Association of Recent Incidence of Foliar Disease in Pine Species in the Southeastern United States with Tree and Climate Variables
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. FIA Data
2.3. Data Analyses
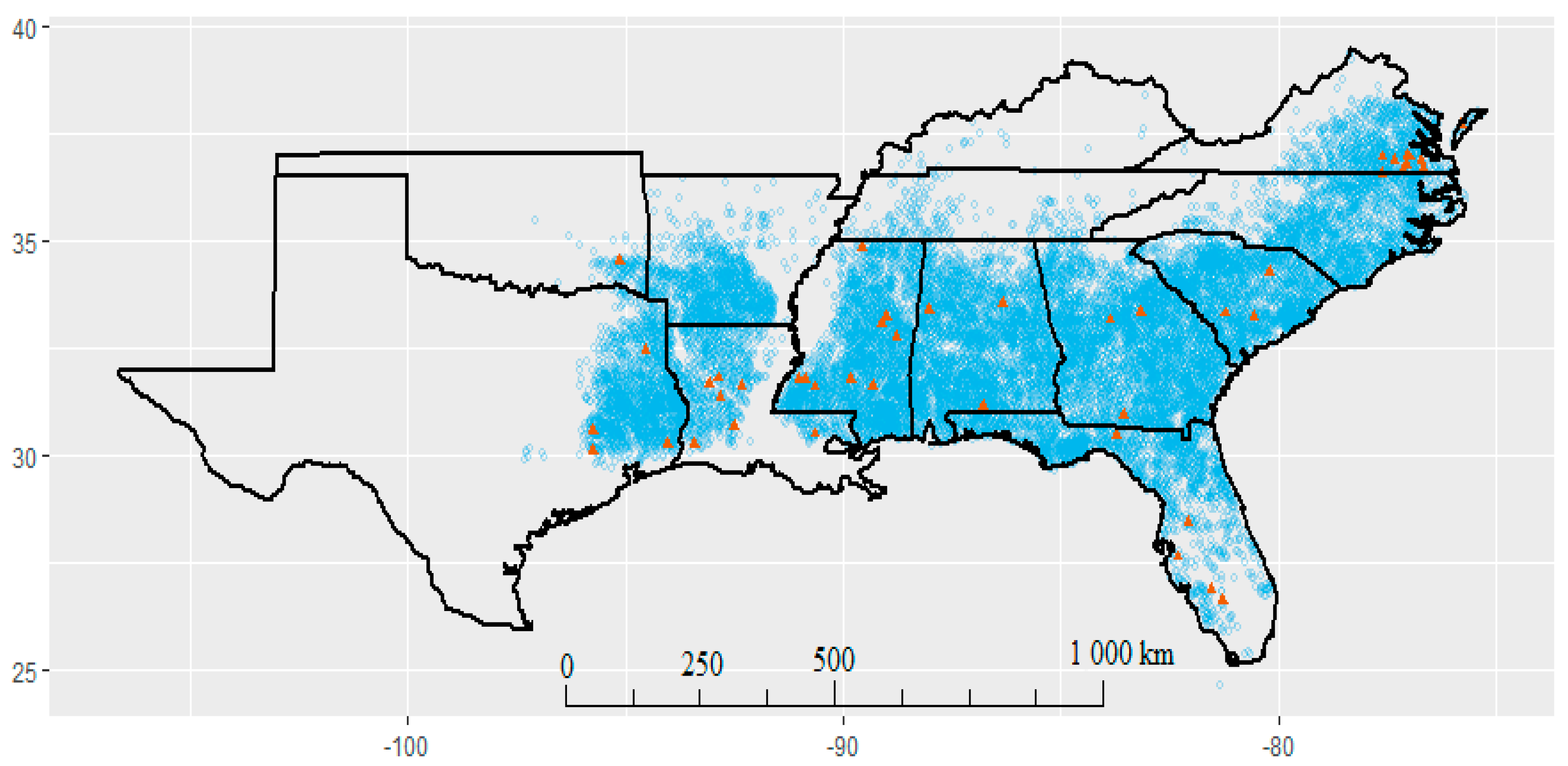
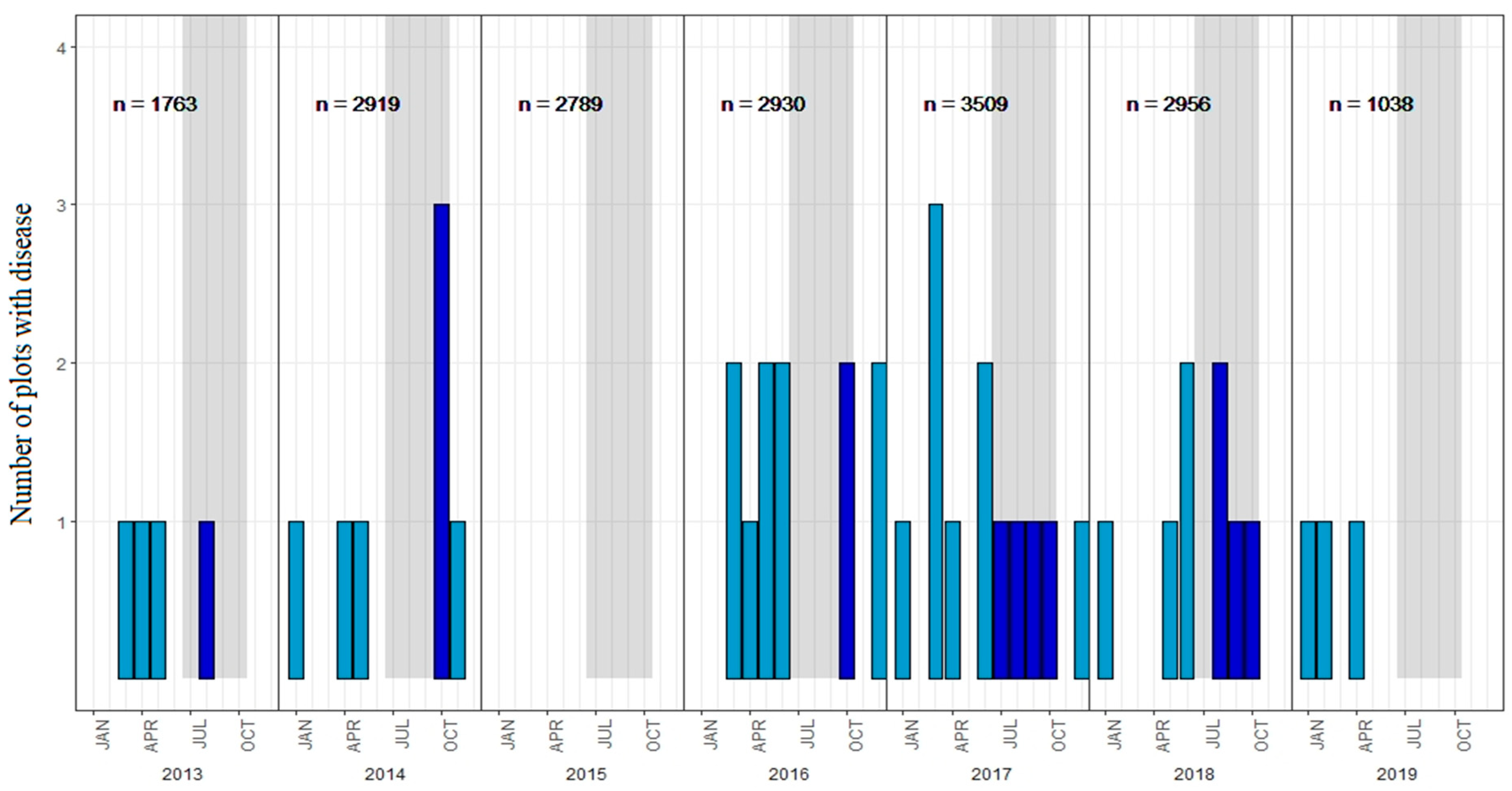
2.3.1. Spatio-Temporal Distribution
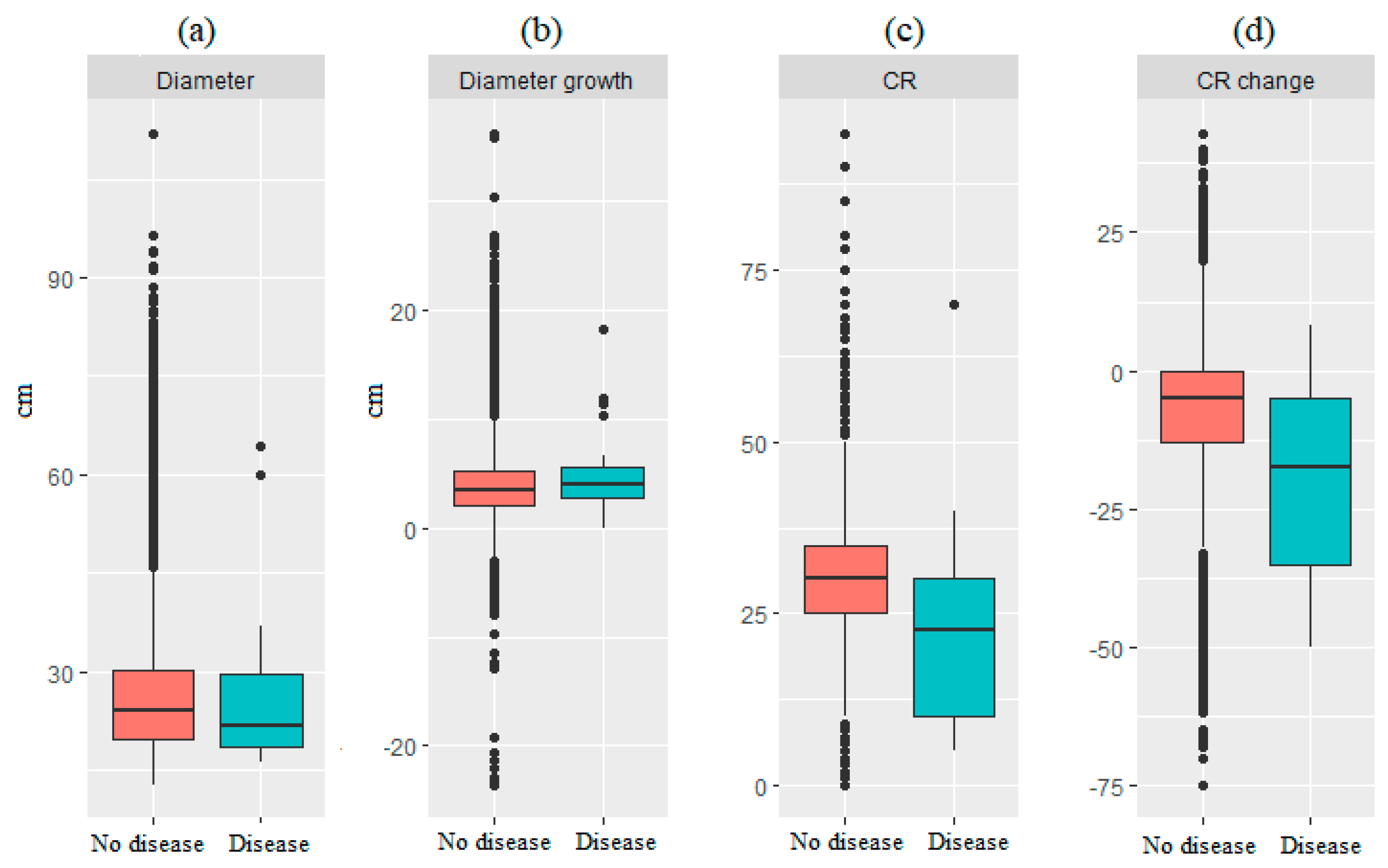
2.3.2. Tree-Level Analysis
2.3.3. Range-Wide Climatic Analysis
3. Results
3.1. Spatio-Temporal Distribution of Pine Foliar Disease
3.2. Tree Level Analysis
3.3. Range-Wide Climatic Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stevens, R.B. Cultural practices in disease control. Chapter 10 in J.G. In Plant Pathology, an Advanced Treatise; Horsfall, J.G., Dimond, A.E., Eds.; Academic Press: New York, NY, USA, 1960; Volume 3, pp. 357–429. [Google Scholar]
- Loehle, C.; Idso, C.; Wigley, T.B. Physiological and ecological factors influencing recent trends in United States forest health responses to climate change. For. Ecol. Manag. 2016, 363, 179–189. [Google Scholar] [CrossRef]
- Moody, M.E.; Mack, R.N. Controlling the Spread of Plant Invasions: The Importance of Nascent Foci. J. Appl. Ecol. 1988, 25, 1009. [Google Scholar] [CrossRef]
- Zamora, D.L.; Thill, D.C. Early detection and eradication of new weed infestations. In Biology and Management of Noxious Rangeland Weeds; Sheley, R.L., Petroff, J.K., Eds.; Oregon State University Press: Corvallis, OR, USA, 1999; pp. 73–84. [Google Scholar]
- Lawrence, R.; Labus, M. Early Detection of Douglas-Fir Beetle Infestation with Subcanopy Resolution Hyperspectral Imagery. West. J. Appl. For. 2003, 18, 202–206. [Google Scholar] [CrossRef]
- Venette, R.C.; Kriticos, D.J.; Magarey, R.D.; Koch, F.H.; Baker, R.H.A.; Worner, S.P.; Raboteaux, N.N.G.; McKenney, D.W.; Dobesberger, E.J.; Yemshanov, D.; et al. Pest Risk Maps for Invasive Alien Species: A Roadmap for Improvement. Bioscience 2010, 60, 349–362. [Google Scholar] [CrossRef]
- Ikegami, M.; Jenkins, T.A.R. Estimate global risks of a forest disease under current and future climates using species distribution model and simple thermal model—Pine Wilt disease as a model case. For. Ecol. Manag. 2018, 409, 343–352. [Google Scholar] [CrossRef]
- Fox, T.R.; Jakela, E.J.; Allen, H.L. The development of pine plantation silviculture in the southern United States. J. Forest. 2007, 105, 337–347. [Google Scholar] [CrossRef]
- McNichol, B.H.; Montes, C.R.; Barnes, B.F.; Nowak, J.T.; Villari, C.; Gandhi, K.J. Interactions between southern Ips bark beetle outbreaks, prescribed fire, and loblolly pine (Pinus taeda L.) mortality. For. Ecol. Manag. 2019, 446, 164–174. [Google Scholar] [CrossRef]
- Prestemon, J.P.; Abt, R.C. Chapter 13: Timber products supply and demand. In Southern Forest Resource Assessment; General Tech. Rpt. SRS-53; Wear, D.N., Greis, J.G., Eds.; U.S. Forest Service, Southern Research Station: Asheville, NC, USA, 2002; pp. 299–325. [Google Scholar]
- Gaby, L.I. The Southern Pines, an American Wood; U.S. Forest Service, F-256: Washington, DC, USA, 1985. [Google Scholar]
- Schultz, R.P. Loblolly Pine: The Ecology and Culture of Loblolly Pine (Pinus taeda L.). In Agricultural Handbook 713; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1997; p. 493. [Google Scholar]
- Wear, D.N.; Greis, J.G. The Southern Forest Futures Project: Summary Report; General Technical Report, SRS-168; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2012. [Google Scholar]
- Hanson, C.; Yonavjak, L.; Clarke, C.; Minnemeyer, S.; Boisrobert, L.; Leach, A.; Schleeweis, K. Southern Forests for the Future; World Resources Institute: Washington, DC, USA, 2010; ISBN 978-1-56973-737-8. [Google Scholar]
- Noss, R.F.; LaRoe, E.T.; Scott, J.M. Endangered Ecosystems of the United States: A Preliminary Assessment of Loss and Degradation; U.S. Department of the Interior, National Biological Service: Washington, DC, USA, 1995. [Google Scholar]
- Mcintyre, R.K.; McCall, B.B.; Wear, D.N.; Kirkman, L.K.; Jack, S.B. The Social and Economic Drivers of the Southeastern Forest Landscape. In Ecological Restoration and Management of Longleaf Pine Forests; Kirkman, L.K., Jack, S.B., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 39–68. [Google Scholar]
- Brown, H.D.; McDowell, W.E. Status of Loblolly Pine Die-Off on the Oakmulgee District, Talladega National Forest, Alabama—1968; USDA Forest Service Rep. 69-2-28; Forest Insect & Disease Management: Pineville, LA, USA, 1968; p. 22. [Google Scholar]
- Hess, N.J.; Otrosina, W.J.; Carter, E.A.; Steinman, J.R.; Jones, J.P.; Eckhardt, L.G.; Weber, A.M.; Walkinshaw, C.H. Assessment of loblolly pine decline in central Alabama. In Eleventh Biennial Southern Silvicultural Research Conference; Outcalt, K.W., Ed.; USDA Forest Service Gen: Asheville, NC, USA, 2002; pp. 558–564. [Google Scholar]
- Coyle, D.R.; Klepzig, K.D.; Koch, F.H.; Morris, L.A.; Nowak, J.T.; Oak, S.W.; Otrosina, W.J.; Smith, W.D.; Gandhi, K.J. A review of southern pine decline in North America. For. Ecol. Manag. 2015, 349, 134–148. [Google Scholar] [CrossRef]
- Coyle, D.R.; Barnes, B.F.; Klepzig, K.D.; Koch, F.H.; Morris, L.A.; Nowak, J.T.; Otrosina, W.J.; Smith, W.D.; Gandhi, K.J.K. Abiotic and Biotic Factors Affecting Loblolly Pine Health in the Southeastern United States. For. Sci. 2019, 66, 145–156. [Google Scholar] [CrossRef]
- Duerr, D.A.; Mistretta, P.A. Invasive pests—Insects and diseases. In The Southern Forest Futures Project: Technical Report; Gen. Tech. Rep. SRS-GTR-178; Wear, D.N., Greis, J.G., Eds.; USDA-Forest Service, Southern Research Station: Asheville, NC, USA, 2013; pp. 457–508. [Google Scholar]
- Pye, J.M.; Holmes, T.P.; Prestemon, J.P.; Wear, D.N. Economic impacts of the southern pine beetle. In Southern Pine Beetle II; Coulson, R.N., Klepzig, K.D., Eds.; US Department of Agriculture Forest Service: Asheville, NC, USA, 2011; pp. 213–222. [Google Scholar]
- Barry, J.E.; Rowe, K.D.; Ford, V.L.; Kirkpartick, T.L. Pine Needle Diseases in Arkansas. Fact Sheet FSA5022. University of Arkansas Research and Extension. 2008. Available online: https://www.uaex.edu/publications/PDF/FSA-5022.pdf (accessed on 16 June 2020).
- Boyce, J.S., Jr. Needle cast of southern pines. In Forest Pest Leaflet 28; USDA Forest Service: Washington, DC, USA, 1969; p. 4. [Google Scholar]
- Barnard, E.L.; Ash, E.C. Needle Cast of Pines in Florida; Plant Pathology Circular No. 368; Florida Department of Agriculture and Consumer Services: Tallahassee, FL, USA, 1998; pp. 1–4. [Google Scholar]
- Boyce, J.S., Jr. Hypoderma needle blight of southern pines. J. For. 1954, 52, 496–498. [Google Scholar]
- Sinclair, W.A.; Lyon, H.H.; Johnson, W.T. Diseases of Trees and Shrubs; Comstock Publishers; Cornell University Press: Ithaca, NY, USA, 1987; p. 574. [Google Scholar]
- Jansons, Ā.; Zeltiņš, P.; Donis, J.; Neimane, U. Long-Term Effect of Lophodermium Needle Cast on The Growth of Scots Pine and Implications for Financial Outcomes. Forests 2020, 11, 718. [Google Scholar] [CrossRef]
- Drenkhan, R. Epidemiological Investigation of Pine Foliage Diseases by the Use of the Needle Trace Method. Ph.D. Thesis, Estonian University of Life Sciences, Tartu, Estonia, 2011. [Google Scholar]
- Bechtold, W.A.; Patterson, P.L. The Enhanced Forest Inventory and Analysis Program-National Sampling Design and Estimation Procedures; Gen. Tech. Rep. SRS-80; US Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2005; p. 85. [Google Scholar]
- USFS. Forest Inventory and Analysis, National Core Field Guide, Volume I: Field Data Collection Procedures for Phase 2 Plots; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 2019. [Google Scholar]
- Getis, A.; Ord, J.K. The Analysis of Spatial Association by Use of Distance Statistics. Geogr. Anal. 2010, 24, 189–206. [Google Scholar] [CrossRef]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press (CUP): Cambridge, UK, 2006; p. 625. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Usinglme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 9 July 2020).
- Phillips, S.J.; Dudík, M.; Schapire, R.E. [Internet] Maxent Software for Modeling Species Niches and Distributions (Version 3.4.1). 2020. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 20 July 2020).
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Anderson, R.P.; Gonzalez, I. Species-specific tuning increases robustness to sampling bias in models of species distributions: An implementation with Maxent. Ecol. Model. 2011, 222, 2796–2811. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Elith, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; NCEAS Predicting Species Distributions Working Group†. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxentmodels of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2013, 41, 629–643. [Google Scholar] [CrossRef]
- Derose, R.J.; Wang, S.-Y.; Shaw, J.D. Feasibility of High-Density Climate Reconstruction Based on Forest Inventory and Analysis (FIA) Collected Tree-Ring Data. J. Hydrometeorol. 2013, 14, 375–381. [Google Scholar] [CrossRef]
- Tinkham, W.T.; Mahoney, P.R.; Hudak, A.T.; Domke, G.M.; Falkowski, M.J.; Woodall, C.; Smith, A.M.S. Applications of the United States Forest Inventory and Analysis dataset: A review and future directions. Can. J. For. Res. 2018, 48, 1251–1268. [Google Scholar] [CrossRef]
- PRISM Climate Group. Annual Dataset Covering the Conterminous U.S. Oregon State University, 2019. Available online: http://prism.oregonstate.edu (accessed on 28 April 2020).
- PRISM Climate Group. Monthly 30-Year “Normal” Dataset Covering the Conterminous U.S, (1981–2010). Oregon State University, 2012. Available online: http://prism.oregonstate.edu (accessed on 28 April 2020).
- Meentemeyer, R.K.; Anacker, B.L.; Mark, W.; Rizzo, D.M. Early detection of emerging forest disease using dispersal estimation and ecological niche modeling. Ecol. Appl. 2008, 18, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.M.; Stone, J.K.; Capitano, B.R.; Rosso, P.; Sutton, W.; Winton, L.; Kanaskie, A.; McWilliams, M.G. Incidence and Impact of Swiss Needle Cast in Forest Plantations of Douglas-fir in Coastal Oregon. Plant Dis. 2000, 84, 773–778. [Google Scholar] [CrossRef]
- Bradshaw, R.E. Dothistroma (red-band) needle blight of pines and the dothistromin toxin: A review. For. Pathol. 2004, 34, 163–185. [Google Scholar] [CrossRef]
- Gearman, M.; Blinnikov, M.S. Mapping the Potential Distribution of Oak Wilt (Bretziella fagacearum) in East Central and Southeast Minnesota Using Maxent. J. For. 2019, 117, 579–591. [Google Scholar] [CrossRef]
- Wyka, S.A.; Smith, C.; Munck, I.A.; Rock, B.N.; Ziniti, B.L.; Broders, K. Emergence of White Pine Needle Damage (WPND) in the northeastern U.S. is associated with changes in pathogen pressure in response to climate change. Glob. Chang. Biol. 2016, 23, 394–405. [Google Scholar] [CrossRef]
- Fokkema, M.; Edbrooke-Childs, J.; Wolpert, M. Generalized linear mixed-model (GLMM) trees: A flexible decision-tree method for multilevel and longitudinal data. Psychother. Res. 2020, 1–13. [Google Scholar] [CrossRef]


| Predictor Variable |
|---|
| Diameter at breast height (cm) |
| Crown ratio, (%) |
| Diameter growth from past inventory (cm) |
| Crown ratio change from past inventory (%) |
| Environment Variable |
|---|
| Mean annual precipitation from previous year (mm) |
| Mean annual temperature from previous year (°C) |
| Mean dew point temperature from previous year (°C) |
| Mean maximum vapor pressure deficit from previous year (hpa) |
| Mean precipitation during coldest months from last season (mm) |
| Mean temperature during coldest months from last season (°C) |
| Random Effect | ||||||
| Name | Variance | Std. Dev | ||||
| Plot (Intercept) | 126.00 | 11.23 | ||||
| Fixed Effects: | ||||||
| Name | Estimate | Std. Error | Z value | Pr (>Z) | LRT | Pr (Chi) |
| Intercept | −15.47 | 0.98 | −15.82 | 0.00 | ||
| Diameter | 0.10 | 0.32 | 0.31 | 0.76 | 0.10 | 0.76 |
| Diameter growth | 0.15 | 0.29 | 0.51 | 0.61 | 0.26 | 0.61 |
| Crown ratio | −0.49 | 0.30 | −1.64 | 0.10 | 2.81 | 0.09 |
| Crown ratio change | −0.22 | 0.23 | −0.94 | 0.35 | 0.87 | 0.35 |
| Description | 2016 | 2017 |
|---|---|---|
| Beta multiplier | 0.90 | 0.10 |
| AUC | 0.73 | 0.70 |
| Mean dew point temperature (%) | 70.90 | 37.40 |
| Maximum vapor pressure deficit (%) | 21.10 | 32.70 |
| Precipitation during cold months (%) | 7.90 | 22.30 |
| Mean temperature during cold months (%) | 0.00 | 7.60 |
| Annual mean temperature (%) | 0.00 | 0.00 |
| Annual mean precipitation (%) | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandit, K.; Smith, J.; Quesada, T.; Villari, C.; Johnson, D.J. Association of Recent Incidence of Foliar Disease in Pine Species in the Southeastern United States with Tree and Climate Variables. Forests 2020, 11, 1155. https://doi.org/10.3390/f11111155
Pandit K, Smith J, Quesada T, Villari C, Johnson DJ. Association of Recent Incidence of Foliar Disease in Pine Species in the Southeastern United States with Tree and Climate Variables. Forests. 2020; 11(11):1155. https://doi.org/10.3390/f11111155
Chicago/Turabian StylePandit, Karun, Jason Smith, Tania Quesada, Caterina Villari, and Daniel J. Johnson. 2020. "Association of Recent Incidence of Foliar Disease in Pine Species in the Southeastern United States with Tree and Climate Variables" Forests 11, no. 11: 1155. https://doi.org/10.3390/f11111155
APA StylePandit, K., Smith, J., Quesada, T., Villari, C., & Johnson, D. J. (2020). Association of Recent Incidence of Foliar Disease in Pine Species in the Southeastern United States with Tree and Climate Variables. Forests, 11(11), 1155. https://doi.org/10.3390/f11111155







