Studies into Fungal Decay of Wood In Ground Contact—Part 1: The Influence of Water-Holding Capacity, Moisture Content, and Temperature of Soil Substrates on Fungal Decay of Selected Timbers
Abstract
1. Introduction
2. Materials and Methods
2.1. Standard Test Requirements
2.2. Preparation of Terrestrial Microcosms (TMC)
2.2.1. Soil Substrates
2.2.2. Determination of the Soil Moisture Content (MCsoil)
2.2.3. Determination of the Soil Water-Holding Capacity (WHCsoil)
2.2.4. Preparation of Mixed Soil Substrates
2.2.5. Preparation of Mixed Soil Substrates to Reach Target Soil Moisture Content (MCsoil,target)
2.2.6. Soil Temperature (Tsoil) Control
2.2.7. Preparation and Exposure of Wood Specimens
3. Results and Discussion
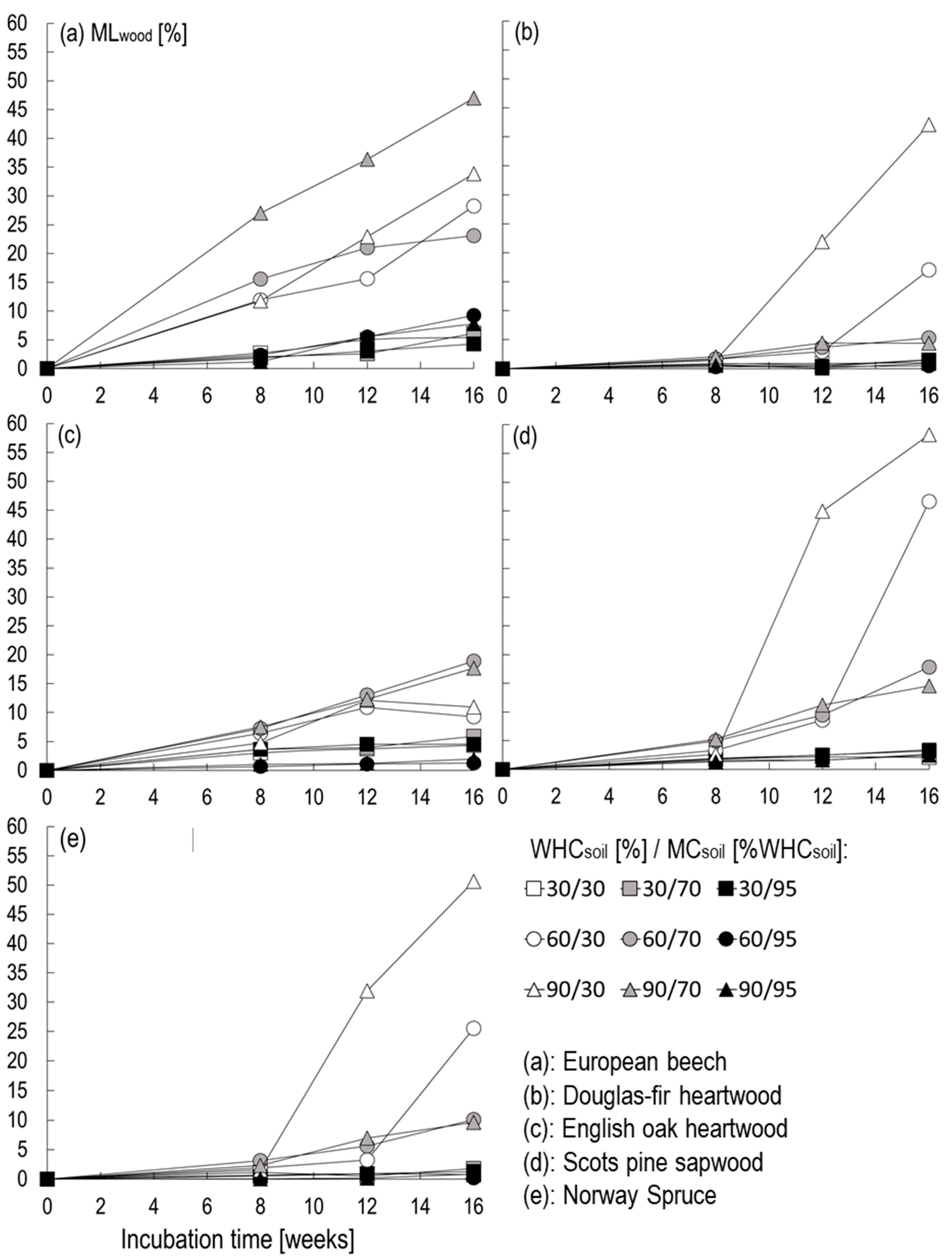
3.1. Impact of WHCsoil and MCsoil on Fungal Decay (Study A)
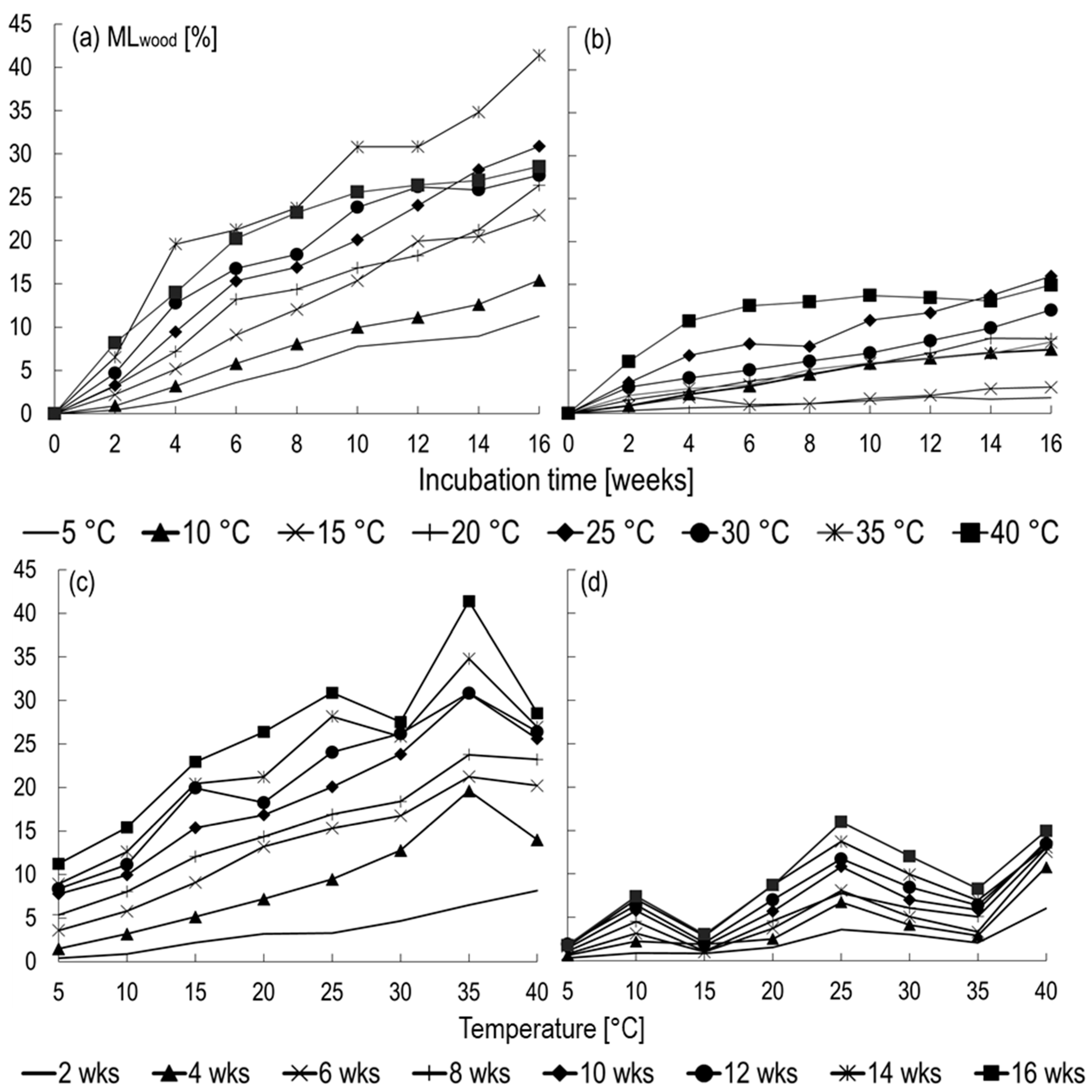
3.2. Impact of Tsoil and MCsoil on Fungal Decay (Study B)
3.2.1. Constant Tsoil
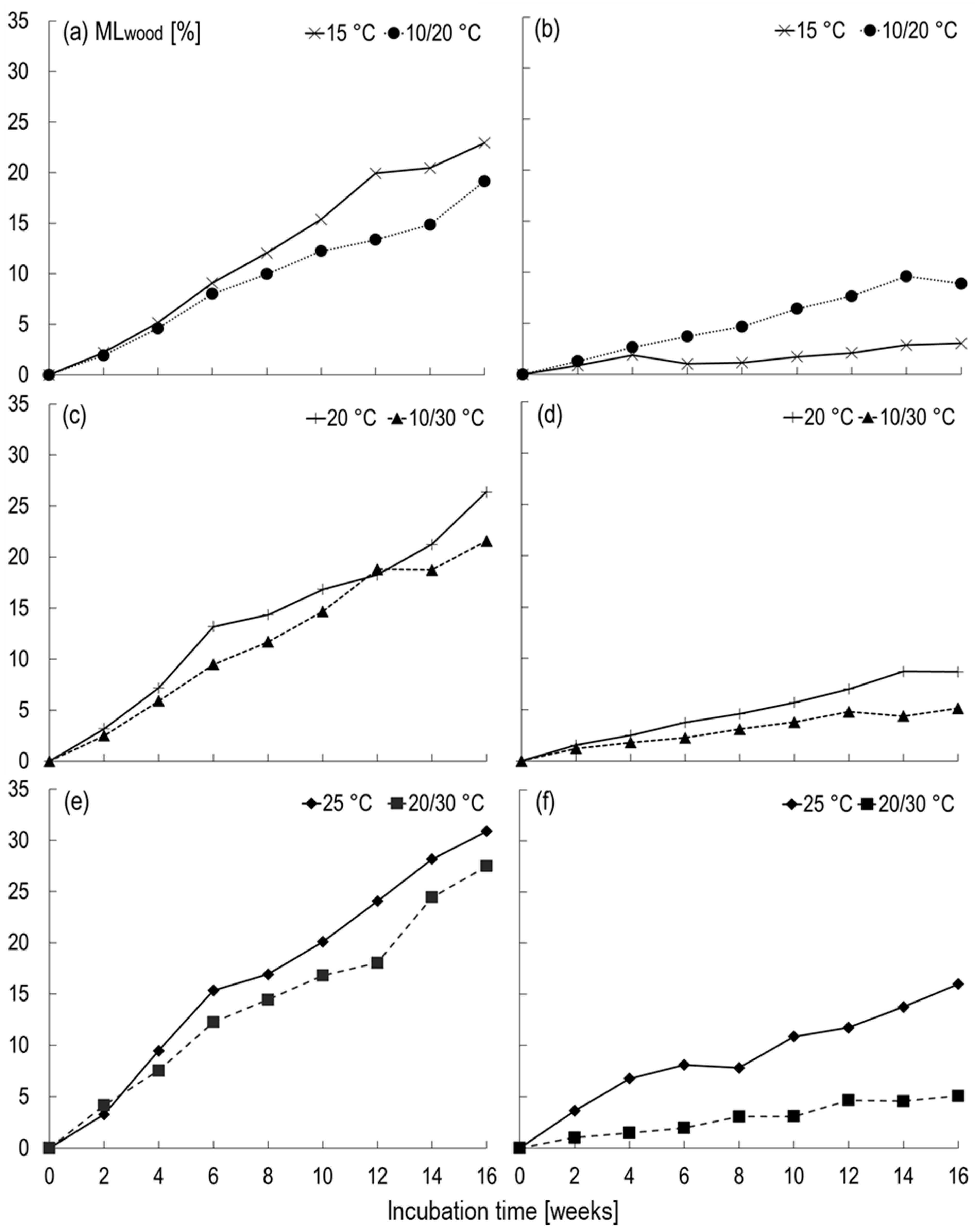
3.2.2. Alternating Tsoil
3.2.3. Comparison: Constant vs. Alternating Tsoil
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Soil Condition WHCsoil[%]/MCsoil[%WHCsoil] | Exposure Interval [Weeks] | ||
|---|---|---|---|
| 8 | 12 | 16 | |
| 30/30 | 2.73 (1.61) | 5.02 (5.28) | 5.45 (5.43) |
| 30/70 | 2.06 (0.35) | 2.59 (2.84) | 6.15 (1.76) |
| 30/95 | 1.83 (0.63) | 3.08 (0.92) | 4.28 (0.83) |
| 60/30 | 11.97 (1.08) | 15.61 (2.76) | 28.26 (10.23) |
| 60/70 | 15.59 (2.73) | 21.01 (3.03) | 23.09 (6.95) |
| 60/95 | 2.35 (0.90) | 5.54 (2.76) | 9.27 (6.76) |
| 90/30 | 11.75 (2.05) | 22.92 (3.21) | 33.85 (7.84) |
| 90/70 | 27.03 (6.00) | 36.38 (7.11) | 46.98 (9.92) |
| 90/95 | 1.18 (9.51) | 5.56 (1.58) | 7.82 (2.18) |
| Soil Condition WHCsoil[%]/MCsoil[%WHCsoil] | Exposure Interval [Weeks] | ||
|---|---|---|---|
| 8 | 12 | 16 | |
| 30/30 | 0.80 (0.12) | 0.92 (0.22) | 1.03 (0.27) |
| 30/70 | 0.85 (0.14) | 0.66 (1.56) | 1.53 (0.65) |
| 30/95 | 0.62 (0.25) | 0.40 (0.85) | 1.50 (0.86) |
| 60/30 | 1.68 (0.53) | 2.91 (1.13) | 17.04 (12.57) |
| 60/70 | 1.69 (0.54) | 3.67 (0.66) | 5.32 (5.06) |
| 60/95 | 0.32 (0.31) | 0.36 (0.66) | 0.51 (0.37) |
| 90/30 | 1.42 (0.64) | 21.91 (8.70) | 42.09 (9.63) |
| 90/70 | 2.04 (0.51) | 4.43 (1.73) | 4.41 (1.85) |
| 90/95 | 0.53 (0.34) | 1.10 (0.57) | 0.92 (0.36) |
| Soil Conditions WHCsoil[%]/MCsoil[%WHCsoil] | Exposure Interval [Weeks] | ||
|---|---|---|---|
| 8 | 12 | 16 | |
| 30/30 | 2.98 (0.14) | 3.71(0.80) | 4.32 (0.67) |
| 30/70 | 3.66 (0.43) | 3.94 (0.73) | 5.88 (0.71) |
| 30/95 | 3.65 (0.75) | 4.52 (0.63) | 4.50 (0.68) |
| 60/30 | 6.42 (1.45) | 10.87 (2.04) | 9.29 (3.13) |
| 60/70 | 7.25 (1.31) | 13.07 (1.55) | 18.95 (2.99) |
| 60/95 | 0.65 (0.22) | 1.07 (1.05) | 1.23 (0.40) |
| 90/30 | 4.75 (0.76) | 12.14 (2.14) | 10.97 (1.37) |
| 90/70 | 7.53 (0.79) | 12.31 (1.41) | 17.69 (1.47) |
| 90/95 | 1.00 (2.15) | 1.21 (0.60) | 1.93 (0.35) |
| Soil Condition WHCsoil[%]/MCsoil[%WHCsoil] | Exposure Interval [Weeks] | ||
|---|---|---|---|
| 8 | 12 | 16 | |
| 30/30 | 1.76 (0.19) | 2.16 (0.49) | 2.10 (0.18) |
| 30/70 | 1.92 (0.29) | 2.50 (1.16) | 3.46 (0.56) |
| 30/95 | 1.86 (0.47) | 2.51 (0.42) | 3.18 (1.69) |
| 60/30 | 3.29 (0.41) | 8.56 (4.64) | 46.66 (7.76) |
| 60/70 | 4.96 (0.58) | 9.47 (0.90) | 17.81 (5.57) |
| 60/95 | 1.51 (0.61) | 1.64 (3.45) | 2.66 (0.56) |
| 90/30 | 2.60 (0.36) | 44.96 (15.16) | 58.24 (4.26) |
| 90/70 | 5.28 (1.08) | 11.21 (1.72) | 14.54 (1.33) |
| 90/95 | 1.36 (0.37) | 1.62 (0.37) | 2.44 (0.27) |
| Soil Condition WHCsoil[%]/MCsoil[%WHCsoil] | Exposure Interval [Weeks] | ||
|---|---|---|---|
| 8 | 12 | 16 | |
| 30/30 | 0.66 (0.32) | 0.87 (0.67) | 0.76 (0.16) |
| 30/70 | 1.17 (1.77) | 0.50 (0.18) | 1.78 (0.60) |
| 30/95 | 0.50 (0.24) | 0.91 (0.41) | 1.24 (0.48) |
| 60/30 | 1.88 (0.30) | 3.21 (1.24) | 25.54 (12.09) |
| 60/70 | 3.11 (1.50) | 5.62 (1.68) | 10.07 (5.62) |
| 60/95 | 0.03 (1.18) | −0.182 (0.443) | 0.20 (0.80) |
| 90/30 | 1.53 (0.98) | 31.92 (22.97) | 50.61 (14.67) |
| 90/70 | 2.33 (1.09) | 6.96 (2.33) | 9.65 (2.81) |
| 90/95 | 0.05 (0.43) | 0.18 (0.36) | 0.84 (0.54) |
| Temp. [°C] | Exposure Interval [Weeks] | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | |
| 5 | 0.41 (0.13) | 1.49 (0.34) | 3.59 (0.55) | 5.38 (0.93) | 7.79 (1.08) | 8.37 (1.06) | 8.93 (0.71) | 11.25 (1.72) |
| 10 | 0.91 (0.34) | 3.19 (0.49) | 5.79 (0.65) | 8.05 (0.89) | 9.97 (0.81) | 11.13 (0.96) | 12.62 (1.92) | 15.41 (3.25) |
| 15 | 2.20 (0.54) | 5.15 (0.49) | 9.10 (1.17) | 12.04 (1.29) | 15.37 (1.64) | 19.93 (3.31) | 20.45 (2.66) | 22.94 (2.25) |
| 20 | 3.18 (0.52) | 7.19 (1.35) | 13.20 (3.67) | 14.36 (3.07) | 16.84 (1.73) | 18.26 (2.70) | 21.23 (4.67) | 26.38 (4.73) |
| 25 | 3.28 (0.48) | 9.46 (0.94) | 15.32 (3.08) | 16.90 (2.16) | 20.07 (2.20) | 24.06 (3.54) | 28.17 (3.97) | 30.89 (5.89) |
| 30 | 4.67 (0.62) | 12.77 (2.64) | 16.76 (2.24) | 18.38 (2.26) | 23.83 (4.57) | 26.19 (5.02) | 25.85 (5.04) | 27.53 (6.49) |
| 35 | 6.52 (1.11) | 19.60 (8.40) | 21.25 (4.64) | 23.77 (5.35) | 30.82 (11.25) | 30.84 (8.17) | 34.81 (8.68) | 41.38 (13.90) |
| 40 | 8.17 (0.95) | 14.00 (1.34) | 20.22 (1.83) | 23.23 (2.80) | 25.59 (4.28) | 26.39 (1.61) | 26.95 (1.68) | 28.54 (5.37) |
| Temp. [°C] | Exposure Interval [Weeks] | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | |
| 10/20 | 1.92 (0.35) | 4.61 (0.39) | 8.02 (1.2) | 9.97 (0.6) | 12.24 (1.31) | 13.37 (1.52) | 14.86 (2.13) | 19.15 (6.64) |
| 10/30 | 2.5 (0.51) | 5.91 (0.53) | 9.48 (1.65) | 11.7 (2.29) | 14.68 (4.5) | 18.8 (4.14) | 18.74 (2.67) | 21.56 (4.72) |
| 20/30 | 4.16 (0.51) | 7.52 (0.99) | 12.24 (2.62) | 14.43 (2.23) | 16.78 (2.58) | 18.03 (1.57) | 24.43 (4.46) | 27.48 (7.78) |
| Temp. [°C] | Exposure Interval [Weeks] | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | |
| 5 | 0.32 (0.37) | 0.62 (0.32) | 0.81 (0.41) | 1.13 (0.55) | 1.46 (0.53) | 1.93 (0.60) | 1.66 (0.77) | 1.81 (0.27) |
| 10 | 0.89 (0.44) | 2.24 (0.29) | 3.16 (0.61) | 4.53 (0.49) | 5.79 (0.73) | 6.43 (0.68) | 7.07 (1.80) | 7.42 (0.95) |
| 15 | 0.87 (0.57) | 1.90 (0.42) | 1.02 (0.54) | 1.14 (0.25) | 1.73 (0.36) | 2.09 (0.43) | 2.87 (0.78) | 3.04 (1.33) |
| 20 | 1.56 (0.36) | 2.52 (0.43) | 3.75 (0.60) | 4.60 (0.85) | 5.70 (1.13) | 7.03 (1.69) | 8.73 (1.48) | 8.70 (1.02) |
| 25 | 3.60 (0.78) | 6.75 (2.13) | 8.09 (1.59) | 7.80 (1.80) | 10.85 (2.08) | 11.72 (3.09) | 13.73 (3.19) | 15.97 (1.76) |
| 30 | 3.05 (0.45) | 4.13 (0.59) | 5.06 (0.76) | 6.08 (0.70) | 7.03 (1.33) | 8.47 (1.69) | 9.95 (1.61) | 12.03 (2.14) |
| 35 | 2.08 (0.41) | 2.91 (1.10) | 3.34 (0.55) | 5.09 (1.19) | 5.87 (1.05) | 6.40 (1.12) | 6.97 (1.86) | 8.29 (2.37) |
| 40 | 6.04 (0.63) | 10.76 (1.92) | 12.53 (1.86) | 12.98 (3.00) | 13.74 (2.42) | 13.47 (1.74) | 13.09 (2.68) | 14.94 (3.61) |
| Temp. [°C] | Exposure Interval [Weeks] | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | |
| 10/20 | 1.27 (0.3) | 2.64 (0.54) | 3.72 (0.69) | 4.67 (1.09) | 6.42 (1.45) | 7.67 (1.18) | 9.61 (2.11) | 8.9 (1.78) |
| 10/30 | 1.23 (0.45) | 1.81 (0.67) | 2.27 (0.36) | 3.12 (0.49) | 3.8 (0.56) | 4.8 (0.91) | 4.39 (1.27) | 5.14 (1.37) |
| 20/30 | 1.00 (0.49) | 1.46 (0.57) | 1.93 (0.65) | 3.04 (3.43) | 3.06 (0.7) | 4.63 (1.96) | 4.54 (1.69) | 5.06 (1.68) |
References
- Ramage, M.H.; Burridge, H.; Busse-Wicher, M.; Fereday, G.; Reynolds, T.; Shah, D.U.; Wu, G.; Yu, L.; Fleming, P.; Densley-Tingley, D.; et al. The wood from the trees: The use of timber in construction. Renew. Sustain. Energy Rev. 2017, 68, 333–359. [Google Scholar] [CrossRef]
- Van Acker, J.; Palanti, S. 5.3 Durability. Europäische Zusammenarbeit auf dem Gebiet der Wissenschaftlichen und Technischen Forschung. In Performance of Bio-Based Building Materials; Brischke, C., Jones, D., Eds.; Woodhead publishing series in civil and structural engineering; WP—Woodhead Publishing, An Imprint of Elsevier: Duxford Cambridge, UK, 2017; pp. 257–277. ISBN 978-0-08-100992-5. [Google Scholar]
- Blanchette, R.A.; Held, B.W.; Jurgens, J.A.; McNew, D.L.; Harrington, T.C.; Duncan, S.M.; Farrell, R.L. Wood-Destroying Soft Rot Fungi in the Historic Expedition Huts of Antarctica. Appl. Environ. Microbiol. 2004, 70, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Zabel, R.A.; Morrell, J.J. 4 Factors Affecting the Growth and Survival of Fungi in Wood. In Wood Microbiology: Decay and Its Prevention; Elsevier Science: Saint Louis, MO, USA, 2014; pp. 90–115. ISBN 978-0-323-13946-5. [Google Scholar]
- Ritschkoff, A.-C. Decay Mechanisms of Brown-Rot Fungi; VTT Technical Research Centre of Finland: Espoo, Finland, 1996; ISBN 978-951-38-4926-9. [Google Scholar]
- Teles, C.D.M.; Do Valle, Â. Wood structures: Acting before deterioration. In Historical constructions 2001: Possibilities of Numerical and Experimental Techniques, Proceedings of the 3rd International Seminar: Gimaraes, Portugal, University of Minho, 7–9 November, 2001; International Seminar Historical Construction; Lourenço, P.B., Roca Fabregat, P., Eds.; University of Minho, Department of Civil Engineering: Gimaraes, Portugal, 2001; pp. 857–866. ISBN 978-972-8692-01-8. [Google Scholar]
- Edlund, M.-L.; Nilsson, T. Testing the durability of wood. Mat. Struct. 1998, 31, 641–647. [Google Scholar] [CrossRef]
- Brischke, C.; Olberding, S.; Meyer, L.; Bornemann, T.; Welzbacher, C.R. Intrasite variability of fungal decay on wood exposed in ground contact. Int. Wood Prod. J. 2013, 4, 37–45. [Google Scholar] [CrossRef]
- van der Wal, A.; de Boer, W.; Smant, W.; van Veen, J.A. Initial decay of woody fragments in soil is influenced by size, vertical position, nitrogen availability and soil origin. Plant Soil 2007, 301, 189–201. [Google Scholar] [CrossRef]
- Torres-Andrade, P.; Morrell, J.J.; Cappellazzi, J.; Stone, J.K. Culture-based identification to examine spatiotemporal patterns of fungal communities colonizing wood in ground contact. Mycologia 2019, 111, 703–718. [Google Scholar] [CrossRef]
- Savory, J.G.; Carey, J.K. Decay in external framed joinery in the United Kingdom. J. Inst. Wood Sci. 1979, 8, 176–180. [Google Scholar]
- Nilsson, T.; Daniel, G. Decay Types Observed in Small Stakes of Pine and Alstonia Scholaris Inserted in Different Types of Unsterile Soil; IRG/WP/1443; IRG Secretariat: Stockholm, Sweden, 1990; p. 10. [Google Scholar]
- Mieß, S. Einfluß des Wasserhaushaltes auf Abbau und Fäuletypen von Holz in Terrestrichen Mikrokosmen (Diplomarbeit). Bachelor’s Thesis, Universität Hamburg ordinariat für Holzbiologie, Hamburg, Germany, 1997. [Google Scholar]
- Baines, E.F.; Dickinson, D.J.; Levy, J.F. Testing Wood in Ground Contact: An Artificial Soil; IRG/WP/280; IRG Secretariat: Stockholm, Sweden, 1977; p. 10. [Google Scholar]
- Gersonde, M.; Kerner, W. Soft Rot Tests with Soils of Different Origins; IRG/WP/2226; IRG Secretariat: Stockholm, Sweden, 1984; p. 8. [Google Scholar]
- Carey, J.K.; Grant, C. Moisture control in laboratory tests wood-rotting fungi. Int. Biodeterior. Bull. 1975, 11, 101–105. [Google Scholar]
- Gray, S.M. Effect of Soil Type and Moisture Content on Soft Rot Testing; IRG/WP/2270; IRG Secretariat: Stockholm, Sweden, 1986; p. 26. [Google Scholar]
- Brischke, C.; Wegener, F.L. Impact of Water Holding Capacity and Moisture Content of Soil Substrates on the Moisture Content of Wood in Terrestrial Microcosms. Forests 2019, 10, 485. [Google Scholar] [CrossRef]
- CEN/TS 15083-2:2005. Durability of Wood and Wood-Based Products—Determination of the Natural Durability of Solid Wood Against Wood-Destroying Fungi, Test Methods—Part 2: Soft Rotting Micro-Fungi; European Committee for Standardization (CEN): Brussels, Belgium, 2005. [Google Scholar]
- CEN/TS 15083-1:2005. Durability of Wood and Wood-Based Products—Determination of the Natural Durability of Solid Wood against Wood-Destroying Fungi, Test Methods—Part 1: Basidiomycetes; European Committee for Standardization (CEN): Brussels, Belgium, 2005. [Google Scholar]
- EN 252:2015. Field Test Methods for Determining the Relative Protective Effectiveness of Wood Preservatives in Ground Contact; European Committee for Standardization (CEN): Brussels, Belgium, 2014. [Google Scholar]
- AWPA E7-15. Standard Field Test for Evaluation of Wood Preservatives to be Used in Ground Contact (UC4A, UC4B, UC4C); Stake Test; American Wood Protection Association (AWPA/WOOD): Hoover, AL, USA, 2015. [Google Scholar]
- Wells, J.M.; Boddy, L. Effect of temperature on wood decay and translocation of soil-derived phosphorus in mycelial cord systems. New Phytol 1995, 129, 289–297. [Google Scholar] [CrossRef]
- Risch, A.C.; Jurgensen, M.F.; Page-Dumroese, D.S.; Schütz, M. Initial turnover rates of two standard wood substrates following land-use change in subalpine ecosystems in the Swiss Alps. Can. J. For. Res. 2013, 43, 901–910. [Google Scholar] [CrossRef]
- Finér, L.; Jurgensen, M.; Palviainen, M.; Piirainen, S.; Page-Dumroese, D. Does clear-cut harvesting accelerate initial wood decomposition? A five-year study with standard wood material. For. Ecol. Manag. 2016, 372, 10–18. [Google Scholar] [CrossRef]
- Wang, C.; Leicester, R.H.; Nguyen, M.N. Manual 3—Decay in Ground Contact; Timber Service Life Design Guide; CSIRO: Victoria, Australia, 2008.
- Lacasse, M.A. Advances in service life prediction—An overview of durability and methods of service life prediction for non-structural building components. In Proceedings of the Annual Australasian Corrosion Association Conference, Wellington Convention Centre, Wellington, New Zealand, 16–19 November 2008; pp. 1–13. [Google Scholar]
- Brischke, C.; Rapp, A.O. Dose–response relationships between wood moisture content, wood temperature and fungal decay determined for 23 European field test sites. Wood Sci. Technol. 2008, 42, 507–518. [Google Scholar] [CrossRef]
- ISO 11268-2. Soil Quality—Effects of Pollutants on Earthworms—Part 2: Determination of Effects on Reproduction of Eisenia Fetida/Eisenia Andrei; International Organisation for Standardization (ISO): Geneva, Switzerland, 2012. [Google Scholar]
- EN 350:2016. Durability of Wood and Wood-Based Products–Testing and Classification of the Durability to Biological Agents of Wood and Wood-Based Materials; European Committee for Standardization (CEN): Brussels, Belgium, 2016. [Google Scholar]
- Weil, R.R.; Brady, N.C. 5 Soil Water: Characteristics and Behaviour. In The Nature and Properties of Soils; Pearson: Columbus, OH, USA, 2016; pp. 188–232. ISBN 978-0-13-325448-8. [Google Scholar]
- Zabel, R.A.; Morrell, J.J. 3 Characteristics and classification of Fungi and Bacteria. In Wood Microbiology: Decay and Its Prevention.; Elsevier Science: Saint Louis, MO, USA, 2014; pp. 52–89. ISBN 978-0-323-13946-5. [Google Scholar]
- Jurgensen, M.; Laks, P.; Reed, D.; Collins, A. Chemical, Physical and Biological Factors Affecting Wood Decomposition in Forest Soils; IRG/WP 03-20281; IRG Secretariat: Stockholm, Sweden, 2003; p. 15. [Google Scholar]
- Edlund, M.-L. Durability of Some Alternatives to Preservative Treated Wood; IRG/WP 04-30353; IRG Secretariat: Stockholm, Sweden, 2004; p. 13. [Google Scholar]
- Wakeling, R. Is Field Test Data from 20 x 20mm Stakes Reliable? Effects of Decay Hazard, Decay Type and Preservative Depletion Hazard; IRG/WP 06-20327; IRG Secretariat: Stockholm, Sweden, 2006. [Google Scholar]
- Schultz, T.P.; Nicholas, D.D. Technical note: Effect of soil on the pH of treated wood in ground contact. Wood Fiber Sci. 2010, 42, 412–416. [Google Scholar]
- Larsson-Brelid, P.; Brischke, C.; Rapp, A.O.; Hansson, M.; Westin, M.; Jermer, J.; Pilgård, A. Methods of Field Data Evaluation—Time Versus Reliability; IRG/WP 11-20466; IRG Secretariat: Stockholm, Sweden, 2011; p. 18. [Google Scholar]
- Stirling, R.; Alfredsen, G.; Brischke, C.; De Windt, I.; Francis, L.P.; Frühwald Hansson, E.; Humar, M.; Jermer, J.; Klamer, M.; Laks, P.E.; et al. Global Survey on Durability Variation–on the Effect of the Reference Species; IRG Secretariat: Stockholm, Sweden, 2016; p. 26. [Google Scholar]
- Krzic, M.; Sanborn, P.; Watson, K.; Bomke, A.A.; Crowley, C.; Doree, A.; Dyanatkar, S. Soil Formation and Soil Processes; The University of British Columbia, Vancouver, University of Northern British Columbia, and Thompson Rivers University: Kamloops, BC, Canada, 2008; Available online: https://processes.soilweb.ca/ (accessed on 9 October 2020).
- Blanchette, R.A.; Nilsson, T.; Daniel, G.; Abad, A. 6 Biological Degradation of Wood. In Archaeological Wood; Rowell, R.M., Barbour, R.J., Eds.; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1989; Volume 225, pp. 141–174. ISBN 978-0-8412-1623-5. [Google Scholar]
- Elam, J.; Björdal, C. A review and case studies of factors affecting the stability of wooden foundation piles in urban environments exposed to construction work. Int. Biodeterior. Biodegrad. 2020, 148, 104913. [Google Scholar] [CrossRef]
- A’Bear, A.D.; Jones, T.H.; Kandeler, E.; Boddy, L. Interactive effects of temperature and soil moisture on fungal-mediated wood decomposition and extracellular enzyme activity. Soil Biol. Biochem. 2014, 70, 151–158. [Google Scholar] [CrossRef]
- Donnelly, P.K.; Entry, J.A.; Crawford, D.L.; Cromack, K. Cellulose and lignin degradation in forest soils: Response to moisture, temperature, and acidity. Microb Ecol 1990, 20, 289–295. [Google Scholar] [CrossRef]
- Donnelly, D.P.; Boddy, L. Development of mycelial systems of Stropharia caerulea and Phanerochaete velutina on soil: Effect of temperature and water potential. Mycol. Res. 1997, 101, 705–713. [Google Scholar] [CrossRef]
- Eaton, R.A.; Hale, M.D.C. Wood: Decay, Pests, and Protection, 1st ed.; Chapman & Hall: London, UK, 1993; ISBN 978-0-412-53120-0. [Google Scholar]
- Hiscox, J.; Clarkson, G.; Savoury, M.; Powell, G.; Savva, I.; Lloyd, M.; Shipcott, J.; Choimes, A.; Amargant Cumbriu, X.; Boddy, L. Effects of pre-colonisation and temperature on interspecific fungal interactions in wood. Fungal Ecol. 2016, 21, 32–42. [Google Scholar] [CrossRef]
- Schmidt, O. 3 Physiology. In Wood and Tree Fungi: Biology, Damage, Protection, and Use; Czeschlik, D., Ed.; Springer: Berlin, Germany, 2006; pp. 53–85. ISBN 978-3-540-32138-5. [Google Scholar]
- Marais, B.N.; Brischke, C.; Militz, H. Wood durability in terrestrial and aquatic environments–A review of biotic and abiotic influence factors. Wood Mater. Sci. Eng. 2020, 1–24. [Google Scholar] [CrossRef]
- Morton, L.H.G.; Eggins, H.O.W. The effect of constant, alternating & fluctuating temperatures on the growth on some wood inhabiting fungi. Int. Biodeterior. Bull. 1977, 13, 116–122. [Google Scholar]
- Morton, L.H.G. Ecological studies of fungi growing on insolated wood. Ph.D. Thesis, University of Aston, Birmingham, UK, 1975. [Google Scholar]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Brischke, C.; Bayerbach, R.; Rapp, A.O. Decay-influencing factors: A basis for service life prediction of wood and wood-based products. Wood Mater. Sci. Eng. 2006, 1, 91–107. [Google Scholar] [CrossRef]
- Scheffer, T.C. A Climate Index for Estimating Potential for Decay in Wood Structures Above Ground. For. Prod. J. 1971, 21, 25–31. [Google Scholar]
| Resultant WHCsoil [%] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (A): Equation (4) | 104 | 95 | 88 | 81 | 74 | 67 | 60 | 53 | 45 | 37 | 30 |
| (B): Equation (5) | 102 | 99 | 94 | 88 | 81 | 74 | 66 | 58 | 49 | 40 | 30 |
| Percentage compost [%] | 100 | 90 | 80 | 70 | 60 | 50 | 40 | 30 | 20 | 10 | 0 |
| Percentage sand [%] | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
| Study | WHCsoil [%] | MCsoil [%WHCsoil] | MCsoil,target [%] |
|---|---|---|---|
| (A) | 30 | 30 | 9 |
| (A) | 30 | 70 | 21 |
| (A) | 30 | 95 | 29 |
| (A) | 60 | 30 | 18 |
| (A) | 60 | 70 | 42 |
| (A) | 60 | 95 | 57 |
| (A) | 90 | 30 | 27 |
| (A) | 90 | 70 | 63 |
| (A) | 90 | 95 | 86 |
| (B) | 60 | 60 | 36 |
| (B) | 60 | 90 | 54 |
| MLwood [%] per Soil Condition WHCsoil[%]/MCsoil[%WHCsoil] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | 30/30 | 30/70 | 30/95 | 60/30 | 60/70 | 60/95 | 90/30 | 90/70 | 90/95 | MLwood* [%] |
| Beech | 5.45 | 6.15 | 4.28 | 28.26 | 23.09 | 9.27 | 33.85 | 46.98 | 7.82 | 18.35 |
| D-fir | 1.03 | 1.53 | 1.50 | 17.04 | 5.32 | 0.51 | 42.09 | 4.41 | 0.92 | 8.26 |
| Oak | 4.32 | 5.88 | 4.50 | 9.29 | 18.95 | 1.23 | 10.97 | 17.69 | 1.93 | 8.31 |
| Spruce | 0.76 | 1.78 | 1.24 | 25.54 | 10.07 | 0.20 | 50.61 | 9.65 | 0.84 | 11.29 |
| Pine | 2.10 | 3.46 | 3.18 | 46.66 | 17.81 | 2.66 | 58.24 | 14.54 | 2.44 | 16.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marais, B.N.; Brischke, C.; Militz, H.; Peters, J.H.; Reinhardt, L. Studies into Fungal Decay of Wood In Ground Contact—Part 1: The Influence of Water-Holding Capacity, Moisture Content, and Temperature of Soil Substrates on Fungal Decay of Selected Timbers. Forests 2020, 11, 1284. https://doi.org/10.3390/f11121284
Marais BN, Brischke C, Militz H, Peters JH, Reinhardt L. Studies into Fungal Decay of Wood In Ground Contact—Part 1: The Influence of Water-Holding Capacity, Moisture Content, and Temperature of Soil Substrates on Fungal Decay of Selected Timbers. Forests. 2020; 11(12):1284. https://doi.org/10.3390/f11121284
Chicago/Turabian StyleMarais, Brendan Nicholas, Christian Brischke, Holger Militz, Johann Hinrich Peters, and Lena Reinhardt. 2020. "Studies into Fungal Decay of Wood In Ground Contact—Part 1: The Influence of Water-Holding Capacity, Moisture Content, and Temperature of Soil Substrates on Fungal Decay of Selected Timbers" Forests 11, no. 12: 1284. https://doi.org/10.3390/f11121284
APA StyleMarais, B. N., Brischke, C., Militz, H., Peters, J. H., & Reinhardt, L. (2020). Studies into Fungal Decay of Wood In Ground Contact—Part 1: The Influence of Water-Holding Capacity, Moisture Content, and Temperature of Soil Substrates on Fungal Decay of Selected Timbers. Forests, 11(12), 1284. https://doi.org/10.3390/f11121284






