0.79–0.92 | 0.87 0.79–0.92 | 0.87 0.79–0.92 |
| (r = 1) | dens(burn_binary + year)-P | Both veg. types | 0.75 0.61–0.88 | 1.23 1.12–1.35 | 1.23 1.12–1.35 | 1.23 1.12–1.35 | |
| Lincoln’s Sparrow | 207 | psi(vegtype + burn_rank) p(.) | Peatland | 0.58 0.48–0.68 | 0.65 0.57–0.73 | 0.72 0.64–0.79 | 0.78 0.69–0.85 |
| (r = 0.71) | Upland | 0.29 0.23–0.37 | 0.36 0.31–0.42 | 0.44 0.38–0.50 | 0.52 0.44–0.60 | ||
| dens(vegtype × burn_cat. + year) − NB | Peatland | 1.15 0.79–1.51 | 2.30 1.70–289 | 1.09 0.76–1.43 | 1.07 0.73–1.41 | ||
| Upland | 0.09 0.04–0.14 | 0.27 0.17–0.37 | 0.27 0.16–0.37 | 0.39 0.29–0.49 | |||
| Olive-Sided Flycatcher | 48 | psi(burn_binary) p(doy) | Both veg. types | 0.01 0–0.07 | 0.15 0.11–0.21 | 0.15 0.11–0.21 | 0.15 0.11–0.21 |
| (r = 0.78) | dens(vegtype + burn_binary) − P | Peatland | 0 | 0.07 0.04–0.09 | 0.07 0.04–0.09 | 0.07 0.04–0.09 | |
| Upland | 0 | 0.03 0.02–0.04 | 0.03 0.02–0.04 | 0.03 0.02–0.04 | |||
| Western Wood-Pewee | 10 | psi(vegtype + burn_rank) p(doy) | Peatland | 0 | 0 | 0 | 0 |
| (r = 0.82) | Upland | 0.02 0.01–0.08 | 0.03 0.01–0.09 | 0.07 0.03–0.12 | 0.12 0.05–0.26 | ||
| dens(vegtype + burn_rank + year) − P | Peatland | 0 | 0 | 0 | 0 | ||
| Upland | 0 | 0.01 0–0.02 | 0.01 0–0.02 | 0.01 0–0.02 | |||
| Species with occupancy rates similar between burned and unburned areas | |||||||
| LeConte’s Sparrow | 27 | psi(vegtype) p(.) | Peatland | 0.10 0.06–0.15 | 0.10 0.06–0.15 | 0.10 0.06–0.15 | 0.10 0.06–0.15 |
| (r = 1) | Upland | 0.03 0.02–0.06 | 0.03 0.02–0.06 | 0.03 0.02–0.06 | 0.03 0.02–0.06 | ||
| dens(vegtype + year) − NB | Peatland | 0.26 0.10–0.41 | 0.26 0.10–0.41 | 0.26 0.10–0.41 | 0.26 0.10–0.41 | ||
| Upland | 0.02 0–0.04 | 0.02 0–0.04 | 0.02 0–0.04 | 0.02 0–0.04 | |||
| Palm Warbler | 93 | psi(vegtype) p(.) | Peatland | 0.35 0.26–0.46 | 0.35 0.26–0.46 | 0.35 0.26–0.46 | 0.35 0.26–0.46 |
| (r = 0.72) | Upland | 0.20 0.15–0.26 | 0.20 0.15–0.26 | 0.20 0.15–0.26 | 0.20 0.15–0.26 | ||
| dens(vegtype + burn_rank + year) | Peatland | 0.68 0.40–0.95 | 0.43 0.29–0.58 | 0.28 0.18–0.38 | 0.18 0.09–0.27 | ||
| Upland | 0.18 0.11–0.25 | 0.11 0.08–0.15 | 0.07 0.05–0.10 | 0.05 0.02–0.07 | |||
| Species with occupancy rates that show non-linear response to burn severity and/or whose response depends on vegetation type | |||||||
| Hermit Thrush | 353 | psi(burn_category) p(doy + year) | Both veg. types | 0.87 0.79–0.92 | 0.97 0.85–1 | 0.91 0.81–0.96 | 0.86 0.78–0.92 |
| (r = −0.32) | dens(vegtype × burn_rank + year) − P | Peatland | 0.37 0.30–0.44 | 0.42 0.37–0.47 | 0.48 0.43–0.53 | 0.55 0.47–0.63 | |
| Upland | 0.63 0.56–0.69 | 0.61 0.56–0.65 | 0.58 0.55–0.62 | 0.56 0.51–0.61 | |||
| White-Crowned Sparrow | 77 | psi(vegtype × burn_cat.) p(doy + year) | Peatland | 0.04 0.01–0.24 | 0.56 0.33–0.77 | 0.54 0.32–0.75 | 0.30 0.14–0.51 |
| (r = 0.72) | Upland | 0 | 0.06 0.02–0.17 | 0.25 0.14–0.42 | 0.34 0.23–0.48 | ||
| dens(vegtype + burn_cat. + year) − NB | Peatland | 0.02 0–0.05 | 0.30 0.16–0.44 | 0.29 0.13–0.43 | 0.43 0.26–0.60 | ||
| Upland | 0 | 0.06 0.03–0.09 | 0.06 0.03–0.09 | 0.09 0.05–0.13 | |||
| White-Throated Sparrow | 225 | psi(vegtype × burn_rank) p(year) | Peatland | 0.74 0.59–0.85 | 0.73 0.64–0.81 | 0.73 0.64–0.81 | 0.73 0.59–0.84 |
| (r = 0.98) | Upland | 0.27 0.20–0.35 | 0.40 0.32–0.47 | 0.55 0.49–0.62 | 0.69 0.61–0.77 | ||
| dens(vegtype × burn_rank + year) − P | Peatland | 0.47 0.35–0.59 | 0.50 0.41–0.58 | 0.53 0.44–0.62 | 0.56 0.42–0.70 | ||
| Upland | 0.08 0.05–0.11 | 0.15 0.11–0.18 | 0.26 0.22–0.31 | 0.47 0.37–0.57 | |||
| Peatland | Upland | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Community Metric | Best Model | Control | Low | Mod. | High | Control | Low | Mod. | High |
| Alpha (visit) 2015 | vegtype × burn_rank + year | 5.6 5.3–6.0 | 5.1 4.9–5.4 | 4.6 4.4–4.9 | 4.2 3.8–4.5 | 3.4 3.1–3.6 | 3.3 3.1–3.5 | 3.2 3.1–3.4 | 3.2 2.9–3.4 |
| 2016 | 7.5 7.1–7.8 | 7.0 6.7–7.2 | 6.5 6.2–6.8 | 6.0 5.6–6.4 | 5.2 4.9–5.5 | 5.1 5.0–5.3 | 5.1 4.9–5.3 | 5.0 4.8–5.3 | |
| Alpha (station) | vegtype × burn_cat. | 11.5 10.7–12.2 | 10.9 10.2–11.6 | 9.5 8.7–10.2 | 9.1 8.3–9.9 | 8.5 7.9–9.1 | 7.8 7.2–8.4 | 8.0 7.4–8.7 | 7.9 7.4–8.4 |
| Gamma | n/a | 30.3 27–32 | 27.8 25–30.5 | 23.4 21–26 | 23.4 21–25 | 22.6 19–26 | 20.7 17–24 | 22.5 19–25 | 22.1 19–25 |
| Functional diversity | burn_cat. | 0.61 0.58–0.64 | 0.53 0.50–0.56 | 0.47 0.44–0.50 | 0.44 0.42–0.47 | 0.61 0.58–0.64 | 0.53 0.50–0.56 | 0.47 0.44–0.50 | 0.44 0.42–0.47 |
| Reference Number | This Study | [68] | [69] | [70] | [30] | [71] | [72] | [37] | [36] | [25] |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2020 | 2012 | 2019 | 2018 | 2011 | 2006 | 2015 | 2016 | 2016 | 2020 |
| Location | NWT | USA | MN | CA | QB | AZ | OR | ID, AZ | MT | AB |
| Alder Flycatcher | − | + | / | +++++ | ||||||
| American Robin | + | / | + | / | / | / | /+//+ | |||
| Canada Jay | + | − | −−/−− | |||||||
| Chipping Sparrow | + | int | + | / | −/+ | + | −−/−− | |||
| Common Yellowthroat | − | / | + | +++++ | ||||||
| Dark-Eyed Junco | + | / | / | int | + | −/ | − | −−/−− | ||
| Hermit Thrush | int | − | / | − | + | − | int | −−−−− | ||
| LeConte’s Sparrow | / | +++++ | ||||||||
| Lincoln’s Sparrow | + | + | − | +++++ | ||||||
| Orange-Crowned Warbler | − | − | −−−−− | |||||||
| Olive-Sided Flycatcher | + | + | / | int | / | + | −−/+− | |||
| Palm Warbler | / | −−−−− | ||||||||
| Ruby-Crowned Kinglet | − | − | − | −−−−− | ||||||
| Ruffed Grouse | − | −−−−− | ||||||||
| Swamp Sparrow | − | + | …// | |||||||
| Swainson’s Thrush | − | − | / | − | −−−−− | |||||
| White-Crowned Sparrow | int/ | …// | ||||||||
| Western Wood-Peewee | + | + | + | ++/++ | ||||||
| White-Throated Sparrow | /+ | + | + | / | +++++ | |||||
| Yellow-Rumped Warbler | − | − | − | − | − | int | − | −/ | int | −−−−− |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knaggs, M.; Haché, S.; Nielsen, S.E.; Pankratz, R.F.; Bayne, E. Avian Response to Wildfire Severity in a Northern Boreal Region. Forests 2020, 11, 1330. https://doi.org/10.3390/f11121330
Knaggs M, Haché S, Nielsen SE, Pankratz RF, Bayne E. Avian Response to Wildfire Severity in a Northern Boreal Region. Forests. 2020; 11(12):1330. https://doi.org/10.3390/f11121330
Chicago/Turabian StyleKnaggs, Michelle, Samuel Haché, Scott E. Nielsen, Rhiannon F. Pankratz, and Erin Bayne. 2020. "Avian Response to Wildfire Severity in a Northern Boreal Region" Forests 11, no. 12: 1330. https://doi.org/10.3390/f11121330
APA StyleKnaggs, M., Haché, S., Nielsen, S. E., Pankratz, R. F., & Bayne, E. (2020). Avian Response to Wildfire Severity in a Northern Boreal Region. Forests, 11(12), 1330. https://doi.org/10.3390/f11121330






