Abstract
Many diseases of date palm are known. However, pathogens that might affect seed germination and seedling emergence from soil are poorly studied, perhaps because date palm cultivars are propagated vegetatively. Here, we first determined the effects of date seed fungi on the germination and emergence of 600 seeds overall (i.e., 200 of each of three cultivars: ‘Thoory’, ‘Halawi’, and ‘Barhi’). In each cultivar, 100 seeds were from Saudi Arabia (part of the native range), and 100 were from the southwestern USA (where the date palm was introduced around 1765). Just four fungal genera (i.e., Alternaria, Aspergillus, Chaetomium, and Penicillium) were isolated from the surface-sterilized date seeds. Aspergillus isolates all belonged to Aspergillus sect. Nigri; collectively they were in the highest relative abundance at 39%, and significantly more common in Saudi Arabian seeds than in American seeds. Aspergillus reduced seed germination and also reduced emergence when germinated and non-germinated seeds were planted in potting mix in a greenhouse. In contrast, Penicillium species were more common in American than in Saudi seeds; Penicillium did not affect germination, although it did have a positive effect on seedling emergence. In a second experiment with 17 seeds of the ‘Halawi’ cultivar, fungus-free seeds were either inoculated with isolates of Aspergillus sect. Nigri or not, and then planted. Controls emerged whereas Aspergillus-inoculated seeds did not. Finally, a third experiment was conducted with Aspergillus tubingensis Mosseray, a sect. Nigri member, as sole inoculum of 100 ‘Halawi’ seeds versus 100 uninoculated controls. Aspergillus tubingensis exerted the same pathogenic effects on germinating and emerging seedlings as the isolates identified only to Aspergillus sect. Nigri. Aspergillus tubingensis is thus a previously unreported, seedborne pathogen affecting date palm seedlings. Our findings also suggest that A. tubingensis may be more common in seeds in the host’s native range than in its introduced range.
1. Introduction
Date palm, Phoenix dactylifera L., is a tree belonging to the family Arecaceae. Cultivars of this species have been cultivated for at least five millennia in desert oases from North Africa to Southwest Asia, including the Persian Gulf [1]. The date palm was introduced to Spain, and the Spanish, who in turn introduced date culture to North America, almost 300 years ago in the desert oasis of Mision San Ignacio of Baja California Sur in Mexico. It was subsequently introduced to other parts of the American Southwest (the states of California and Arizona) as well as to Australia, India, Pakistan, South Africa, and South America [2]. Over the years, the United States have imported much propagative material of date palm including seeds for the selection of cultivars [3,4]. Cultivars, once selected, are then propagated vegetatively. Many popular date cultivars are now grown in both the native and introduced ranges.
Plants often host fewer diseases where they are introduced versus where they are native; this is known as enemy release. Mitchell and Power [5] showed that this was true for certain obligate parasites that might be left behind in the native range when the plant is introduced elsewhere, such as rust fungi (Uredinales), powdery mildew fungi (Erysiphales), smut fungi (Ustilaginales), and viruses. Date palms are not known to host any rust or powdery mildew fungi anywhere in the world, but they do host a widespread, ‘falsesmut’ fungus (in Exobasidiales rather than Ustilaginales) [6]. However, other pathogens might also be more common in date palm’s native range than in its introduced range. Given vegetative propagation, the same cultivars (genotypes) can be contrasted in the two ranges, and here we consider that comparison for seed-borne pathogens of three cultivars of date palm.
We obtained seeds from the same cultivars sampled in both the native range and the American Southwest. Seeds of date palm are relatively large (from 0.5 to 4 g per seed) [7], and they can remain viable for an extraordinary period of up to 2000 years under certain climatic conditions of high temperatures and low precipitation [8]. Overall, 13 genera of fungi have been reported from seeds (Alternaria, Aspergillus, Bipolaris, Chaetomium, Curvularia, Fusarium, Penicillium, Phialophora, Rhizopus, Scytalidium, Thielavia, Trichoderma, and Ulocladium) [6], but none have been investigated for effects on seed germination and seedling emergence [9,10]. The objectives of this study were to determine the seedborne fungi in date palm seed of the same cultivars in Saudi Arabia and the American Southwest and then to determine the effects of these fungi on germination and the emergence of seedlings.
2. Materials and Methods
2.1. Seed Germination and Seedling Emergence
Seeds of three date palm cultivars (cv.) (‘Thoory’, ‘Halawi’, and ‘Barhi’) were obtained from local markets in Riyadh, Madinah, Albir, and Thadq in Saudi Arabia, and ordered online in the United States (seeds of cv. ‘Thoory’ from Oasis Date Gardens in Thermal, CA, USA; ‘Halawi’ from Sun Organic Farm in San Marcos, CA, USA; ‘Barhi’ from Fresh Date by Anderson in Thermal, CA, USA). All seeds were from fruits harvested in 2015. One hundred seeds of each of the three cultivars were from Saudi Arabia and 100 were from the American source. Thus, the total number of seeds was 600. All seeds were surface-disinfested by being dipped in 1% sodium hypochlorite for two minutes, and then washed in sterile distilled water (SDW) three times. Ten to 13 seeds were placed in each 25 cm Petri dish depending on the size of the seeds. In each dish, seeds were placed on a sterilized paper towel that had been moistened with 20 mL of SDW. SDW was also added as needed over time to prevent seeds from drying out and to allow seeds to germinate [9]. The plates were incubated at room temperature (22 °C) with continuous white fluorescent lights. After 10 days, seed germination and genus of fungal isolates were recorded every two days for 24 days. Representative isolates of fungal genera were recovered from each cultivar and maintained on potato dextrose agar (PDA). Ungerminated seeds and germinants were then moved to the greenhouse where they were planted in ‘Cone-tainers’ filled with Sunshine #1 potting mix (from Sungro Horticulture, 770 Silver Street, Agawam, MA 01001-2907). Seeds and germinants were kept in the greenhouse for 115 more days to observe seedling emergence. The greenhouse temperatures were maintained between 20 °C and 24 °C, with a 16 h:8 h, day:night cycle.
2.2. Identification of Seedborne Fungi
Fungi emerging from, or present on, surface-disinfested seeds were recorded and examined first by dissecting and then by a compound microscope to identify each to a genus. Appropriate mycological texts were consulted. A representative Aspergillus isolate from a Saudi Arabian date was subsequently identified to species level [11,12]. In short, the total genomic DNA was extracted and a part of the calmodulin (CaM) and β-tubulin (BenA) gene amplified and sequenced according to the method described in Samson et al. [12]. The generated sequences were compared against an in-house sequence database containing all Aspergillus and Penicillium reference sequences [11]. The isolate of A. tubingensis Mosseray used in our final experiment in this study was deposited in the CBS culture collection housed at the Westerdijk Fungal Biodiversity Institute under accession number CBS 144784.
2.3. Koch’s Postulates/Initial Aspergillus Experiment
After first observing the effects of fungi on seed germination and emergence in the initial, 600-seed experiment described above and identifying Aspergillus sect. Nigri isolates as putative pathogens, the latter were the focus of two experiments to test Koch’s Postulates. In this initial experiment, 17 seeds of ‘Halawi’ cultivar and 14 different isolates of Aspergillus sect. Nigri were used. Three seeds were uninoculated controls and 14 seeds were inoculated with the isolates; seeds were without any other seed-borne fungi. All seeds were initially surface-sterilized by dipping in 1% sodium hypochlorite for two minutes and then washed in SDW three times. Each seed was then placed in a 10-cm diameter Petri dish over a sterilized paper towel with 10 mL of SDW; more SDW was added as needed to prevent seeds from drying out and to allow seeds to germinate. Each of the 14 isolates was added to one of these 14 seeds as spore suspension (1 × 106 conidia per ml). The spore suspension was made by adding SDW by transfer pipette to the Aspergillus PDA plate and then the transfer pipette was used to add a small amount of spore suspension directly applied to the surface of each seed. Then, after 30 days, the seeds were transferred to the greenhouse. One of the control seeds had to be removed at this point in the experiment because of the appearance of another fungus (Penicillium) on the surface of the seed; one of the 14 inoculated seeds had to be removed for the same reason. The total number of seeds transferred to the greenhouse was thus 15 seeds: two controls and 13 inoculated with isolates of Aspergillus sect. Nigri. Seeds were planted in Cone-tainers with potting mix, and each seed was labeled. To observe emergence, seeds were kept in the greenhouse for 121 days with watering four days a week and the greenhouse conditions were kept at 20 to 24 °C, with a 16:8 day:night cycle.
2.4. Experiment with Aspergillus tubingensis
Koch’s Postulates were then repeated using 200 seeds of the ‘Halawi’ cultivar and A. tubingensis CBS 144,784 as the inoculum. One hundred seeds were used as controls and 100 seeds were inoculated with A. tubingensis (CBS 144784). All seeds were surface-sterilized by being dipped in 1% sodium hypochlorite for two minutes and then washed in SDW three times. Ten seeds were placed in each 25 cm Petri dish over a sterilized paper towel with 10 mL of SDW; more SDW was added as needed to prevent seeds from drying out and to allow seeds to germinate. The A. tubingensis isolate was added to these 100 seeds as spore suspension (1 × 106 conidia per ml). The spore suspension was prepared by adding SDW by transfer pipette to the A. tubingensis PDA plate and then with the transfer pipette a small amount of spore suspension was directly applied to the surface of each seed. Then, after 30 days, all seeds were transferred to the greenhouse to test the effects of A. tubingensis. Seeds were planted in Cone-tainers with potting mix, and each seed was labeled. Seeds were kept in the greenhouse for 121 days with watering four days a week and the greenhouse conditions were set to 20 to 24 °C with a 16:8 day:night cycle.
2.5. Statistical Analyses
Survival analysis was carried out to determine how fungi affected seedling emergence over time using RStudio version 3.6.0. Chi-square analyses were performed for 2 × 2 contingencies.
3. Results
One fungal species per seed was isolated in 54.5% or 327 of the 600 seeds. A further 238 seeds, or 39.7%, yielded no fungi. Just 35 (5.8%) of the seeds yielded two species per seed. A total of 397 fungal isolates were thus obtained, and these belonged to four genera: Alternaria, Aspergillus, Chaetomium, and Penicillium (Table 1). The relative abundance of each genus was as follows: 39.0% Aspergillus, 33.8% Penicillium, 18.4% Chaetomium and 8.6% Alternaria. The two most abundant genera, Aspergillus and Penicillium, were recorded to a varying extent in all cultivars from both Saudi Arabia and the American Southwest. Alternaria was relatively rare, and was only found in one cultivar, ‘Barhi’, in American seeds. Phenotypic diversity was observed in the detected Alternaria isolates, and 79% would have been identified as Ulocladium using the classical, phenotype-based classification system of those genera [13]. At this stage, the isolates of Aspergillus had been identified only as Aspergillus section Nigri.

Table 1.
Number of isolates of fungi per cultivar (Th, cv. ‘Thoory’; HA, cv. ‘Halawi’; Ba, cv. ‘Barhi’) and range (SA, Saudi Arabia; USA, American Southwest) for a total of 397 isolates per 600 seeds. Relative abundances are shown on the bottom line as percentages of the total number of isolates. Aspergillus here refers to Aspergillus sect. Nigri.
Aspergillus section Nigri species were more common in Saudi (116 seeds) than American (39 seeds) sources (χ2 = 51.58, p < 0.0001). Penicillium sp. was the reverse: more common in American seed (i.e., 97 seeds) than Saudi (37 seeds) (χ2 = 14.57, p = 0.0001). Chaetomium sp., the genus with the third highest relative abundance (73 seeds from which isolates were obtained) was equally common in American (45) and Saudi (28) seed (χ2 = 2.02, p = 0.15).
Seed germination was recorded in the lab prior to planting all seeds, both germinated and non-germinated, in potting mix in the greenhouse to determine emergence. Germination in Petri dishes in the lab was affected by fungi associated with the seeds (Table 2; Figure 1). The growth of Aspergillus sect. Nigri reduced germination of seeds (χ2 = 22.13, p < 0.0001) and then also strongly reduced the emergence (Figure 2) when germinated and non-germinated seeds were planted in potting mix in a greenhouse (χ2 = 77.42, p < 0.0001). In contrast, the Penicillium species present did not affect germination (χ2 = 3.43, p = 0.06) although it did have a marginally positive effect on seedling emergence (χ2 = 5.52, p = 0.019). The third most abundant fungus in date seeds was a Chaetomium sp., which represented a third pattern of effects on germination and emergence. It slowed the germination of seeds (χ2 = 20.19, p < 0.0001) but did not reduce emergence (χ2 = 3.80, p = 0.051).

Table 2.
Effects of fungal status on germination of the 600 seeds in the lab followed by seedling emergence in potting mix in the greenhouse. Aspergillus here refers to Aspergillus sect. Nigri.

Figure 1.
Lack of germination and slow development caused by Aspergillus sect. Nigri (left) versus a typical, fungus-free seedling at same time (right).
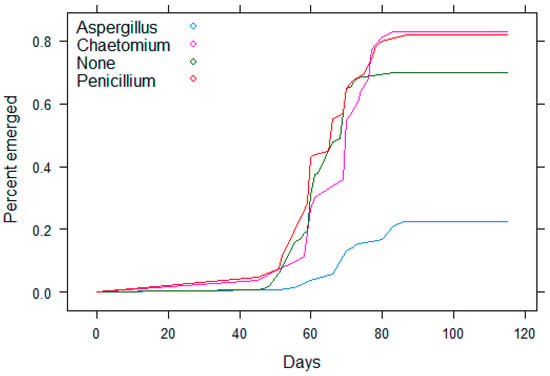
Figure 2.
Survival analysis of emergence of date palm seedlings with or without fungi in the 600-seed experiment. Note delayed emergence due to Aspergillus sect. Nigri.
3.1. Koch’s Postulates/Initial Aspergillus Experiment
Seeds inoculated with different isolates of Aspergillus sect. Nigri were slower to emerge or did not emerge. The difference between the controls and the seeds with Aspergillus was as clear as it had previously been in the 600-seed experiment (Figure 1). Delayed emergence caused by Aspergillus sect. Nigri was again apparent after four months of observation, as it had been previously (Figure 2).
3.2. Experiment with Aspergillus tubingensis
One representative isolate of Aspergillus sect. Nigri (CBS 144784) was then identified as Aspergillus tubingensis using partial calmodulin and tubulin gene sequencing (BenA 99.8%; CaM 99.5%) (similarity percentages with the type strain of the species). When inoculated with this A. tubingensis isolate (CBS 144784), 100 seeds and then seedlings of cv. ‘Halawi’ were again negatively affected as seeds and seedlings had been in the 600-seed experiment or in the initial, ‘Koch’s Postulates’ experiment, where inoculation was with isolates identified only as Aspergillus sect. Nigri (Figure 3). Delayed emergence, in particular, was just as it had been before in the 600-seed experiment (Figure 3).
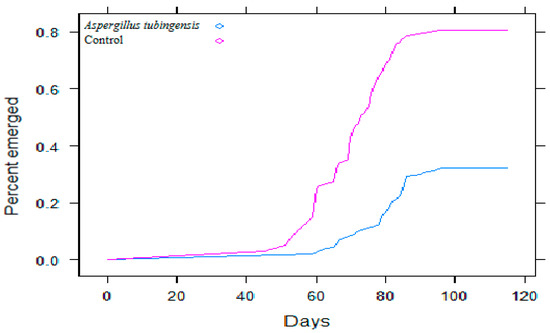
Figure 3.
Emergence of date palm seedlings from 100 seeds of cv. ‘Halawi’ inoculated with a single isolate of A. tubingensis, versus 100 seeds without A. tubingensis (controls). Note that the effect of A. tubingensis reproduces the effect on emergence that we saw in the prior experiment, which was attributed more loosely to Aspergillus sect. Nigri (Figure 2).
4. Discussion
Aspergillus tubingensis has never been reported from date palm before in any disease context. Our proof of Koch’s Postulates took several steps in the discovery, as just outlined. Briefly, we first saw that there was a cryptic pathogen among the black Aspergillus isolates. We then showed that single isolates from the group could reduce germination and emergence. Then, we showed that a representative isolate, expertly identified as A. tubingensis, and deposited in the world’s largest collection of living fungi as CBS 144784, could again reduce germination and emergence.
This is the first report of A. tubingensis as a pathogen of Phoenix dactylifera, to be added to the list of pathogens of date palm [14]. Aspergillus niger, on the other hand, another member of Aspergillus section Nigri [12,15], has been reported as a postharvest pathogen causing fruit rot in Spain [16,17]. In cases where A. tubingensis is known to be a plant pathogen, it has also been associated with fruit rot [18]. In a study of endophytes in maturing date palm fruit, Aspergillus becomes common in the pulp of the later stages, and A. tubingensis is the most common species [19,20]. This is in any case the second report of A. tubingensis as a pathogen beyond the association with fruit rot; the first report was that A. tubingensis is a leaf spot pathogen of Jatropha curcas in China [21]. If more emergence assays were performed with seedborne microbes it seems probable that more ‘cryptic pathogens’ would be discovered, even in genera such as Aspergillus. A similar example, albeit from a different genus, is the recent finding that Sydowia polyspora can act as a pre-emergent pathogen of Pinus ponderosa [22].
Many fungi have been implicated in fruit rot of date palm [9,23], including Aspergillus species belonging to the A. niger clade [17], and Penicillium spp. [23]. Bacteria appear to be less important, perhaps due to antibacterial compounds in date palm seeds [24], although some bacteria can have beneficial effects on the germination of date palm [25,26]. Yet, with respect to seed germination and seedling emergence, A. tubingensis and Penicillium sp. had opposing effects; the former with decidedly negative effects versus those of the latter that trended positively. This suggests that members of the fruit rot community switch to more specific and varied roles when it comes to seed germination and seedling performance.
Differences in the communities of seedborne fungi in native and introduced ranges have been reported before [27]. That the pathogenic A. tubingensis was more common in Saudi seeds than American counterparts of three date cultivars confirms the expectation of enemy release for a cryptic, pre-emergent pathogen. It is interesting that Penicillium species were more common in American seeds and exerted a positive effect on seedling emergence. Novel mutualisms such as that are sometimes seen in plants in their introduced ranges e.g., [28].
Most of the 600 seeds (94.2%) yielded either one fungus per seed (54.5% or 327 of the 600 seeds) or were fungus-free (238 seeds, or 39.7%). Only 5.8% yielded two fungi per seed. These findings confirm the hypothetical bottleneck in the plant microbiome that was recently proposed [29]. Two reasons have been invoked to explain such a bottleneck: optimal defense theory and exclusionary interactions among seed-infecting fungi [30]. According to the Primary Symbiont Hypothesis that is based on the bottleneck, the identity of each microbe in a seed then matters as effects on seedlings can change with primary symbiont identity. That is, in effect, what we saw in this study, as A. tubingensis had pathogenic effects that distinguished it from the other three genera of fungi isolated from seeds of date palm.
Author Contributions
Conceptualization, G.N.; Methodology, G.N., M.A. and J.H.; Investigation, M.A. and J.H.; Resources, J.H. and G.N.; Data Curation, M.A. and G.N.; Writing—Original Draft Preparation, M.A.; Writing—Review and Editing, G.N., and J.H; Visualization, M.A.; Supervision, G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no traditional, external funding but it was supported in that MA was funded by the government of Saudi Arabia.
Acknowledgments
We wish to thank Tim Prather, Frank Dugan and Rula Awwad-Rafferty for their helpful contributions to this project as members of the PhD committee of Maryam Alomran.
Conflicts of Interest
The authors declare no competing interests.
References
- Abdelmonem, A.M.; Rasmy, M.R. Major diseases of date palm and their control. Commun. Inst. For. Bohem. 2007, 23, 9–23. [Google Scholar]
- Chao, C.T.; Krueger, R.R. The Date Palm (Phoenix dactylifera L.): Overview of Biology, Uses, and Cultivation. HortScience 2007, 42, 1077–1082. [Google Scholar] [CrossRef]
- Fairchild, D. Seeds and Plants Imported during the Period from July 1, 1906 to December 1, 1907; Inventory No. 13: Nos. 19058 to 21730; U.S. Government Publishing Office: Washington, DC, USA, 1908.
- McCarthy, M.A. Date Palms in the Desert: Reimagining and Cooperating with Nature in Arid Arizona. Ariz. J. Interdiscip. Stud. 2012, 1, 39. [Google Scholar]
- Mitchell, C.E.; Power, A.G. Release of invasive plants from fungal and viral pathogens. Nat. Cell Biol. 2003, 421, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, Systematic Mycology and Microbiology Laboratory, ARS, USDA. Available online: http://nt.ars-grin.gov/fungaldatabases/ (accessed on 12 October 2020).
- Zaid, A.; Arias-Jimenez, A.J. Date Palm Cultivation; FAO Plant Production and Protection Paper 156. 2017. Available online: http://www.fao.org/docrep/006/Y4360E/y4360e0g.htm#bm16 (accessed on 12 October 2020).
- Sallon, S.; Solowey, E.; Cohen, Y.; Korchinsky, R.; Egli, M.; Woodhatch, I.; Simchoni, O.; Kislev, M. Germination, Genetics, and Growth of an Ancient Date Seed. Science 2008, 320, 1464. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheikh, H. Date-Palm Fruit Spoilage and Seed-Borne Fungi of Saudi Arabia. Res. J. Microbiol. 2009, 4, 208–213. [Google Scholar] [CrossRef][Green Version]
- Bokhary, H. Seed-borne fungi of date-palm, Phoenix dactylifera L. from Saudi Arabia. Saudi J. Biol. Sci. 2010, 17, 327–329. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.-C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.; et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef]
- Samson, R.A.; Visagie, C.; Houbraken, J.; Hong, S.-B.; Hubka, V.; Klaassen, C.; Perrone, G.; Seifert, K.; Susca, A.; Tanney, J.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef]
- Simmons, E.G. Alternaria: An Identification Manual; Series No.6, Utrecht; CBS Fungal Biodiversity Centre: Utrecht, The Netherlands, 2007. [Google Scholar]
- Haq, I.U.; Khan, N.A. Fungal Diseases of Date Palm (Phoenix dactylifera): Etiology and Management. In Sustainability in Plant and Crop Protection; Springer: Cham, Switzerland, 2020; pp. 169–196. [Google Scholar]
- Varga, J.; Frisvad, J.; Kocsubé, S.; Brankovics, B.; Tóth, B.; Szigeti, G.; Samson, R. New and revisited species in Aspergillus section Nigri. Stud. Mycol. 2011, 69, 1–17. [Google Scholar] [CrossRef]
- Abdullah, S.K.; Monfort, E.; Asensio, L.; Salinas, J.; Llorca, L.V.L.; Jansson, H.B. Soil mycobiota of date palm plantations in Elche, SE Spain. Czech Mycol. 2010, 61, 149–162. [Google Scholar] [CrossRef]
- Palou, L.; Rosales, R.; Taberner, V.; Vilella-Espla, J. Incidence and etiology of postharvest diseases of fresh fruit of date palm (Phoenix dactylifera L.) in the grove of Elx (Spain). Phytopathol. Mediterr. 2017, 55, 391–400. [Google Scholar]
- Andersen, B.; Thrane, U. Food-borne fungi in fruit and cereals and their production of mycotoxins. In Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2006; Volume 571, pp. 137–152. [Google Scholar]
- Piombo, E.; Abdelfattah, A.; Danino, Y.; Salim, S.; Feygenberg, O.; Spadaro, D.; Wisniewski, M.; Droby, S. Characterizing the Fungal Microbiome in Date (Phoenix dactylifera) Fruit Pulp and Peel from Early Development to Harvest. Microorganisms 2020, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, M.; Santinelli, M.; Sulyok, M.; Onofri, A.; Covarelli, L.; Beccari, G. Aspergillus, Penicillium and Cladosporium species associated with dried date fruits collected in the Perugia (Umbria, Central Italy) market. Int. J. Food Microbiol. 2020, 322, 108585. [Google Scholar] [CrossRef]
- Guo, J.-W.; Gao, Y.; Li, C.-Y.; Yang, L.-F.; Tian, X.-J.; Hong, L.; Kong, Q.; Zhang, Y.-G.; Li, W.-J. First Report of Leaf Spot Disease Caused by Aspergillus tubingensis on Jatropha curcas in Yunnan, China. Plant Dis. 2017, 101, 505. [Google Scholar] [CrossRef]
- Ridout, M.; Newcombe, G. Sydowia polyspora is both a foliar endophyte and a pre-emergent seed pathogen in Pinus ponderosa. Plant Dis. 2018, 102, 640–644. [Google Scholar] [CrossRef]
- Palou, L.; Montesinos-Herrero, C.; Taberner, V.; Vilella-Esplá, J. First Report of Alternaria alternata Causing Postharvest Black Spot of Fresh Date Palm Fruit in Spain. Plant Dis. 2013, 97, 286. [Google Scholar] [CrossRef]
- Metoui, M.; Essid, A.; Bouzoumita, A.; Ferchichi, A. Chemical Composition, Antioxidant and Antibacterial Activity of Tunisian Date Palm Seed. Pol. J. Environ. Stud. 2018, 28, 267–274. [Google Scholar] [CrossRef]
- AbdelGawad, H.; Saleh, A.M.; Al Jaouni, S.; Selim, S.; Hassan, M.O.; Wadaan, M.A.; Shuikan, A.M.; Mohamed, H.S.; Hozzein, W.N. Utilization of actinobacteria to enhance the production and quality of date palm (Phoenix dactylifera L.) fruits in a semi-arid environment. Sci. Total Environ. 2019, 665, 690–697. [Google Scholar] [CrossRef]
- Boutheina, Z.-E.; Aya, H.; Naima, B.; Ahmed, N. Responses of date Palm Seedling to co-Inoculation with Phosphate Solubilizing Bacteria and Mycorrhizal Arbuscular Fungi. Int. J. Environ. Agric. Biotechnol. 2019, 4, 581–588. [Google Scholar] [CrossRef]
- Shipunov, A.; Newcombe, G.; Raghavendra, A.K.H.; Anderson, C.L. Hidden diversity of endophytic fungi in an invasive plant. Am. J. Bot. 2008, 95, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Baynes, M.; Newcombe, G.; Dixon, L.; Castlebury, L.; O’Donnell, K. A novel plant–fungal mutualism associated with fire. Fungal Biol. 2012, 116, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, G.; Harding, A.; Ridout, M.; Busby, P.E. A Hypothetical Bottleneck in the Plant Microbiome. Front. Microbiol. 2018, 9, 1645. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.K.H.; Newcombe, G.; Shipunov, A.; Baynes, M.; Tank, D. Exclusionary interactions among diverse fungi infecting developing seeds of Centaurea stoebe. FEMS Microbiol. Ecol. 2013, 84, 143–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).