Influence of Climate on Carbon Sequestration in Conifers Growing under Contrasting Hydro-Climatic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Species Studied and Field Sampling
2.3. Total Ring Width
2.4. Estimation of Biomass and Carbon Accumulation
2.5. Statistical Analysis
3. Results
3.1. Growth Characteristics and Carbon Accumulation
3.2. Internal and External Influences on Carbon Accumulation
3.3. Climate Sensitivity in Carbon Accumulation
3.4. Relationships between Drought and Carbon Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- OCDE. Informe de la Cooperación para el Desarrollo 2014: Movilizar Recursos para un Desarrollo Sostenible, Ediciones OCDE; OCDE: Paris, France, 2014. [Google Scholar]
- González-Elizondo, M.S.; González-Elizondo, M.; López-Enríquez, I.L.; Tena-Flores, J.A.; Márquez-Linares, M.A. Cambios y tendencias sucesionales en ecosistemas de Durango. Vidsupra 2005, 1, 5–11. [Google Scholar]
- Woodall, C.W.; Westfall, J.A.; D’Amato, A.W.; Foster, J.R.; Walters, B.F. Decadal changes in tree range stability across forests of the eastern U.S. For. Ecol. Manag. 2018, 429, 503–510. [Google Scholar] [CrossRef]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D.; et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang. 2012, 3, 292–297. [Google Scholar] [CrossRef]
- Freeman, A.M. The measurement of environmental and resource values. In Theory and Methods; Resources for the Future Press: Washington, DC, USA, 2003. [Google Scholar]
- Hassan, R.; Scholes, N.; Ash, N. (Eds.) Ecosystems and Human Well-Being: Current State and Trends; Island Press: Washington, DC, USA, 2005; Volume 1. [Google Scholar]
- Brockerhoff, E.G.; Barbaro, L.; Castagneyrol, B.; Forrester, D.I.; Gardiner, B.; González-Olabarria, J.R.; Lyver, P.; Meurisse, N.; Oxbrough, A.; Taki, H.; et al. Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodivers. Conserv. 2017, 26, 3005–3035. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Brienen, R.J.W.; Phillips, L.; Feldpausch, R.; Gloor, E.; Baker, R.; Lloyd, J.; Lopez-Gonzalez, G.; Monteagudo-Mendoza, A.; Malhi, Y.; Lewis, S.L.; et al. Long-term decline of the Amazon carbon sink. Nature 2015, 519, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Sabine, C.L.; Feely, R.A.; Gruber, N.; Key, R.M.; Lee, K.; Bullister, J.L.; Wanninkhof, R.; Wong, C.S.; Wallace, D.W.R.; Tilbrook, B.; et al. The oceanic sink for anthropogenic CO2. Science 2004, 305, 367–371. [Google Scholar] [CrossRef]
- Solomon, S. Climate Change 2007-The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Shimamoto, C.Y.; Botosso, P.C.; Marques, M. How much carbon is sequestered during the restoration of tropical forests? Estimates from tree species in the Brazilian Atlantic forest. For. Ecol. Manag. 2014, 329, 1–9. [Google Scholar] [CrossRef]
- Gernandt, D.S.; Pérez-de la Rosa, J.A. Biodiversidad de Pinophyta (coníferas) en México. Rev. Mex. Biodivers. 2014, 85, 126–133. [Google Scholar] [CrossRef]
- Cartus, O.; Kellndorfer, J.; Walker, W.; Franco, C.; Bishop, J.; Santos, L.; Fuentes, J.M. A national, detailed map of forest aboveground carbon stocks in Mexico. Remote Sens. 2014, 6, 5559–5588. [Google Scholar] [CrossRef]
- González-Elizondo, M.S.; González-Elizondo, M.; Tena-Flores, J.A.; Ruacho-González, L.; López-Enríquez, I.L. Vegetación de la Sierra Madre Occidental, México: Una síntesis. Acta Bot. Mex. 2012, 100, 351–403. [Google Scholar] [CrossRef]
- Douterlungne, D.; Herrera-Gorocica, A.M.; Ferguson, B.G.; Siddique, I.; Soto-Pinto, L. Ecuaciones alométricas para estimar biomasa y carbono de cuatro especies leñosas neotropicales con potencial para la restauración. Agrociencia 2013, 47, 385–397. [Google Scholar]
- Gómez-Díaz, J.D.; Etchevers-Barra, J.D.; Monterrosos-Rivas, A.I.; Campo-Alvez, J.; Tinoco-Rueda, J.A. Ecuaciones alométricas para estimar biomasa y carbono en Quercus magnoliaefolia. Rev. Chapingo Ser. Cienc. For. Ambiente 2011, 17, 261–272. [Google Scholar] [CrossRef]
- Navar, J. Allometric equations for tree species and carbon stocks for forests of northwestern Mexico. For. Ecol. Manag. 2009, 257, 427–434. [Google Scholar] [CrossRef]
- Pompa-García, M.; Sigala-Rodríguez, J.A.; Jurado, E.; Flores, J. Tissue carbón concentration of 175 Mexican forest species. iForest 2017, 10, 754–758. [Google Scholar] [CrossRef]
- Sánchez-González, A. Una visión actual de la diversidad y distribución de los pinos de México. Madera Bosques 2008, 14, 107–120. [Google Scholar] [CrossRef]
- Van der Putten, W.H. Climate change, aboveground-belowground interactions, and species range shifts. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 365–383. [Google Scholar] [CrossRef]
- Suarez, L.M.; Kitzberger, T. Differential effects of climate variability on forest dynamics along a precipitation gradient in northern Patagonia. J. Ecol. 2010, 98, 1023–1034. [Google Scholar] [CrossRef]
- Babst, F.; Alexander, M.R.; Szejner, P.; Bouriaud, O.; Klesse, S.; Roden, J.; Ciais, P.; Poulter, B.; Frank, D.; Moore, D.J.P.; et al. A tree-ring perspective on the terrestrial carbon cycle. Oecologia 2014, 176, 307–322. [Google Scholar] [CrossRef]
- Babst, F.; Bouriaud, O.; Papale, D.; Gielen, B.; Janssens, A.; Nikinmaa, E.; Ibrom, A.; Wu, J.; Bernhofer, C.; Kostner, B.; et al. Aboveground woody carbon sequestration measured from tree rings is coherent with net ecosystem productivity at five eddy covariance sites. New Phytol. 2014, 201, 1289–1303. [Google Scholar] [CrossRef]
- Pompa-García, M.; Sigala, R.J.A. Variation of carbon uptake from forest species in Mexico: A review. Madera Bosques 2017, 23, 225–235. [Google Scholar] [CrossRef][Green Version]
- Acosta-Hernández, A.C.; Pompa-García, M.; Camarero, J.J. An updated review of dendrochronological investigations in Mexico, a megadiverse Country with a high potential for tree-ring sciences. Forests 2017, 8, 160. [Google Scholar] [CrossRef]
- Pompa-García, M.; Venegas-González, A.; Júnior, A.A.; Sigala-Rodríguez, J.A. Dendroecological approach to assessing carbon accumulation dynamics in two Pinus species from northern Mexico. Tree Ring Res. 2018, 74, 196–209. [Google Scholar] [CrossRef]
- Descroix, L.; Barrios, J.L.G.; Ávalos, J.E. La Sierra Madre Occidental: Una Fuente de Agua Amenazada; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias: Gómez Palacio, México, 2004. [Google Scholar]
- Rzedowski, J. Vegetación de México; Limusa: Ciudad de México, Mexico, 1978. [Google Scholar]
- García-Arévalo, A. Vegetación y flora de un bosque relictual de Picea chihuahuana Martínez del norte de México. Polibotánica 2008, 25, 45–68. [Google Scholar]
- González-Cásares, M.; Pompa-García, M.; Camarero, J.J. Differences in climate–growth relationship indicate diverse drought tolerances among five pine species coexisting in Northwestern Mexico. Trees 2016, 31, 531–544. [Google Scholar] [CrossRef]
- Yocom, L.L.; Fulé, P.Z.; Falk, D.A.; García-Domínguez, C.; Cornejo-Oviedo, E.; Brown, P.M.; Villanueva-Díaz, J.; Cerano, J.; Cortés, C. Fine-scale factors influence fire regimes in mixed-conifer forests on three high mountains in Mexico. Int. J. Wildland Fire 2014, 23, 959–968. [Google Scholar] [CrossRef]
- Farjon, A.; Styles, B.T. Flora Neotropica. Pinus (Pinaceae); New York Botanical Garden: New York, NY, USA, 1997. [Google Scholar]
- Richardson, D.M.; Rundel, P.W. Ecology and biogeography of Pinus: An introduction. In Ecology and Biogeography of Pinus; Cambridge University Press: Cambridge, UK, 1998; pp. 3–46. [Google Scholar]
- García, A.A.; González, E.M.S. Pináceas de Durango; Comisión Nacional Forestal, Instituto de Ecología A.C.: Zapopan, Mexico, 2003. [Google Scholar]
- Nehrbass-Ahles, C.; Babst, F.; Klesse, S.; Nötzli, M.; Bouriaud, O.; Neukom, R.; Dobbertin, M.; Franket, D. The influence of sampling design on tree-ring-based quantification of forest growth. Glob. Chang. Biol. 2014, 20, 2867–2885. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.A.; Smiley, T.L. Tree-Ring Dating; The University of Chicago Press: Chicago, IL, USA, 1968. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in treering dating and measurement. Tree Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Peters, R.L.; Klesse, S.; Fonti, P.; Frank, D.C. Contribution of climate vs. larch budmoth outbreaks in regulating biomass accumulation in high-elevation forests. For. Ecol. Manag. 2017, 401, 147–158. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Linear mixed-effects models: Basic concepts and examples. In Mixed-Effects Models in S and S-Plus; Springer: Berlin/Heidelberg, Germany, 2000; pp. 3–56. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Version 3.0.1; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org (accessed on 3 November 2018).
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Bunn, A.G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Merian, P.; Qeadan, F. dplR: Dendrochronology Program Library in R. R Package Version 1.6.3. 2015. Available online: https://CRAN.R-project.org/package=dplR (accessed on 21 January 2015).
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Beguería, S.; Vicente-Serrano, S.M.; Reig, F.; Latorre, B. Standardized precipitation evapotranspiration index (SPEI) revisited: Parameter fitting, evapotranspiration models, tools, datasets and drought monitoring. Int. J. Climatol. 2014, 34, 3001–3023. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Shuman, J.K.; Shugart, H.H.; Krankina, O.N. Assessment of carbon stores in tree biomass for two management scenarios in Russia. Environ. Res. Lett. 2013, 8, 045019. [Google Scholar] [CrossRef]
- Chang, K.; Price, D.T.; Chena, J.M.; Kurzc, W.A.; Boisvenuec, C.; Hogg, E.H.; Black, T.A.; Gonsamoa, A.; Wua, C.; Hember, R.A. Simulating impacts of water stress on woody biomass in the southern boreal region of western Canada using a dynamic vegetation model. Agric. For. Meteorol. 2014, 198, 142–154. [Google Scholar] [CrossRef]
- Pompa-García, M.; González-Cásares, M.; Acosta-Hernández, A.C.; Camarero, J.J.; Rodríguez-Catón, M. Drought influence over radial growth of mexican conifers inhabiting mesic and xeric sites. Forests 2017, 8, 175. [Google Scholar] [CrossRef]
- Rodríguez-Catón, M.; Villalba, R.; Morales, M.; Srur, A. Influence of droughts on Nothofagus pumilio forest decline across northern Patagonia, Argentina. Ecosphere 2016, 7, e01390. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Juday, G.P.; Alix, C.; Grant, T.A. Spatial coherence and change of opposite white spruce temperature sensitivities on floodplains in Alaska confirms early-stage boreal biome shift. For. Ecol. Manag. 2015, 350, 46–61. [Google Scholar] [CrossRef]
- Looney, C.E.; Waring, K.M. Pinus strobiformis (southwestern white pine) stand dynamics, regeneration, and disturbance ecology: A review. For. Ecol. Manag. 2013, 287, 90–102. [Google Scholar] [CrossRef]
- Cram, D.; Saud, P.; Baker, T. Structure and Composition of a Dry Mixed-Conifer Forest in Absence of Contemporary Treatments, Southwest, USA. Forests 2017, 8, 349. [Google Scholar] [CrossRef]
- Thomas, C.D. Translocation of species, climate change, and the end of trying to recreate past ecological communities. Trends Ecol. Evol. 2011, 26, 216–221. [Google Scholar] [CrossRef]
- Campelo, F.; Vieira, J.; Nabais, C. Tree-ring growth and intra-annual density fluctuations of Pinus pinaster responses to climate: Does size matter? Trees 2013, 27, 763–772. [Google Scholar] [CrossRef]
- Rehfeldt, G.E.; Jaquish, B.C.; López-Upton, J.; Sáenz-Romero, C.; St Clair, J.B.; Leites, L.P.; Joyce, D.G. Comparative genetic responses to climate for the varieties of Pinus ponderosa and Pseudotsuga menziesii: Realized climate niches. For. Ecol. Manag. 2014, 324, 126–137. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Dragoni, D.; Schmid, H.P.; Rahman, A.F.; Sims, D.; Wayson, C.A.; Johnson, D.; Phillips, R.P. Chronic water stress reduces tree growth and the carbon sink of deciduous hardwood forests. Glob. Chang. Biol. 2014, 20, 2531–2539. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef]
- Pompa-García, M.; Venegas-González, A. Temporal variation of wood density and carbon in two elevational sites of Pinus cooperi in relation to climate response in northern Mexico. PLoS ONE 2016, 11, e0156782. [Google Scholar] [CrossRef] [PubMed]
- Britez, M.R.D.; Sergent, A.S.; Martinez, A.M.; Bréda, N.; Rozenberg, P. Wood density proxies of adaptive traits linked with resistance to drought in Douglas fir (Pseudotsuga menziesii (Mirb.) Franco). Trees 2014, 28, 1289–1304. [Google Scholar] [CrossRef]
- Goodrich, B.A.; Waring, K.M.; Kolb, T.E. Genetic variation in Pinus strobiformis growth and drought tolerance from southwestern US populations. Tree Physiol. 2016, 36, 1219–1235. [Google Scholar] [CrossRef] [PubMed]
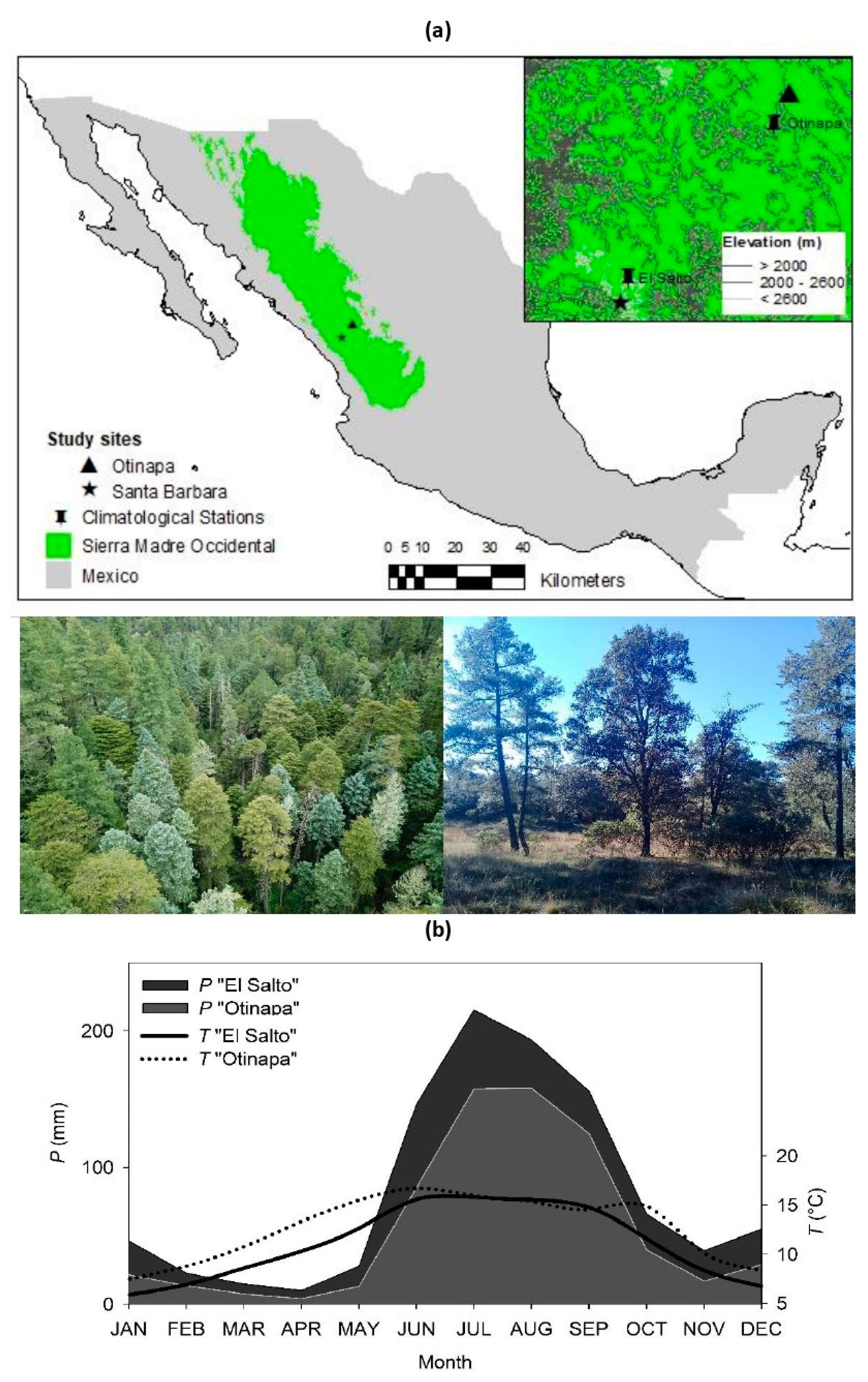

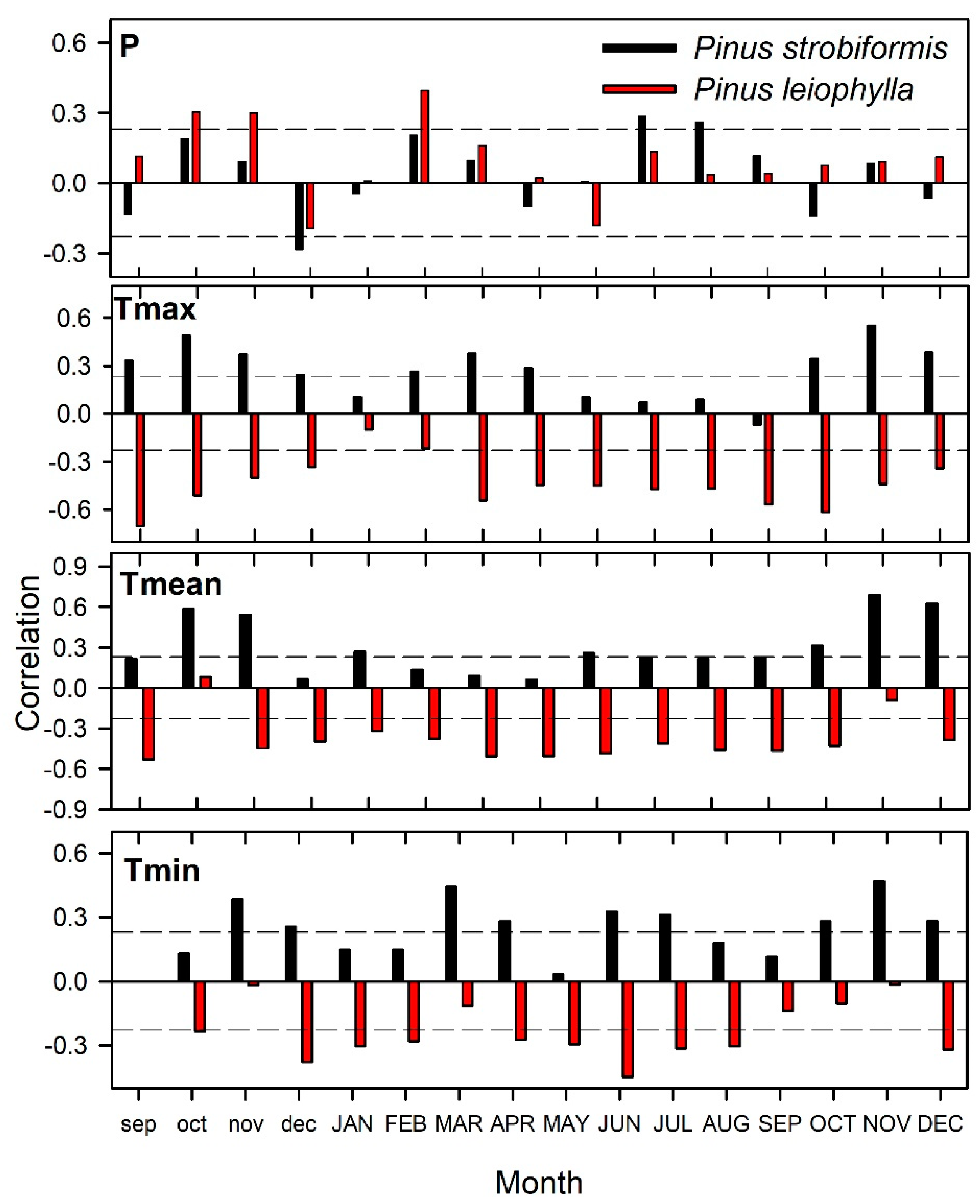
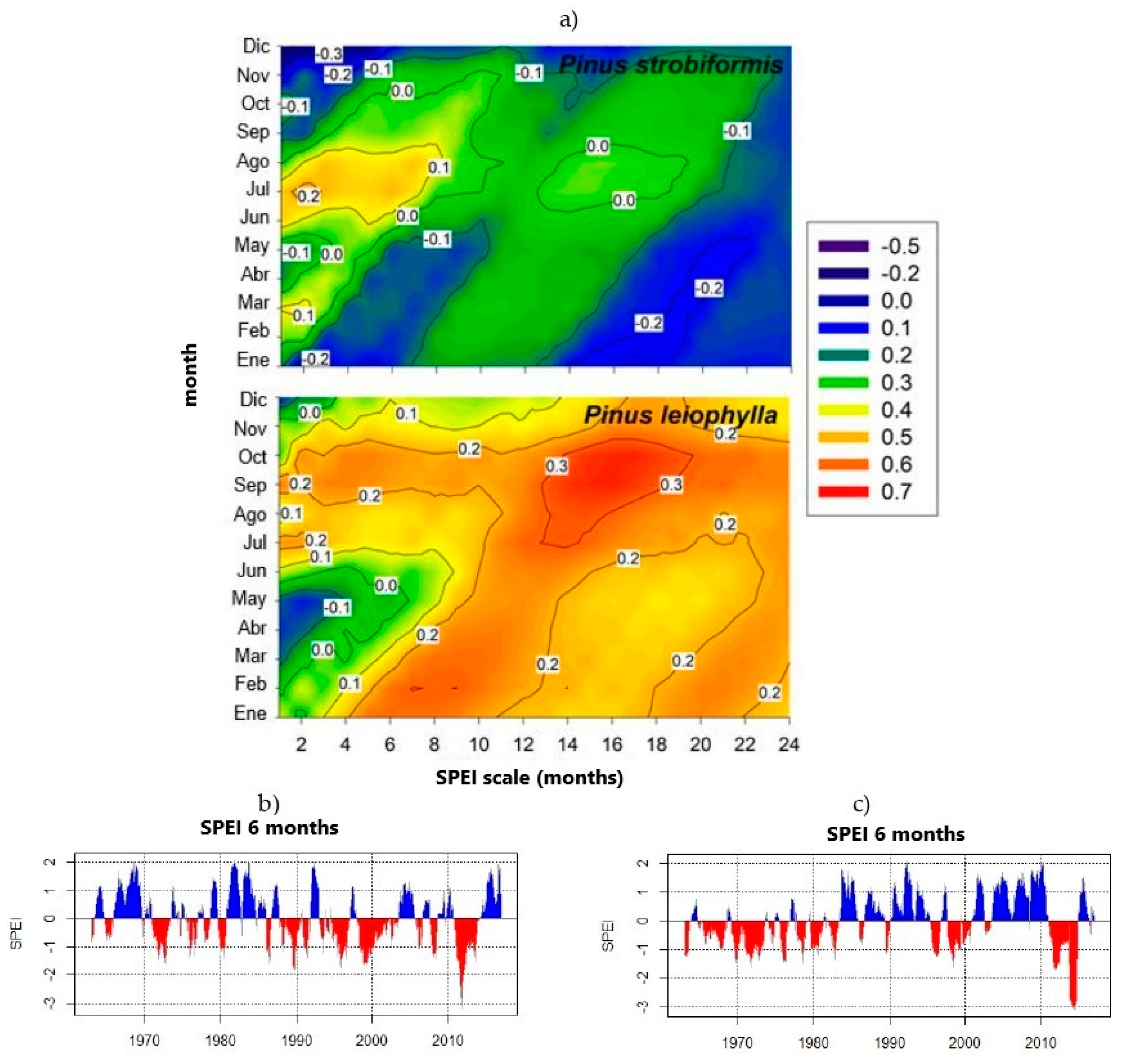
| Species | Pinus strobiformis | Pinus leiophylla |
|---|---|---|
| Site | Santa Bárbara | Otinapa |
| Coordinates | 23°41′ N 105°25′ W | 23°58′ N 104°57′ W |
| Elevation (m) | 2844 | 2430 |
| Diameter at breast height (cm) | 38.13 ± 1.59 | 34.93 ± 1.56 |
| Height (m) | 13.67 ± 0.58 | 11.33 ± 0.75 |
| Amplitude of the chronology | 1945–2016 | 1934–2016 |
| Age (years) | 49 ± 3.11 | 52 ± 1.85 |
| Ring width (mm) | 3.23 ± 0.20 | 2.49 ± 0.11 |
| Accumulated carbon (g cm−2) | 4.65 ± 0.13 | 3.05 ± 0.07 |
| Pinus strobiformis | Pinus leiophylla | |||
|---|---|---|---|---|
| Estimator | SE | Estimator | SE | |
| Fixed parameters | ||||
| Intercept | −3.83 × 10−3 | 3.22 × 10−1 | −6.16 × 10−1 d | 2.64 × 10−1 |
| TRW | 2.725 a | 8.00 × 10−2 | 2.46 a | 9.07 × 10−2 |
| Age | 3.67 × 10−2 a | 7.11 × 10−4 | 2.94 × 10−2 a | 1.10 × 10−3 |
| Pp | −2.65 × 10−5 | 3.94 × 10−5 | 1.59 × 10−4 d | 6.25 × 10−5 |
| Tmax | −2.08 × 10−3 | 1.30 × 10−2 | 1.08 × 10−2 | 9.46 × 10−3 |
| Tmed | −1.58 × 10−4 | 2.02 × 10−2 | −2.07 × 10−2 c | 6.87 × 10−3 |
| Tmin | 1.32 × 10−3 | 1.34 × 10−2 | −1.83 × 10−2 d | 8.57 × 10−3 |
| PpPY | −2.3 × 10−6 | 3.97 × 10−5 | 2.62 × 10−4 a | 5.88 × 10−5 |
| TmaxPY | −1.81 × 10−3 | 1.31 × 10−2 | 6.35 × 10−3 | 1.04 × 10−2 |
| TmedPY | −3.50 × 10−2 | 1.93 × 10−2 | 1.15 × 10−2 | 6.81 × 10−3 |
| TminPY | −8.34 × 10−3 | 1.34 × 10−2 | 1.87 × 10−2 d | 8.55 × 10−3 |
| Covariance parameters | ||||
| AIC | 106.1082 | 576.2677 | ||
| BIC | 168.1582 | 638.7421 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Hernández, A.C.; Padilla-Martínez, J.R.; Hernández-Díaz, J.C.; Prieto-Ruiz, J.A.; Goche-Telles, J.R.; Nájera-Luna, J.A.; Pompa-García, M. Influence of Climate on Carbon Sequestration in Conifers Growing under Contrasting Hydro-Climatic Conditions. Forests 2020, 11, 1134. https://doi.org/10.3390/f11111134
Acosta-Hernández AC, Padilla-Martínez JR, Hernández-Díaz JC, Prieto-Ruiz JA, Goche-Telles JR, Nájera-Luna JA, Pompa-García M. Influence of Climate on Carbon Sequestration in Conifers Growing under Contrasting Hydro-Climatic Conditions. Forests. 2020; 11(11):1134. https://doi.org/10.3390/f11111134
Chicago/Turabian StyleAcosta-Hernández, Andrea Cecilia, Jaime Roberto Padilla-Martínez, José Ciro Hernández-Díaz, José Angel Prieto-Ruiz, José Rodolfo Goche-Telles, Juan Abel Nájera-Luna, and Marín Pompa-García. 2020. "Influence of Climate on Carbon Sequestration in Conifers Growing under Contrasting Hydro-Climatic Conditions" Forests 11, no. 11: 1134. https://doi.org/10.3390/f11111134
APA StyleAcosta-Hernández, A. C., Padilla-Martínez, J. R., Hernández-Díaz, J. C., Prieto-Ruiz, J. A., Goche-Telles, J. R., Nájera-Luna, J. A., & Pompa-García, M. (2020). Influence of Climate on Carbon Sequestration in Conifers Growing under Contrasting Hydro-Climatic Conditions. Forests, 11(11), 1134. https://doi.org/10.3390/f11111134






