Predicting the Potential Distribution of Apple Canker Pathogen (Valsa mali) in China under Climate Change
Abstract
:1. Introduction
2. Materials and Methods
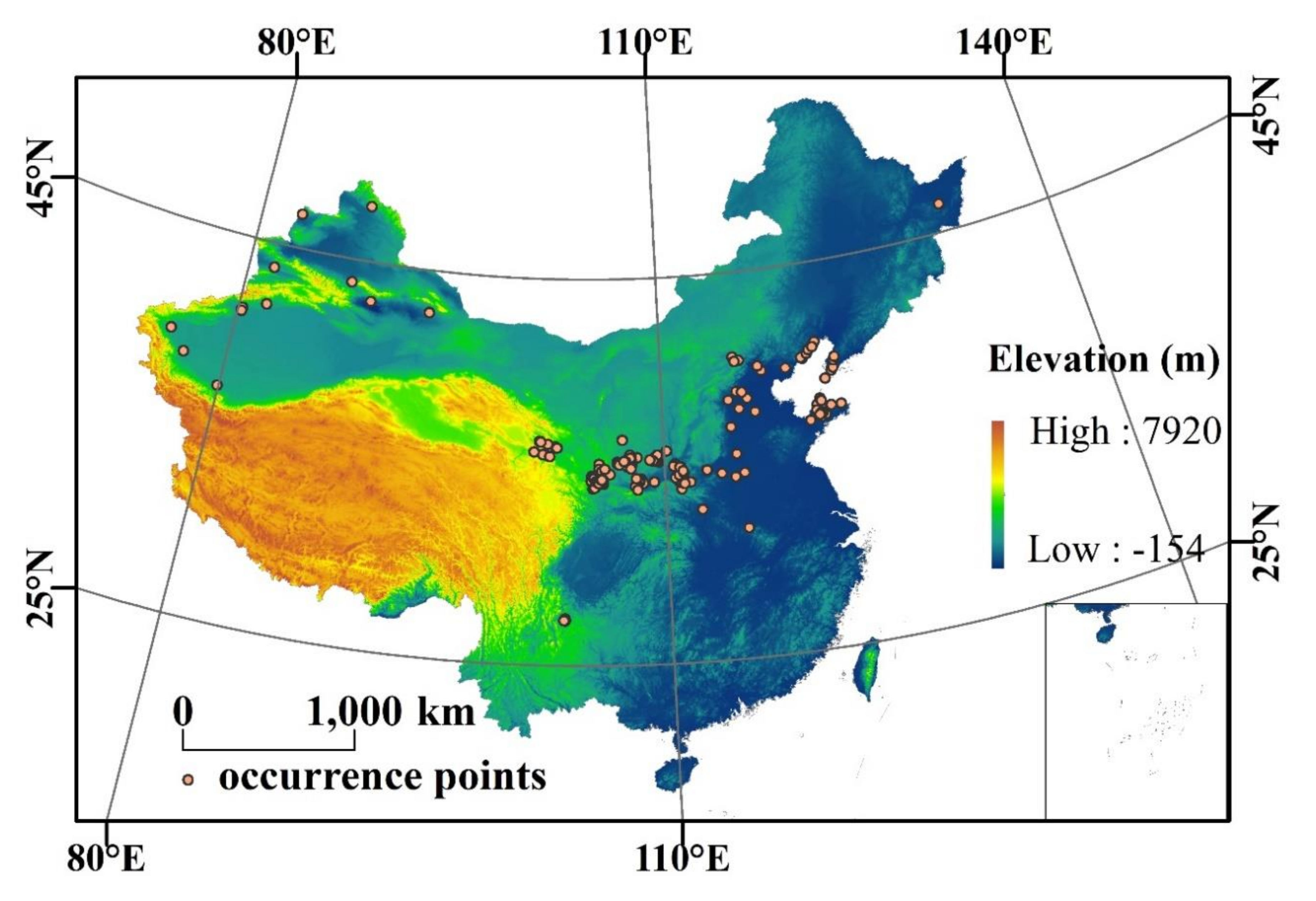
2.1. Species Occurrence Data and Environmental Variables
2.2. Climate Change Scenarios
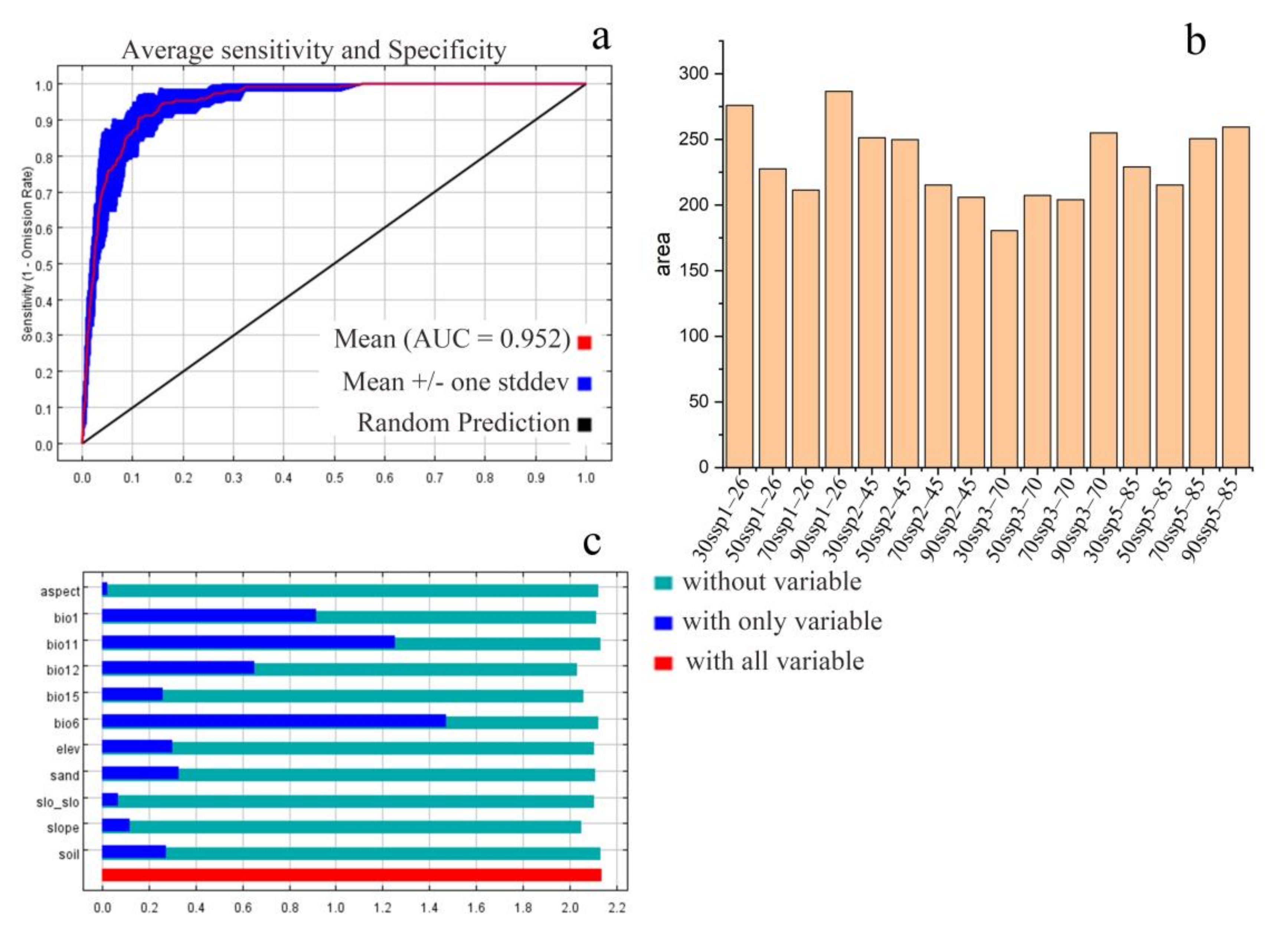
2.3. Species Distribution Model Evaluation
2.4. Spatial and Statistical Analysis
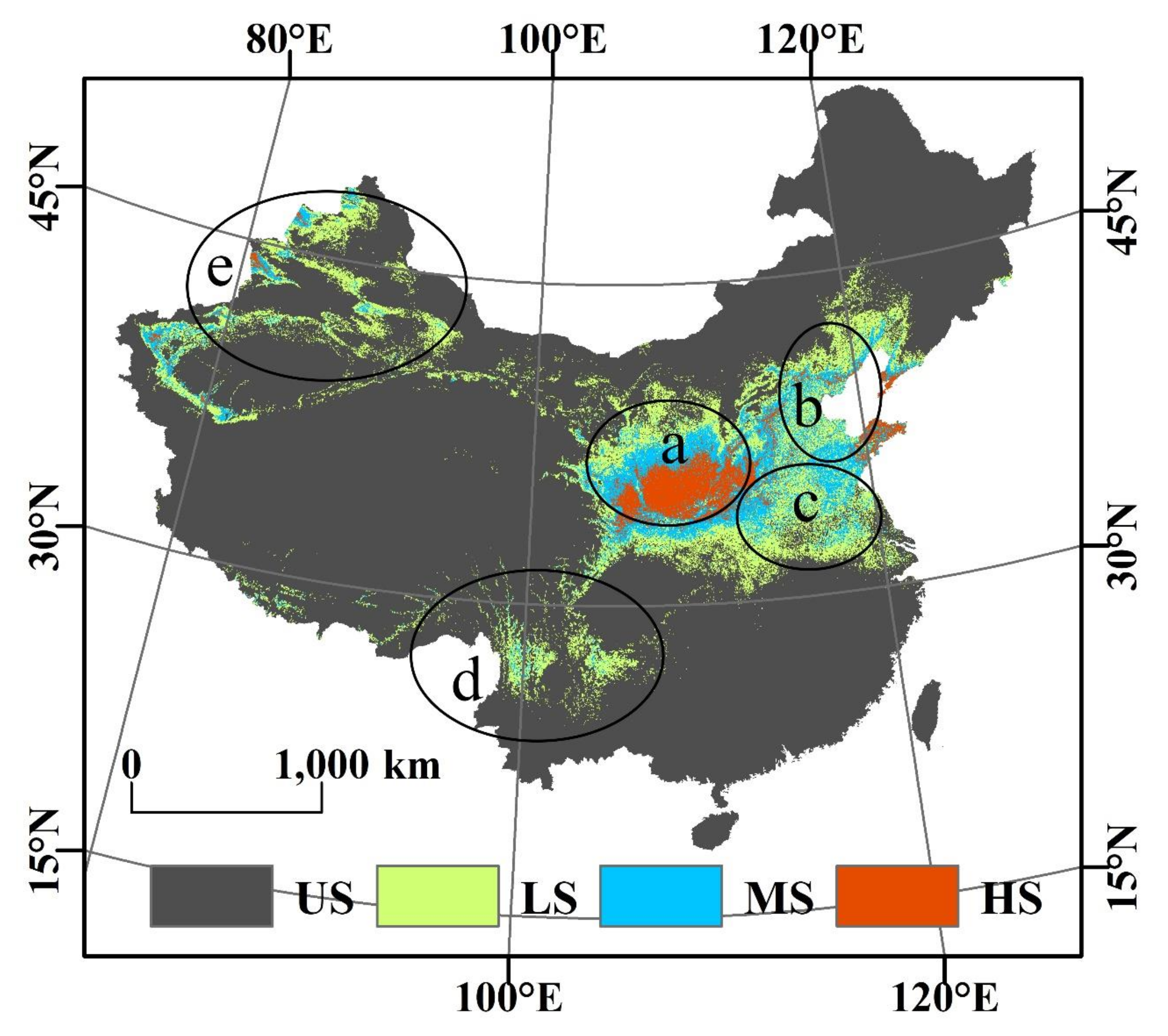
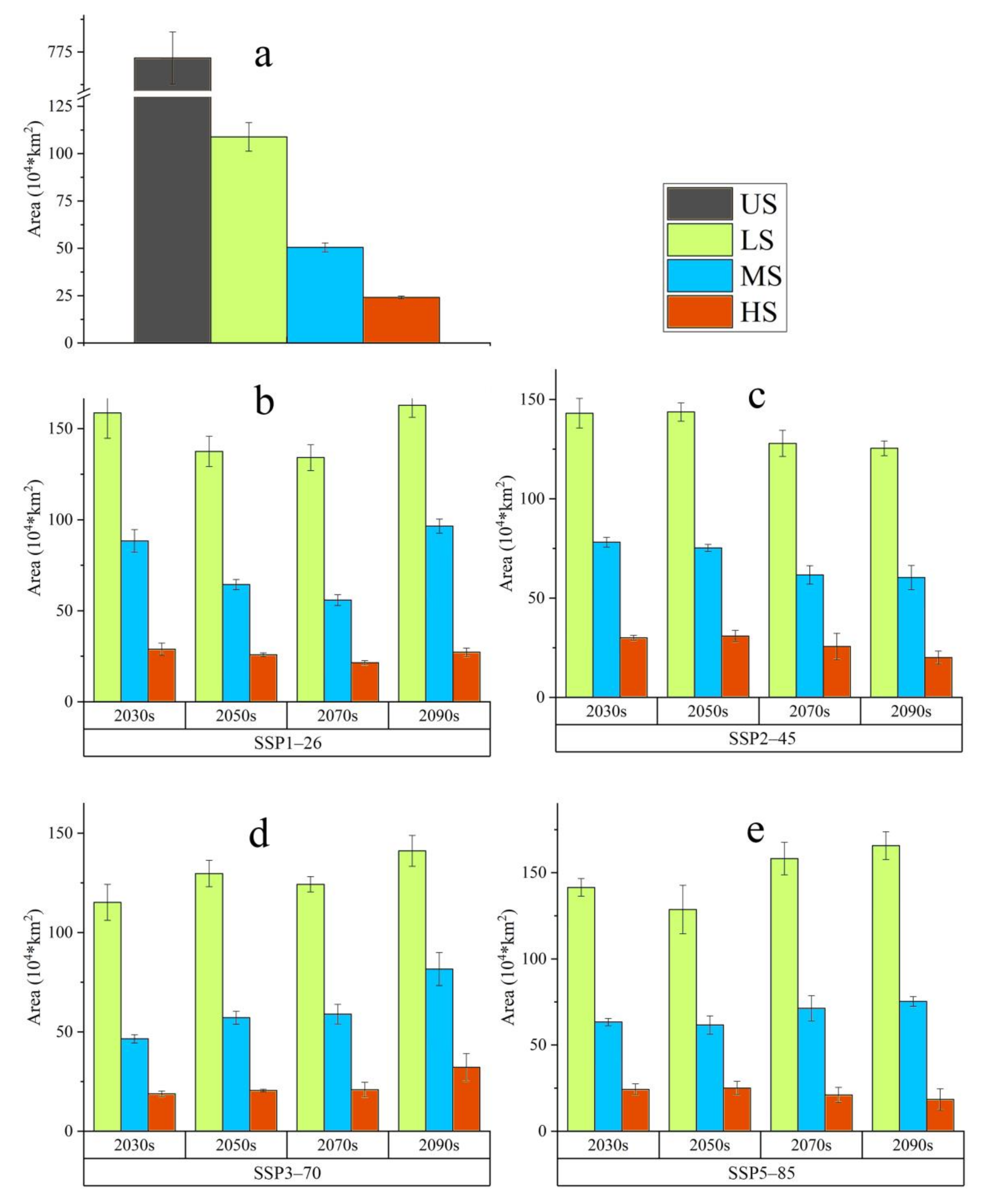
3. Results
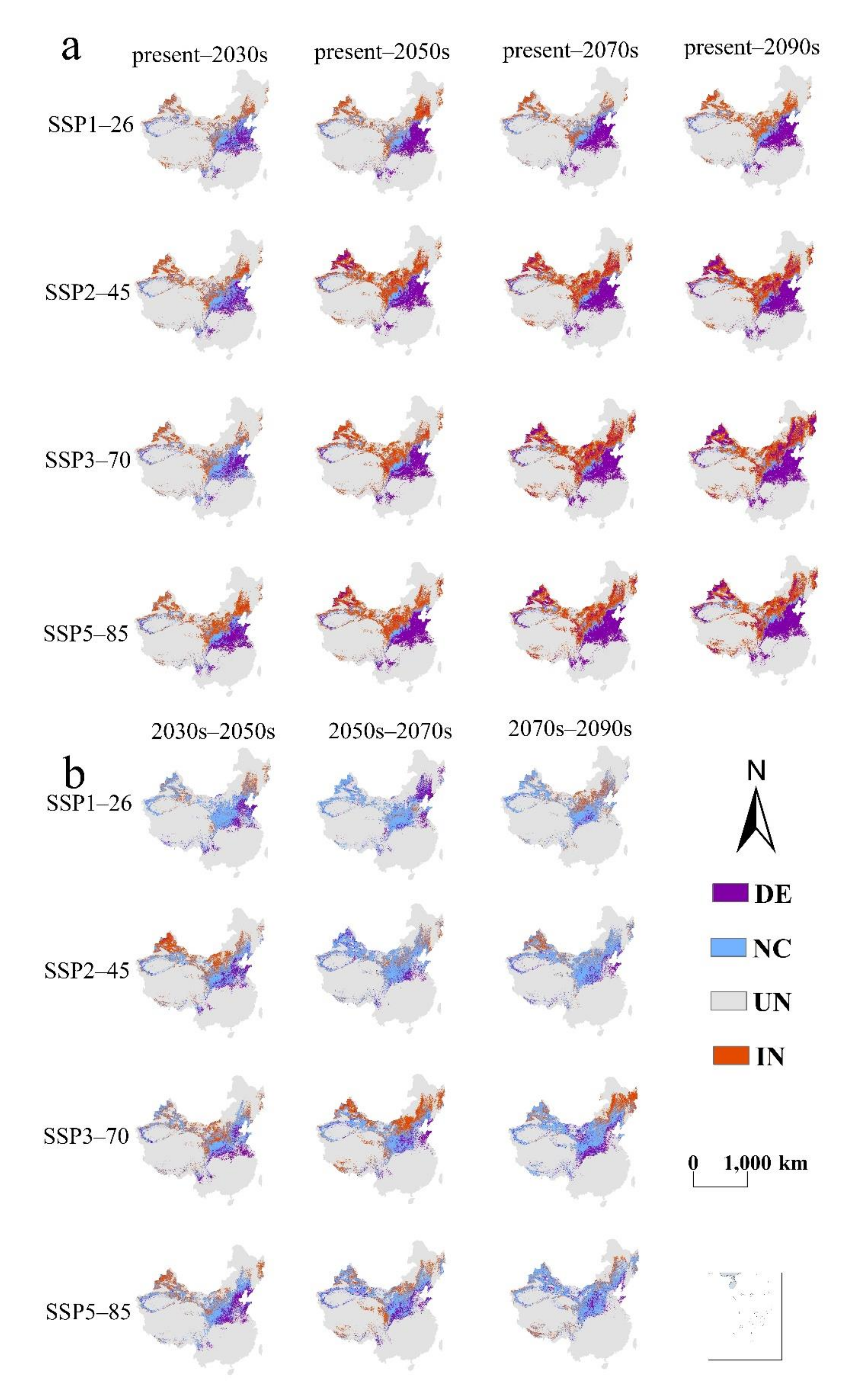
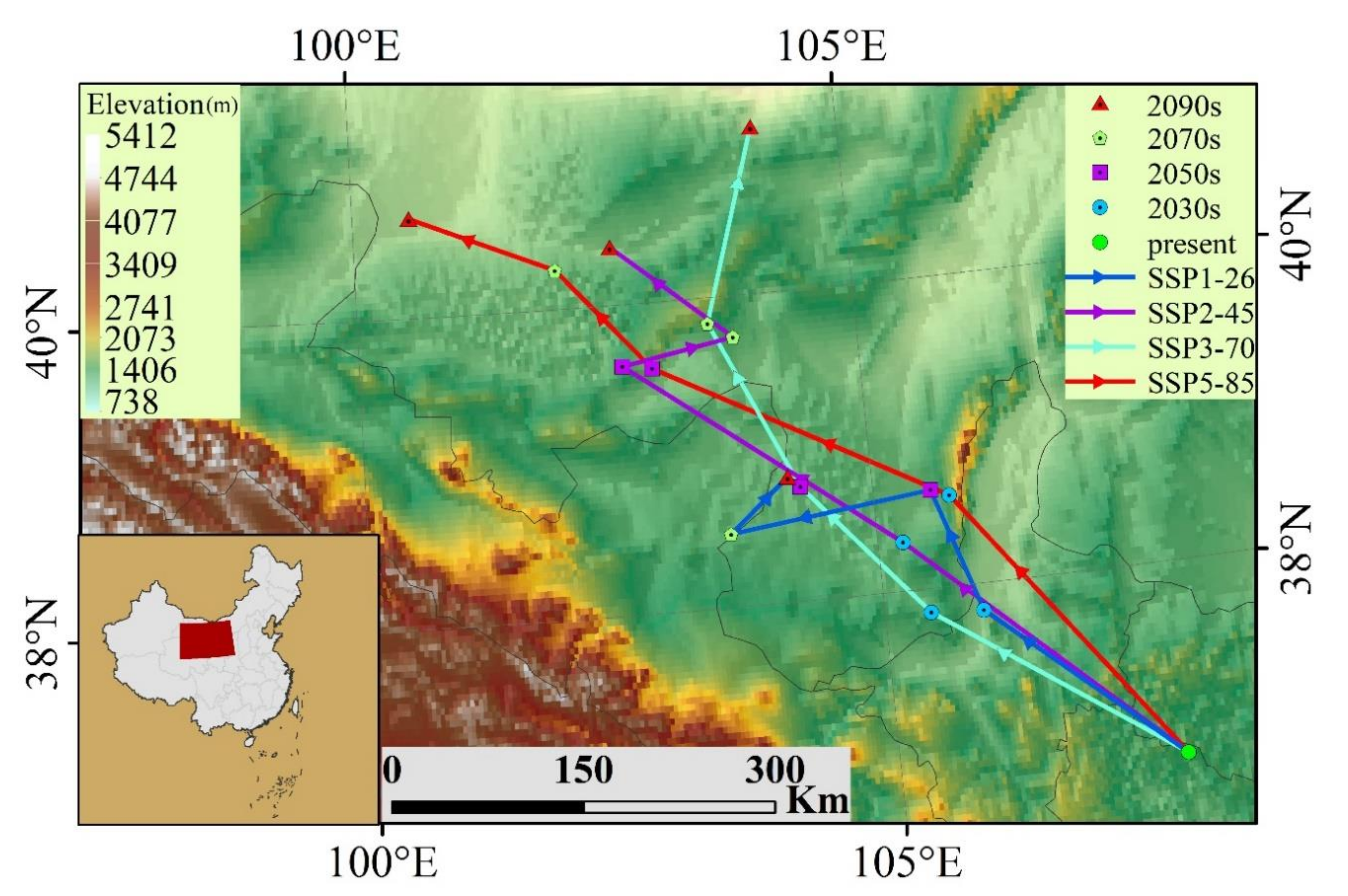
3.1. The Projected Distribution of V. mali
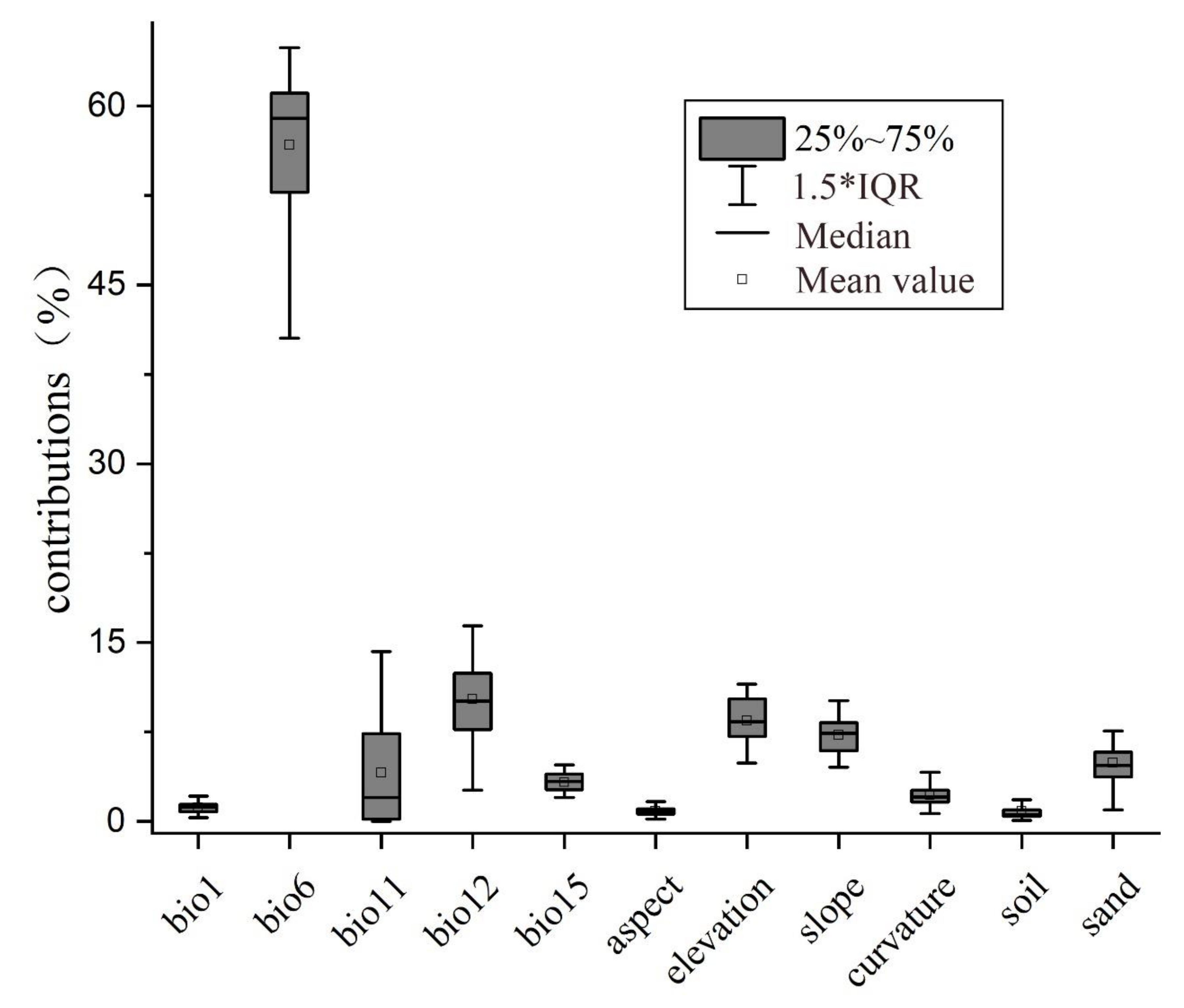
3.2. Contributions of Environmental Factors to the Distribution of V. mali
4. Discussion
4.1. Changes on the Suitable Habitat Area of V. mali
4.2. The Role of Environmental Variables in Effecting the Distribution of V. mali and Corresponding Strategies for Preventing AVC
4.3. Limitations of SDMs in Projecting the Distribution of Species
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Occurrence Point | Longitude | Latitude | Occurrence Point | Longitude | Latitude | Occurrence Point | Longitude | Latitude |
|---|---|---|---|---|---|---|---|---|
| Valsa mali | 105.2459 | 34.85065 | Valsa mali | 108.2079 | 34.33978 | Valsa mali | 105.8128 | 34.68276 |
| Valsa mali | 105.2061 | 34.59958 | Valsa mali | 108.0673 | 34.2826 | Valsa mali | 105.9914 | 34.63819 |
| Valsa mali | 105.3594 | 34.7649 | Valsa mali | 108.2037 | 34.10844 | Valsa mali | 105.9015 | 34.64976 |
| Valsa mali | 107.2143 | 35.48161 | Valsa mali | 109.294 | 34.48988 | Valsa mali | 111.1127 | 34.41605 |
| Valsa mali | 106.93 | 35.55836 | Valsa mali | 108.1357 | 34.69016 | Valsa mali | 110.9476 | 34.42388 |
| Valsa mali | 107.6031 | 35.29842 | Valsa mali | 110.169 | 36.05018 | Valsa mali | 110.9404 | 34.53704 |
| Valsa mali | 105.769 | 35.31032 | Valsa mali | 132.0207 | 46.44701 | Valsa mali | 110.8449 | 34.52789 |
| Valsa mali | 105.729 | 35.25686 | Valsa mali | 120.7195 | 36.97991 | Valsa mali | 111.6608 | 34.38018 |
| Valsa mali | 105.7741 | 35.16865 | Valsa mali | 120.8476 | 37.33181 | Valsa mali | 111.2493 | 34.33246 |
| Valsa mali | 105.7183 | 35.08535 | Valsa mali | 120.4855 | 37.63883 | Valsa mali | 112.7443 | 34.94146 |
| Valsa mali | 105.7232 | 35.46861 | Valsa mali | 120.7312 | 37.92108 | Valsa mali | 114.7795 | 35.63555 |
| Valsa mali | 105.7219 | 35.21247 | Valsa mali | 120.7951 | 37.79816 | Valsa mali | 113.7061 | 34.71211 |
| Valsa mali | 106.9138 | 35.48206 | Valsa mali | 121.4352 | 37.50221 | Valsa mali | 115.1119 | 31.77896 |
| Valsa mali | 107.0432 | 35.41133 | Valsa mali | 122.1074 | 37.50032 | Valsa mali | 114.5726 | 34.46878 |
| Valsa mali | 105.4765 | 34.26437 | Valsa mali | 120.4145 | 37.34365 | Valsa mali | 115.1885 | 34.63789 |
| Valsa mali | 105.3243 | 34.19708 | Valsa mali | 80.25808 | 41.22645 | Valsa mali | 110.6651 | 35.34699 |
| Valsa mali | 105.4158 | 34.24378 | Valsa mali | 80.30529 | 41.32977 | Valsa mali | 112.2942 | 32.938 |
| Valsa mali | 105.9995 | 34.44763 | Valsa mali | 80.27194 | 41.15483 | Valsa mali | 110.7377 | 35.20919 |
| Valsa mali | 105.8874 | 34.47668 | Valsa mali | 115.1034 | 40.60481 | Valsa mali | 110.5999 | 35.24551 |
| Valsa mali | 105.8586 | 34.55483 | Valsa mali | 115.5246 | 40.41747 | Valsa mali | 110.6806 | 34.66079 |
| Valsa mali | 105.7872 | 34.50759 | Valsa mali | 115.2086 | 40.37381 | Valsa mali | 110.9314 | 34.78846 |
| Valsa mali | 107.9775 | 35.65879 | Valsa mali | 114.5499 | 38.42962 | Valsa mali | 111.0413 | 35.19894 |
| Valsa mali | 107.8532 | 35.37529 | Valsa mali | 115.2296 | 37.92998 | Valsa mali | 111.2458 | 34.87806 |
| Valsa mali | 107.8967 | 35.61616 | Valsa mali | 114.5627 | 37.04473 | Valsa mali | 111.2006 | 34.9765 |
| Valsa mali | 108.2307 | 35.76626 | Valsa mali | 117.0065 | 39.75833 | Valsa mali | 119.9172 | 36.86932 |
| Valsa mali | 106.4282 | 34.92928 | Valsa mali | 116.7915 | 40.01876 | Valsa mali | 120.996 | 37.08069 |
| Valsa mali | 105.8358 | 35.04372 | Valsa mali | 116.2845 | 37.69512 | Valsa mali | 120.7892 | 37.19028 |
| Valsa mali | 107.8059 | 35.82949 | Valsa mali | 118.7029 | 39.73591 | Valsa mali | 120.7011 | 37.18603 |
| Valsa mali | 107.8288 | 35.78574 | Valsa mali | 115.8298 | 38.42281 | Valsa mali | 120.5786 | 37.11801 |
| Valsa mali | 107.7535 | 35.89863 | Valsa mali | 115.1384 | 38.83805 | Valsa mali | 81.89624 | 41.79683 |
| Valsa mali | 107.8337 | 35.65728 | Valsa mali | 115.5052 | 38.77561 | Valsa mali | 75.86719 | 39.37309 |
| Valsa mali | 107.5964 | 35.69838 | Valsa mali | 111.0549 | 34.05162 | Valsa mali | 77.13329 | 38.36349 |
| Valsa mali | 107.7583 | 35.74343 | Valsa mali | 110.892 | 34.5089 | Valsa mali | 81.8431 | 43.72794 |
| Valsa mali | 107.4724 | 35.55178 | Valsa mali | 111.0961 | 34.71984 | Valsa mali | 79.92551 | 37.11503 |
| Valsa mali | 107.3565 | 35.58652 | Valsa mali | 111.189 | 34.77284 | Valsa mali | 82.99566 | 46.75049 |
| Valsa mali | 105.8828 | 35.09036 | Valsa mali | 102.3337 | 35.88342 | Valsa mali | 87.60832 | 43.80811 |
| Valsa mali | 106.1066 | 35.19679 | Valsa mali | 101.7788 | 36.60275 | Valsa mali | 88.14282 | 47.84309 |
| Valsa mali | 109.6404 | 35.50803 | Valsa mali | 102.832 | 36.35028 | Valsa mali | 89.2124 | 42.96021 |
| Valsa mali | 109.6078 | 35.58897 | Valsa mali | 102.9515 | 36.28235 | Valsa mali | 93.52182 | 42.8085 |
| Valsa mali | 109.591 | 35.70494 | Valsa mali | 102.0257 | 35.94011 | Valsa mali | 103.646 | 27.45558 |
| Valsa mali | 109.4409 | 35.77218 | Valsa mali | 102.3207 | 36.47386 | Valsa mali | 103.5911 | 27.37106 |
| Valsa mali | 109.5688 | 35.85828 | Valsa mali | 101.8536 | 36.58037 | Valsa mali | 103.6441 | 27.35729 |
| Valsa mali | 108.4528 | 34.64583 | Valsa mali | 102.4791 | 35.84351 | Valsa mali | 121.3041 | 38.90109 |
| Valsa mali | 108.6023 | 34.43616 | Valsa mali | 101.4475 | 36.05149 | Valsa mali | 121.8041 | 39.26661 |
| Valsa mali | 107.2692 | 36.69266 | Valsa mali | 105.9089 | 34.53902 | Valsa mali | 121.9638 | 39.39049 |
| Valsa mali | 109.2573 | 35.56844 | Valsa mali | 105.5558 | 34.56248 | Valsa mali | 122.065 | 39.78451 |
| Valsa mali | 109.0599 | 35.61743 | Valsa mali | 105.5009 | 34.5597 | Valsa mali | 122.1585 | 39.94054 |
| Valsa mali | 107.7961 | 35.20038 | Valsa mali | 105.644 | 34.60327 | Valsa mali | 119.9495 | 40.09864 |
| Valsa mali | 108.3657 | 34.53922 | Valsa mali | 105.7104 | 34.47127 | Valsa mali | 119.8566 | 40.14749 |
| Valsa mali | 120.7382 | 40.61335 | Valsa mali | 120.949 | 40.85481 | Valsa mali | 120.2928 | 40.31371 |
| Valsa mali | 120.4404 | 40.49809 | Valsa mali | 120.8195 | 40.72228 |
| Variable | Percent of Eigenvalues (%) | Accumulative of Eigenvalues (%) |
|---|---|---|
| bio1 | 81.90 | 81.90 |
| bio12 | 17.63 | 99.53 |
| bio6 | 0.37 | 99.91 |
| bio16 | 0.03 | 99.94 |
| bio7 | 0.03 | 99.97 |
| bio18 | 0.01 | 99.98 |
| bio19 | 0.01 | 99.99 |
| bio17 | 0.00 | 99.99 |
| bio15 | 0.00 | 99.99 |
| bio14 | 0.00 | 99.99 |
| bio13 | 0.00 | 99.99 |
| bio11 | 0.00 | 99.99 |
| bio10 | 0.00 | 99.99 |
| bio9 | 0.00 | 99.99 |
| bio8 | 0.00 | 99.99 |
| bio5 | 0.00 | 99.99 |
| bio4 | 0.00 | 99.99 |
| bio3 | 0.00 | 99.99 |
| bio2 | 0.00 | 100.00 |
| Soil | Slop | Aspect | Curvature | Sand | Silt | Clay | |
|---|---|---|---|---|---|---|---|
| soil | 1 | ||||||
| slop | −0.01208 | 1 | |||||
| aspect | −0.00173 | 0.01011 | 1 | ||||
| curvature | −0.07395 | −0.0869 | −0.07472 | 1 | |||
| sand | −0.00915 | 0.00656 | −0.00549 | 0.08697 | 1 | ||
| silt | −0.07583 | −0.00715 | 0.0032 | −0.09976 | −0.88074 | 1 | |
| clay | 0.08828 | −0.00493 | 0.00646 | −0.05828 | −0.84 | 0.56778 | 1 |
References
- Qu, Z.; Zhou, G. Possible Impact of Climate Change on the Quality of Apples from the Major Producing Areas of China. Atmosphere 2016, 7, 113. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zang, R.; Huang, L.L.; Xie, F.Q.; Gao, X.N. The investigation of apple tree Valsa canker in Guanzhong region of Shaanxi Province. J. Northwest Sci Tech Univ. Agric. For. (Nat. Sci. Ed.) 2005, 33, 98–100. [Google Scholar]
- Peng, H.X.; Wei, X.Y.; Xiao, Y.X.; Sun, Y.; Biggs, A.R.; Gleason, M.L.; Shang, S.P.; Zhu, M.Q.; Guo, Y.Z.; Sun, G.Y. Management of Valsa Canker on Apple with Adjustments to Potassium Nutrition. Plant Dis. 2016, 100, 884–889. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Li, B.-H.; Dong, X.-L.; Wang, C.-X.; Lian, S.; Liang, W.-X. Effects of Temperature, Humidity, and Wound Age on Valsa mali Infection of Apple Shoot Pruning Wounds. Plant Dis. 2016, 100, 2394–2401. [Google Scholar] [CrossRef] [Green Version]
- Bessho, H.; Tsuchiya, S.; Soejima, J. Screening methode of apple–trees for resistance to Valsa canker. Euphytica 1994, 77, 15–18. [Google Scholar] [CrossRef]
- Yin, Z.; Ke, X.; Kang, Z.; Huang, L. Apple resistance responses against Valsa mali revealed by transcriptomics analyses. Physiol. Mol. Plant Pathol. 2016, 93, 85–92. [Google Scholar] [CrossRef]
- Wang, C.; Guan, X.; Wang, H.; Li, G.; Dong, X.; Wang, G.; Li, B. Agrobacterium tumefaciens–Mediated Transformation of Valsa mali: An Efficient Tool for Random Insertion Mutagenesis. Sci. World J. 2013. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zang, R.; Yin, Z.; Kang, Z.; Huang, L. Delimiting cryptic pathogen species causing apple Valsa canker with multilocus data. Ecol. Evol. 2014, 4, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Nabi, S.U.; Raja, W.H.; Mir, J.I.; Sharma, O.C.; Singh, D.B.; Sheikh, M.A.; Yousuf, N.; Kamil, D. First Report Ofdiplodia Bulgarica a New Species Causing Canker Disease of Apple (Malus Domestica Borkh) in India. J. Plant Pathol. 2020, 102, 555–556. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, X.; Bozorov, T.A.; Ma, R.; Ma, J.; Zhang, Y.; Yang, H.; Li, L.; Zhang, D. Characterization and Pathogenicity of Six Cytospora Strains Causing Stem Canker of Wild Apple in the Tianshan Forest, China. For. Pathol. 2020. [Google Scholar] [CrossRef]
- Wang, S.T.; Hu, T.L.; Wang, Y.A.; Luo, Y.; Michailides, T.J.; Cao, K.Q. New understanding on infection processes of Valsa canker of apple in China. Eur. J. Plant Pathol. 2016, 146, 531–540. [Google Scholar] [CrossRef]
- Chen, C.; Liu, F.; Xing, Z.; Zham, X.; Shi, X.; Guo, J.; Chen, Y. On the resistance of apple trees to the invasion by Valsa mali Miyabe et Yamada: Observations on the relationship between wound healing capacity of apple bark and the leave of resistance. Acta Phytopathol. Sin. 1982, 12, 49–55. [Google Scholar]
- Ke, X.W.; Huang, L.L.; Han, Q.M.; Gao, X.N.; Kang, Z.S. Histological and cytological investigations of the infection and colonization of apple bark by Valsa mali var. mali. Australas. Plant Pathol. 2013, 42, 85–93. [Google Scholar] [CrossRef]
- Yu, C.; Li, T.; Shi, X.; Saleem, M.; Li, B.; Liang, W.; Wang, C. Deletion of Endo–beta–1,4–Xylanase VmXyl1 Impacts the Virulence of Valsa mali in Apple Tree. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, S.; Du, Y.; Hou, X.; Yan, N.; Dong, W.; Mao, X.; Zhang, Z. Chemical Basis of the Fungicidal Activity of Tobacco Extracts against Valsa mali. Molecules 2016, 21, 1743. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Dai, Q.; Liu, Y.; Yang, Z.; Song, N.; Gao, X.; Voegele, R.T.; Kang, Z.; Huang, L. Agrobacterium tumefaciens–Mediated Transformation of the Causative Agent of Valsa canker of Apple Tree Valsa mali var. mali. Curr. Microbiol. 2014, 68, 769–776. [Google Scholar] [CrossRef]
- Ma, R.; Liu, Y.M.; Yin, Y.X.; Tian, C.M. A canker disease of apple caused by Cytospora parasitica recorded in China. For. Pathol. 2018, 48. [Google Scholar] [CrossRef]
- Liu, C.; Fan, D.; Li, Y.; Chen, Y.; Huang, L.; Yan, X. Transcriptome analysis of Valsa mali reveals its response mechanism to the biocontrol actinomycete Saccharothrix yanglingensis Hhs.015. BMC Microbiol. 2018, 18, 90. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Ke, X.; Li, Z.; Chen, J.; Gao, X.; Huang, L. Unconventional Recombination in the Mating Type Locus of Heterothallic Apple Canker Pathogen Valsa mali. G3–Genes Genomes Genet. 2017, 7, 1259–1265. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Xu, L.; Liu, J.; Yin, Z.; Gao, X.; Feng, H.; Huang, L. A mitogen–activated protein kinase gene (VmPmk1) regulates virulence and cell wall degrading enzyme expression in Valsa mall. Microb. Pathog. 2017, 111, 298–306. [Google Scholar] [CrossRef]
- Wang, N.N.; Yan, X.; Gao, X.N.; Niu, H.J.; Kang, Z.S.; Huang, L.L. Purification and characterization of a potential antifungal protein from Bacillus subtilis E1R–J against Valsa mali. World J. Microbiol. Biotechnol. 2016, 32, 63. [Google Scholar] [CrossRef]
- Nouri, M.T.; Lawrence, D.P.; Holland, L.A.; Doll, D.A.; Kallsen, C.E.; Culumber, C.M.; Trouillas, F.P. Identification and Pathogenicity of Fungal Species Associated with Canker Diseases of Pistachio in California. Plant Dis. 2019, 103, 2397–2411. [Google Scholar] [CrossRef] [PubMed]
- Kamiri, L.K.; Laemmlen, F.F. Epidemiology of cytospora canker caused in Colorado blue spruce by Valsa kunzei [Picea pungens]. Phytopathology 1981, 71, 941–947. [Google Scholar] [CrossRef]
- Abolmaali, S.M.-R.; Tarkesh, M.; Bashari, H. MaxEnt modeling for predicting suitable habitats and identifying the effects of climate change on a threatened species, Daphne mucronata, in central Iran. Ecol. Inform. 2018, 43, 116–123. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Halvorsen, R.; Mazzoni, S.; Dirksen, J.W.; Næsset, E.; Gobakken, T.; Ohlson, M. How important are choice of model selection method and spatial autocorrelation of presence data for distribution modelling by MaxEnt? Ecol. Model. 2016, 328, 108–118. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence–only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Narouei-Khandan, H.A.; Worner, S.P.; Viljanen, S.L.H.; van Bruggen, A.H.C.; Jones, E.E. Projecting the suitability of global and local habitats for myrtle rust (Austropuccinia psidii) using model consensus. Plant Pathol. 2020, 69, 17–27. [Google Scholar] [CrossRef]
- Narouei-Khandan, H.A. Ensemble Models to Assess the Risk of Exotic Plant Pathogens in a Changing Climate, in Lincoln; Lincoln University: Lincoln, New Zealand, 2014; pp. 220–221. [Google Scholar]
- Arteaga, V.L.; Lima, C.H.R. Analysis of CMIP 5 simulations of key climate indices associated with the South America monsoon system. Int. J. Climatol. 2020, 19. [Google Scholar] [CrossRef]
- Pascoe, C.; Lawrence, B.N.; Guilyardi, E.; Juckes, M.; Taylor, K.E. Documenting numerical experiments in support of the Coupled Model Intercomparison Project Phase 6 (CMIP6). Geosci. Model Dev. 2020, 13, 2149–2167. [Google Scholar] [CrossRef]
- Papes, M.; Gaubert, P. Modelling ecological niches from low numbers of occurrences: Assessment of the conservation status of poorly known viverrids (Mammalia, Carnivora) across two continents. Divers. Distrib. 2007, 13, 890–902. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Yue, J.; Xie, T.; Xu, Y.; Tian, Y. Predicting Shifts in the Suitable Climatic Distribution of Walnut (Juglans regia L.) in China: Maximum Entropy Model Paves the Way to Forest Management. Forests 2018, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.Y.; Wang, T.J.; Groen, T.A.; Skidmore, A.K.; Yang, X.F.; Ma, K.P.; Wu, Z.F. Climate and land use changes will degrade the distribution of Rhododendrons in China. Sci. Total Environ. 2019, 659, 515–528. [Google Scholar] [CrossRef]
- Guo, Y.L.; Li, X.; Zhao, Z.F.; Nawaz, Z. Predicting the impacts of climate change, soils and vegetation types on the geographic distribution of Polyporus umbellatus in China. Sci. Total Environ. 2019, 648, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhou, G. Climatic Suitability of the Geographic Distribution of Stipa breviflora in Chinese Temperate Grassland under Climate Change. Sustainability 2018, 10, 3767. [Google Scholar] [CrossRef] [Green Version]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Bracegirdle, T.J.; Krinner, G.; Tonelli, M.; Haumann, F.A.; Naughten, K.A.; Rackow, T.; Roach, L.A.; Wainer, I. Twenty first century changes in Antarctic and Southern Ocean surface climate in CMIP6. Atmos. Sci. Lett. 2020, 14, e984. [Google Scholar] [CrossRef]
- Overpeck, J.T.; Rind, D.; Goldberg, R.J.N. Climate–induced changes in forest disturbance and vegetation. Nature 1990, 343, 51–53. [Google Scholar] [CrossRef]
- Cornelissen, B.; Neumann, P.; Schweiger, O. Global warming promotes biological invasion of a honey bee pest. Glob. Chang. Biol. 2019, 25, 3642–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Gegout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A Significant Upward Shift in Plant Species Optimum Elevation During the 20th Century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, X.; Shrestha, N.; Zimmermann, N.E.; Wang, Z. Response of spatial vegetation distribution in China to climate changes since the Last Glacial Maximum (LGM). PLoS ONE 2017, 12, e0175742. [Google Scholar] [CrossRef] [Green Version]
- Perry, C.T.; Alvarez-Filip, L. Changing geo–ecological functions of coral reefs in the Anthropocene. Funct. Ecol. 2019, 33, 976–988. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Sun, J. Predicting the distribution of Stipa purpurea across the Tibetan Plateau via the MaxEnt model. BMC Ecol. 2018, 18, 10. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.K.; Rana, H.K.; Ghimire, S.K.; Shrestha, K.K.; Ranjitkar, S. Predicting the impact of climate change on the distribution of two threatened Himalayan medicinal plants of Liliaceae in Nepal. J. Mt. Sci. 2017, 14, 558–570. [Google Scholar] [CrossRef]
- Porta, N.L.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; Weissenberg, K.V. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, Y.M.; Salama, M.S.; Su, Z.B. Assessment of vegetation dynamics and their response to variations in precipitation and temperature in the Tibetan Plateau. Clim. Chang. 2010, 103, 519–535. [Google Scholar] [CrossRef]
- Na, L.; Wang, G.; Yan, Y.; Gao, Y.; Liu, G. Plant production, and carbon and nitrogen source pools, are strongly intensified by experimental warming in alpine ecosystems in the Qinghai–Tibet Plateau. Soil Biol. Biochem. 2011, 43, 942–953. [Google Scholar] [CrossRef]
- Guo, L.; Dai, J.H.; Wang, M.C.; Xu, J.C.; Luedeling, E. Responses of spring phenology in temperate zone trees to climate warming: A case study of apricot flowering in China. Agric. For. Meteorol. 2015, 201, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Chen, L.; Tang, W.; Peng, S.; Li, M.; Deng, N.; Chen, Y. Predicting Potential Distribution and Evaluating Suitable Soil Condition of Oil Tea Camellia in China. Forests 2018, 9, 487. [Google Scholar] [CrossRef] [Green Version]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1–km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, H.; Zhao, Z.; Liu, J.; Ran, Q.; Yu, J.; Gu, W. Optimization of the Fuzzy Matter Element Method for Predicting Species Suitability Distribution Based on Environmental Data. Sustainability 2018, 10, 3444. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, I.P.; Ormerod, S.J. The Continuing Challenges of Testing Species Distribution Models. J. Appl. Ecol. 2005, 42, 720–730. [Google Scholar] [CrossRef]
- Touré, D.; Ge, J. The Response of Plant Species Diversity to the Interrelationships between Soil and Environmental Factors in the Limestone Forests of Southwest China. J. Environ. Earth Sci. 2014, 4, 105–122. [Google Scholar]
- Riahi, K.; van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Chang. Hum. Policy Dimens. 2017, 42, 153–168. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, B.C.; Tebaldi, C.; van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.F.; Lowe, J.; et al. The Scenario Model Intercomparison Project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef] [Green Version]
- Vuuren, D.P.V.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.F. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Xin, X.G.; Zhang, L.; Zhang, J.; Wu, T.W.; Fang, Y.J. Climate Change Projections over East Asia with BCC_CSM1.1 Climate Model under RCP Scenarios. J. Meteorol. Soc. Jpn. 2013, 91, 413–429. [Google Scholar] [CrossRef] [Green Version]
- Dudik, M. Maximum Entropy Density Estimation and Modeling Geographic Distributions of Species; Princeton University: Princeton, NJ, USA, 2007. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudik, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Khanum, R.; Mumtaz, A.S.; Kumar, S. Predicting impacts of climate change on medicinal asclepiads of Pakistan using Maxent modeling. Acta Oecologica Int. J. Ecol. 2013, 49, 23–31. [Google Scholar] [CrossRef]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Garcia, K.; Lasco, R.; Ines, A.; Lyon, B.; Pulhin, F. Predicting geographic distribution and habitat suitability due to climate change of selected threatened forest tree species in the. Philippines. Appl. Geogr. 2013, 44, 12–22. [Google Scholar] [CrossRef]
- Guisan, A.; Graham, C.H.; Elith, J.; Huettmann, F.; Distri, N.S. Sensitivity of predictive species distribution models to change in grain size. Divers. Distrib. 2007, 13, 332–340. [Google Scholar] [CrossRef]
- Lv, W.; Li, Z.; Wu, X.; Ni, W.; Qv, W. Maximum Entropy Niche–Based Modeling (Maxent) of Potential Geographical Distributions of Lobesia Botrana (Lepidoptera: Tortricidae) in China; Computer and Computing Technologies in Agriculture; Springer: Berlin/Heidelberg, Germany, 2011; pp. 239–246. [Google Scholar]
- Hu, X.; Wu, F.; Guo, W.; Liu, N. Identification of Potential Cultivation Region fie Santalum album in China by the MaxEnt Ecologic Niche Model. Sci. Silvae Sin. 2014, 50, 27–33. [Google Scholar]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence–only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.L. SDMtoolbox: A python–based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Lenoir, J.; Svenning, J.C. Climate–related range shifts—A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Liu, S.H.; Li, J.G.; Sun, L.; Wang, G.H.; Tang, D.L.; Huang, P.; Yan, H.; Gao, S.; Liu, C.; Gao, Z.Q.; et al. Basin–wide responses of the South China Sea environment to Super Ty–phoon Mangkhut (2018). Sci. Total Environ. 2020, 731, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nielsen, S.E.; Chen, Y.H.; Georges, D.; Qin, Y.C.; Wang, S.S.; Svenning, J.C.; Thuiller, W. Extinction risk of North American seed plants elevated by climate and land–use change. J. Appl. Ecol. 2017, 54, 303–312. [Google Scholar] [CrossRef]
- Li, Z.; Liu, G.; Zhang, X. Review of reaearches on the Valsa canker of apple trees. North Fruits 2013, 4, 1–3. [Google Scholar]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate change effects on plant disease: Genomes to ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef] [Green Version]
- Xiang-Long, M.; Xing-Hua, Q.; Ze-Yuan, H.; Yong-Bin, G.; Ya-Nan, W.; Tong-le, H.; Li-Ming, W.; Ke-Qiang, C.; Shu-Tong, W. Latent Infection of Valsa mali in the Seeds, Seedlings and Twigs of Crabapple and Apple Trees is a Potential Inoculum Source of Valsa Canker. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Suo, G.-D.; Xie, Y.-S.; Zhang, Y.; Cai, M.-Y.; Wang, X.-S.; Chuai, J.-F. Crop load management (CLM) for sustainable apple production in China. Sci. Hortic. 2016, 211, 213–219. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]


| Category | Variable | Description | Unit |
|---|---|---|---|
| Bioclimate | bio1 | Annual Mean Temperature | °C |
| bio6 | Min Temperature of Coldest Month | °C | |
| bio11 | Mean Temperature of Coldest Quarter | °C | |
| bio12 | Annual Precipitation | mm | |
| bio15 | Precipitation Seasonality | ||
| Topographic | aspect | Aspect | |
| curvature | Curvature | ||
| elevation | Elevation | m | |
| slope | Slope | ° | |
| Soil | sand | Texture of Soil | |
| soil | Type of Soil |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Sun, H.; Jin, J.; Cheng, J. Predicting the Potential Distribution of Apple Canker Pathogen (Valsa mali) in China under Climate Change. Forests 2020, 11, 1126. https://doi.org/10.3390/f11111126
Xu W, Sun H, Jin J, Cheng J. Predicting the Potential Distribution of Apple Canker Pathogen (Valsa mali) in China under Climate Change. Forests. 2020; 11(11):1126. https://doi.org/10.3390/f11111126
Chicago/Turabian StyleXu, Wei, Hongyun Sun, Jingwei Jin, and Jimin Cheng. 2020. "Predicting the Potential Distribution of Apple Canker Pathogen (Valsa mali) in China under Climate Change" Forests 11, no. 11: 1126. https://doi.org/10.3390/f11111126
APA StyleXu, W., Sun, H., Jin, J., & Cheng, J. (2020). Predicting the Potential Distribution of Apple Canker Pathogen (Valsa mali) in China under Climate Change. Forests, 11(11), 1126. https://doi.org/10.3390/f11111126





