Comparison of Soil Greenhouse Gas Fluxes during the Spring Freeze–Thaw Period and the Growing Season in a Temperate Broadleaved Korean Pine Forest, Changbai Mountains, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil GHG Flux Measurements
2.3. Environmental Variable Measurements
2.4. Division of the SFT Period and the GS
2.5. GWP100 Calculation
2.6. Data Analysis and Statistics
3. Results
3.1. Environmental Factors
3.2. Seasonal Patterns of Soil CO2, CH4, and N2O Fluxes
3.3. Correlations between Soil CO2, CH4, and N2O Fluxes
3.4. Responses of Soil CO2, CH4, and N2O Fluxes to Environmental Factors
3.5. GWP100 of Soil CO2, CH4, and N2O Fluxes
4. Discussion
4.1. Comparison of Soil GHG Fluxes between the SFT Period and the GS
4.1.1. CO2
4.1.2. CH4
4.1.3. N2O
4.2. Relationships among Soil GHG Fluxes
4.3. Impacts of Ts and Soil VWC on Soil GHG Fluxes
4.3.1. CO2
4.3.2. CH4
4.3.3. N2O
4.4. Contributions of Soil GHG Fluxes to the Greenhouse Effect
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirschbaum, M.U.F. Will changes in soil organic carbon act as a positive or negative feedback on global warming? Biogeochemistry 2000, 48, 21–51. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Lloyd, J.; Taylor, J.A. On the Temperature Dependence of Soil Respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Fuglestvedt, J.S.; Berntsen, T.K.; Godal, O.; Skodvin, T. Climate implications of GWP-based reductions in greenhouse gas emissions. Geophys. Res. Lett. 2000, 27, 409–412. [Google Scholar] [CrossRef]
- Le Treut, H.; Somerville, R. Chapter 1: Historical overview of climate change science. In IPCC Fourth Assessment Review: Climate Change Science; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Feng, H.; Guo, J.; Han, M.; Wang, W.; Peng, C.; Jin, J.; Song, X.; Yu, S. A review of the mechanisms and controlling factors of methane dynamics in forest ecosystems. For. Ecol. Manag. 2020, 455, 117702. [Google Scholar] [CrossRef]
- Sa, M.M.F.; Schaefer, C.; Loureiro, D.C.; Simas, F.N.B.; Alves, B.J.R.; de Sa Mendonca, E.; de Figueiredo, E.B.; La Scala, N., Jr.; Panosso, A.R. Fluxes of CO2, CH4, and N2O in tundra-covered and Nothofagus forest soils in the Argentinian Patagonia. Sci. Total Environ. 2019, 659, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Dixon, R.K. Carbon pools and flux of global forest ecosystems (Vol 263, Pg 185, 1994). Science 1994, 265, 171. [Google Scholar]
- Cole, D.W.; Rapp, M. Elemental cycling in forest ecosystems. In Dynamic Properties of Forest Ecosystems; Reichle, D.E., Ed.; Cambridge University Press: Cambridge, UK, 2009; pp. 341–375. [Google Scholar]
- Huang, G.; Li, Y.; Su, Y.G. Effects of increasing precipitation on soil microbial community composition and soil respiration in a temperate desert, Northwestern China. Soil Biol. Biochem. 2015, 83, 52–56. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Högberg, P.; Nordgren, A.; Buchmann, N.; Taylor, A.F.S.; Ekblad, A.; Högberg, M.N.; Nyberg, G.; Ottosson-Löfvenius, M.; Read, D.J. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 2001, 411, 789–792. [Google Scholar] [CrossRef]
- Luo, G.J.; Kiese, R.; Wolf, B.; Butterbach-Bahl, K. Effects of soil temperature and moisture on methane uptake and nitrous oxide emissions across three different ecosystem types. Biogeosciences 2013, 10, 3205–3219. [Google Scholar] [CrossRef]
- Liu, L.; Estiarte, M.; Penuelas, J. Soil moisture as the key factor of atmospheric CH4 uptake in forest soils under environmental change. Geoderma 2019, 355, 113920. [Google Scholar] [CrossRef]
- Petrakis, S.; Seyfferth, A.; Kan, J.; Inamdar, S.; Vargas, R. Influence of experimental extreme water pulses on greenhouse gas emissions from soils. Biogeochemistry 2017, 133, 147–164. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Müller, C.; Martin, M.; Stevens, R.J.; Laughlin, R.J.; Kammann, C.; Ottow, J.C.G.; Jäger, H.J. Processes leading to N2O emissions in grassland soil during freezing and thawing. Soil Biol. Biochem. 2002, 34, 1325–1331. [Google Scholar] [CrossRef]
- McCalley, C.K.; Woodcroft, B.J.; Hodgkins, S.B.; Wehr, R.A.; Kim, E.H.; Mondav, R.; Crill, P.M.; Chanton, J.P.; Rich, V.I.; Tyson, G.W.; et al. Methane dynamics regulated by microbial community response to permafrost thaw. Nature 2014, 514, 478–481. [Google Scholar] [CrossRef]
- Fang, Y.; Gundersen, P.; Zhang, W.; Zhou, G.; Christiansen, J.R.; Mo, J.; Dong, S.; Zhang, T. Soil–atmosphere exchange of N2O, CO2 and CH4 along a slope of an evergreen broad-leaved forest in southern China. Plant Soil 2009, 319, 37–48. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Baldocchi, D.D.; Gershenson, A.; Goldstein, A.; Misson, L.; Wong, S. Microbial soil respiration and its dependency on carbon inputs, soil temperature and moisture. Glob. Chang. Biol. 2007, 13, 2018–2035. [Google Scholar] [CrossRef]
- Tu, C.; Li, F.D. Responses of greenhouse gas fluxes to experimental warming in wheat season under conventional tillage and no-tillage fields. J. Environ. Sci. 2017, 54, 314–327. [Google Scholar] [CrossRef]
- Zang, H.; Blagodatskaya, E.; Wen, Y.; Shi, L.; Cheng, F.; Chen, H.; Zhao, B.; Zhang, F.; Fan, M.; Kuzyakov, Y. Temperature sensitivity of soil organic matter mineralization decreases with long-term N fertilization: Evidence from four Q10 estimation approaches. Land Degrad. Dev. 2020, 31, 683–693. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Prior, S.A.; Runion, G.B.; Torbert, H.A.; Tian, H.; Lu, C.; Venterea, R.T. Effects of elevated carbon dioxide and increased temperature on methane and nitrous oxide fluxes: Evidence from field experiments. Front. Ecol. Environ. 2012, 10, 520–527. [Google Scholar] [CrossRef]
- Wu, X.; Wang, F.; Li, T.; Fu, B.; Lv, Y.; Liu, G. Nitrogen additions increase N2O emissions but reduce soil respiration and CH4 uptake during freeze–thaw cycles in an alpine meadow. Geoderma 2020, 363, 114157. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Brown, J.R.; Dijkstra, P.; Allwright, M.C.; Hungate, B.A. Response of Terrestrial CH4 Uptake to Interactive Changes in Precipitation and Temperature Along a Climatic Gradient. Ecosystems 2010, 13, 1157–1170. [Google Scholar] [CrossRef]
- Sponseller, R.A. Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Glob. Chang. Biol. 2007, 13, 426–436. [Google Scholar] [CrossRef]
- Wen, Y.; Zang, H.; Freeman, B.; Ma, Q.; Chadwick, D.R.; Jones, D.L. Rye cover crop incorporation and high watertable mitigate greenhouse gas emissions in cultivated peatland. Land Degrad. Dev. 2019, 30, 1928–1938. [Google Scholar] [CrossRef]
- Carey, J.C.; Tang, J.W.; Templer, P.H.; Kroeger, K.D.; Crowther, T.W.; Burton, A.J.; Dukes, J.S.; Emmett, B.; Frey, S.D.; Heskel, M.A.; et al. Temperature response of soil respiration largely unaltered with experimental warming. Proc. Natl. Acad. Sci. USA 2016, 113, 13797–13802. [Google Scholar] [CrossRef]
- Fang, C.; Moncrieff, J.B. The dependence of soil CO2 efflux on temperature. Soil Biol. Biochem. 2001, 33, 155–165. [Google Scholar] [CrossRef]
- Luo, G.J.; Brüggemann, N.; Wolf, B.; Gasche, R.; Grote, R.; Butterbach-Bahl, K. Decadal variability of soil CO2, NO, N2O, and CH4 fluxes at the Höglwald Forest, Germany. Biogeosciences 2012, 9, 1741–1763. [Google Scholar] [CrossRef]
- Zou, J.; Tobin, B.; Luo, Y.; Osborne, B. Differential responses of soil CO2 and N2O fluxes to experimental warming. Agric. For. Meteorol. 2018, 259, 11–22. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, L.; Liu, J.; Peng, B.; Fan, Z.; Dai, W.; Jiang, P.; Bai, E. Responses of terrestrial nitrogen pools and dynamics to different patterns of freeze-thaw cycle: A meta-analysis. Glob. Chang. Biol. 2018, 24, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, S.-q.; Zhang, A.-p.; Jing, X.; Song, W.-m.; Mi, Z.-r.; Zhang, Q.-w.; Wang, W.-y.; Yang, Z.-l. Nitrous oxide emissions following seasonal freeze-thaw events from arable soils in Northeast China. J. Integr. Agric. 2018, 17, 231–246. [Google Scholar] [CrossRef]
- Zhang, T.; Barry, R.; Knowles, K.; Ling, F.; Armstrong, R. Distribution of seasonally and perennially frozen ground in the Northern Hemisphere. In Proceedings of the 8th International Conference on Permafrost, Zürich, Switzerland, 21–25 July 2003; Volume 2. [Google Scholar]
- Wang, C.; Han, Y.; Chen, J.; Wang, X.; Zhang, Q.; Bond-Lamberty, B. Seasonality of soil CO2 efflux in a temperate forest: Biophysical effects of snowpack and spring freeze–thaw cycles. Agric. For. Meteorol. 2013, 177, 83–92. [Google Scholar] [CrossRef]
- Han, C.; Gu, Y.; Kong, M.; Hu, L.; Jia, Y.; Li, F.; Sun, G.; Siddique, K.H.M. Responses of soil microorganisms, carbon and nitrogen to freeze–thaw cycles in diverse land-use types. Appl. Soil Ecol. 2018, 124, 211–217. [Google Scholar] [CrossRef]
- Song, Y.; Zou, Y.; Wang, G.; Yu, X. Altered soil carbon and nitrogen cycles due to the freeze-thaw effect: A meta-analysis. Soil Biol. Biochem. 2017, 109, 35–49. [Google Scholar] [CrossRef]
- Kim, D.G.; Vargas, R.; Bond-Lamberty, B.; Turetsky, M.R. Effects of soil rewetting and thawing on soil gas fluxes: A review of current literature and suggestions for future research. Biogeosci. Discuss. 2011, 8, 9847–9899. [Google Scholar] [CrossRef]
- Wagner-Riddle, C.; Congreves, K.A.; Abalos, D.; Berg, A.A.; Brown, S.E.; Ambadan, J.T.; Gao, X.; Tenuta, M. Globally important nitrous oxide emissions from croplands induced by freeze–thaw cycles. Nat. Geosci. 2017, 10, 279–283. [Google Scholar] [CrossRef]
- Mastepanov, M.; Sigsgaard, C.; Dlugokencky, E.J.; Houweling, S.; Ström, L.; Tamstorf, M.P.; Christensen, T.R. Large tundra methane burst during onset of freezing. Nature 2008, 456, 628–630. [Google Scholar] [CrossRef]
- Kurganova, I.; Teepe, R.; Loftfield, N. Influence of freeze-thaw events on carbon dioxide emission from soils at different moisture and land use. Carbon Balance Manag. 2007, 2, 2. [Google Scholar] [CrossRef]
- Wu, G.; Chen, X.-M.; Ling, J.; Li, F.; Li, F.-Y.; Peixoto, L.; Wen, Y.; Zhou, S.-L. Effects of soil warming and increased precipitation on greenhouse gas fluxes in spring maize seasons in the North China Plain. Sci. Total Environ. 2020, 734, 139269. [Google Scholar] [CrossRef]
- Cooper, M.D.A.; Estop-Aragonés, C.; Fisher, J.P.; Thierry, A.; Garnett, M.H.; Charman, D.J.; Murton, J.B.; Phoenix, G.K.; Treharne, R.; Kokelj, S.V.; et al. Limited contribution of permafrost carbon to methane release from thawing peatlands. Nat. Clim. Chang. 2017, 7, 507–511. [Google Scholar] [CrossRef]
- Congreves, K.A.; Wagner-Riddle, C.; Si, B.C.; Clough, T.J. Nitrous oxide emissions and biogeochemical responses to soil freezing-thawing and drying-wetting. Soil Biol. Biochem. 2018, 117, 5–15. [Google Scholar] [CrossRef]
- Peng, B.; Sun, J.; Liu, J.; Dai, W.; Sun, L.; Pei, G.; Gao, D.; Wang, C.; Jiang, P.; Bai, E. N2O emission from a temperate forest soil during the freeze-thaw period: A mesocosm study. Sci. Total Environ. 2019, 648, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Wu, J.; Zhao, X.; Han, S.; Yu, G.; Sun, X.; Jin, C. CO2 fluxes over an old, temperate mixed forest in northeastern China. Agric. For. Meteorol. 2006, 137, 138–149. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, L.; Sun, X.; Fu, Y.; Wen, X.; Wang, Q.; Li, S.; Ren, C.; Song, X.; Liu, Y.; et al. Environmental controls over carbon exchange of three forest ecosystems in eastern China. Glob. Chang. Biol. 2008, 14, 2555–2571. [Google Scholar] [CrossRef]
- Zhou, Y.; Hagedorn, F.; Zhou, C.; Jiang, X.; Wang, X.; Li, M.-H. Experimental warming of a mountain tundra increases soil CO2 effluxes and enhances CH4 and N2O uptake at Changbai Mountain, China. Sci. Rep. 2016, 6, 21108. [Google Scholar] [CrossRef]
- Chen, Z.; Setälä, H.; Geng, S.; Han, S.; Wang, S.; Dai, G.; Zhang, J. Nitrogen addition impacts on the emissions of greenhouse gases depending on the forest type: A case study in Changbai Mountain, Northeast China. J. Soils Sediments 2017, 17, 23–34. [Google Scholar] [CrossRef]
- Wang, M.; Guan, D.-X.; Han, S.-J.; Wu, J.-L. Comparison of eddy covariance and chamber-based methods for measuring CO2 flux in a temperate mixed forest. Tree Physiol. 2009, 30, 149–163. [Google Scholar] [CrossRef]
- Wu, J.; Guan, D.; Wang, M.; Pei, T.; Han, S.; Jin, C. Year-round soil and ecosystem respiration in a temperate broad-leaved Korean Pine forest. For. Ecol. Manag. 2006, 223, 35–44. [Google Scholar] [CrossRef]
- Bai, E.; Li, W.; Li, S.; Sun, J.; Peng, B.; Dai, W.; Jiang, P.; Han, S. Pulse increase of soil N2O emission in response to N addition in a temperate forest on Mt Changbai, northeast China. PLoS ONE 2014, 9, e102765. [Google Scholar] [CrossRef]
- Wu, B.; Mu, C.C. Effects on Greenhouse Gas (CH4, CO2, N2O) Emissions of Conversion from Over-Mature Forest to Secondary Forest and Korean Pine Plantation in Northeast China. Forests 2019, 10, 788. [Google Scholar] [CrossRef]
- Phillips, S.C.; Varner, R.K.; Frolking, S.; Munger, J.W.; Bubier, J.L.; Wofsy, S.C.; Crill, P.M. Interannual, seasonal, and diel variation in soil respiration relative to ecosystem respiration at a wetland to upland slope at Harvard Forest. J. Geophys. Res. Biogeosci. 2010, 115. [Google Scholar] [CrossRef]
- Wan, Q.; Feng, X.; Lu, J.; Zheng, W.; Song, X.; Li, P.; Han, S.; Xu, H. Atmospheric mercury in Changbai Mountain area, northeastern China II. The distribution of reactive gaseous mercury and particulate mercury and mercury deposition fluxes. Environ. Res. 2009, 109, 721–727. [Google Scholar] [CrossRef]
- Wang, Z.; Gallet, J.; Pedersen, C.; Zhang, X.; Ström, J.; Ci, Z. Elemental carbon in snow at Changbai Mountain, northeastern China: Concentrations, scavenging ratios, and dry deposition velocities. Atmos. Chem. Phys. 2014, 14. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Hao, Z.; Zhang, J.; Song, B.; Ye, J.; Li, B. Vertical structure and spatial associations of dominant tree species in an old-growth temperate forest. For. Ecol. Manag. 2007, 252, 1–11. [Google Scholar] [CrossRef]
- Zheng, X.; Mei, B.; Wang, Y.; Xie, B.; Wang, Y.; Dong, H.; Xu, H.; Chen, G.; Cai, Z.; Yue, J.; et al. Quantification of N2O fluxes from soil–plant systems may be biased by the applied gas chromatograph methodology. Plant Soil 2008, 311, 211–234. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Yang, X.; Gong, Y.; Zheng, X.; Fan, M.; Kuzyakov, Y. Annual methane uptake from different land uses in an agro-pastoral ecotone of northern China. Agric. For. Meteorol. 2017, 236, 67–77. [Google Scholar] [CrossRef]
- Zheng, Z.M.; Yu, G.R.; Sun, X.M.; Li, S.G.; Wang, Y.S.; Wang, Y.H.; Fu, Y.L.; Wang, Q.F. Spatio-temporal variability of soil respiration of forest ecosystems in China: Influencing factors and evaluation model. Environ. Manag. 2010, 46, 633–642. [Google Scholar] [CrossRef]
- Yang, X.; Chen, H.; Gong, Y.; Zheng, X.; Fan, M.; Kuzyakov, Y. Nitrous oxide emissions from an agro-pastoral ecotone of northern China depending on land uses. Agric. Ecosyst. Environ. 2015, 213, 241–251. [Google Scholar] [CrossRef]
- Streiner, D.L. Maintaining Standards: Differences between the Standard Deviation and Standard Error, and When to Use Each. Can. J. Psychiatry 1996, 41, 498–502. [Google Scholar] [CrossRef]
- Brinkmann, W.A.R. Growing season length as an indicator of climatic variations? Clim. Chang. 1979, 2, 127–138. [Google Scholar] [CrossRef]
- Segura, J.H.; Nilsson, M.B.; Haei, M.; Sparrman, T.; Mikkola, J.P.; Grasvik, J.; Schleucher, J.; Oquist, M.G. Microbial mineralization of cellulose in frozen soils. Nat. Commun. 2017, 8, 1154. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.D.; Grogan, P.; Templer, P.H.; Groffman, P.; Öquist, M.G.; Schimel, J. Carbon and Nitrogen Cycling in Snow-Covered Environments. Geogr. Compass 2011, 5, 682–699. [Google Scholar] [CrossRef]
- Arslantaş, E.E.; Yeşilırmak, E. Changes in the climatic growing season in western Anatolia, Turkey. Meteorol. Appl. 2020, 27, e1897. [Google Scholar] [CrossRef]
- Kunkel, K.E.; Easterling, D.R.; Hubbard, K.; Redmond, K. Temporal variations in frost-free season in the United States: 1895–2000. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Raich, J.W.; Potter, C.S.; Bhagawati, D. Interannual variability in global soil respiration, 1980–94. Glob. Chang. Biol. 2002, 8, 800–812. [Google Scholar] [CrossRef]
- Raich, J.W.; Potter, C.S. Global patterns of carbon dioxide emissions from soils. Glob. Biogeochem. Cycles 1995, 9, 23–36. [Google Scholar] [CrossRef]
- Dong, Y.; Scharffe, D.; Lobert, J.M.; Crutzen, P.J.; Sanhueza, E. Fluxes of CO2, CH4 and N2O from a temperate forest soil: The effects of leaves and humus layers. Tellus B 1998, 50, 243–252. [Google Scholar] [CrossRef]
- Wu, X.; Brüggemann, N.; Gasche, R.; Shen, Z.; Wolf, B.; Butterbach-Bahl, K. Environmental controls over soil-atmosphere exchange of N2O, NO, and CO2 in a temperate Norway spruce forest. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Guckland, A.; Corre, M.D.; Flessa, H. Variability of soil N cycling and N2O emission in a mixed deciduous forest with different abundance of beech. Plant Soil 2010, 336, 25–38. [Google Scholar] [CrossRef][Green Version]
- Smith, J.; Wagner-Riddle, C.; Dunfield, K. Season and management related changes in the diversity of nitrifying and denitrifying bacteria over winter and spring. Appl. Soil Ecol. 2010, 44, 138–146. [Google Scholar] [CrossRef]
- Öquist, M.G.; Nilsson, M.; Sörensson, F.; Kasimir-Klemedtsson, Å.; Persson, T.; Weslien, P.; Klemedtsson, L. Nitrous oxide production in a forest soil at low temperatures – processes and environmental controls. FEMS Microbiol. Ecol. 2004, 49, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.D.; Borken, W.; Gebauer, G. N2O emission in a Norway spruce forest due to soil frost: Concentration and isotope profiles shed a new light on an old story. Biogeochemistry 2010, 97, 21–30. [Google Scholar] [CrossRef]
- Dalal, R.; Allen, D. Greenhouse gas fluxes from natural ecosystems. Aust. J. Bot. 2008, 56, 396–407. [Google Scholar] [CrossRef]
- Xing, G.X.; Zhu, Z.L. Preliminary studies on N2O emission fluxes from upland soils and paddy soils in China. Nutr. Cycl. Agroecosyst. 1997, 49, 17–22. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Chen, G. Major factors controlling nitrous oxide emission and methane uptake from forest soil. J. For. Res. 2001, 12, 239–242. [Google Scholar] [CrossRef]
- Ferry, J.G. Methane: Small molecule, big impact. Science 1997, 278, 1413–1414. [Google Scholar] [CrossRef]
- Whalen, S.C.; Reeburgh, W.S. Methane oxidation, production, and emission at contrasting sites in a boreal bog. Geomicrobiol. J. 2000, 17, 237–251. [Google Scholar]
- Garcia-Montiel, D.; Melillo, J.; Steudler, P.A.; Cerri, C.C.; Piccolo, M.C. Carbon limitations to nitrous oxide emissions in a humid tropical forest of the Brazilian Amazon. Biol. Fertil. Soils 2003, 38, 267–272. [Google Scholar] [CrossRef]
- Kettunen, R.; Saarnio, S.; Martikainen, P.J.; Silvola, J. Can a mixed stand of N2-fixing and non-fixing plants restrict N2O emissions with increasing CO2 concentration? Soil Biol. Biochem. 2007, 39, 2538–2546. [Google Scholar] [CrossRef]
- Azam, F.; Gill, S.; Farooq, S. Availability of CO2 as a factor affecting the rate of nitrification in soil. Soil Biol. Biochem. 2005, 37, 2141–2144. [Google Scholar] [CrossRef]
- Megmw, S.; Knowles, R. Active methanotrophs suppress nitrification in a humisol. Biol. Fertil. Soils 1987, 4, 205–212. [Google Scholar] [CrossRef]
- Zhou, J.; Zang, H.; Loeppmann, S.; Gube, M.; Kuzyakov, Y.; Pausch, J. Arbuscular mycorrhiza enhances rhizodeposition and reduces the rhizosphere priming effect on the decomposition of soil organic matter. Soil Biol. Biochem. 2020, 140, 107641. [Google Scholar] [CrossRef]
- Huxman, T.E.; Snyder, K.A.; Tissue, D.; Leffler, A.J.; Ogle, K.; Pockman, W.T.; Sandquist, D.R.; Potts, D.L.; Schwinning, S. Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia 2004, 141, 254–268. [Google Scholar] [CrossRef]
- Stark, J.M.; Firestone, M.K. Mechanisms for soil moisture effects on activity of nitrifying bacteria. Appl. Environ. Microbiol. 1995, 61, 218–221. [Google Scholar] [CrossRef]
- Gao, D.; Hagedorn, F.; Zhang, L.; Liu, J.; Qu, G.; Sun, J.; Peng, B.; Fan, Z.; Zheng, J.; Jiang, P.; et al. Small and transient response of winter soil respiration and microbial communities to altered snow depth in a mid-temperate forest. Appl. Soil Ecol. 2018, 130, 40–49. [Google Scholar] [CrossRef]
- Wu, H.; Xu, X.; Duan, C.; Li, T.; Cheng, W. Synergistic effects of dissolved organic carbon and inorganic nitrogen on methane uptake in forest soils without and with freezing treatment. Sci. Rep. 2016, 6, 32555. [Google Scholar] [CrossRef][Green Version]
- Gulledge, J.; Hrywna, Y.; Cavanaugh, C.; Steudler, P.A. Effects of long-term nitrogen fertilization on the uptake kinetics of atmospheric methane in temperate forest soils. FEMS Microbiol. Ecol. 2004, 49, 389–400. [Google Scholar] [CrossRef]
- Ni, X.; Groffman, P.M. Declines in methane uptake in forest soils. Proc. Natl. Acad. Sci. USA 2018, 115, 8587–8590. [Google Scholar] [CrossRef]
- Macdonald, J.A.; Eggleton, P.; Bignell, D.E.; Forzi, F.; Fowler, D. Methane emission by termites and oxidation by soils, across a forest disturbance gradient in the Mbalmayo Forest Reserve, Cameroon. Glob. Chang. Biol. 1998, 4, 409–418. [Google Scholar] [CrossRef]
- Sgouridis, F.; Ullah, S. Soil greenhouse gas fluxes, environmental controls, and the partitioning of N2O sources in UK natural and seminatural land use types. J. Geophys. Res. Biogeosci. 2017, 122, 2617–2633. [Google Scholar] [CrossRef]
- Borken, W.; Davidson, E.A.; Savage, K.; Sundquist, E.T.; Steudler, P. Effect of summer throughfall exclusion, summer drought, and winter snow cover on methane fluxes in a temperate forest soil. Soil Biol. Biochem. 2006, 38, 1388–1395. [Google Scholar] [CrossRef]
- Gao, B.; Ju, X.; Su, F.; Meng, Q.; Oenema, O.; Christie, P.; Chen, X.; Zhang, F. Nitrous oxide and methane emissions from optimized and alternative cereal cropping systems on the North China Plain: A two-year field study. Sci. Total Environ. 2014, 472, 112–124. [Google Scholar] [CrossRef]
- Song, W.; Wang, H.; Guangshuai, W.; Chen, L.; Jin, Z.; Zhuang, Q.; He, J.-S. Methane emissions from an alpine wetland on the Tibetan Plateau: Neglected but vital contribution of non-growing season. J. Geophys. Res. Biogeosci. 2015. [Google Scholar] [CrossRef]
- Granberg, G.; Ottosson-Löfvenius, M.; Grip, H.; Sundh, I.; Nilsson, M. Effect of climatic variability from 1980 to 1997 on simulated methane emission from a boreal mixed mire in northern Sweden. Glob. Biogeochem. Cycles 2001, 15, 977–991. [Google Scholar] [CrossRef]
- Kitzler, B.; Zechmeister-Boltenstern, S.; Holtermann, C.; Skiba, U.; Butterbach-Bahl, K. Nitrogen oxides emission from two beech forests subjected to different nitrogen loads. Biogeosciences 2006, 3, 293–310. [Google Scholar] [CrossRef]
- Braker, G.; Conrad, R. Chapter 2—Diversity, Structure, and Size of N2O-Producing Microbial Communities in Soils—What Matters for Their Functioning? In Advances in Applied Microbiology; Laskin, A.I., Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 75, pp. 33–70. [Google Scholar]
- Wertz, S.; Goyer, C.; Zebarth, B.J.; Burton, D.L.; Tatti, E.; Chantigny, M.H.; Filion, M. Effects of temperatures near the freezing point on N2O emissions, denitrification and on the abundance and structure of nitrifying and denitrifying soil communities. FEMS Microbiol. Ecol. 2013, 83, 242–254. [Google Scholar] [CrossRef]
- Smith, K.A. Changing views of nitrous oxide emissions from agricultural soil: Key controlling processes and assessment at different spatial scales. Eur. J. Soil Sci. 2017, 68, 137–155. [Google Scholar] [CrossRef]
- Davidson, E.A.; Vitousek, P.M.; Matson, P.A.; Riley, R.; García-Méndez, G.; Maass, J.M. Soil emissions of nitric oxide in a seasonally dry tropical forest of México. J. Geophys. Res. Atmos. 1991, 96, 15439–15445. [Google Scholar] [CrossRef]
- Shine, K.P.; Fuglestvedt, J.S.; Hailemariam, K.; Stuber, N. Alternatives to the Global Warming Potential for comparing climate impacts of emissions of greenhouse gases. Clim. Chang. 2005, 68, 281–302. [Google Scholar] [CrossRef]

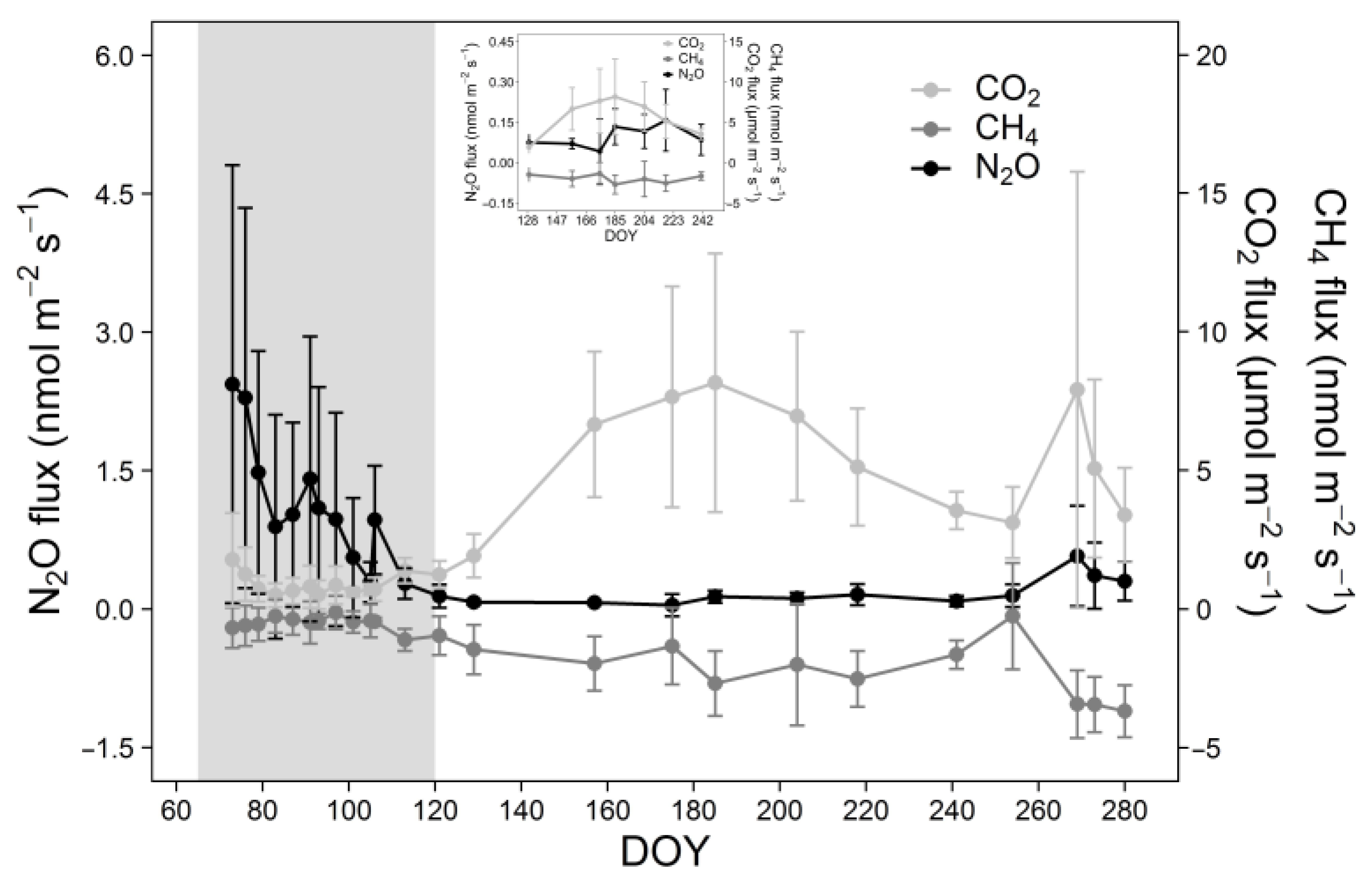

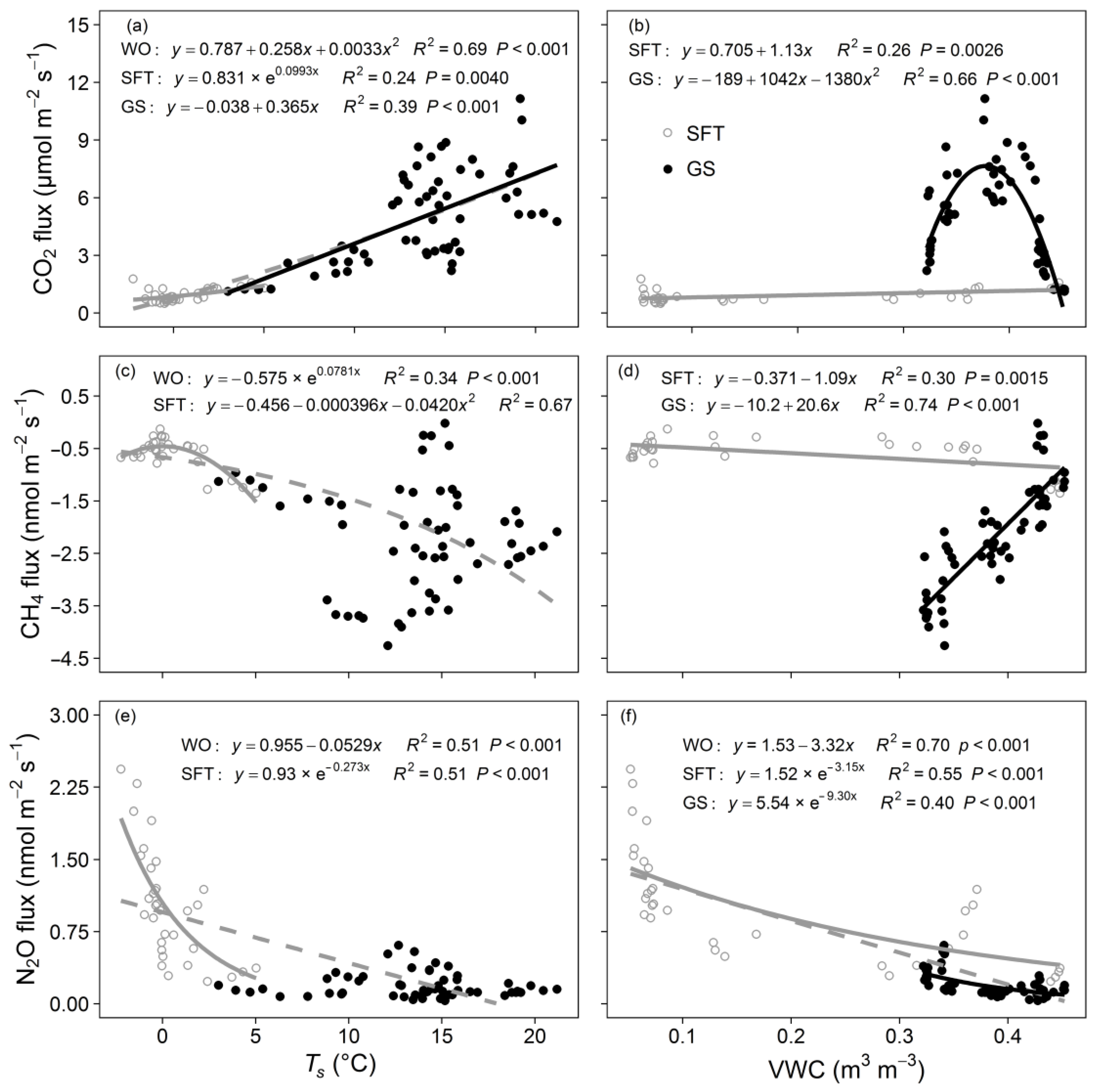

| GHG | Average Flux (nmol m−2 s−1) | Cumulative Fluxes (mmol m−2) | ||
|---|---|---|---|---|
| Spring Freeze—Thaw (SFT) Period | Growing Season (GS) | SFT Period | GS | |
| CO2 | 888.61 ± 423.98 | 5476.38 ± 2609.98 | 4299.48 (5.2%) | 79,017.50 (94.8%) |
| CH4 | −0.50 ± 0.28 | −2.24 ± 0.78 | −2.43 (7.0%) | −32.29 (93.0%) |
| N2O | 1.15 ± 0.82 | 0.22 ± 0.14 | 5.57 (64.7%) | 3.05 (35.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Zhang, L.; Li, S.; Li, Q.; Dai, G. Comparison of Soil Greenhouse Gas Fluxes during the Spring Freeze–Thaw Period and the Growing Season in a Temperate Broadleaved Korean Pine Forest, Changbai Mountains, China. Forests 2020, 11, 1135. https://doi.org/10.3390/f11111135
Guo C, Zhang L, Li S, Li Q, Dai G. Comparison of Soil Greenhouse Gas Fluxes during the Spring Freeze–Thaw Period and the Growing Season in a Temperate Broadleaved Korean Pine Forest, Changbai Mountains, China. Forests. 2020; 11(11):1135. https://doi.org/10.3390/f11111135
Chicago/Turabian StyleGuo, Chuying, Leiming Zhang, Shenggong Li, Qingkang Li, and Guanhua Dai. 2020. "Comparison of Soil Greenhouse Gas Fluxes during the Spring Freeze–Thaw Period and the Growing Season in a Temperate Broadleaved Korean Pine Forest, Changbai Mountains, China" Forests 11, no. 11: 1135. https://doi.org/10.3390/f11111135
APA StyleGuo, C., Zhang, L., Li, S., Li, Q., & Dai, G. (2020). Comparison of Soil Greenhouse Gas Fluxes during the Spring Freeze–Thaw Period and the Growing Season in a Temperate Broadleaved Korean Pine Forest, Changbai Mountains, China. Forests, 11(11), 1135. https://doi.org/10.3390/f11111135






