An Assessment of the Spatial Variability of Tropical Swamp Forest along a 300 km Long Transect in the Usumacinta River Basin, Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Analysis
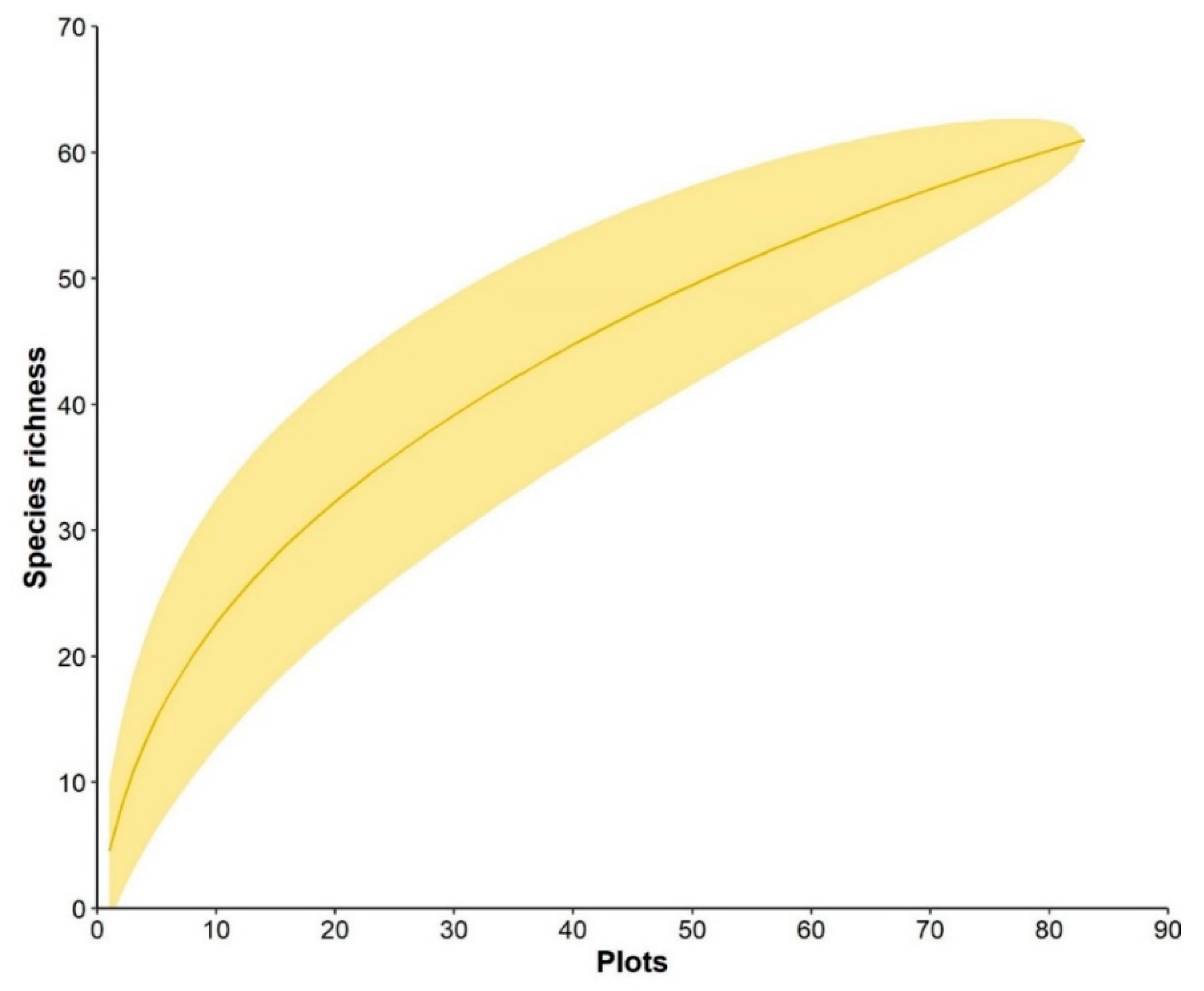
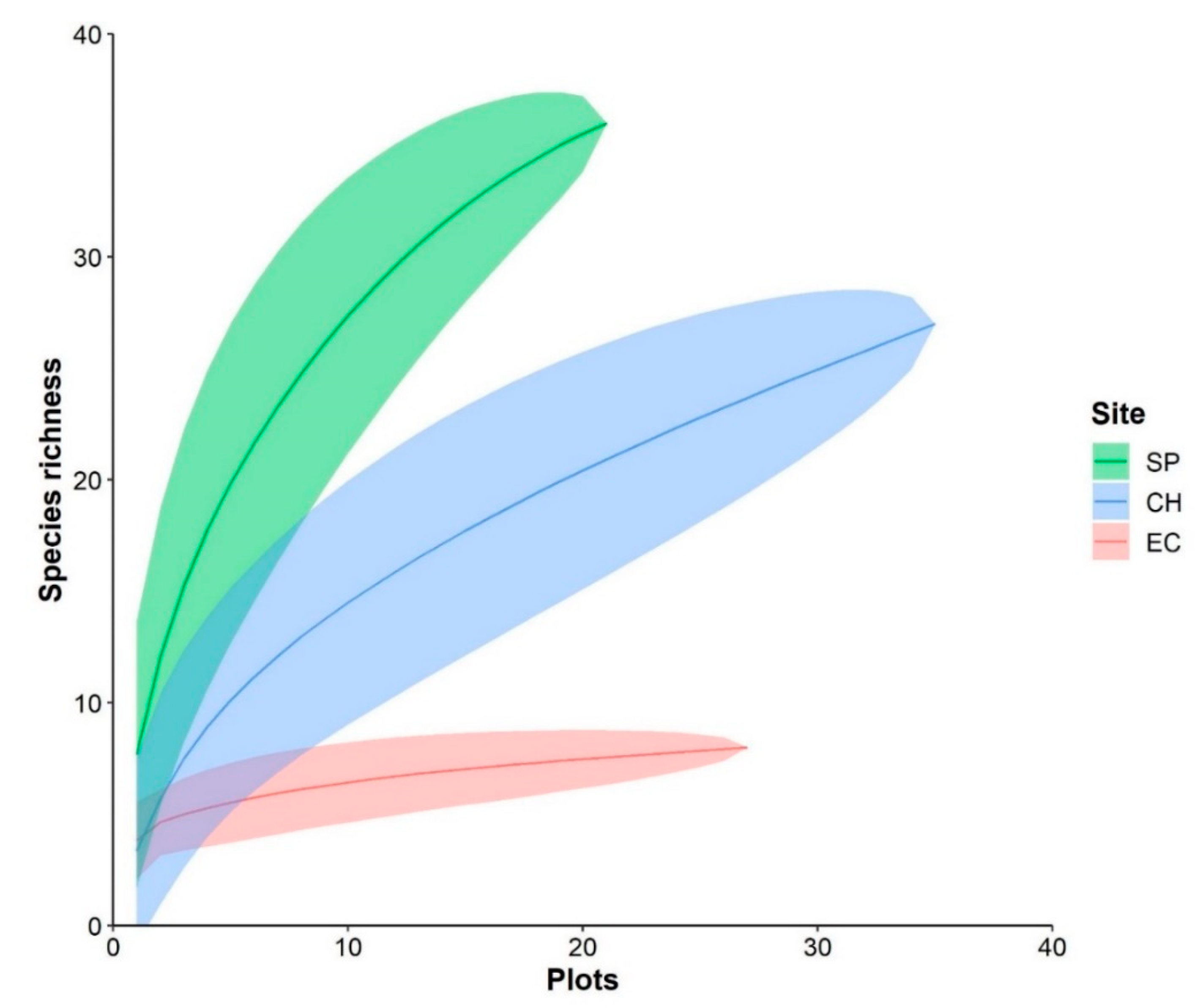
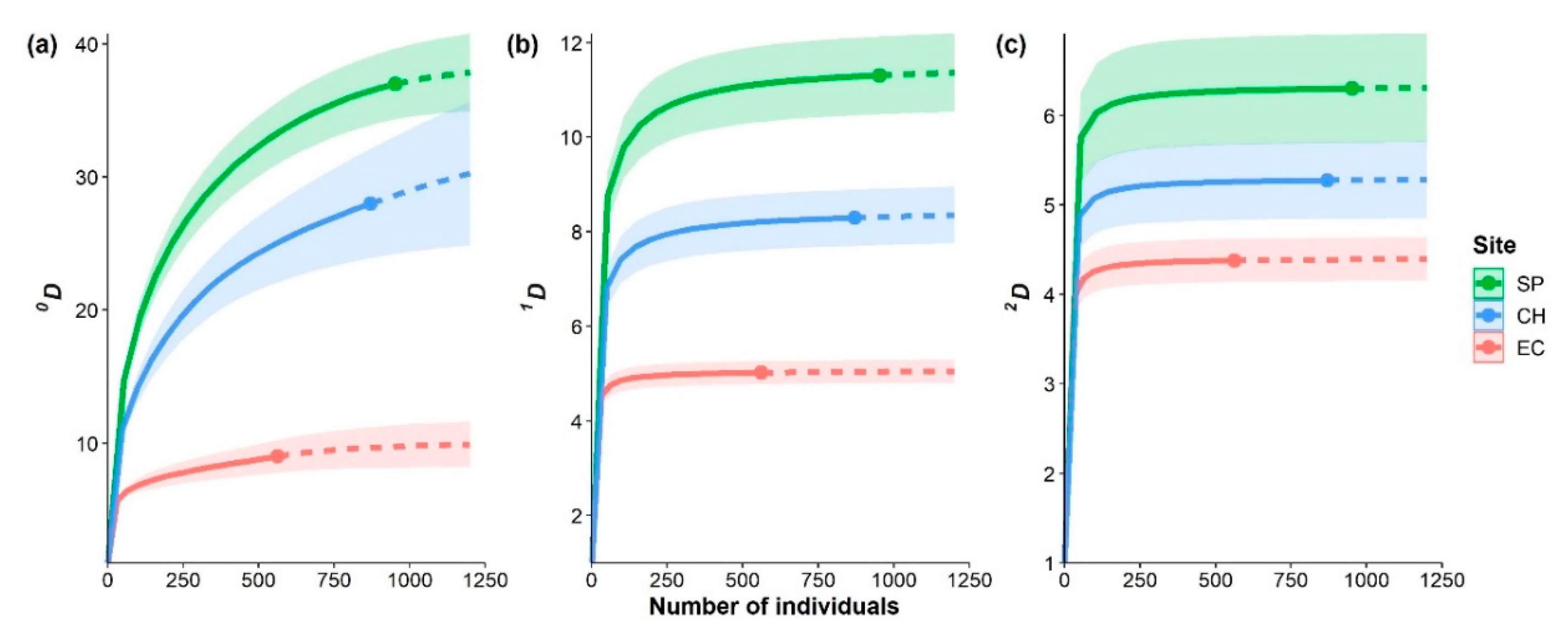
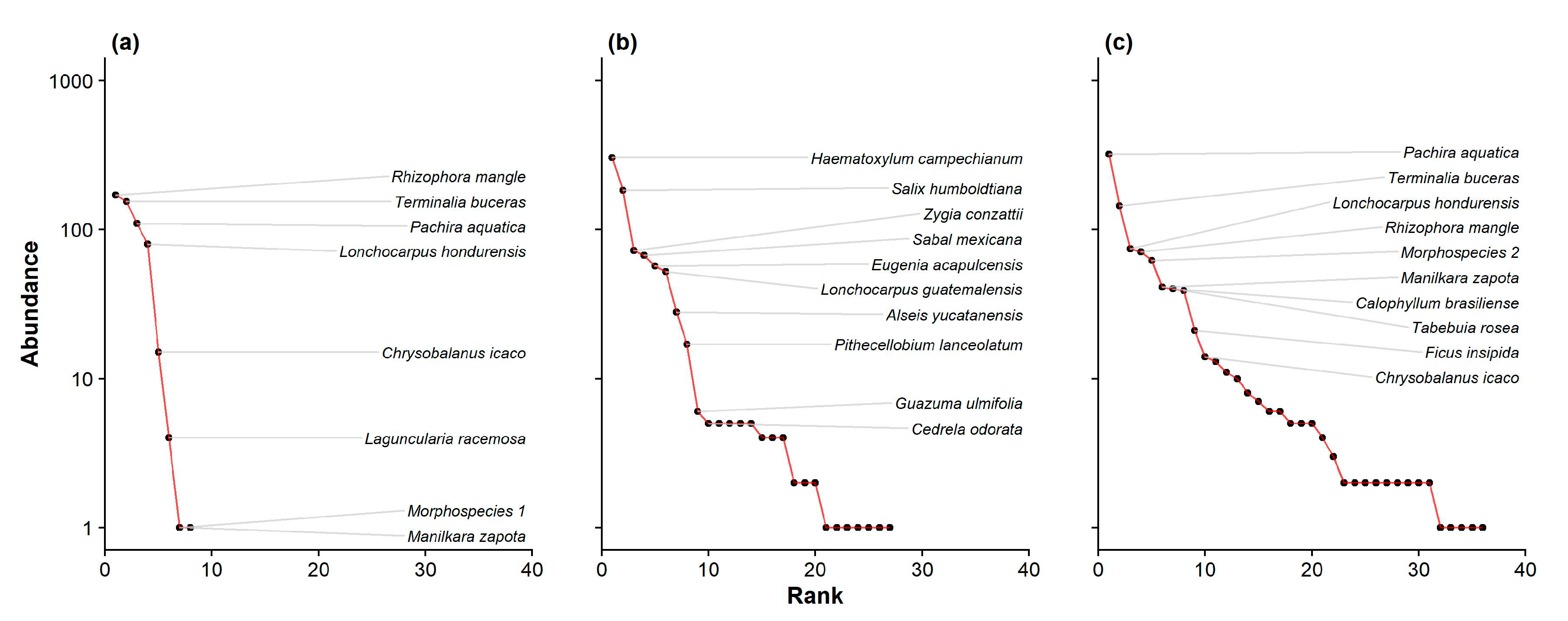
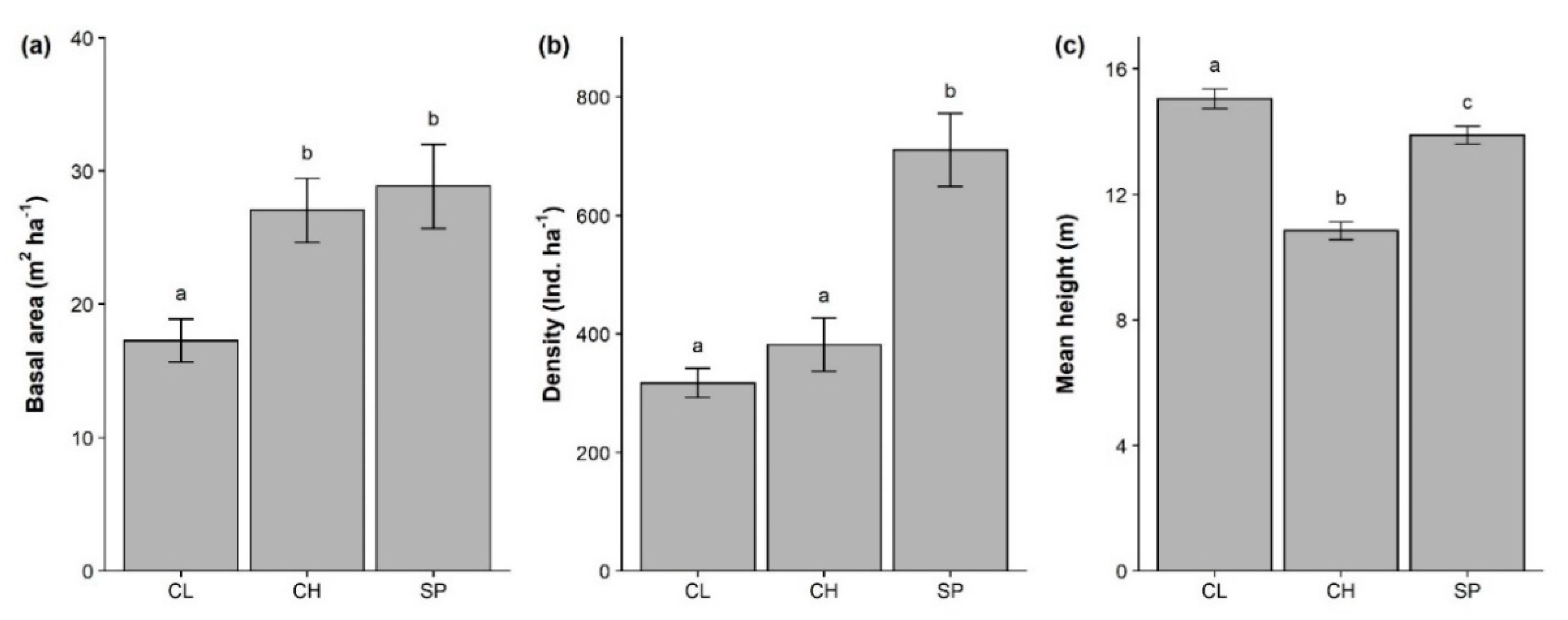
3. Results
3.1. Overall Community Characterization
3.2. Diversity and Dominance
3.3. Forest Structure
4. Discussion
4.1. Diversity and Dominance
4.2. Community Structure
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bruland, G.L. Coastal wetlands: Function and role in reducing impact of land-based management. In Coastal Watershed Management; Fares, A., El-Kadi, A.I., Eds.; WIT Press: Southampton, UK, 2008; pp. 1–40. [Google Scholar]
- Flores-Verdugo, F.J.; Moreno-Casasola, P.; Lanza-Espino, G.; Agraz-Hernández, C. El manglar, otros humedales costeros y el cambio climático. In Vulnerabilidad de las Zonas Costeras Mexicanas Ante el Cambio Climático; Botello, A.V., Villanueva-Fragoso, S., Gutiérrez, J., Rojas-Galaviz, J.L., Eds.; SEMARNAT-INE-Universidad Nacional Autónoma de México-Universidad Autónoma de Campeche: Campeche, Mexico, 2010; pp. 165–188. [Google Scholar]
- Piadade, M.T.F.; Ferreira, C.S.; Franco, A.C. Estrategias reproductivas de la vegetación y sus respuestas al pulso de la inundación en las zonas inundables de la Amazonía Central. Ecosistemas 2010, 19, 52–66. [Google Scholar]
- Davidson, N.C. How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar. Freshw. Res. 2014, 65, 934–941. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Taillardat, P.; Clendenning, J.N.; Cameron, C.; Friess, D.A.; Murdiyarso, D.; Hutley, L.B. Effect of land-use and land-cover change on mangrove blue carbon: A systematic review. Glob. Chang. Biol. 2019, 25, 4291–4302. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Casasola, P.; Infante-Mata, D.; López-Rosas, H. Tropical freshwater marshes and swamps of North America. In Wetland habitats of North America: Ecology and Conservation Concerns; Batzer, D., Ed.; University of California Press: Berkeley, CA, USA, 2012; pp. 267–282. [Google Scholar]
- López-Portillo, J.; Ezcurra, E. Los manglares de México: Una revisión. Madera Bosques 2002, 8, 27–51. [Google Scholar] [CrossRef]
- Duke, N.C.; Ball, M.C.; Ellison, J.C. Factors influencing biodiversity and distributional gradients in mangroves. Glob. Ecol. Biogeogr. Lett. 1998, 7, 27–47. [Google Scholar] [CrossRef]
- Campo, J.T.F.; Olvera-Vargas, M.; Figueroa-Rangel, B.L.; Cuevas-Guzmán, R.; Iñiguez-Dávalos, L.I. Patterns of spatial diversity and structure of mangrove vegetation in Pacific West-Central Mexico. Wetlands 2018, 38, 919–931. [Google Scholar]
- Schaeffer-Novelli, Y.; Cintrón-Molero, G.; Adamie, R.R.; Camargo, T.M. Variability of mangrove ecosystems along the Brazilian coast. Estuaries 1990, 13, 204–218. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Latham, R.E. Global patterns of diversity in mangrove floras. In Species Diversity in Ecological Communities: Historical and Geographical Perspectives; Ricklefs, R.E., Schluter, D., Eds.; University of Chicago Press: Chicago, IL, USA, 1993; pp. 215–229. [Google Scholar]
- Murray, M.R.; Zisman, S.A.; Furley, P.A.; Munro, D.M.; Gibson, J.; Ratter, J.; Bridgewater, S.; Minty, C.D.; Place, C.J. The mangroves of Belize Part 1. distribution, composition and classification. For. Ecol. Manag. 2003, 174, 265–279. [Google Scholar] [CrossRef]
- Agraz-Hernández, C.M.; García-Zaragoza, C.; Iriarte-Vivar, S.; Flores-Verdugo, F.J.; Moreno-Casasola, P. Forest structure, productivity and species phenology of mangroves in the La Mancha lagoon in the Atlantic coast of Mexico. Wetl. Ecol. Manag. 2011, 19, 273–293. [Google Scholar] [CrossRef]
- Duque-Estrada, G.C.; Gómez-Soares, M.L.; Oliveira-Chavez, F.; Fernández-Cavalcanti, V. Analysis of the structural variability of mangrove forests through the physiographic types approach. Aquat. Bot. 2013, 111, 135–143. [Google Scholar] [CrossRef]
- Costa, P.; Dórea, A.; Mariano-Neto, E.; Barros, F. Are there general spatial patterns of mangrove structure and composition along estuarine salinity gradients in Todos os Santos Bay? Estuar. Coast. Shelf Sci. 2015, 166, 83–91. [Google Scholar] [CrossRef]
- Gomes-Soares, M.L.; Oliveira-Chaves, F.; Duque-Estrada, G.D.; Fernandez, V. Mangrove forests associated with salt flats: A case study from southeast Brazil. Braz. J. Oceanogr. 2017, 65, 102–115. [Google Scholar] [CrossRef]
- Nguyen, L.T.M.; Hoang, H.T.; Ta, H.V.; Park, P.S. Comparison of mangrove stand development on accretion and erosion sites in Ca Mau, Vietnam. Forests 2020, 11, 615. [Google Scholar] [CrossRef]
- Mifsut, I.M.; Castro, M. La cuenca del río Usumacinta: Perfil y perspectivas para su conservación y desarrollo sustentable. In Las Cuencas Hidrográficas de México, Diagnóstico y Priorización; Cotler-Ávalos, H., Ed.; SEMARNAT: México City, Mexico, 2010; pp. 193–197. [Google Scholar]
- Carabias, J.; Zorrilla, M.; Escobedo, A.H.; Gallardo, A.; Rodríguez, Y.; Fernández, A.I.; Charruau, P.; Martínez, M.; Rodríguez, A. Diagnóstico Integral de la Cuenca Baja del Río Usumacinta en Tabasco; Centro del Cambio Global y La Sustentabilidad A.C.: Villahermosa, Mexico, 2015. [Google Scholar]
- Kolb, M.; Galicia, L. Challenging the linear forestation narrative in the Neo-tropic: Regional patterns and processes of deforestation and regeneration in southern Mexico. Geogr. J. 2011, 178, 147–161. [Google Scholar] [CrossRef]
- Thom, B.G. Mangrove ecology and deltaic geomorphology: Tabasco, Mexico. J. Ecol. 1967, 55, 301–343. [Google Scholar] [CrossRef]
- Chávez, D.; Gallardo-Cruz, J.A.; Solórzano, J.V.; Peralta-Carreta, C.; Enríquez, M.; Meave, J.A. Spatial correlates of floristic and structural variation in a Neotropical wetland forest. Wetl. Ecol. Manag. 2020, 28, 341–356. [Google Scholar] [CrossRef]
- Solórzano, J.V.; Gallardo-Cruz, J.A.; Peralta-Carreta, C.; Martínez-Camilo, R.; de Oca, A.F.M. Plant community composition patterns in relation to microtopography and distance to water bodies in a tropical forested wetland. Aquat. Bot. 2020, 167, 103295. [Google Scholar] [CrossRef]
- Barba-Macías, E.; Alva-Juárez, M.A.; Calva-Benítez, L.G. Guía Ilustrada para la Identificación de Plantas Acuáticas en Humedales de Tabasco; El Colegio de la Frontera Sur: Villahermosa, Mexico, 2013. [Google Scholar]
- De la Maza, J.; Carabias, J. Usumacinta: Bases para una Política de Sustentabilidad Ambiental; Instituto Mexicano de Tecnología del Agua, Natura-Ecosistemas Mexicanos A.C.: Mexico City, Mexico, 2011.
- Lot, A.; Novelo, A. El pantano de Tabasco y Campeche: La reserva más importante de plantas acuáticas de Mesoamérica. In Memorias del Simposio Internacional Sobre Ecología y Conservación del Delta de los Ríos Usumacinta y Grijalva; Instituto Nacional de Investigaciones sobre Recursos Bióticos-Gobierno del Estado de Tabasco: Villahermosa, Mexico, 1988; pp. 537–547. [Google Scholar]
- Méndez-H, C.A.; Flores, M.C.; Calderón, C.; Hernández, S. Inventario Nacional de los Humedales de Guatemala; UICN Mesoamérica-Consejo Nacional de Áreas Protegidas-Universidad de San Carlos-Corredor Biológico Mesoamericano: San José, Costa Rica, 2001. [Google Scholar]
- Novelo, A.; Ramos, L. Vegetación acuática. In Biodiversidad del Estado de Tabasco; Bueno, J., Álvarez, F., Santiago, S., Eds.; Universidad Nacional Autónoma de México-Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2005; pp. 111–144. [Google Scholar]
- Kauffman, J.B.; Hernandez-Trejo, H.; Jesus-García, M.C.; Heider, C.; Contreras, W.M. Carbon stocks of mangroves and losses arising from their conversion to cattle pastures in the Pantanos de Centla, Mexico. Wetl. Ecol. Manag. 2016, 24, 203–216. [Google Scholar] [CrossRef]
- Esqueda-Lara, K.; Sánchez, A.J.; Valdés-Lagunes, G.; Salcedo, M.A.; Franco-Torres, A.E.; Florido, R. Fitoplancton en el humedal tropical Chaschoc en la cuenca baja del río Usumacinta. Rev. Mex. Biodivers. 2016, 87, 1177–1188. [Google Scholar] [CrossRef]
- West, R.C. The natural vegetation of the Tabascan Lowlands, Mexico. Rev. Geogr. 1966, 64, 108–122. [Google Scholar]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Salas-Morales, S.H.; González, E.J.; Meave, J.A. Canopy height variation and environmental heterogeneity in the tropical dry forests of coastal Oaxaca, Mexico. Biotropica 2018, 50, 26–38. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing 2019; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Infante-Mata, D.; Moreno-Casasola, P.; Madero-Vega, C. Pachira aquatica, ¿un indicador del límite del manglar? Rev. Mex. Biodivers. 2014, 85, 143–160. [Google Scholar] [CrossRef]
- Castillo-Elías, B.; Gervacio-Jiménez, H.; Bedolla-Solano, R. Estructura forestal de una zona de manglar en la laguna de Coyuca de Benítez, Guerrero. Rev. Mex. Cienc. For. 2018, 9, 66–93. [Google Scholar] [CrossRef][Green Version]
- Duke, N.C. Mangrove floristics and biogeography. In Coastal and Stuarine Studies Series; Robertson, A.I., Alongi, D.M., Eds.; American Geophysical Union: Washington, DC, USA, 1992; pp. 63–100. [Google Scholar]
- Torres, J.R.; Barba, E.; Choix, F.J. Mangrove productivity and phenology in relation to hydroperiod and physical–chemistry properties of water and sediment in Biosphere Reserve; Centla Wetland, Mexico. Trop. Conserv. Sci. 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Secretaría de Medio Ambiente, Recursos Naturales y Pesca [SEMARNAP]. Programa de Manejo de la Reserva de la Biosfera Pantanos de Centla; Instituto Nacional de Ecología: Mexico City, Mexico, 2000.
- Infante-Mata, D.; Moreno-Casasola, P.; Madero-Vega, C.; Castillo-Campos, G.; Warner, B.G. Floristic composition and soil characteristics of tropical freshwater forested wetlands of Veracruz on the coastal plain of the Gulf of Mexico. For. Ecol. Manag. 2011, 262, 1514–1531. [Google Scholar] [CrossRef]
- Spalding, M.D.; Blasco, F.; Field, C.D. World Mangrove Atlas; The International Society for Mangrove Ecosystems: Okinawa, Japan, 1997. [Google Scholar]
- US Fish and Wildlife Service. South Florida Multi-Species Recovery Plan; U.S. Fish and Wildlife Service: Atlanta, GA, USA, 1999.
- Yáñez-Arancibia, A.; Lara-Domínguez, A.L. Ecosistemas de Manglar en América Tropical; Instituto de Ecología A.C.: Xalapa, México, 1999. [Google Scholar]
- Burnham, R.J. Relationships between standing vegetation and leaf litter in a paratropical forest: Implications for paleobotany. Rev. Palaeobot. Palynol. 1989, 58, 5–32. [Google Scholar] [CrossRef]
- Comisión Nacional para el Conocimiento y Uso de la Biodiversidad [CONABIO]. Manglares de México: Extensión y Distribución; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2009.
- Estrada-Loreto, F. Indicadores Ecológicos de la Zona Riparia del San Río Pedro, Tabasco, México. Master’s Thesis, El Colegio de La Frontera Sur, Villahermosa, Mexico, 2011. [Google Scholar]
- González-Zamorano, P.; Lluch-Cota, S.E.; Nava-Sánchez, E.H. Relation between the structure of mangrove forests and geomorphic types of lagoons of the Baja California Peninsula. J. Coast. Res. 2013, 29, 173–181. [Google Scholar]
- Tovilla-Hernández, C.; Romero-Berny, E. Diagnóstico estructural de los manglares de Chiapas y Oaxaca. In Recursos Acuáticos Costeros del Sureste; Sánchez, A.J., Chiappa-Carrara, X., Brito-Pérez, R., Eds.; Universidad Nacional Autónoma de México: Merida, Mexico, 2012; Volume 1, pp. 257–279. [Google Scholar]
- Romero-Berny, E.I.; Tovilla-Hernández, C.; Torrescano-Valle, N.; Schmook, B. Heterogeneidad estructural del manglar como respuesta a factores ambientales y antrópicos en el Soconusco, Chiapas, México. Polibotánica 2019, 47, 39–58. [Google Scholar] [CrossRef]
- Ramírez-García, P.; Lot, A. La distribución del manglar y de los pastos marinos en el Golfo de California, México. An. Inst. Biol. Univ. Nac. Auton. Mex. Bot. 1994, 65, 63–72. [Google Scholar]
- Pacheco-Ruiz, I.; Zertuche-González, J.A.; Meling-López, A.E.; Riosmena-Rodríguez, R.; Orduña-Rojas, J. El límite norte de Rhizophora mangle L. en el golfo de California, México. Cienc. Mar. 2006, 28, 19–22. [Google Scholar]

| Family | Species | Site | ||
|---|---|---|---|---|
| EC | CH | SP | ||
| Anacardiaceae | Metopium brownei (Jacq.) Urb. | 2 | ||
| Spondias mombin L. | 5 | |||
| Annonaceae | Annona reticulata L. | 2 | ||
| Apocynaceae | Tabernaemontana longipes Donn.Sm. | 1 | ||
| Arecaceae | Attalea butyracea (Mutis ex L.f.) Wess.Boer | 1 | ||
| Sabal mexicana Mart. | 67 | |||
| Bignoniaceae | Crescentia cujete L. | 2 | ||
| Tabebuia rosea (Bertol.) Bertero ex A.DC. | 2 | 39 | ||
| Burseraceae | Bursera sp. | 6 | ||
| Capparaceae | Crateva tapia L. | 1 | ||
| Chrysobalanaceae | Chrysobalanus icaco L. | 15 | 14 | |
| Couepia polyandra (Kunth) Rose | 1 | |||
| Clusiaceae | Calophyllum brasiliense Cambess. | 40 | ||
| Combretaceae | Terminalia buceras (L.) C.Wright | 154 | 1 | 144 |
| Laguncularia racemosa (L.) C.F.Gaertn. | 4 | |||
| Fabaceae | Acacia cornigera (L.) Willd. | 2 | ||
| Albizia lebbeck (L.) Benth. | 1 | |||
| Cynometra retusa Britton & Rose | 4 | |||
| Enterolobium cyclocarpum (Jacq.) Griseb. | 4 | |||
| Haematoxylum campechianum L. | 303 | 13 | ||
| Inga vera Willd. | 2 | |||
| Lonchocarpus guatemalensis Benth. | 52 | |||
| Lonchocarpus hondurensis Benth. | 80 | 74 | ||
| Lonchocarpus sp. | 6 | |||
| Pithecellobium lanceolatum (Willd.) Benth. | 17 | |||
| Swartzia cubensis (Britton & Wilson) Standl. | 2 | |||
| Zygia conzattii (Standl.) Britton & Rose | 72 | |||
| Zygia recordii Britton & Rose | 8 | |||
| Fagaceae | Quercus oleoides Schltdl. & Cham. | 5 | ||
| Malpighiaceae | Malpighia sp1. | 5 | ||
| Malvaceae | Guazuma ulmifolia Lam. | 6 | ||
| Pachira aquatica Aubl. | 110 | 320 | ||
| Meliaceae | Cedrela odorata L. | 5 | ||
| Trichilia havanensis Jacq. | 2 | |||
| Moraceae | Ficus insipida Willd. | 21 | ||
| Myrtaceae | Eugenia acapulcensis Steud. | 57 | ||
| Myrtaceae sp1. | 2 | |||
| Polygonaceae | Coccoloba barbadensis Jacq. | 5 | 11 | |
| Primulaceae | Bonellia macrocarpa (Cav.) B.Ståhl & Källersjö | 4 | ||
| Rhizophoraceae | Rhizophora mangle L. | 170 | 71 | |
| Rubiaceae | Alseis yucatanensis Standl. | 28 | ||
| Salicaceae | Salix humboldtiana Willd. | 183 | ||
| Xylosma sp. | 1 | |||
| Sapindaceae | Sapindus saponaria L. | 5 | ||
| Sapotaceae | Manilkara zapota (L.) P.Royen | 1 | 41 | |
| Solanaceae | Cestrum nocturnum L. | 1 | ||
| Not determined | Morphospecies 1 | 1 | ||
| Morphospecies 2 | 62 | |||
| Morphospecies 3 | 10 | |||
| Morphospecies 4 | 4 | |||
| Morphospecies 5 | 5 | |||
| Morphospecies 6 | 5 | |||
| Morphospecies 7 | 2 | |||
| Morphospecies 8 | 3 | |||
| Morphospecies 9 | 1 | |||
| Morphospecies 10 | 2 | |||
| Morphospecies 11 | 2 | |||
| Morphospecies 12 | 2 | |||
| Morphospecies 13 | 1 | |||
| Morphospecies 14 | 1 | |||
| Morphospecies 15 | 1 | |||
| Site | Plots | Abundance | Families | Species |
|---|---|---|---|---|
| El Cometa Lagoon | 27 | 535 | 6 | 8 |
| Chaschoc Lagoon complex | 35 | 835 | 19 | 27 |
| San Pedro River | 21 | 932 | 19 | 35 |
| Site | Species | Basal Area (m2 ha−1) | Density (Ind. ha−1) | Height (m) |
|---|---|---|---|---|
| El Cometa Lagoon | Rhizophora mangle | 210.58 | 100.74 | 17.42 |
| Terminalia buceras | 200.97 | 91.26 | 12.66 | |
| Lonchocarpus hondurensis | 26.06 | 47.41 | 8.19 | |
| Pachira aquatica | 21.39 | 65.19 | 5.76 | |
| Chrysobalanus icaco | 5.02 | 8.89 | 5.96 | |
| Laguncularia racemosa | 1.84 | 2.37 | 10.32 | |
| Morphospecies 1 | 0.25 | 0.59 | 6.47 | |
| Manilkara zapota | 0.14 | 0.59 | 6.19 | |
| Chaschoc Lagoon complex | Haematoxylum campechianum | 420.42 | 138.51 | 11.13 |
| Sabal mexicana | 122.07 | 30.63 | 8.48 | |
| Lonchocarpus guatemalensis | 79.12 | 23.77 | 12.15 | |
| Salix humboldtiana | 56.68 | 83.66 | 9.79 | |
| Alseis yucatanensis | 47.62 | 12.80 | 11.36 | |
| Enterolobium cyclocarpum | 40.65 | 1.83 | 22.00 | |
| Eugenia acapulcensis | 40.05 | 26.06 | 10.68 | |
| Zygia conzattii | 38.19 | 32.91 | 8.69 | |
| Cedrela odorata | 30.75 | 2.29 | 25.00 | |
| Cynometra retusa | 10.77 | 1.83 | 14.00 | |
| Coccoloba barbadensis | 9.18 | 2.29 | 12.60 | |
| Quercus oleoides | 8.67 | 2.29 | 16.0 | |
| Spondias mombin | 7.90 | 2.29 | 12.60 | |
| Pithecellobium lanceolatum | 7.81 | 7.77 | 8.59 | |
| Guazuma ulmifolia | 5.21 | 2.74 | 11.83 | |
| Crescentia cujete | 4.41 | 0.91 | 7.50 | |
| Crateva tapia | 2.92 | 0.46 | 8.00 | |
| Couepia polyandra | 2.80 | 0.46 | 15.00 | |
| Attalea butyracea | 2.48 | 0.46 | 12.00 | |
| Albizia lebbeck | 2.12 | 0.46 | 15.00 | |
| Sapindus saponaria | 1.70 | 2.29 | 8.20 | |
| Bonellia macrocarpa | 1.62 | 1.83 | 8.00 | |
| Tabebuia rosea | 1.02 | 0.91 | 11.50 | |
| Annona reticulata | 0.89 | 0.91 | 10.00 | |
| Cestrum nocturnum | 0.48 | 0.46 | 8.00 | |
| Terminalia buceras | 0.42 | 0.46 | 15.00 | |
| Tabernaemontana longipes | 0.25 | 0.46 | 10.00 | |
| San Pedro River | Terminalia buceras | 219.68 | 109.71 | 12.84 |
| Pachira aquatica | 188.71 | 243.81 | 10.54 | |
| Lonchocarpus hondurensis | 31.29 | 56.38 | 10.42 | |
| Rhizophora mangle | 24.77 | 54.10 | 8.25 | |
| Manilkara zapota | 22.36 | 31.24 | 9.39 | |
| Morphospecies 2 | 19.43 | 47.24 | 9.46 | |
| Tabebuia rosea | 17.38 | 29.71 | 10.83 | |
| Calophyllum brasiliense | 14.90 | 30.48 | 12.02 | |
| Ficus insipida | 11.18 | 16.00 | 9.71 | |
| Haematoxylum campechianum | 10.93 | 9.90 | 9.00 | |
| Swartzia cubensis | 9.99 | 1.52 | 12.64 | |
| Chrysobalanus icaco | 7.11 | 10.67 | 6.41 | |
| Morphospecies 3 | 4.34 | 7.62 | 11.55 | |
| Coccoloba barbadensis | 3.69 | 8.38 | 9.10 | |
| Zygia recordii | 1.92 | 6.10 | 7.88 | |
| Morphospecies 4 | 1.91 | 3.05 | 10.31 | |
| Morphospecies 5 | 1.75 | 3.81 | 9.89 | |
| Malpighia sp. | 1.70 | 3.81 | 7.20 | |
| Bursera sp. | 1.51 | 4.57 | 10.61 | |
| Lonchocarpus sp. | 1.16 | 4.57 | 9.49 | |
| Metopium brownei | 1.05 | 1.52 | 10.24 | |
| Inga vera | 0.98 | 1.52 | 7.08 | |
| Morphospecies 6 | 0.92 | 3.81 | 9.01 | |
| Acacia cornigera | 0.68 | 1.52 | 9.16 | |
| Morphospecies 7 | 0.52 | 1.52 | 5.38 | |
| Morphospecies 8 | 0.50 | 2.29 | 8.40 | |
| Trichilia havanensis | 0.43 | 1.52 | 7.05 | |
| Morphospecies 9 | 0.42 | 0.76 | 17.88 | |
| Morphospecies 10 | 0.40 | 1.52 | 8.25 | |
| Morphospecies 11 | 0.39 | 1.52 | 11.19 | |
| Morphospecies 12 | 0.33 | 1.52 | 6.01 | |
| Morphospecies 13 | 0.23 | 0.76 | 10.04 | |
| Morphospecies 14 | 0.18 | 0.76 | 10.42 | |
| Xylosma sp. | 0.15 | 0.76 | 8.62 | |
| Morphospecies 15 | 0.13 | 0.76 | 9.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Camilo, R.; Gallardo-Cruz, J.A.; Solórzano, J.V.; Peralta-Carreta, C.; Jiménez-López, D.A.; Castillo-Acosta, O.; Sánchez-González, M.; Meave, J.A. An Assessment of the Spatial Variability of Tropical Swamp Forest along a 300 km Long Transect in the Usumacinta River Basin, Mexico. Forests 2020, 11, 1238. https://doi.org/10.3390/f11121238
Martínez-Camilo R, Gallardo-Cruz JA, Solórzano JV, Peralta-Carreta C, Jiménez-López DA, Castillo-Acosta O, Sánchez-González M, Meave JA. An Assessment of the Spatial Variability of Tropical Swamp Forest along a 300 km Long Transect in the Usumacinta River Basin, Mexico. Forests. 2020; 11(12):1238. https://doi.org/10.3390/f11121238
Chicago/Turabian StyleMartínez-Camilo, Rubén, José Alberto Gallardo-Cruz, Jonathan V. Solórzano, Candelario Peralta-Carreta, Derio Antonio Jiménez-López, Ofelia Castillo-Acosta, Miguelina Sánchez-González, and Jorge A. Meave. 2020. "An Assessment of the Spatial Variability of Tropical Swamp Forest along a 300 km Long Transect in the Usumacinta River Basin, Mexico" Forests 11, no. 12: 1238. https://doi.org/10.3390/f11121238
APA StyleMartínez-Camilo, R., Gallardo-Cruz, J. A., Solórzano, J. V., Peralta-Carreta, C., Jiménez-López, D. A., Castillo-Acosta, O., Sánchez-González, M., & Meave, J. A. (2020). An Assessment of the Spatial Variability of Tropical Swamp Forest along a 300 km Long Transect in the Usumacinta River Basin, Mexico. Forests, 11(12), 1238. https://doi.org/10.3390/f11121238







